Application of Micro/Nanomotors in Environmental Remediation: A Review
Abstract
1. Introduction
2. From Materials to MNMs
2.1. Propulsion Mechanism
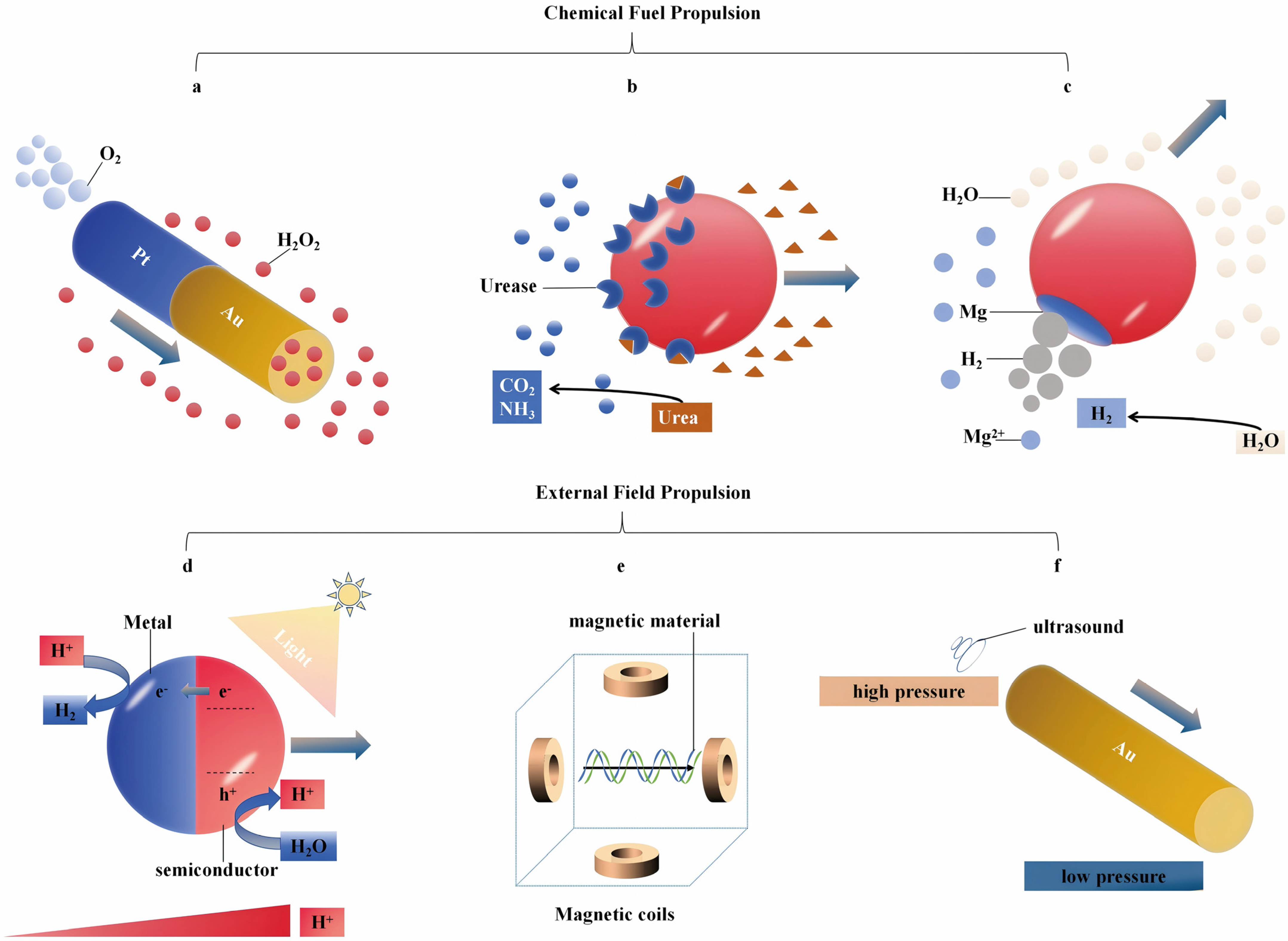
2.1.1. Fuel-Driven MNMs
2.1.2. Externally Driven MNMs
2.2. Motion Control
2.2.1. Speed Control
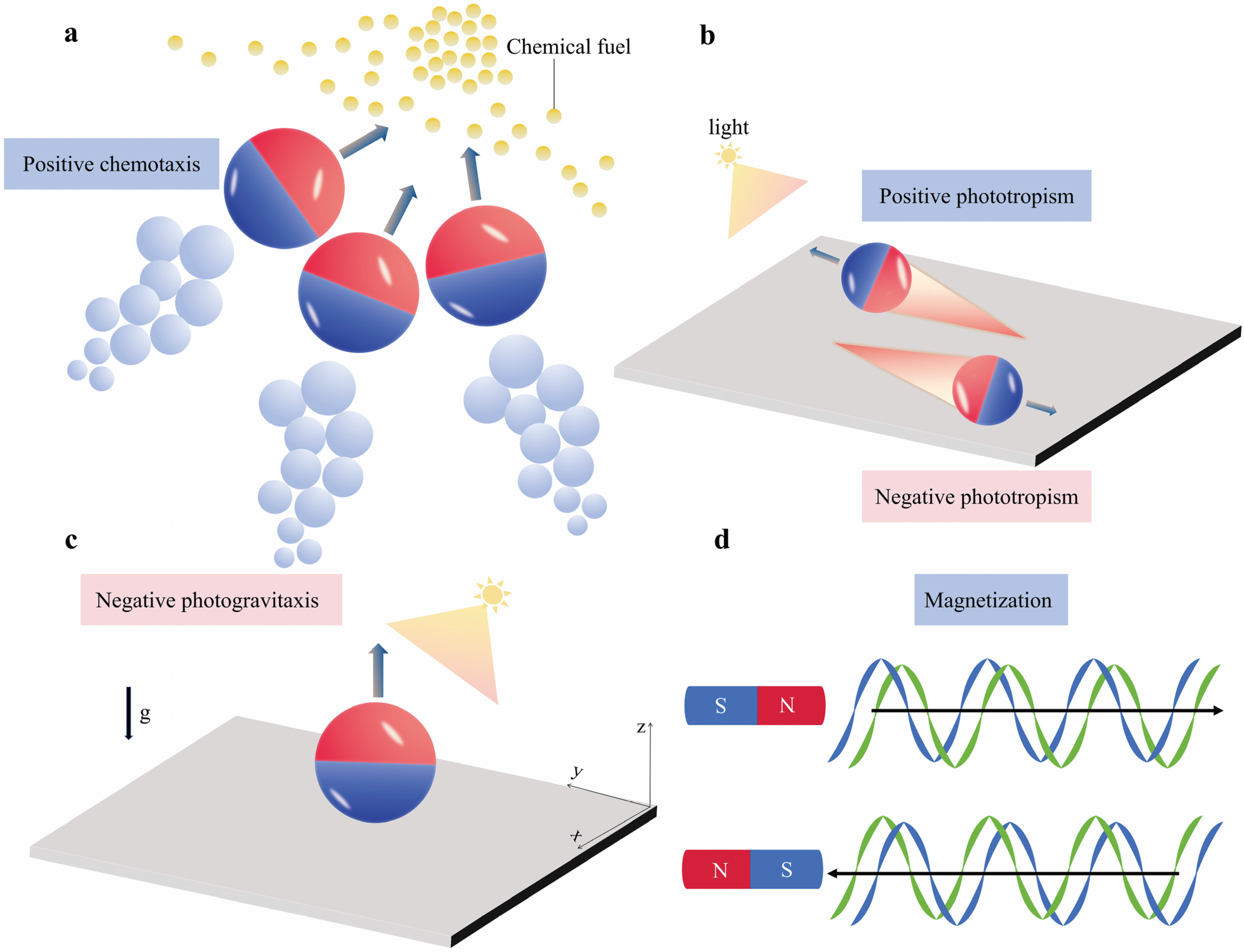
2.2.2. Directional Control
2.3. Multifunctionality
2.4. Swarm Behavior
3. Environmental Remediation
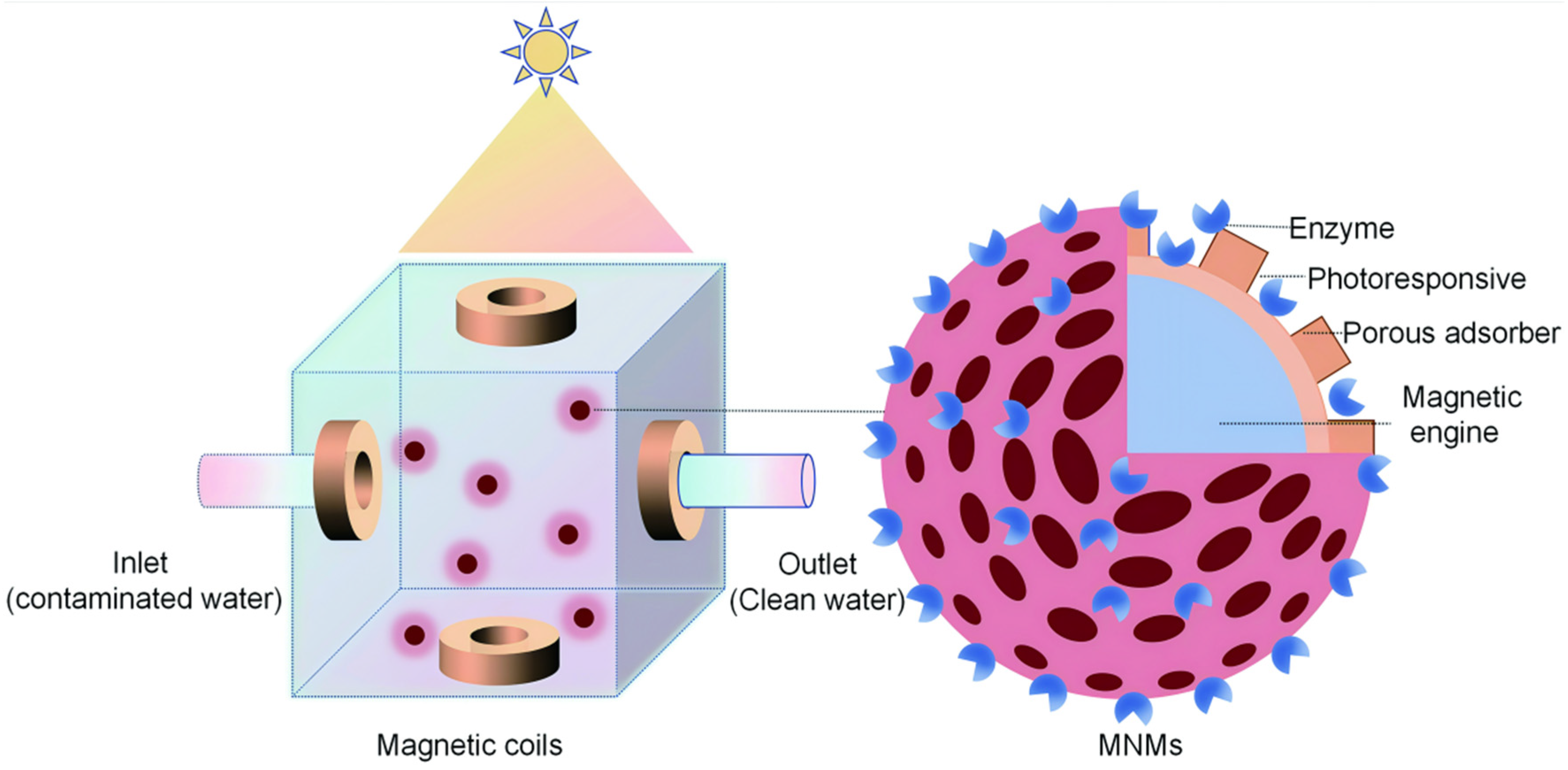
3.1. MNM-Based Environmental Remediation Systems
3.2. MNMs for Environmental Sensing and Monitoring

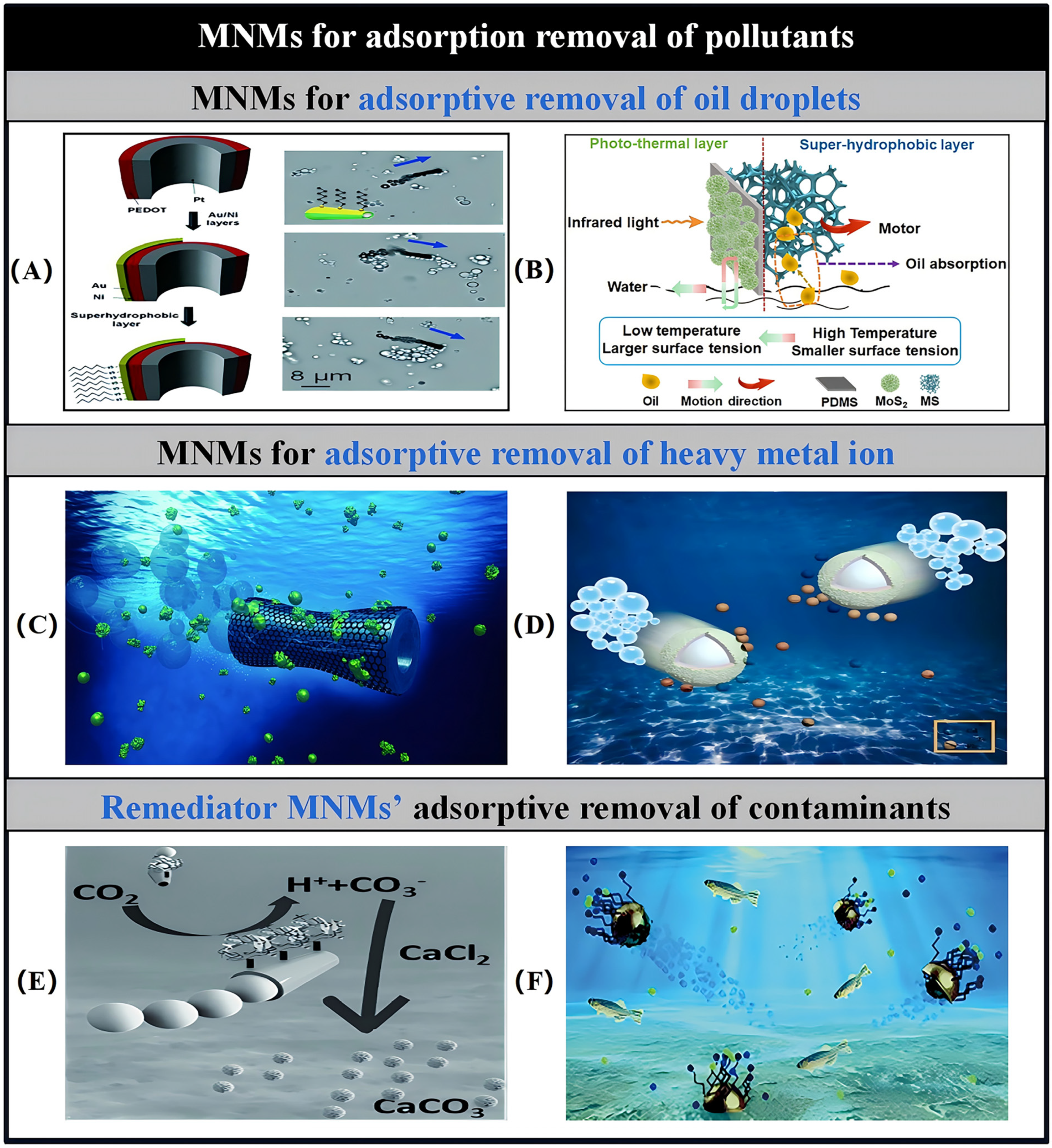
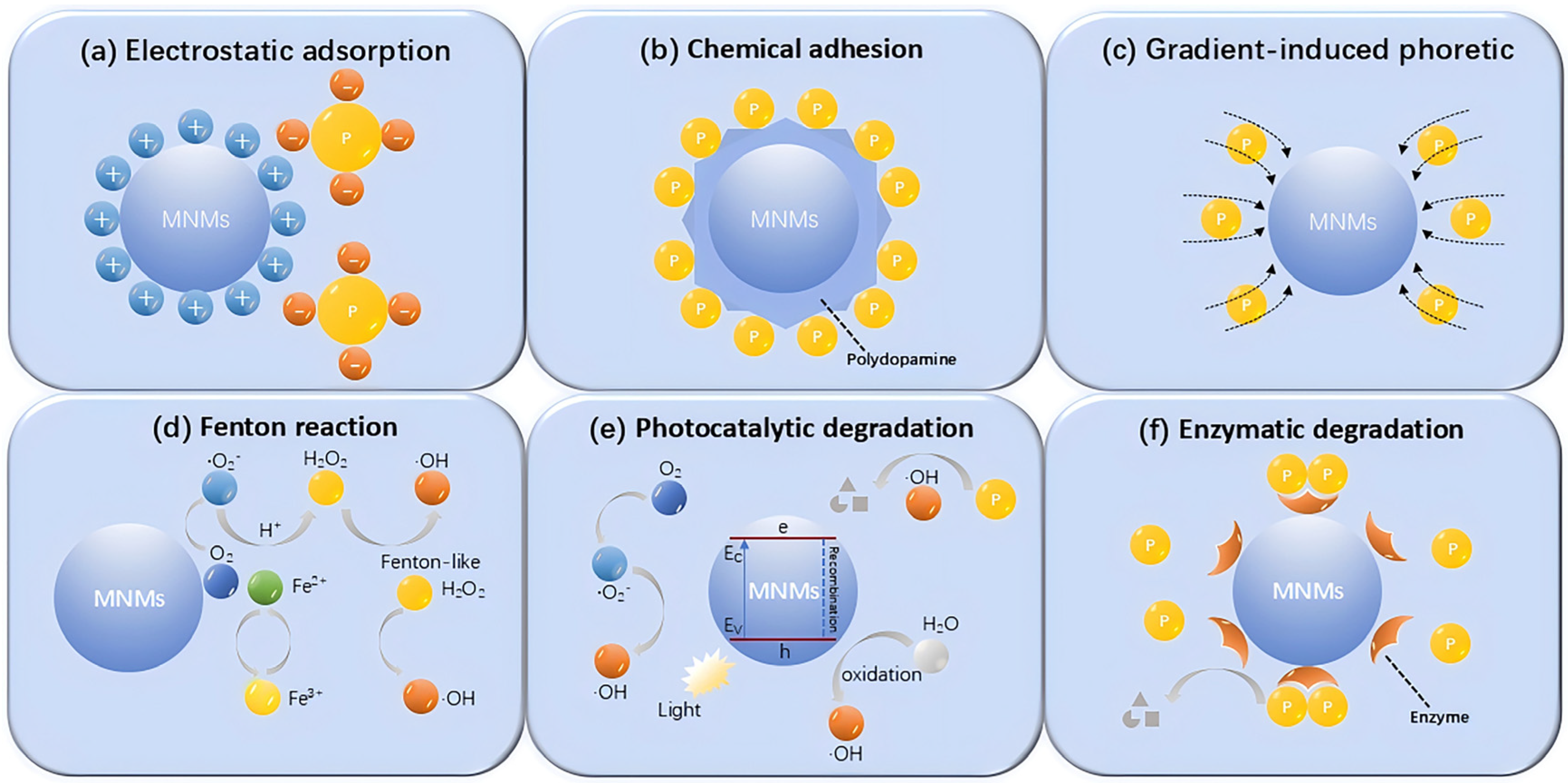
3.3. MNMs for Adsorption Removal of Pollutants
3.4. MNMs for Accelerated Pollutant Degradation
3.4.1. Self-Propelled MNMs in Advanced Oxidation Processes for Pollutant Degradation

3.4.2. Self-Propelled MNMs for Biocatalytic Pollutant Degradation
3.4.3. Chemical and Light-Driven MNMs for Photocatalytic Pollutant Degradation
3.5. Self-Propelled MNMs for Pathogenic Bacteria Removal
4. Limitations of MNMs in Environmental Remediation
5. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Tratnyek, P.G.; Johnson, R.L. Nanotechnologies for environmental cleanup. Nano Today 2006, 1, 44–48. [Google Scholar] [CrossRef]
- Ebbens, S.J.; Howse, J.R. In pursuit of propulsion at the nanoscale. Soft Matter 2010, 6, 726–738. [Google Scholar] [CrossRef]
- Mei, Y.; Solovev, A.A.; Sanchez, S.; Schmidt, O.G. Rolled-up nanotech on polymers: From basic perception to self-propelled catalytic microengines. Chem. Soc. Rev. 2011, 40, 2109–2119. [Google Scholar] [CrossRef] [PubMed]
- Ozin, G.A.; Manners, I.; Fournier-Bidoz, S.; Arsenault, A. Dream nanomachines. Adv. Mater. 2005, 17, 3011–3018. [Google Scholar] [CrossRef]
- Sanchez, S.; Pumera, M. Nanorobots: The ultimate wireless self-propelled sensing and actuating devices. Chem.-Asian J. 2009, 4, 1402–1410. [Google Scholar] [CrossRef]
- Sengupta, S.; Ibele, M.E.; Sen, A. Fantastic voyage: Designing self-powered nanorobots. Angew. Chem. Int. Ed. 2012, 51, 8434–8445. [Google Scholar] [CrossRef]
- Wang, J. Nanomachines: Fundamentals and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Wang, J.; Manesh, K.M. Motion control at the nanoscale. Small 2010, 6, 338–345. [Google Scholar] [CrossRef]
- Karshalev, E.; Esteban-Fernández de Ávila, B.; Wang, J. Micromotors for “Chemistry-on-the-Fly”. J. Am. Chem. Soc. 2018, 140, 3810–3820. [Google Scholar] [CrossRef]
- Orozco, J.; Jurado-Sánchez, B.; Wagner, G.; Gao, W.; Vazquez-Duhalt, R.; Sattayasamitsathit, S.; Galarnyk, M.; Cortés, A.; Saintillan, D.; Wang, J. Bubble-propelled micromotors for enhanced transport of passive tracers. Langmuir 2014, 30, 5082–5087. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Chiang, T.Y.; Velegol, D.; Mallouk, T.E. Understanding the efficiency of autonomous nano- and microscale motors. J. Am. Chem. Soc. 2013, 135, 10557–10565. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Seah, T.H.; Pumera, M. External-energy-independent polymer capsule motors and their cooperative behaviors. Chem.–Eur. J. 2011, 17, 12020–12026. [Google Scholar] [CrossRef]
- Ibele, M.; Mallouk, T.E.; Sen, A. Schooling behavior of light-powered autonomous micromotors in water. Angew. Chem. Int. Ed. 2009, 48, 3308–3312. [Google Scholar] [CrossRef]
- Tottori, S.; Zhang, L.; Qiu, F.; Krawczyk, K.K.; Franco-Obregón, A.; Nelson, B.J. Magnetic helical micromachines: Fabrication, controlled swimming, and cargo transport. Adv. Mater. 2012, 24, 811–816. [Google Scholar] [CrossRef]
- Gao, W.; Feng, X.; Pei, A.; Kane, C.R.; Tam, R.; Hennessy, C.; Wang, J. Bioinspired helical microswimmers based on vascular plants. Nano Lett. 2014, 14, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, M.; Mayorga-Martinez, C.C.; Escarpa, A.; Pumera, M. Micellar Polymer Magnetic Microrobots as Efficient Nerve Agent Microcleaners. ACS Appl. Mater. Interfaces 2022, 14, 26128–26134. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Wang, L.; Wang, J.; Wang, S.; Wang, Y.; Jin, D.; Chen, P.; Zhang, L.; Liu, B.-F. Graphene-Based Helical Micromotors Constructed by ‘‘Microscale Liquid Rope-Coil Effect’’ with Microfluidics. ACS Nano 2020, 14, 16600–16613. [Google Scholar] [CrossRef]
- Chen, X.; Chen, X.; Elsayed, M.; Edwards, H.; Liu, J.; Peng, Y.; Zhang, H.P.; Zhang, S.; Wang, W.; Wheeler, A.R. Steering Micromotors via Reprogrammable Optoelectronic Paths. ACS Nano 2023, 17, 5894–5904. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, R.; Zhou, D.; Chang, X.; Mo, Y.; Zhang, G.; Li, L. Alternating Current Electric Field Driven Topologically Defective Micro/nanomotors. Appl. Mater. Today 2022, 26, 101314. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, W.; Wang, Z.; Lyu, D.; Mu, Y.; Duan, W.; Wang, Y. Polyhedral Micromotors of Metal–Organic Frame-works: Symmetry Breaking and Propulsion. J. Am. Chem. Soc. 2021, 143, 19881–19892. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Li, F.; Li, T.; Zhang, W.; Li, B.; Liu, K.; Lun, X.; Guo, Y. Self-actuated biomimetic nanocomposites for photothermal therapy and PD-L1 immunosuppression. Front. Chem. 2023, 11, 1167586. [Google Scholar] [CrossRef]
- Safdar, M.; Simmchen, J.; Jänis, J. Light-Driven Micro- and Nanomotors for Environmental Remediation. Environ. Sci. Nano 2017, 4, 1602–1616. [Google Scholar] [CrossRef]
- Guo, Y.; Cao, X.; Zheng, X.; Abbas, S.K.J.; Li, J.; Tan, W. Construction of Nanocarriers Based on Nucleic Acids and Their Applications in Nanobiology Delivery Systems. Natl. Sci. Rev. 2022, 9, nwac006. [Google Scholar] [CrossRef]
- Lin, F.; Shao, Y.; Wu, Y.; Zhang, Y. NIR Light-Propelled Janus-Based Nanoplatform for Cytosolic-Fueled microRNA Imaging. ACS Appl. Mater. Interfaces 2021, 13, 3713–3721. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Wang, Y.; Pan, S.; Ma, E.; Jin, S.; Jiao, M.; Wang, W.; Li, J.; Xu, K.; Wang, H. Biocompatible Nanomotors as Active Diagnostic Imaging Agents for Enhanced Magnetic Resonance Imaging of Tumor Tissues In Vivo. Adv. Funct. Mater. 2021, 31, 2100936. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Z.; Lyu, Y.; Li, J.; Song, K.; Xing, N.; Ng, D.H. NIR Light-Powered Halloysite-Based Nanomotors for CT Imaging Diagnosis and Synergistic Chemo-Photothermal Cancer Therapy. J. Ind. Eng. Chem. 2022, 116, 180–190. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Q.; Tan, L.; Liu, C.; Shang, L. Metal–Organic Frameworks-Mediated Assembly of Gold Nanoclusters for Sensing Applications. J. Anal. Test. 2022, 6, 163–177. [Google Scholar] [CrossRef]
- Qian, B.; Montiel, D.; Bregulla, A.; Cichos, F.; Yang, H. Harnessing thermal fluctuations for purposeful activities: The manipulation of single micro-swimmers by adaptive photon nudging. Chem. Sci. 2013, 4, 1420–1429. [Google Scholar] [CrossRef]
- Dong, R.; Hu, Y.; Wu, Y.; Gao, W.; Ren, B.; Wang, Q.; Cai, Y. Visible-light-driven BiOI-based Janus micromotor in pure water. J. Am. Chem. Soc. 2017, 139, 1722–1725. [Google Scholar] [CrossRef] [PubMed]
- Jang, B.; Hong, A.; Kang, H.E.; Alcantara, C.; Charreyron, S.; Mushtaq, F.; Pellicer, E.; Büchel, R.; Sort, J.; Lee, S.S.; et al. Multiwavelength light-responsive Au/B-TiO2 Janus micromotors. ACS Nano 2017, 11, 6146–6154. [Google Scholar] [CrossRef]
- Zhang, Q.; Dong, R.; Wu, Y.; Gao, W.; He, Z.; Ren, B. Light-driven Au-WO3@C Janus micromotors for rapid photodegradation of dye pollutants. ACS Appl. Mater. Interfaces 2017, 9, 4674–4683. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Castro, L.A.; Hoyos, M.; Mallouk, T.E. Autonomous motion of metallic microrods propelled by ultrasound. ACS Nano 2012, 6, 6122–6132. [Google Scholar] [CrossRef]
- Garcia-Gradilla, V.; Orozco, J.; Sattayasamitsathit, S.; Soto, F.; Kuralay, F.; Pourazary, A.; Katzenberg, A.; Gao, W.; Shen, Y.; Wang, J. Ultrasound-propelled magnetically guided nanomotors: Toward practical biomedical applications. ACS Nano 2013, 7, 9232–9240. [Google Scholar] [CrossRef]
- Kiristi, M.; Singh, V.V.; de Ávila, B.E.-F.; Uygun, M.; Soto, F.; Uygun, D.A.; Wang, J. Lysozyme-based antibacterial nanomotors. ACS Nano 2015, 9, 9252–9259. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Gao, C.; Zhou, C.; Lin, Z.; He, Q. Leukocyte membrane-coated liquid metal nanoswimmers for actively targeted delivery and synergistic chemophotothermal therapy. Research 2020, 2020, 3676954. [Google Scholar] [CrossRef]
- Guo, Y.; Li, W.; Liu, S.; Jing, D.; Wang, Y.; Feng, Q.; Zhang, K.; Xu, J. Construction of Nanocarriers Based on Endogenous Cell Membrane and Their Application in Nanomedicine. Chin. J. Chem. 2022, 40, 1623–1640. [Google Scholar] [CrossRef]
- Zhang, F.; Zhuang, J.; Esteban Fernández de Ávila, B.; Tang, S.; Zhang, Q.; Fang, R.H.; Zhang, L.; Wang, J. A Nanomotor-Based Active Delivery System for Intracellular Oxygen Transport. ACS Nano 2019, 13, 11996–12005. [Google Scholar] [CrossRef] [PubMed]
- Valdez-Gardun, M.; Leal-Estrada, M.; Oliveros-Mata, E.S.; Sandoval-Bojorquez, D.I.; Soto, F.; Wang, J.; Garcia-Gradilla, V. Density Asymmetry Driven Propulsion of Ultrasound-Powered Janus Micromotors. Adv. Funct. Mater. 2020, 30, 2004043. [Google Scholar] [CrossRef]
- Lu, X.; Ou, H.; Wei, Y.; Ding, X.; Wang, X.; Zhao, C.; Bao, J.; Liu, W. Superfast Fuel-free Tubular Hydrophobic Micromotors Powered by Ultrasound. Sens. Actuators B 2022, 372, 132667. [Google Scholar] [CrossRef]
- Stanton, M.M.; Park, B.-W.; Miguel-López, A.; Ma, X.; Sitti, M.; Sánchez, S. Biohybrid microtube swimmers driven by single captured bacteria. Small 2017, 13, 1603679. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Medina-Sánchez, M.; Magdanz, V.; Schwarz, L.; Hebenstreit, F.; Schmidt, O.G. Sperm-hybrid micromotor for targeted drug delivery. ACS Nano 2018, 12, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Esteban-Fernández de Ávila, B.; Angsantikul, P.; Li, J.; Gao, W.; Zhang, L.; Wang, J. Micromotors go in vivo: From test tubes to live animals. Adv. Funct. Mater. 2018, 28, 1705640. [Google Scholar] [CrossRef]
- Peng, F.; Tu, Y.; Wilson, D.A. Micro/nanomotors towards in vivo application: Cell, tissue and biofluid. Chem. Soc. Rev. 2017, 46, 5289–5310. [Google Scholar] [CrossRef] [PubMed]
- Jurado-Sánchez, B.; Escarpa, A. Janus micromotors for electrochemical sensing and biosensing applications: A review. Electroanalysis 2017, 29, 14–23. [Google Scholar] [CrossRef]
- Duan, W.; Wang, W.; Das, S.; Yadav, V.; Mallouk, T.E.; Sen, A. Synthetic nano- and micromachines in analytical chemistry: Sensing, migration, capture, delivery, and separation. Annu. Rev. Anal. Chem. 2015, 8, 311–333. [Google Scholar] [CrossRef] [PubMed]
- Campuzano, S.; Kagan, D.; Orozco, J.; Wang, J. Motion-driven sensing and biosensing using electrochemically propelled nanomotors. Analyst 2011, 136, 4621–4630. [Google Scholar] [CrossRef]
- Singh, V.V.; Soto, F.; Kaufmann, K.; Wang, J. Micromotor-based energy generation. Angew. Chem. Int. Ed. 2015, 54, 6896–6899. [Google Scholar] [CrossRef]
- Medina-Sánchez, M.; Schwarz, L.; Meyer, A.K.; Hebenstreit, F.; Schmidt, O.G. Cellular cargo delivery: Toward assisted fertilization by sperm-carrying micromotors. Nano Lett. 2016, 16, 555–561. [Google Scholar] [CrossRef]
- Kagan, D.; Calvo-Marzal, P.; Balasubramanian, S.; Sattayasamitsathit, S.; Manesh, K.M.; Flechsig, G.-U.; Wang, J. Chemical sensing based on catalytic nanomotors: Motion-based detection of trace silver. J. Am. Chem. Soc. 2009, 131, 12082–12083. [Google Scholar] [CrossRef]
- Guix, M.; Orozco, J.; García, M.; Gao, W.; Sattayasamitsathit, S.; Merkoçi, A.; Escarpa, A.; Wang, J. Superhydrophobic alkanethiol-coated microsubmarines for effective removal of oil. ACS Nano 2012, 6, 4445–4451. [Google Scholar] [CrossRef] [PubMed]
- Soler, L.; Magdanz, V.; Fomin, V.M.; Sanchez, S.; Schmidt, O.G. Self-propelled micromotors for cleaning polluted water. ACS Nano 2013, 7, 9611–9620. [Google Scholar] [CrossRef]
- Chen, X.-Z.; Jang, B.; Ahmed, D.; Hu, C.; De Marco, C.; Hoop, M.; Mushtaq, F.; Nelson, B.J.; Pané, S. Small-scale machines driven by external power sources. Adv. Mater. 2018, 30, 1705061. [Google Scholar] [CrossRef] [PubMed]
- Beladi-Mousavi, S.M.; Hermanova, S.; Ying, Y.; Plutnar, J.; Pumera, M. A maze in plastic wastes: Autonomous motile photocatalytic microrobots against microplastics. ACS Appl. Mater. Interfaces 2021, 13, 25102–25110. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Arqué, X.; Patiño, T.; Guillerm, V.; Blersch, P.-R.; Pérez-Carvajal, J.; Imaz, I.; Maspoch, D.; Sánchez, S. Enzyme-powered porous micromotors built from a hierarchical micro-and mesoporous UiO-type metal–organic framework. J. Am. Chem. Soc. 2020, 142, 20962–20967. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Huang, Y.; Xu, H.; Chen, S.; Chen, H.; Lin, Y.; Wang, X.; Liu, X.; Sánchez, S.; Huang, X. Contaminants-fueled laccase-powered Fe3O4@ SiO2 nanomotors for synergistical degradation of multiple pollutants. Mater. Today Chem. 2022, 26, 101059. [Google Scholar] [CrossRef]
- Wang, J. Can man-made nanomachines compete with nature biomotors? ACS Nano 2009, 3, 4–9. [Google Scholar] [CrossRef]
- Mallouk, T.E.; Sen, A. Powering nanorobots. Sci. Am. 2009, 300, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yuan, K.; Zhang, L. Micro/nanomachines: From functionalization to sensing and removal. Adv. Mater. Technol. 2019, 4, 1800636. [Google Scholar] [CrossRef]
- Wang, B.; Kostarelos, K.; Nelson, B.J.; Zhang, L. Trends in micro-/nanorobotics: Materials development, actuation, localization, and system integration for biomedical applications. Adv. Mater. 2021, 33, 2002047. [Google Scholar] [CrossRef]
- Zhu, S.; Chen, Y.; Liu, G.; Qian, H.; Niu, F.; Wang, Y.; Zhao, Y.; Luo, T.; Yang, R. External Field-Driven Untethered Microrobots for Targeted Cargo Delivery. Adv. Mater. Technol. 2022, 7, 2101256. [Google Scholar] [CrossRef]
- Jurado-Sánchez, B.; Campuzano, S.; Pingarrón, J.M.; Escarpa, A. Janus particles and motors: Unrivaled devices for mastering (bio) sensing. Microchim. Acta 2021, 188, 416. [Google Scholar] [CrossRef] [PubMed]
- Venugopalan, P.L.; Esteban-Fernández de Ávila, B.; Pal, M.; Ghosh, A.; Wang, J. Fantastic voyage of nanomotors into the cell. ACS Nano 2020, 14, 9423–9439. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, C.K.; Medina-Sánchez, M.; Edmondson, R.J.; Schmidt, O.G. Engineering microrobots for targeted cancer therapies from a medical perspective. Nat. Commun. 2020, 11, 5618. [Google Scholar] [CrossRef]
- Yuan, K.; Bujalance-Fernández, J.; Jurado-Sánchez, B.; Escarpa, A. Light-driven nanomotors and micromotors: Envisioning new analytical possibilities for bio-sensing. Microchim. Acta 2020, 187, 581. [Google Scholar] [CrossRef]
- Ussia, M.; Pumera, M. Towards micromachine intelligence: Potential of polymers. Chem. Soc. Rev. 2022, 51, 1558–1572. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.Y.; Gu, H.; Nelson, B.J. Increasingly intelligent micromachines. Annu. Rev. Control. Robot. Auton. Syst. 2022, 5, 279–310. [Google Scholar] [CrossRef]
- Wang, H.; Pumera, M. Fabrication of micro/nanoscale motors. Chem. Rev. 2015, 115, 8704–8735. [Google Scholar] [CrossRef] [PubMed]
- Men, Y.; Peng, F.; Wilson, D.A. Micro/nanomotors via self-assembly. Sci. Lett. J. 2016, 5, 219. [Google Scholar]
- Wu, Z.; Lin, X.; Si, T.; He, Q. Recent progress on bioinspired self-propelled micro/nanomotors via controlled molecular self-assembly. Small 2016, 12, 3080–3093. [Google Scholar] [CrossRef] [PubMed]
- Shivalkar, S.; Gautam, P.K.; Verma, A.; Maurya, K.; Sk, M.P.; Samanta, S.K.; Sahoo, A.K. Autonomous magnetic microbots for environmental remediation developed by organic waste derived carbon dots. J. Environ. Manag. 2021, 297, 113322. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Mou, F.; Wu, Z.; Tang, S.; Xie, H.; You, M.; Liang, X.; Xu, L.; Guan, J. Simple-structured micromotors based on inherent asymmetry in crystalline phases: Design, large-scale preparation, and environmental application. ACS Appl. Mater. Interfaces 2019, 11, 16639–16646. [Google Scholar] [CrossRef] [PubMed]
- Bail, R.; Lee, D.H. Displacement Mapping as a Highly Flexible Surface Texturing Tool for Additively Photopolymerized Components. Micromachines 2024, 15, 575. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Medina, M.; Ramos-Docampo, M.A.; Hovorka, O.; Salgueiriño, V. Recent advances in nano-and micromotors. Adv. Funct. Mater. 2020, 30, 1908283. [Google Scholar] [CrossRef]
- Soler, L.; Sánchez, S. Catalytic nanomotors for environmental monitoring and water remediation. Nanoscale 2014, 6, 7175–7182. [Google Scholar] [CrossRef] [PubMed]
- Dey, K.K.; Wong, F.; Altemose, A.; Sen, A. Catalytic motors-quo vadimus? Curr. Opin. Colloid Interface Sci. 2016, 21, 4–13. [Google Scholar] [CrossRef]
- Wilson, D.A.; Nolte, R.J.M.; Van Hest, J.C.M. Autonomous movement of platinum-loaded stomatocytes. Nat. Chem. 2012, 4, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Tu, Y.; Peng, F.; Sui, X.; Men, Y.; White, P.B.; van Hest, J.C.; Wilson, D.A. Self-propelled supramolecular nanomotors with temperature-responsive speed regulation. Nat. Chem. 2017, 9, 480–486. [Google Scholar] [CrossRef] [PubMed]
- Moran, J.; Posner, J. Microswimmers with no moving parts. Phys. Today 2019, 72, 44–50. [Google Scholar] [CrossRef]
- Li, J.; Rozen, I.; Wang, J. Rocket science at the nanoscale. ACS Nano 2016, 10, 5619–5634. [Google Scholar] [CrossRef]
- Urso, M.; Iffelsberger, C.; Mayorga-Martinez, C.C.; Pumera, M. Nickel Sulfide Microrockets as Self-Propelled Energy Storage Devices to Power Electronic Circuits “On-Demand”. Small Methods 2021, 5, 2100511. [Google Scholar] [CrossRef]
- Mathesh, M.; Sun, J.; Wilson, D.A. Enzyme catalysis powered micro/nanomotors for biomedical applications. J. Mater. Chem. B 2020, 8, 7319–7334. [Google Scholar] [CrossRef]
- Yang, Q.; Gao, Y.; Xu, L.; Hong, W.; She, Y.; Yang, G. Enzyme-driven micro/nanomotors: Recent advances and biomedical applications. Int. J. Biol. Macromol. 2021, 167, 457–469. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Liu, X.; Wang, L.; Ma, X. Fundamentals and applications of enzyme powered micro/nano-motors. Bioact. Mater. 2021, 6, 1727–1749. [Google Scholar] [CrossRef]
- Hortelao, A.C.; Simó, C.; Guix, M.; Guallar-Garrido, S.; Julián, E.; Vilela, D.; Rejc, L.; Ramos-Cabrer, P.; Cossío, U.; Gómez-Vallejo, V.; et al. Swarming behavior and in vivo monitoring of enzymatic nanomotors within the bladder. Sci. Robot. 2021, 6, eabd2823. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Ou, J.; Wang, S.; Gao, J.; Liu, L.; Ye, Y.; Wilson, D.A.; Hu, Y.; Peng, F.; Tu, Y. Magnesium-based micromotors for enhanced active and synergistic hydrogen chemotherapy. Appl. Mater. Today 2020, 20, 100694. [Google Scholar] [CrossRef]
- Yang, J.; Liu, Y.; Li, J.; Zuo, M.; Li, W.; Xing, N.; Wang, C.; Li, T. γ-Fe2O3@Ag-mSiO2NH2 magnetic Janus micromotor for active water remediation. Appl. Mater. Today 2021, 25, 101190. [Google Scholar] [CrossRef]
- Nourhani, A.; Karshalev, E.; Soto, F.; Wang, J. Multigear bubble propulsion of transient micromotors. Research 2020, 2020, 7823615. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yang, Y.; Yang, X.; Yang, L.; Shen, Y.; Shang, W. Multi-functionalized micro-helical capsule robots with superior loading and releasing capabilities. J. Mater. Chem. B 2021, 9, 1441–1451. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.M.; Wang, W.; Zheng, W.C.; Ju, X.J.; Xie, R.; Zerrouki, D.; Deng, N.N.; Chu, L.Y. Hydrogel-based microactuators with remote-controlled locomotion and fast Pb2+-response for micromanipulation. ACS Appl. Mater. Interfaces 2013, 5, 7219–7226. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Deng, M.; Chen, J.; Duan, Y.; Zhang, J.; Mu, D.; Dong, S.; Luo, J.; Jin, H.; Kakio, S. Rational Design of a Surface Acoustic Wave Device for Wearable Body Temperature Monitoring. Micromachines 2024, 15, 555. [Google Scholar] [CrossRef] [PubMed]
- Bi, M.; Zhang, H.; Ma, Y.; Wang, H.; Wang, W.; Shi, Y.; Sheng, W.; Li, Q.; Gao, G.; Cai, L. Modulation Steering Motion by Quantitative Electrical Stimulation in Pigeon Robots. Micromachines 2024, 15, 595. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Xiong, Z.; Wang, J.; Tang, J.; Li, D. Light hybrid micro/nano-robots: From propulsion to functional signals. Nano Res. 2022, 15, 5355–5375. [Google Scholar] [CrossRef]
- Zhou, D.; Zhuang, R.; Chang, X.; Li, L. Enhanced light-harvesting efficiency and adaptation: A review on visible-light-driven micro/nanomotors. Research 2020, 2020, 6821595. [Google Scholar] [CrossRef] [PubMed]
- Villa, K.; Pumera, M. Fuel-free light-driven micro/nanomachines: Artificial active matter mimicking nature. Chem. Soc. Rev. 2019, 48, 4966–4978. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Mayorga-Martinez, C.C.; Guan, J.; Pumera, M. Photocatalytic micromotors activated by UV to visible light for environmental remediation, micropumps, reversible assembly, transportation, and biomimicry. Small 2020, 16, 1903179. [Google Scholar] [CrossRef] [PubMed]
- Che, S.; Zhang, J.; Mou, F.; Guo, X.; Kauffman, J.E.; Sen, A.; Guan, J. Light-programmable assemblies of isotropic micromotors. Research 2022, 2020, 9816562. [Google Scholar] [CrossRef]
- Oral, C.M.; Ussia, M.; Yavuz, D.K.; Pumera, M. Shape Engineering of TiO2 Microrobots for “On-the-Fly” Optical Brake. Small 2022, 18, 2106271. [Google Scholar] [CrossRef]
- Wu, Y.; Dong, R.; Zhang, Q.; Ren, B. Dye-enhanced self-electrophoretic propulsion of light-driven TiO2-Au Janus micromotors. Nano-Micro Lett. 2017, 9, 30. [Google Scholar] [CrossRef]
- Chattopadhyay, P.; Heckel, S.; Irigon Pereira, F.; Simmchen, J. A path toward inherently asymmetric micromotors. Adv. Intell. Syst. 2023, 5, 2200091. [Google Scholar] [CrossRef]
- Chen, X.Z.; Hoop, M.; Mushtaq, F.; Siringil, E.; Hu, C.; Nelson, B.J.; Pané, S. Recent developments in magnetically driven micro-and nanorobots. Appl. Mater. Today 2017, 9, 37–48. [Google Scholar] [CrossRef]
- Zhou, H.; Mayorga-Martinez, C.C.; Pané, S.; Zhang, L.; Pumera, M. Magnetically driven micro and nanorobots. Chem. Rev. 2021, 121, 4999–5041. [Google Scholar] [CrossRef]
- Wang, X.; Chen, X.Z.; Alcântara, C.C.J.; Sevim, S. MOFBOTS: Metal-organic-framework-based biomedical microrobots. Adv. Mater. 2019, 31, 1901592. [Google Scholar] [CrossRef]
- Sitti, M.; Wiersma, D.S. Pros and cons: Magnetic versus optical microrobots. Adv. Mater. 2020, 32, 1906766. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Xu, L.; Zhong, W.; Yan, Q.; Gao, Y.; Hong, W.; She, Y.; Yang, G. Recent advances in motion control of micro/nanomotors. Adv. Intell. Syst. 2020, 2, 2000049. [Google Scholar] [CrossRef]
- Li, J.; Mayorga-Martinez, C.C.; Ohl, C.D.; Pumera, M. Ultrasonically propelled micro-and nanorobots. Adv. Funct. Mater. 2022, 32, 2102265. [Google Scholar] [CrossRef]
- Rao, K.J.; Li, F.; Meng, L.; Zheng, H.; Cai, F.; Wang, W. A force to be reckoned with: A review of synthetic microswimmers powered by ultrasound. Small 2015, 11, 2836–2846. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Xu, L.P.; Zhang, X. Ultrasound propulsion of micro-/nanomotors. Appl. Mater. Today 2017, 9, 493–503. [Google Scholar] [CrossRef]
- Li, D.; Zheng, Y.; Zhang, Z.; Zhang, Q.; Huang, X.; Dong, R.; Cai, Y.; Wang, L. Single-metal hybrid micromotor. Front. Bioeng. Biotechnol. 2022, 10, 844328. [Google Scholar] [CrossRef] [PubMed]
- Esteban-Fernández de Ávila, B.; Angell, C.; Soto, F.; Lopez-Ramirez, M.A.; Báez, D.F.; Xie, S.; Wang, J.; Chen, Y. Acoustically propelled nanomotors for intracellular siRNA delivery. Acs Nano 2016, 10, 4997–5005. [Google Scholar] [CrossRef] [PubMed]
- Esteban-Fernández de Ávila, B.; Martín, A.; Soto, F.; Lopez-Ramirez, M.A.; Campuzano, S.; Vasquez-Machado, G.M.; Gao, W.; Zhang, L.; Wang, J. Single cell real-time miRNAs sensing based on nanomotors. ACS Nano 2015, 9, 6756–6764. [Google Scholar] [CrossRef] [PubMed]
- Ceylan, H.; Yasa, I.C.; Yasa, O.; Tabak, A.F.; Giltinan, J.; Sitti, M. 3D-Printed Biodegradable Microswimmer for Theranostic Cargo Delivery and Release. ACS Nano 2019, 13, 3353–3362. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, Z.; Gao, C.; Fan, X.; Pang, Y.; Li, T.; Wu, Z.; Xie, H.; He, Q. Dual-responsive biohybrid neutrobots for active target delivery. Sci. Robot. 2021, 6, eaaz9519. [Google Scholar] [CrossRef] [PubMed]
- Al-Fandi, M.; Alshraiedeh, N.; Oweis, R.; Alshdaifat, H.; AlMahaseneh, O.; Al-Tall, K.; Alawneh, R. Novel Selective Detection Method of Tumor Angiogenesis Factors Using Living Nano-Robots. Sensors 2017, 17, 1580. [Google Scholar] [CrossRef]
- Liu, F.; Liu, X.; Shi, Q.; Maffeo, C.; Kojima, M.; Dong, L.; Aksimentiev, A.; Huang, Q.; Fukuda, T.; Arai, T. A tetrahedral DNA nanorobot with conformational change in response to molecular trigger. Nanoscale 2021, 13, 15552–15559. [Google Scholar] [CrossRef]
- Esplandiu, M.J.; Afshar Farniya, A.; Reguera, D. Key parameters controlling the performance of catalytic motors. J. Chem. Phys. 2016, 144, 124702. [Google Scholar] [CrossRef]
- Esplandiu, M.J.; Reguera, D.; Romero-Guzmán, D.; Gallardo-Moreno, A.M.; Fraxedas, J. From radial to unidirectional water pumping in zeta-potential modulated Nafion nanostructures. Nat. Commun. 2022, 13, 2812. [Google Scholar] [CrossRef]
- Fraxedas, J.; Reguera, D.; Esplandiu, M.J. Collective motion of Nafion-based micromotors in water. Faraday Discuss. 2024, 249, 424–439. [Google Scholar] [CrossRef] [PubMed]
- Terzopoulou, A.; Wang, X.; Chen, X.Z.; Palacios-Corella, M.; Pujante, C.; Herrero-Martín, J.; Qin, X.H.; Sort, J.; deMello, A.J.; Nelson, B.J.; et al. Biodegradable metal–organic framework-based microrobots (MOFBOTs). Adv. Healthc. Mater. 2020, 9, 2001031. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Qin, X.H.; Hu, C.; Terzopoulou, A.; Chen, X.Z.; Huang, T.Y.; Maniura-Weber, K.; Pané, S.; Nelson, B.J. 3D printed enzymatically biodegradable soft helical microswimmers. Adv. Funct. Mater. 2018, 28, 1804107. [Google Scholar] [CrossRef]
- Mathesh, M.; Bhattarai, E.; Yang, W. 2D Active Nanobots Based on Soft Nanoarchitectonics Powered by an Ultralow Fuel Concentration. Angew. Chem. Int. Ed. 2022, 61, e202113801. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Sen, A. Autonomous nanomotor based on copper–platinum segmented nanobattery. J. Am. Chem. Soc. 2011, 133, 20064–20067. [Google Scholar] [CrossRef]
- Moran, J.L.; Wheat, P.M.; Posner, J.D. Locomotion of electrocatalytic nanomotors due to reaction induced charge autoelectrophoresis. Phys. Rev. E 2010, 81, 065302. [Google Scholar] [CrossRef] [PubMed]
- Paxton, W.F.; Baker, P.T.; Kline, T.R.; Wang, Y.; Mallouk, T.E.; Sen, A. Catalytically induced electrokinetics for motors and micropumps. J. Am. Chem. Soc. 2006, 128, 14881–14888. [Google Scholar] [CrossRef] [PubMed]
- Kline, T.R.; Paxton, W.F.; Wang, Y.; Velegol, D.; Mallouk, T.E.; Sen, A. Catalytic micropumps: Microscopic convective fluid flow and pattern formation. J. Am. Chem. Soc. 2005, 127, 17150–17151. [Google Scholar] [CrossRef] [PubMed]
- Ibele, M.E.; Wang, Y.; Kline, T.R.; Mallouk, T.E.; Sen, A. Hydrazine fuels for bimetallic catalytic microfluidic pumping. J. Am. Chem. Soc. 2007, 129, 7762–7763. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Huang, G.; Ye, M.; Li, M.; Liu, R.; Mei, Y. Dynamics of catalytic tubular microjet engines: Dependence on geometry and chemical environment. Nanoscale 2011, 3, 5083–5089. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Li, J.; Yuan, M.; Yang, H.; Zhao, Y.; Ying, Y.; Wang, S. Large-Scale Self-Assembly of MOFs Colloidosomes for Bubble-Propelled Micromotors and Stirring-Free Environmental Remediation. Angew. Chem. Int. Ed. 2022, 61, e202211163. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanian, S.; Kagan, D.; Manesh, K.M.; Calvo-Marzal, P.; Flechsig, G.U.; Wang, J. Thermal modulation of nanomotor movement. Small 2009, 5, 1569–1574. [Google Scholar] [CrossRef]
- Yoshida, K.; Onoe, H. Soft Spiral-Shaped Microswimmers for Autonomous Swimming Control by Detecting Surrounding Environments. Adv. Intell. Syst. 2020, 2, 2000095. [Google Scholar] [CrossRef]
- Soler, L.; Martínez-Cisneros, C.; Swiersy, A.; Sánchez, S.; Schmidt, O.G. Thermal activation of catalytic microjets in blood samples using microfluidic chips. Lab Chip 2013, 13, 4299–4303. [Google Scholar] [CrossRef]
- Wang, X.; Wang, C.; Zhang, Q.; Cheng, Y. Near infrared light-responsive and injectable supramolecular hydrogels for on-demand drug delivery. Chem. Commun. 2016, 52, 978–981. [Google Scholar] [CrossRef]
- Li, L.; Scheiger, J.M.; Levkin, P.A. Design and applications of photoresponsive hydrogels. Adv. Mater. 2019, 31, 1807333. [Google Scholar] [CrossRef]
- Luo, R.-C.; Lim, Z.H.; Li, W.; Shi, P.; Chen, C.-H. Near-infrared light triggerable deformation-free polysaccharide double network hydrogels. Chem. Commun. 2014, 50, 7052–7055. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Zhang, B.; Yi, S.; Sun, K.; Pei, G.; Shang, Y.; Liu, X.; Ren, S.; Liu, P.; Zhao, J. Promising advances in physically propelled micro/nanoscale robots. Nano Mater. Sci. 2024, in press. [CrossRef]
- Zhou, Q.; Kong, Y.; Zhou, X.; Ren, L.; Jiang, H.; Lou, D.; Xiao, J.; Bian, R. Navigating Therapeutic Opportunities and Challenges with Micro/Nanomotors in Translational Medicine: A Review. ACS Appl. Nano Mater. 2024, 7, 23321–23336. [Google Scholar] [CrossRef]
- Feng, Y.; Gao, C.; Xie, D.; Liu, L.; Chen, B.; Liu, S.; Yang, H.; Gao, Z.; Wilson, D.A.; Tu, Y.; et al. Directed Neural Stem Cells Differentiation via Signal Communication with Ni–Zn Micromotors. Adv. Mater. 2023, 35, 2301736. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, S.; Solovev, A.A.; Harazim, S.M.; Schmidt, O.G. Microbots Swimming in the Flowing Streams of Microfluidic Channels. J. Am. Chem. Soc. 2011, 133, 701–703. [Google Scholar] [CrossRef]
- Yang, J.; Li, J.; Yan, X.; Lyu, Y.; Xing, N.; Yang, P.; Song, P.; Zuo, M. Three-dimensional hierarchical HRP-MIL-100 (Fe)@ TiO2@ Fe3O4 Janus magnetic micromotor as a smart active platform for detection and degradation of hydroquinone. ACS Appl. Mater. Interfaces 2022, 14, 6484–6498. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Liu, D.; Guo, R.; Wang, B.; Liu, Z.; Guo, Y.; Dong, J.; Lu, Y. Magnetic-controlled dandelion-like nanocatalytic swarm for targeted biofilm elimination. Nanoscale 2022, 14, 6497–6506. [Google Scholar] [CrossRef]
- Muñoz, J.; Urso, M.; Pumera, M. Self-Propelled Multifunctional Microrobots Harboring Chiral Supramolecular Selectors for “Enantiorecognition-on-the-Fly”. Angew. Chem. Int. Ed. 2022, 61, e202116090. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Tang, S.; Cong, Z.; Lu, D.; Yang, Q.; Chen, Q.; Zhang, X.; Wu, S. Biohybrid bacterial microswimmers with metal-organic framework exoskeletons enable cytoprotection and active drug delivery in a harsh environment. Mater. Today Chem. 2022, 23, 100609. [Google Scholar] [CrossRef]
- Urso, M.; Pumera, M. Micro-and Nanorobots Meet DNA. Adv. Funct. Mater. 2022, 32, 2200711. [Google Scholar] [CrossRef]
- Wang, J. Will future microbots be task-specific customized machines or multi-purpose “all in one” vehicles? Nat. Commun. 2021, 12, 7125. [Google Scholar] [CrossRef] [PubMed]
- Mattingly, H.H.; Kamino, K.; Machta, B.B.; Emonet, T. Escherichia coli chemotaxis is information limited. Nat. Phys. 2021, 17, 1426–1431. [Google Scholar] [CrossRef] [PubMed]
- Giometto, A.; Altermatt, F.; Maritan, A.; Stocker, R.; Rinaldo, A. Generalized receptor law governs phototaxis in the phytoplankton Euglena gracilis. Proc. Natl. Acad. Sci. USA 2015, 112, 7045–7050. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Feng, Y.; Wilson, D.A.; Tu, Y.; Peng, F. Micro-nano motors with taxis behavior: Principles, designs, and biomedical applications. Small 2022, 18, 2106263. [Google Scholar] [CrossRef] [PubMed]
- You, M.; Chen, C.; Xu, L.; Mou, F.; Guan, J. Intelligent micro/nanomotors with taxis. Acc. Chem. Res. 2018, 51, 3006–3014. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Tong, F.; Wang, S.; Jiang, J.; Gao, J.; Liu, L.; Liu, K.; Wang, F.; Wang, Z.; Ou, J.; et al. Apoptotic tumor DNA activated nanomotor chemotaxis. Nano Lett. 2021, 21, 8086–8094. [Google Scholar] [CrossRef]
- Xu, D.; Hu, J.; Pan, X.; Sanchez, S.; Yan, X.; Ma, X. Enzyme-powered liquid metal nanobots endowed with multiple biomedical functions. ACS Nano 2021, 15, 11543–11554. [Google Scholar] [CrossRef] [PubMed]
- Frank, B.D.; Djalali, S.; Baryzewska, A.W.; Giusto, P.; Seeberger, P.H.; Zeininger, L. Reversible morphology-resolved chemotactic actuation and motion of Janus emulsion droplets. Nat. Commun. 2022, 13, 2562. [Google Scholar] [CrossRef]
- Cao, S.; Shao, J.; Wu, H.; Song, S.; De Martino, M.T.; Pijpers, I.A.; Friedrich, H.; Abdelmohsen, L.K.; Williams, D.S.; van Hest, J.C. Photoactivated nanomotors via aggregation induced emission for enhanced phototherapy. Nat. Commun. 2021, 12, 2077. [Google Scholar] [CrossRef] [PubMed]
- Lin, G.; Richardson, J.J.; Ahmed, H.; Besford, Q.A.; Christofferson, A.J.; Beyer, S.; Lin, Z.; Rezk, A.R.; Savioli, M.; Zhou, J.; et al. Programmable phototaxis of metal–phenolic particle microswimmers. Adv. Mater. 2021, 33, 2006177. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Jiang, H.; Li, J.; Ma, Y.; Fu, B.; Hu, C. Dipole-moment induced phototaxis and fuel-free propulsion of ZnO/Pt Janus micromotors. Small 2021, 17, 2101388. [Google Scholar] [CrossRef]
- Mou, F.; Xie, Q.; Liu, J.; Che, S.; Bahmane, L.; You, M.; Guan, J. ZnO-based micromotors fueled by CO2: The first example of self-reorientation-induced biomimetic chemotaxis. Natl. Sci. Rev. 2021, 8, nwab066. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Mou, F.; Xu, L.; Wang, S.; Guan, J.; Feng, Z.; Wang, Q.; Kong, L.; Li, W.; Wang, J.; et al. Semiconductors: Light-Steered Isotropic Semiconductor Micromotors (Adv. Mater. 3/2017). Adv. Mater. 2017, 29. [Google Scholar] [CrossRef]
- Dai, B.; Wang, J.; Xiong, Z.; Zhan, X.; Dai, W.; Li, C.C.; Feng, S.P.; Tang, J. Programmable artificial phototactic microswimmer. Nat. Nanotechnol. 2016, 11, 1087–1092. [Google Scholar] [CrossRef]
- Zhang, J.; Mou, F.; Tang, S.; Kauffman, J.E.; Sen, A.; Guan, J. Photochemical micromotor of eccentric core in isotropic hollow shell exhibiting multimodal motion behavior. Appl. Mater. Today 2022, 26, 101371. [Google Scholar] [CrossRef]
- Zhou, C.; Zhang, H.P.; Tang, J.; Wang, W. Photochemically powered AgCl Janus micromotors as a model system to understand ionic self-diffusiophoresis. Langmuir 2018, 34, 3289–3295. [Google Scholar] [CrossRef] [PubMed]
- Urso, M.; Ussia, M.; Novotný, F.; Pumera, M. Trapping and detecting nanoplastics by MXene-derived oxide microrobots. Nat. Commun. 2022, 13, 3573. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.W.; Huang, T.Y.; Charilaou, M.; Lyttle, S.; Zhang, Q.; Pane, S.; Nelson, B.J. Investigation of Magnetotaxis of Reconfigurable Micro-Origami Swimmers with Competitive and Cooperative Anisotropy. Adv. Funct. Mater. 2018, 28, 1802110. [Google Scholar] [CrossRef]
- Santomauro, G.; Singh, A.V.; Park, B.W.; Mohammadrahimi, M.; Erkoc, P.; Goering, E.; Schütz, G.; Sitti, M.; Bill, J. Incorporation of terbium into a microalga leads to magnetotactic swimmers. Adv. Biosyst. 2018, 2, 1800039. [Google Scholar] [CrossRef]
- Li, Q.; Chen, H.; Feng, X.; Yu, C.; Feng, F.; Chai, Y.; Lu, P.; Song, T.; Wang, X.; Yao, L. Nanoparticle-Regulated Semiartificial Magnetotactic Bacteria with Tunable Magnetic Moment and Magnetic Sensitivity. Small 2019, 15, 1900427. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Pumera, M. Coordinated behaviors of artificial micro/nanomachines: From mutual interactions to interactions with the environment. Chem. Soc. Rev. 2020, 49, 3211–3230. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Jiang, J.; Gao, X.; Wang, Q.; Dou, Q.; Zhang, L. Autonomous environment-adaptive microrobot swarm navigation enabled by deep learning-based real-time distribution planning. Nat. Mach. Intell. 2022, 4, 480–493. [Google Scholar] [CrossRef]
- Zhang, J.; Mou, F.; Wu, Z.; Song, J.; Kauffman, J.E.; Sen, A.; Guan, J. Cooperative transport by flocking phototactic micromotors. Nanoscale Adv. 2021, 3, 6157–6163. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Jiang, J.; Wang, X.; Zhang, H.; Song, B.; Dong, B. Visible light-regulated BiVO4-based micromotor with biomimetic ‘predator-bait’behavior. J. Mater. Sci. 2022, 57, 4092–4103. [Google Scholar] [CrossRef]
- Shen, Y.; Zhang, W.; Li, G.; Ning, P.; Li, Z.; Chen, H.; Wei, X.; Pan, X.; Qin, Y.; He, B.; et al. Adaptive control of nanomotor swarms for magnetic-field-programmed cancer cell destruction. ACS Nano 2021, 15, 20020–20031. [Google Scholar] [CrossRef]
- Yue, H.; Chang, X.; Liu, J.; Zhou, D.; Li, L. Wheel-like magnetic-driven microswarm with a band-aid imitation for patching up microscale intestinal perforation. ACS Appl. Mater. Interfaces 2022, 14, 8743–8752. [Google Scholar] [CrossRef]
- Lu, X.; Wei, Y.; Ou, H.; Zhao, C.; Shi, L.; Liu, W. Universal control for micromotor swarms with a hybrid sonoelectrode. Small 2021, 17, 2104516. [Google Scholar] [CrossRef]
- Yang, M.; Mou, F.; Xiong, K.; Li, L.; Zhang, S.; Wang, F.; Gao, T.; Zhao, Z.; Guan, J. Tumbleweed-like aggregation-induced-emission microrobots: Swarming for ultra-tracing of hydrazine. Sens. Actuators B Chem. 2024, 412, 135794. [Google Scholar] [CrossRef]
- Peng, X.; Urso, M.; Ussia, M.; Pumera, M. Shape-controlled self-assembly of light-powered microrobots into ordered microchains for cells transport and water remediation. ACS Nano 2022, 16, 7615–7625. [Google Scholar] [CrossRef] [PubMed]
- Reinmüller, A.; Schöpe, H.J.; Palberg, T. Self-organized cooperative swimming at low Reynolds numbers. Langmuir 2013, 29, 1738–1742. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gardi, G.; Ceron, S.; Wang, W.; Petersen, K.; Sitti, M. Microrobot collectives with reconfigurable morphologies, behaviors, and functions. Nat. Commun. 2022, 13, 2239. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Moran, J.L.; Wang, W. Designing chemical micromotors that communicate-A survey of experiments. JCIS Open 2021, 2, 100006. [Google Scholar] [CrossRef]
- Cheng, Y.; Mou, F.; Yang, M.; Liu, S.; Xu, L.; Luo, M.; Guan, J. Long-range hydrodynamic communication among synthetic self-propelled micromotors. Cell Rep. Phys. Sci. 2022, 3, 100739. [Google Scholar] [CrossRef]
- Cui, X.; Li, J.; Ng, D.H.L.; Liu, J.; Liu, Y.; Yang, W. 3D Hierarchical ACFs-Based Micromotors as Efficient Photo-Fenton-Like Catalysts. Carbon 2020, 158, 738–748. [Google Scholar] [CrossRef]
- Ussia, M.; Urso, M.; Oral, C.M.; Peng, X.; Martin, P. Magnetic Microrobot Swarms with Polymeric Hands Catching Bacteria and Microplastics in Water. ACS Nano 2024, 18, 13171–13183. [Google Scholar] [CrossRef]
- Vilela, D.; Guix, M.; Parmar, J.; Blanco-Blanes, À.; Sánchez, S. Micromotor-in-Sponge Platform for Multicycle Large-Volume Degradation of Organic Pollutants. Small 2022, 18, 2107619. [Google Scholar] [CrossRef] [PubMed]
- Asunción Nadal, V.; Solano Rodríguez, E.; Jurado Sánchez, B.; Alberto, E. Photophoretic MoS2-Fe2O3 Piranha Micromotors for Collective Dynamic Microplastics Removal. ACS Appl. Mater. Interfaces 2024, 16, 47396–47405. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Ren, H.; Qin, H.; Liu, X.; Li, B.; Zheng, X. Light-Armed Nitric Oxide-Releasing Micromotor In Vivo. Nano Lett. 2024, 24, 12452–12460. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, L.; Fu, L.; Feng, K.; Gong, J.; Qu, J.; Niu, R. Dual-functional metal-organic frameworks-based hydrogel micromotor for uranium detection and removal. J. Hazard. Mater. 2024, 467, 133654. [Google Scholar] [CrossRef] [PubMed]
- Yin, B.; Wan, X.; Qian, C.; Sohan, A.M.; Zhou, T.; Yue, W. Enzyme method-based microfluidic chip for the rapid detection of copper ions. Micromachines 2021, 12, 1380. [Google Scholar] [CrossRef]
- Oral, C.M.; Ussia, M.; Pumera, M. Hybrid enzymatic/Photocatalytic degradation of antibiotics via morphologically programmable light-driven ZnO microrobots. Small 2022, 18, 2202600. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Tian, M.; Wang, Z.; Ma, H.; Du, Y.; Si, W.; Zhang, W.; Yang, H.Y.; Chen, S. Engineering Heterointerface to Synergistically Regulate Kinetics and Stress of Copper–Cobalt Selenide toward Reversible Magnesium/Lithium Hybrid Batteries. Nano Lett. 2024. [Google Scholar] [CrossRef]
- Cai, L.; Xu, D.; Zhang, Z.; Li, N.; Zhao, Y. Tailoring functional micromotors for sensing. Research 2023, 6, 0044. [Google Scholar] [CrossRef] [PubMed]
- Sen, A.; Ibele, M.; Hong, Y.; Velegol, D. Chemo and phototactic nano/microbots. Faraday Discuss. 2009, 143, 15–27. [Google Scholar] [CrossRef]
- Dey, K.K.; Bhandari, S.; Bandyopadhyay, D.; Basu, S.; Chattopadhyay, A. The pH taxis of an intelligent catalytic microbot. Small 2013, 9, 1916–1920. [Google Scholar] [CrossRef] [PubMed]
- Ezhilan, B.; Gao, W.; Pei, A.; Rozen, I.; Dong, R.; Jurado-Sanchez, B.; Wang, J.; Saintillan, D. Motion-based threat detection using microrods: Experiments and numerical simulations. Nanoscale 2015, 7, 7833–7840. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Balasubramanian, S.; Kagan, D.; Manesh, K.M.; Campuzano, S.; Wang, J. Motion-based DNA detection using catalytic nanomotors. Nat. Commun. 2010, 1, 36. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Pei, A.; Dong, R.; Wang, J. Catalytic iridium-based janus micromotors powered by ultralow levels of chemical fuels. J. Am. Chem. Soc. 2014, 136, 2276–2279. [Google Scholar] [CrossRef] [PubMed]
- Dong, R.; Li, J.; Rozen, I.; Ezhilan, B.; Xu, T.; Christianson, C.; Gao, W.; Saintillan, D.; Ren, B.; Wang, J. Vapor-driven propulsion of catalytic micromotors. Sci. Rep. 2015, 5, 13226. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.V.; Kaufmann, K.; de Avila, B.E.-F.; Uygun, M.; Wang, J. Nanomotors responsive to nerve-agent vapor plumes. Chem. Commun. 2016, 52, 3360–3363. [Google Scholar] [CrossRef]
- Orozco, J.; García-Gradilla, V.; D’Agostino, M.; Gao, W.; Cortés, A.; Wang, J. Artificial enzyme-powered microfish for water-quality testing. ACS Nano 2013, 7, 818–824. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Sun, Y.; Wang, T.; Ye, Z.; Zhang, H.; Dong, B.; Li, C.Y. A biodegradable, all-polymer micromotor for gas sensing applications. J. Mater. Chem. C 2016, 4, 5945–5952. [Google Scholar] [CrossRef]
- Moo, J.G.S.; Wang, H.; Zhao, G.; Pumera, M. Biomimetic artificial inorganic enzyme-free self-propelled microfish robot for selective detection of Pb2+ in water. Chem.-Eur. J. 2014, 20, 4292–4296. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Sanchez, S.; Schmidt, O.G.; Pumera, M. Poisoning of bubble propelled catalytic micromotors: The chemical environment matters. Nanoscale 2013, 5, 2909–2914. [Google Scholar] [CrossRef]
- Jurado-Sanchez, B.; Escarpa, A.; Wang, J. Lighting up micromotors with quantum dots for smart chemical sensing. Chem. Commun. 2015, 51, 14088–14091. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.L.; Ji, Y.Y.; Zou, X.; Chen, Q.M.; Zhang, S.L.; Gong, Z.J. N, P Co-doped carbon dots as multifunctional fluorescence nano-sensor for sensitive and selective detection of Cr (VI) and ascorbic acid. J. Anal. Test. 2022, 6, 335–345. [Google Scholar] [CrossRef]
- Singh, V.V.; Kaufmann, K.; Orozco, J.; Li, J.; Galarnyk, M.; Arya, G.; Wang, J. Micromotor-based on-off fluorescence detection of sarin and soman simulants. Chem. Commun. 2015, 51, 11190–11193. [Google Scholar] [CrossRef] [PubMed]
- De Ávila, B.E.-F.; Lopez-Ramirez, M.A.; Báez, D.F.; Jodra, A.; Singh, V.V.; Kaufmann, K.; Wang, J. Aptamer-modified Graphene-based catalytic micromotors: Off–on fluorescent detection of ricin. ACS Sens. 2016, 1, 217–221. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, J.; Fu, L.; Liu, D.; Chen, L. Magnetic molecularly imprinted microsensor for selective recognition and transport of fluorescent phycocyanin in seawater. J. Mater. Chem. A 2015, 3, 7437–7444. [Google Scholar] [CrossRef]
- Wang, J. Self-propelled affinity biosensors: Moving the receptor around the sample. Biosens. Bioelectron. 2016, 76, 234–242. [Google Scholar] [CrossRef] [PubMed]
- SCampuzano; Orozco, J.; Kagan, D.; Guix, M.; Gao, W.; Sattayasamitsathit, S.; Claussen, J.C.; Merkoci, A.; Wang, J. Bacterial isolation by lectin-modified microengines. Nano Lett. 2012, 12, 396–401. [Google Scholar] [CrossRef]
- Orozco, J.; Pan, G.; Sattayasamitsathit, S.; Galarnyk, M.; Wang, J. Micromotors to capture and destroy anthrax simulant spores. Analyst 2015, 140, 1421–1427. [Google Scholar] [CrossRef] [PubMed]
- Cinti, S.; Valdes-Ramirez, G.; Gao, W.; Li, J.; Palleschi, G.; Wang, J. Microengine-assisted electrochemical measurements at printable sensor strips. Chem. Commun. 2015, 51, 8668–8671. [Google Scholar] [CrossRef] [PubMed]
- Rojas, D.; Jurado-Sanchez, B.; Escarpa, A. “Shoot and Sense” Janus micromotors-based strategy for the simultaneous degradation and detection of persistent organic pollutants in food and biological samples. Anal. Chem. 2016, 88, 4153–4160. [Google Scholar] [CrossRef]
- Karshalev, E.; Kumar, R.; Jeerapan, I.; Castillo, R.; Campos, I.; Wang, J. Multistimuli-responsive camouflage swimmers. Chem. Mater. 2018, 30, 1593–1601. [Google Scholar] [CrossRef]
- Xing, N.; Lyu, Y.; Li, J.; Ng, D.H.; Zhang, X.; Zhao, W. 3D hierarchical LDHs-based Janus micro-actuator for detection and degradation of catechol. J. Hazard. Mater. 2023, 442, 129914. [Google Scholar] [CrossRef] [PubMed]
- Xing, N.; Lyu, Y.; Yang, J.; Zhang, X.; Han, Y.; Zhao, W.; Ng, D.H.; Li, J. Motion-based phenol detection and degradation using 3D hierarchical AA-NiMn-CLDHs@ HNTs-Ag nanomotors. Environ. Sci. Nano 2022, 9, 2815–2826. [Google Scholar] [CrossRef]
- Mou, F.; Pan, D.; Chen, C.; Gao, Y.; Xu, L.; Guan, J. Magnetically modulated pot-like MnFe2O4 micromotors: Nanoparticle assembly fabrication and their capability for direct oil removal. Adv. Funct. Mater. 2015, 25, 6173–6181. [Google Scholar] [CrossRef]
- Baptista-Pires, L.; Orozco, J.; Guardia, P.; Merkoçi, A. Architecting graphene oxide rolled-up micromotors: A simple paper-based manufacturing technology. Small 2018, 14, 1702746. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Feng, X.; Pei, A.; Gu, Y.; Li, J.; Wang, J. Seawater-driven magnesium based Janus micromotors for environmental remediation. Nanoscale 2013, 5, 4696–4700. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.D.; Yang, H.; Liang, Y.; Yan, K.; Liu, L.; Gao, D.; Ma, J. Light-Propelled Super-Hydrophobic Sponge Motor and its Application in Oil–Water Separation. ACS Appl. Mater. Interfaces 2023, 15, 43205–43215. [Google Scholar] [CrossRef] [PubMed]
- Maria-Hormigos, R.; Jurado-Sanchez, B.; Vazquez, L.; Escarpa, A. Carbon allotrope nanomaterials based catalytic micromotors. Chem. Mater. 2016, 28, 8962–8970. [Google Scholar] [CrossRef]
- Martín, A.; Jurado-Sánchez, B.; Escarpa, A.; Wang, J. Template electrosynthesis of high-performance graphene microengines. Small 2015, 11, 3568–3574. [Google Scholar] [CrossRef] [PubMed]
- Jurado-Sanchez, B.; Sattayasamitsathit, S.; Gao, W.; Santos, L.; Fedorak, Y.; Singh, V.V.; Orozco, J.; Galarnyk, M.; Wang, J. Self-propelled activated carbon Janus micromotors for efficient water purification. Small 2015, 11, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Orozco, J.; Mercante, L.A.; Pol, R.; Merkoçi, A. Graphene-based Janus micromotors for the dynamic removal of pollutants. J. Mater. Chem. A 2016, 4, 3371–3378. [Google Scholar] [CrossRef]
- Singh, V.V.; Martin, A.; Kaufmann, K.; de Oliveira, S.D.S.; Wang, J. Zirconia/graphene oxide hybrid micromotors for selective capture of nerve agents. Chem. Mater. 2015, 27, 8162–8169. [Google Scholar] [CrossRef]
- Vilela, D.; Parmar, J.; Zeng, Y.; Zhao, Y.; Sanchez, S. Graphene-based microbots for toxic heavy metal removal and recovery from water. Nano Lett. 2016, 16, 2860–2866. [Google Scholar] [CrossRef]
- Yang, W.; Qiang, Y.; Du, M.; Cao, Y.; Wang, Y.; Zhang, X.; Yue, T.; Huang, J.; Li, Z. Self-propelled nanomotors based on hierarchical metal-organic framework composites for the removal of heavy metal ions. J. Hazard. Mater. 2022, 435, 128967. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.V.; Jurado-Sánchez, B.; Sattayasamitsathit, S.; Orozco, J.; Li, J.; Galarnyk, M.; Fedorak, Y.; Wang, J. Multifunctional silver-exchanged zeolite micromotors for catalytic detoxification of chemical and biological threats. Adv. Funct. Mater. 2015, 25, 2147–2155. [Google Scholar] [CrossRef]
- Li, W.; Wu, C.; Xiong, Z.; Liang, C.; Li, Z.; Liu, B.; Cao, Q.; Wang, J.; Tang, J.; Li, D. Self-driven magnetorobots for recyclable and scalable micro/nanoplastic removal from nonmarine waters. Sci. Adv. 2022, 8, eade1731. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Mayorga-Martinez, C.C.; Pumera, M. Microplastic removal and degradation by mussel-inspired adhesive magnetic/enzymatic microrobots. Small Methods 2021, 5, 2100230. [Google Scholar] [CrossRef]
- Uygun, M.; Singh, V.V.; Kaufmann, K.; Uygun, D.A.; de Oliveira, S.D.S.; Wang, J. Micromotor-based biomimetic carbon dioxide sequestration: Towards mobile microscrubbers. Angew. Chem. Int. Ed. 2015, 54, 12900–12904. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Khezri, B.; Pumera, M. Self-Propelled micromachines for environmental remediation. Chem 2016, 1, 473–481. [Google Scholar] [CrossRef]
- Uygun, D.A.; Jurado-Sánchez, B.; Uygun, M.; Wang, J. Self-propelled chelation platforms for efficient removal of toxic metals. Environ. Sci. Nano 2016, 3, 559–566. [Google Scholar] [CrossRef]
- El-Naggar, K.; Yang, Y.; Tian, W.; Zhang, H.; Sun, H.; Wang, S. Metal-Organic Framework-Based Micro-/Nanomotors for Wastewater Remediation. Small Sci. 2024, 4, 2400110. [Google Scholar] [CrossRef]
- Vaghasiya, J.V.; Mayorga-Martinez, C.C.; Matějková, S.; Pumera, M. Pick up and dispose of pollutants from water via temperature-responsive micellar copolymers on magnetite nanorobots. Nat. Commun. 2022, 13, 1026. [Google Scholar] [CrossRef] [PubMed]
- Geng, Z.; Li, J.; Deng, T.; Nie, X.; Meng, X.; Han, W.; Wei, K.; Qu, L. Recyclable Nanomotors for Dynamic Enrichment and Detection of Low-Concentration Emerging Pollutants. Adv. Funct. Mater. 2024, 34, 2404097. [Google Scholar] [CrossRef]
- Parmar, J.; Vilela, D.; Pellicer, E.; los Ojos, D.E.-D.; Sort, J.; Sánchez, S. Reusable and long-lasting active microcleaners for heterogeneous water remediation. Adv. Funct. Mater. 2016, 26, 4152–4161. [Google Scholar] [CrossRef]
- Lee, C.-S.; Gong, J.; Oh, D.-S.; Jeon, J.-R.; Chang, Y.-S. Zerovalent-iron/platinum Janus micromotors with spatially separated functionalities for efficient water decontamination. ACS Appl. Nano Mater. 2018, 1, 768–776. [Google Scholar] [CrossRef]
- Hao, J.; Yang, W.; Zhang, Z.; Tang, J. Surfactant-assisted fabrication of 3D Prussian blue-reduced graphene oxide hydrogel as a self-propelling motor for water treatment. Nanoscale 2015, 7, 10498–10503. [Google Scholar] [CrossRef]
- Parmar, J.; Villa, K.; Vilela, D.; Sánchez, S. Platinum-free cobalt ferrite based micromotors for antibiotic removal. Appl. Mater. Today 2017, 9, 605–611. [Google Scholar] [CrossRef]
- Wang, R.; Guo, W.; Li, X.; Liu, Z.; Liu, H.; Ding, S. Highly efficient MOF-based self-propelled micromotors for water purification. RSC Adv. 2017, 7, 42462–42467. [Google Scholar] [CrossRef]
- Orozco, J.; Cheng, G.; Vilela, D.; Sattayasamitsathit, S.; Vazquez-Duhalt, R.; Valdes-Ramirez, G.; Pak, O.S.; Escarpa, A.; Kan, C.; Wang, J. Micromotor-based high-yielding fast oxidative detoxification of chemical threats. Angew. Chem. Int. Ed. 2013, 52, 13276–13279. [Google Scholar] [CrossRef] [PubMed]
- Wani, O.M.; Safdar, M.; Kinnunen, N.; Janis, J. Dual effect of manganese oxide micromotors: Catalytic degradation and adsorptive bubble separation of organic pollutants. Chem.–Eur. J. 2016, 22, 1244–1247. [Google Scholar] [CrossRef]
- Srivastava, S.K.; Guix, M.; Schmidt, O.G. Wastewater mediated activation of micromotors for efficient water cleaning. Nano Lett. 2016, 16, 817–821. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Torres, J.; Serra, A.; Tierno, P.; Alcobe, X.; Valles, E. Magnetic propulsion of recyclable catalytic nanocleaners for pollutant degradation. ACS Appl. Mater. Interfaces 2017, 9, 23859–23868. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Ma, E.; Cui, H.; Hu, Z.; Wang, H. Bioinspired Self-Propelled Micromotors with Improved Transport Efficiency in the Subsurface Environment for Soil Decontamination. Adv. Funct. Mater. 2023, 33, 2307632. [Google Scholar] [CrossRef]
- Li, Y.; Wu, Y.; Wang, H. Self-propelled micromotors based on natural pyrolusite for dynamic remediation of organic contaminated soil. J. Environ. Chem. Eng. 2023, 11, 110068. [Google Scholar] [CrossRef]
- Li, J.; Singh, V.V.; Sattayasamitsathit, S.; Orozco, J.; Kaufmann, K.; Dong, R.; Gao, W.; Jurado-Sanchez, B.; Fedorak, Y.; Wang, J. Water-driven micromotors for rapid photocatalytic degradation of biological and chemical warfare agents. ACS Nano 2014, 8, 11118–11125. [Google Scholar] [CrossRef]
- Wang, S.; Jiang, Z.; Ouyang, S.; Dai, Z.; Wang, T. Internally/externally bubble-propelled photocatalytic tubular nanomotors for efficient water cleaning. ACS Appl. Mater. Interfaces 2017, 9, 23974–23982. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhao, A.; Wang, F.; Ren, J.; Qu, X. Design of a plasmonic micromotor for enhanced photo-remediation of polluted anaerobic stagnant waters. Chem. Commun. 2016, 52, 5550–5553. [Google Scholar] [CrossRef] [PubMed]
- Orozco, J.; Vilela, D.; Valdes-Ramirez, G.; Fedorak, Y.; Escarpa, A.; Vazquez-Duhalt, R.; Wang, J. Efficient biocatalytic degradation of pollutants by enzyme-releasing self-propelled motors. Chem.–Eur. J. 2014, 20, 2866–2871. [Google Scholar] [CrossRef]
- Sattayasamitsathit, S.; Kaufmann, K.; Galarnyk, M.; Vazquez-Duhalt, R.; Wang, J. Dual-enzyme natural motors incorporating decontamination and propulsion capabilities. RSC Adv. 2014, 4, 27565–27570. [Google Scholar] [CrossRef]
- Gao, S.; Liu, C.; Yang, X.; Lan, Z.; Zuo, M.; Yang, P.; Li, J. New synthetic strategy toward a natural enzyme–nanozyme hybrid dual-function nanomotor and its application in environmental remediation. Catal. Sci. Technol. 2024, 14, 1239–1254. [Google Scholar] [CrossRef]
- Campos, R.F.; Bachimanchi, H.; Volpe, G.; Villa, K. Bubble-propelled micromotors for ammonia generation. Nanoscale 2023, 15, 15785–15793. [Google Scholar] [CrossRef] [PubMed]
- Andreozzi, R.; Caprio, V.; Insola, A.; Marotta, R. Advanced oxidation processes (AOP) for water purification and recovery. Catal. Today 1999, 53, 51–59. [Google Scholar] [CrossRef]
- Qu, Y.; Duan, X. Progress, challenge and perspective of heterogeneous photocatalysts. Chem. Soc. Rev. 2013, 42, 2568–2580. [Google Scholar] [CrossRef] [PubMed]
- Mushtaq, F.; Asani, A.; Hoop, M.; Chen, X.-Z.; Ahmed, D.; Nelson, B.J.; Pané, S. Highly efficient coaxial TiO2-PtPd tubular nanomachines for photocatalytic water purification with multiple locomotion strategies. Adv. Funct. Mater. 2016, 26, 6995–7002. [Google Scholar] [CrossRef]
- Jurado-Sánchez, B.; Wang, J.; Escarpa, A. Ultrafast nanocrystals decorated micromotors for on-site dynamic chemical processes. ACS Appl. Mater. Interfaces 2016, 8, 19618–19625. [Google Scholar] [CrossRef] [PubMed]
- Tesař, J.; Ussia, M.; Alduhaish, O.; Pumera, M. Autonomous self-propelled MnO2 micromotors for hormones removal and degradation. Appl. Mater. Today 2022, 26, 101312. [Google Scholar] [CrossRef]
- Enachi, M.; Guix, M.; Postolache, V.; Ciobanu, V.; Fomin, V.M.; Schmidt, O.G.; Tiginyanu, I. Light-induced motion of microengines based on microarrays of TiO2 nanotubes. Small 2016, 12, 5497–5505. [Google Scholar] [CrossRef] [PubMed]
- Mou, F.; Kong, L.; Chen, C.; Chen, Z.; Xu, L.; Guan, J. Light-controlled propulsion, aggregation and separation of water-fuelled TiO2/Pt Janus submicromotors and their “on-the-fly” photocatalytic activities. Nanoscale 2016, 8, 4976–4983. [Google Scholar] [CrossRef] [PubMed]
- Dong, R.; Zhang, Q.; Gao, W.; Pei, A.; Ren, B. Highly efficient light-driven TiO2–Au Janus micromotors. ACS Nano 2016, 10, 839–844. [Google Scholar] [CrossRef] [PubMed]
- Simmchen, J.; Baeza, A.; Miguel-Lopez, A.; Stanton, M.M.; Vallet-Regi, M.; Ruiz-Molina, D.; Sánchez, S. Dynamics of novel photoactive AgCl microstars and their environmental applications. ChemNanoMat 2017, 3, 65–71. [Google Scholar] [CrossRef]
- Mushtaq, F.; Chen, X.; Torlakcik, H.; Steuer, C.; Hoop, M.; Siringil, E.C.; Marti, X.; Limburg, G.; Stipp, P.; Nelson, B.J.; et al. Magnetoelectrically Driven Catalytic Degradation of Organics. Adv. Mater. 2019, 31, 1901378. [Google Scholar] [CrossRef]
- Delezuk, J.A.; Ramirez-Herrera, D.E.; Avila, B.E.-F.; Wang, J. Chitosan-based water-propelled micromotors with strong antibacterial activity. Nanoscale 2017, 9, 2195–2200. [Google Scholar] [CrossRef] [PubMed]
- Vilela, D.; Stanton, M.M.; Parmar, J.; Sánchez, S. Microbots decorated with silver nanoparticles kill bacteria in aqueous media. ACS Appl. Mater. Interfaces 2017, 9, 22093–22100. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Potroz, M.G.; Jackman, J.A.; Khezri, B.; Marić, T.; Cho, N.J.; Pumera, M. Bioinspired Spiky Micromotors Based on Sporopollenin Exine Capsules. Adv. Funct. Mater. 2017, 27, 1702338. [Google Scholar] [CrossRef]
- Zhe, T.W.; Radek, Z.; Ivo, M.; Pumera, M. Fe0 Nanomotors in Ton Quantities (1020 Units) for Environmental Remediation. Chem.–Eur. J. 2016, 22, 4789–4793. [Google Scholar]
- Jurado-Sánchez, B.; Pacheco, M.; Maria-Hormigos, R.; Escarpa, A. Perspectives on Janus micromotors: Materials and applications. Appl. Mater. Today 2017, 9, 407–418. [Google Scholar] [CrossRef]




| Fabrication | Technique | Structure | Advantages | Limitations |
|---|---|---|---|---|
| Electrochemical | Membrane template-assisted electrochemical deposition | Nanowires, Nanorods, Microtubes | Low cost, prepared from inorganic or organic materials | Large-scale production and MNMs’ geometry are limited by the characteristics of the membrane (pore size, shape) |
| Chemical | Polymerization, hydrothermal reaction, precipitation, solvent extraction and evaporation, functionalization, reactive ion etching | Symmetric and asymmetric MNMs | Simple equipment, low-cost and large-scale production | Reproducibility |
| Physical | Physical vapor deposition (sputtering, evaporation, atomic layer deposition), lithography | Anisotropic structures, coatings for Janus structures | Highly controllable and reusable | Expensive equipment |
| Biohybrid | Bio-templated MNM fabrication, bio-hybrids (microorganisms modified with MNMs) | Organic and inorganic microstructures, self-motile structures for hybrid MNMs | Low cost, sustainability, and abundance of bio-templates | Short MNM lifespan |
| Self-assembly | Layer-by-layer assembly, macromolecular assembly, shape transformation | Vesicles, Janus capsules, polymersomes | Simple equipment, low-cost, sustainability, versatility, bio- inspired and biodegradable materials preparation | Mostly fuel driven |
| 3D printing | Fused deposition modeling, selective laser sintering, direct ink writing | 3D and 4D structures | Low-cost and large-scale production, high control and reproducibility | Limited resolution and range of suitable materials |
| Propulsion Approach | Performance | Span | Safety | Limitations | |
|---|---|---|---|---|---|
| External Field | Magnetic fields | Enables precise 3D movement | Better persistence; MNMs can sustain motion guided by the external field | Good biocompatibility; strong magnetic fields may affect the human body | Electromagnetic drive with low energy efficiency and limited working space |
| Light | On–off control and directional motion can be realized with fast motion speeds | UV light is harmful to human body; other light is basically safe | Driving in liquids requires high power; limited light focus size and range of motion | ||
| Ultrasound | Fast motion response | Safer | Lack of operational precision | ||
| Electric field | Chemotaxis | Strong electric fields may have an effect on the human body | Small range of motion | ||
| Chemical | Bubble | Dependent on bubble size or vibration frequency | Related to the way the bubbles are generated | Lack of operational precision and efficiency | |
| Self-phoresis | Fast movement speed | Poor sustained performance; as chemical fuels are gradually consumed, motility performance will decline | Enzyme-initiated biocatalytic reactions are safe and biocompatible, but H2O2 is harmful | Dependent on fuel concentration; poor motion continuity; uncontrollable motion direction and accuracy | |
| Ionic diffusiophoresis | Dependent on catalytic reaction rate, the strength of the ion concentration gradient, and the electrodynamic effect on the particle surface | Affected by fuel consumption, catalyst stability, and environmental conditions | Depends on the biocompatibility of the fuel, catalyst, and its by-products | In solutions with high salt concentrations, the ion shielding effect weakens the concentration gradient and affects the movement efficiency [115,116,117] | |
| Enzymatic reactions | The speed is relatively stable and can maintain the movement for a longer period of time | Depends on the catalytic efficiency of the enzyme, rate of diffusion of reaction products | Better biocompatibility | Enzymes are easily inactivated by environmental changes (temperature, pH), resulting in cessation of movement [111,118,119] | |
| Biologically driven | Relying on the motor properties of individual organisms | Influenced by biological cell activity | Good biocompatibility | Cells, bacteria, etc. need specific nutrient solutions as well as an environment to survive | |
| Challenges | Strategies |
|---|---|
| Scalability | Laser-based 3D printing |
| Frictional hindrance | Surface modification and lubrication strategies; adaptive advancement mechanisms |
| Swarm control | Applying speed matching, cohesion, and consistency rules |
| Sustained energy supply | Pollutant-fueled power systems or hybrid energy systems |
| Motion and migration efficiency | Optimized structural design |
| Selectivity and interference resistance | Multi-functional composite design; surface modification and functionalization |
| Stability and durability | Protective coatings or self-healing materials |
| Degradability and safety | Degradable material design; self-destruction or disintegration mechanisms; recyclable systems |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, T.; Liu, S.; Yang, Y.; Chen, X. Application of Micro/Nanomotors in Environmental Remediation: A Review. Micromachines 2024, 15, 1443. https://doi.org/10.3390/mi15121443
He T, Liu S, Yang Y, Chen X. Application of Micro/Nanomotors in Environmental Remediation: A Review. Micromachines. 2024; 15(12):1443. https://doi.org/10.3390/mi15121443
Chicago/Turabian StyleHe, Tao, Shishuo Liu, Yonghui Yang, and Xuebo Chen. 2024. "Application of Micro/Nanomotors in Environmental Remediation: A Review" Micromachines 15, no. 12: 1443. https://doi.org/10.3390/mi15121443
APA StyleHe, T., Liu, S., Yang, Y., & Chen, X. (2024). Application of Micro/Nanomotors in Environmental Remediation: A Review. Micromachines, 15(12), 1443. https://doi.org/10.3390/mi15121443






