Construction and Application of an Electrochemical Sensor for Determination of D-Penicillamine Based on Modified Carbon Paste Electrode
Abstract
:1. Introduction
2. Experimental Design
2.1. Equipment and Materials
2.2. Preparation of MWCNT-Co3O4/BF/ILCPE
2.3. Preparation of Pharmaceutical (D-PA Capsules) and Biological (Urine) Samples
3. Results and Discussion
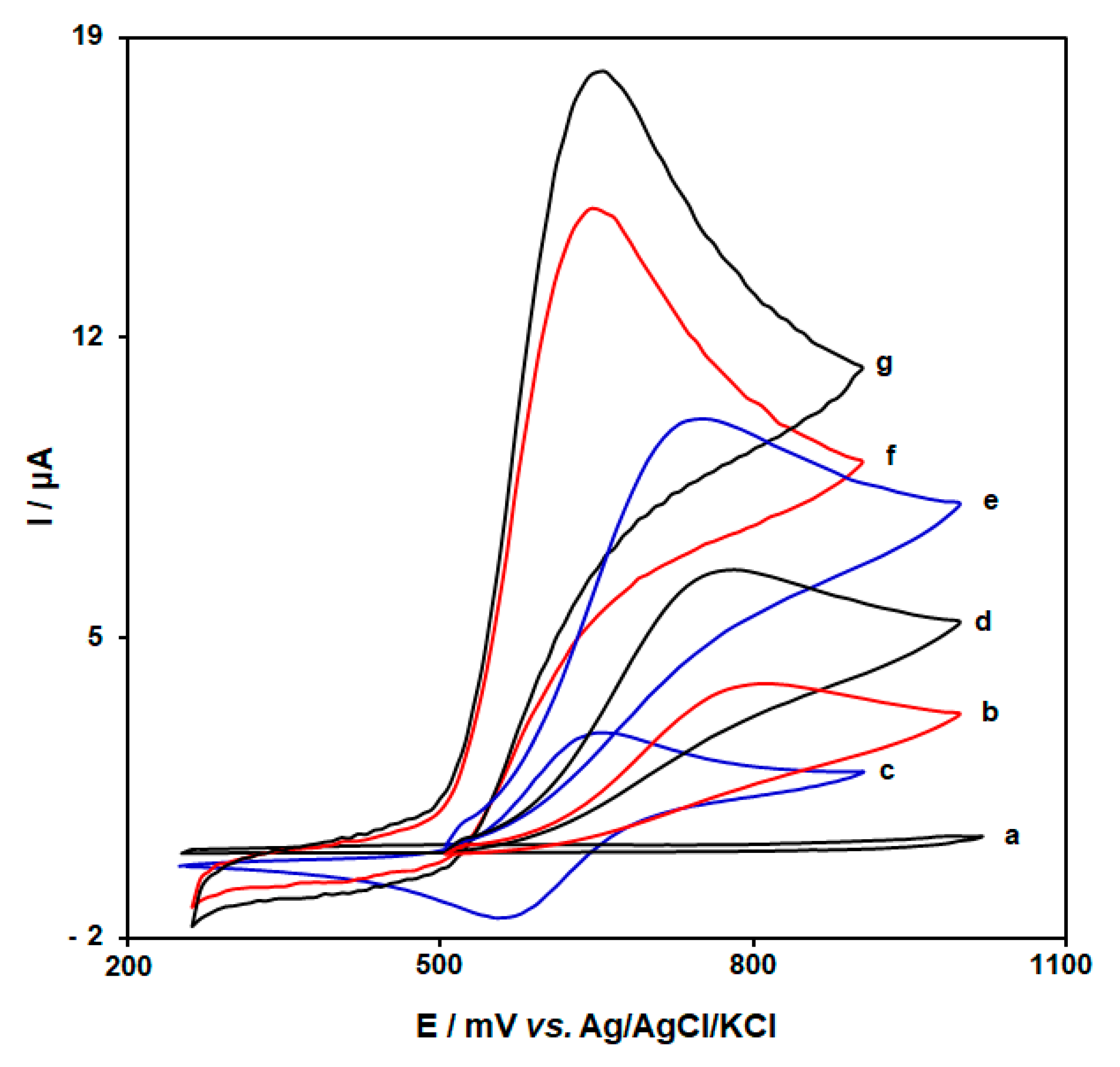
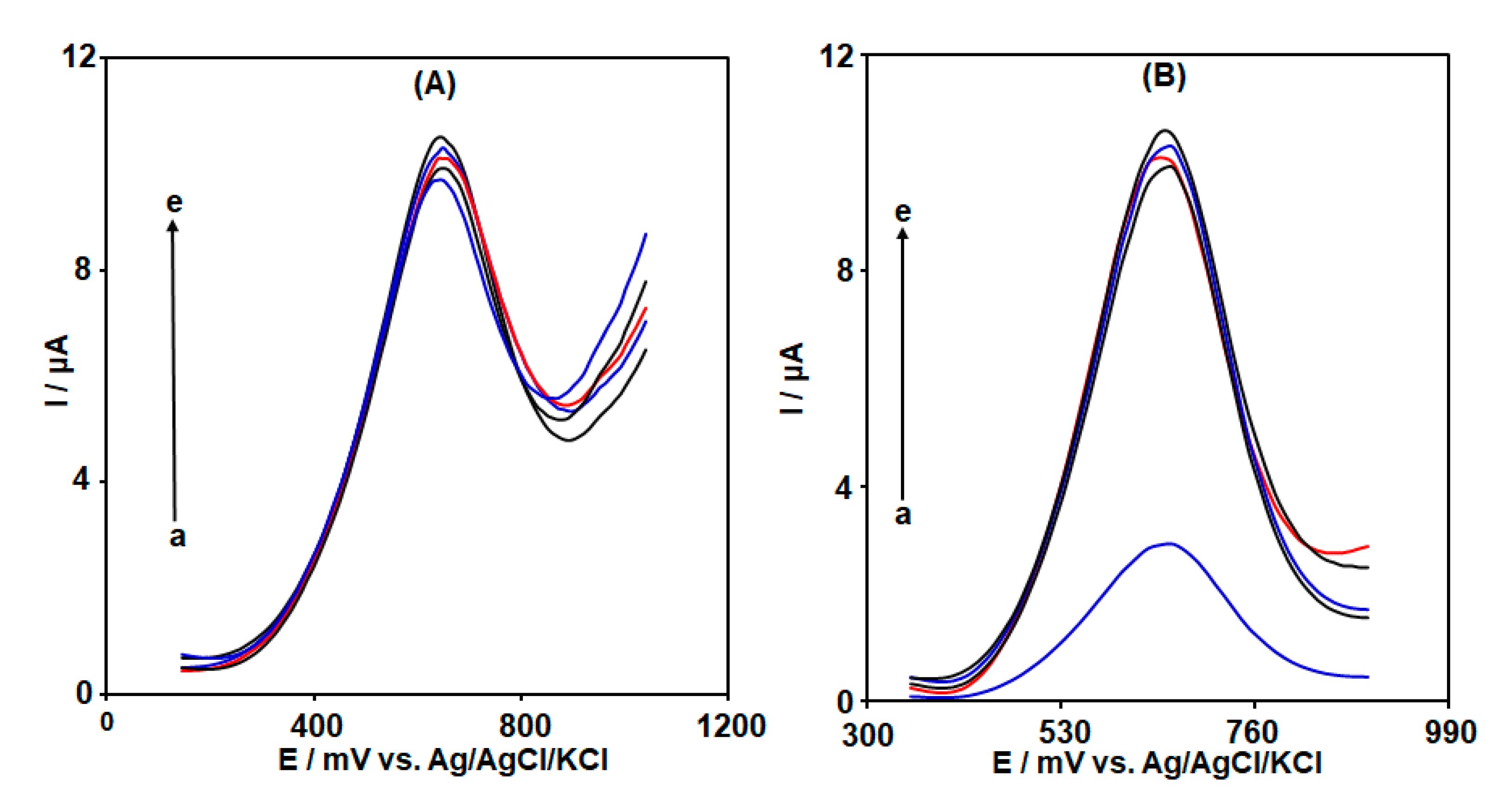
3.1. Evaluation of the Electrocatalytic Activity of MWCNT-Co3O4/BF/ILCPE towards D-PA
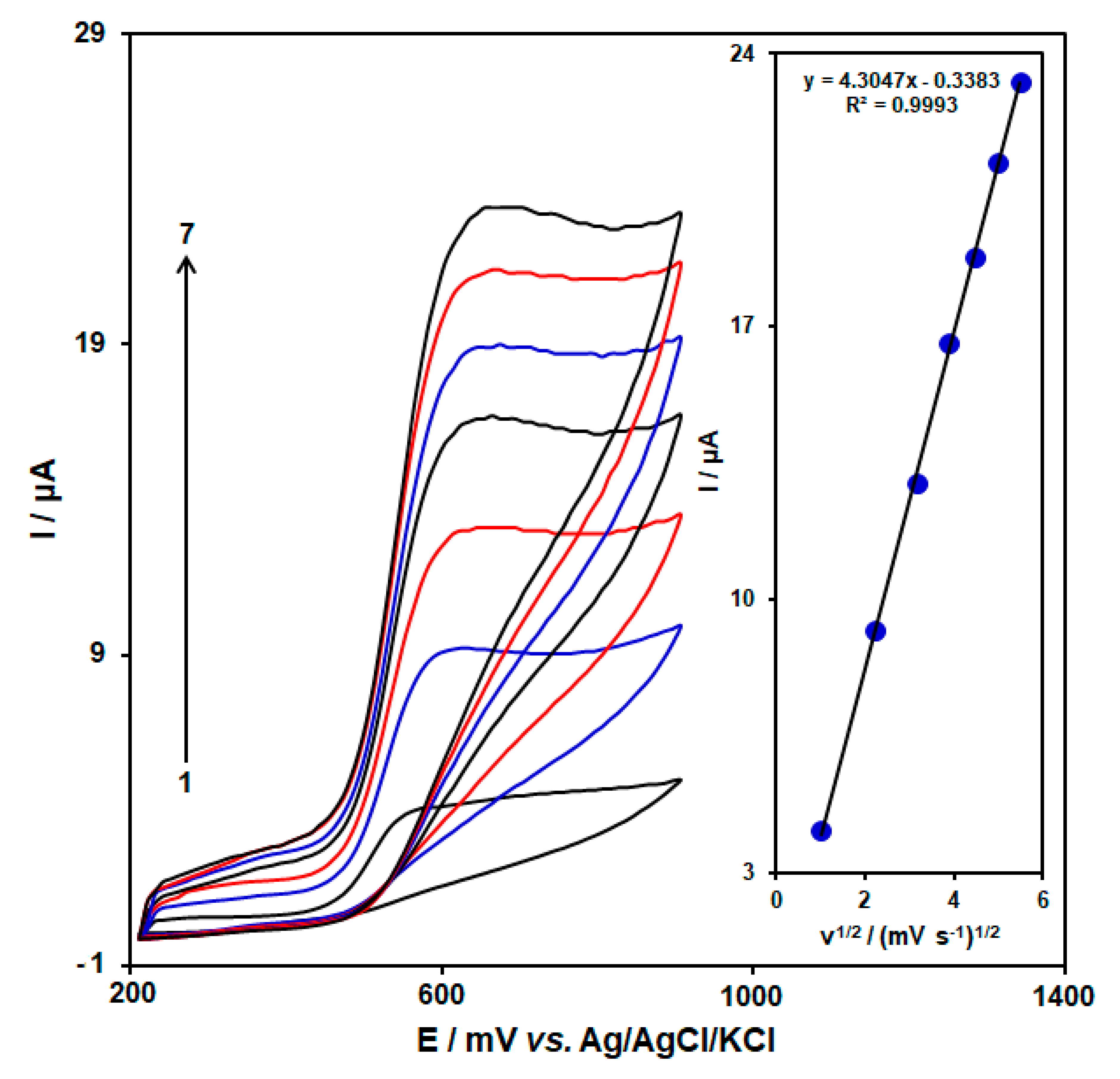
3.2. Influence of Scan Rate
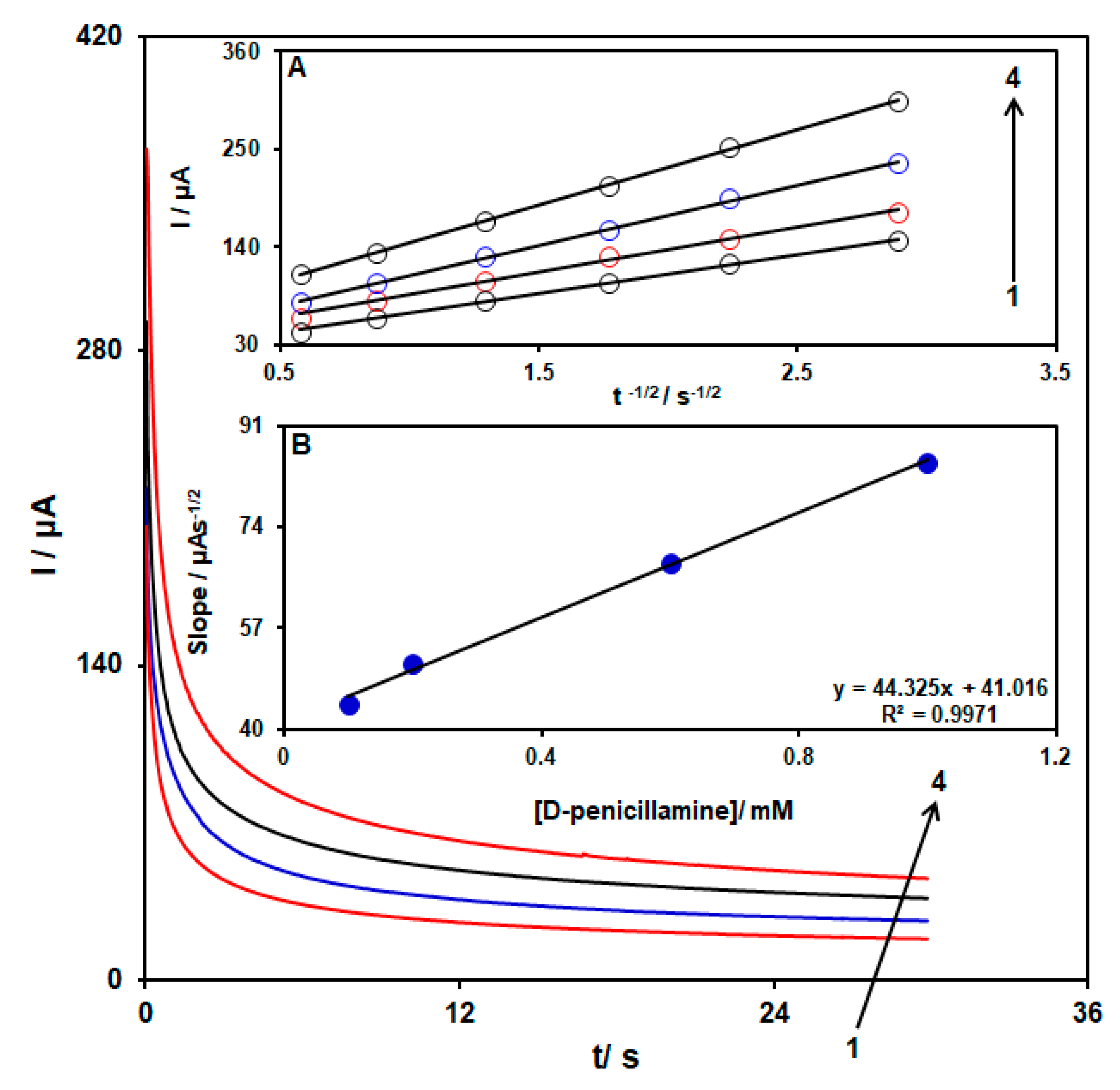
3.3. Chronoamperometric Analysis
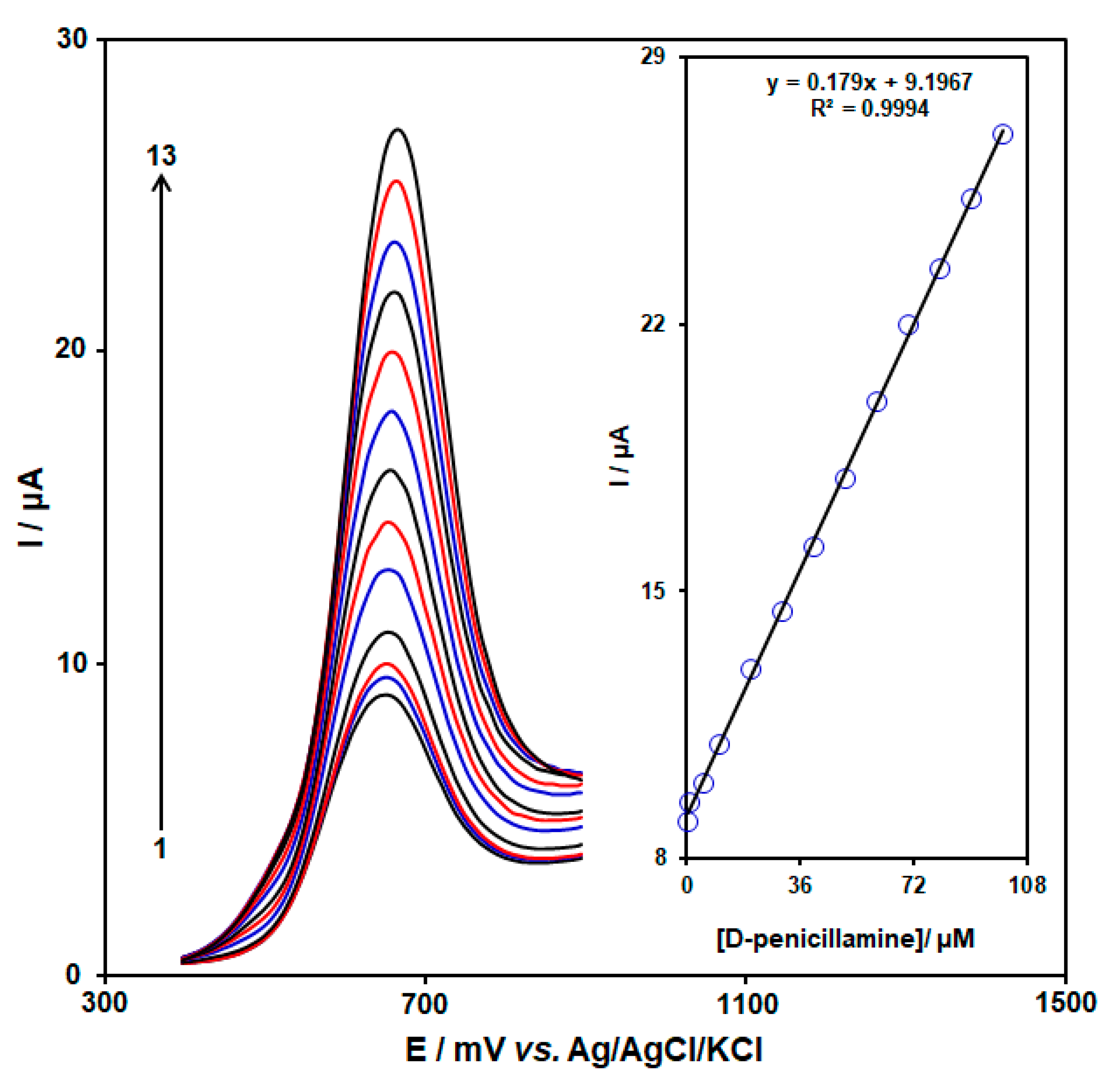
3.4. Quantitative Analysis of D-PA by DPV
3.5. Stability, Repeatability, and Reproducibility of MWCNT-Co3O4/BF/ILCPE for the Determination of D-PA
3.6. D-PA Analysis in D-PA Capsules and Urine Samples
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, Q.; Lei, M.; Kong, F.; Yang, Y. A water-stable homochiral luminescent MOF constructedfrom an achiral acylamide-containing dicarboxylate ligand for enantioselective sensing of penicillamine. Chem. Commun. 2018, 54, 10901–10904. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Yang, J.; Yuan, H.; Guo, Y.; Zeng, X.; Cheng, J.; Zhang, Y. A novel competitive-displacement fluorescence assay for l-penicillamine based on the reaction between the target and N-acetyl-l-cysteine-capped CdTe quantum dots for copper ions. Anal. Methods 2018, 10, 2263–2271. [Google Scholar] [CrossRef]
- Neri, I.; Gurioli, C.; Raggi, M.A.; Saracino, M.A.; Morganti, E.; Bugamelli, F.; de Ponti, F.; Vaccari, S.; Patrizi, A.; Balestri, R. Detection of D-penicillamine in skin lesions in a case of dermal elastosis after a previous long-term treatment for Wilson’s disease. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 383–386. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Ràfols, C.; Serrano, N.; Díaz-Cruz, J.M.; Ariño, C.; Esteban, M. Penicillamine-modified sensor for the voltammetric determination of Cd (II) and Pb (II) ions in natural samples. Talanta 2015, 144, 569–573. [Google Scholar] [CrossRef]
- Ranjith Kumar, D.; Rajesh, K.; Sayed, M.S.; Milton, A.; Shim, J.J. Development of Polydiphenylamine@ Electrochemically Reduced Graphene Oxide Electrode for the D-Penicillamine Sensor from Human Blood Serum Samples Using Amperometry. Polymers 2023, 15, 577. [Google Scholar] [CrossRef]
- Weigert, W.M.; Offermanns, H.; Degussa, P.S. D-Penicillamine—Production and Properties. Angew. Chem. Int. Ed. Engl. 1975, 14, 330–336. [Google Scholar] [CrossRef]
- Joly, D.; Rieu, P.; Méjean, A.; Gagnadoux, M.F.; Daudon, M.; Jungers, P. Treatment of cystinuria. Pediatr. Nephrol. 1999, 13, 945–950. [Google Scholar] [CrossRef]
- Dunea, G. Kidney stones: Medical and surgical management. JAMA 1996, 276, 577. [Google Scholar] [CrossRef]
- Ma, L.; Fan, M.; Xu, X.; Kang, W.; Shi, H. 2011 Utilization of a novel Ag (III)-luminol chemiluminescence system for determination of d-penicillamine in human urine samples. J. Braz. Chem. Soc. 1996, 22, 1463–1469. [Google Scholar]
- Pawar, S.P.; Gore, A.H.; Walekar, L.S.; Anbhule, P.V.; Patil, S.R.; Kolekar, G.B. Turn-on fluorescence probe for selective and sensitive detection of d-penicillamine by CdS quantum dots in aqueous media: Application to pharmaceutical formulation. Sens. Actuators B Chem. 2015, 209, 911–918. [Google Scholar] [CrossRef]
- Saracino, M.A.; Cannistraci, C.; Bugamelli, F.; Morganti, E.; Neri, I.; Balestri, R.; Raggi, M.A. A novel HPLC-electrochemical detection approach for the determination of d-penicillamine in skin specimens. Talanta 2013, 103, 355–360. [Google Scholar] [CrossRef]
- Cao, L.; Wei, T.; Shi, Y.; Tan, X.; Meng, J. Determination of D-penicillamine and tiopronin in human urine and serum by HPLC-FLD and CE-LIF with 1,3,5,7-tetramethyl-8-bromomethyl-difluoroboradiaza-s-indacene. J. Liq. Chromatogr. Relat. Technol. 2018, 41, 58–65. [Google Scholar] [CrossRef]
- Nazifi, M.; Ramezani, A.M.; Absalan, G.; Ahmadi, R. Colorimetric determination of D-penicillamine based on the peroxidase mimetic activity of hierarchical hollow MoS2 nanotubes. Sens. Actuators B Chem. 2021, 332, 129459. [Google Scholar] [CrossRef]
- Wang, Q.; Li, L.; Wu, T.; Kong, X.; Ma, Q.; Ma, C. A graphene quantum dots-Pb2+ based fluorescent switch for selective and sensitive determination of D-penicillamine. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2020, 229, 117924. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Li, B.L.; Li, N.B.; Luo, H.Q. A fluorescence detection of d-penicillamine based on Cu2+-induced fluorescence quenching system of protein-stabilized gold nanoclusters. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 135, 198–202. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.R.; Baynosa, M.L.; Dhakal, G.; Shim, J.J. Sphere-like Ni3S4/NiS2/MoOx composite modified glassy carbon electrode for the electrocatalytic determination of d-penicillamine. J. Mol. Liq. 2020, 301, 112447. [Google Scholar] [CrossRef]
- Jafari, M.; Tashkhourian, J.; Absalan, G. Electrochemical sensing of D-penicillamine on modified glassy carbon electrode by using a nanocomposite of gold nanoparticles and reduced graphene oxide. J. Iran. Chem. Soc. 2017, 14, 1253–1262. [Google Scholar] [CrossRef]
- Safari, F.; Keyvanfard, M.; Karimi-Maleh, H.; Alizad, K. Voltammetric determination of penicillamine using a carbon paste electrode modified with multiwall carbon nanotubes in the presence of methyldopa as a mediator. Iran. J. Pharm. Res. 2017, 16, 1019. [Google Scholar]
- Raoof, J.B.; Ojani, R.; Majidian, M.; Chekin, F. Voltammetric determination of D-penicillamine based on its homogeneous electrocatalytic oxidation with potassium iodide at the surface of glassy carbon electrode. Russ. J. Electrochem. 2010, 46, 1395–1401. [Google Scholar] [CrossRef]
- Raoof, J.B.; Ojani, R.; Amiri-Aref, M.; Chekin, F. Catechol as an electrochemical indicator for voltammetric determination of D-penicillamine in aqueous media at the surface of carbon paste electrode. Russ. J. Electrochem. 2012, 48, 450–456. [Google Scholar] [CrossRef]
- Raoof, J.B.; Ojani, R.; Chekin, F. Electrochemical analysis of d-Penicillamine using a carbon paste electrode modified with ferrocene carboxylic acid. Electroanalysis 2007, 19, 1883–1889. [Google Scholar] [CrossRef]
- Pourtaheri, E.; Taher, M.A.; Beitollahi, H.; Ranjbar, M. ZnIn2S4 Nanoparticles Modified Carbon Paste Electrode for Voltammetric Determination of Penicillamine. Anal. Bioanal. Electrochem. 2016, 8, 803–813. [Google Scholar]
- Shahrokhian, S.; Souri, A.; Khajehsharifi, H. Electrocatalytic oxidation of penicillamine at a carbon paste electrode modified with cobalt salophen. J. Electroanal. Chem. 2004, 565, 95–101. [Google Scholar] [CrossRef]
- Keyvanfard, M.; Najjarian, N.; Alizad, K. Electrocatalytic determination of penicillamine using multiwall carbon nanotubes paste electrode and chlorpromazine as a mediator. J. Anal. Chem. 2017, 72, 1045–1050. [Google Scholar] [CrossRef]
- Han, C.; Yi, W.; Li, Z.; Dong, C.; Zhao, H.; Liu, M. Single-atom palladium anchored N-doped carbon enhanced electrochemical detection of furazolidone. Electrochim. Acta 2023, 447, 142083. [Google Scholar] [CrossRef]
- Amudi, K.; Yiğit, A.; Menges, N.; Pınar, P.T. Highly selective electrochemical sensor for new generation targeted-anticancer drug Ibrutinib using newly synthesized nanomaterial GO-NH-B(OH)2@AgNPs modified glassy carbon electrode. Measurement 2023, 216, 112978. [Google Scholar] [CrossRef]
- Ariavand, S.; Ebrahimi, M.; Foladi, E. Design and construction of a novel and an efficient potentiometric sensor for determination of sodium ion in urban water samples. Chem. Methodol. 2022, 6, 886–904. [Google Scholar]
- Garkani Nejad, F.; Beitollahi, H.; Sheikhshoaie, I. Graphene oxide–PAMAM nanocomposite and ionic liquid modified carbon paste electrode: An efficient electrochemical sensor for simultaneous determination of catechol and resorcinol. Diagnostics 2023, 13, 632. [Google Scholar] [CrossRef] [PubMed]
- Karimi-Maleh, H.; Liu, Y.; Li, Z.; Darabi, R.; Orooji, Y.; Karaman, C.; Karimi, F.; Baghayeri, M.; Rouhi, J.; Fu, L. Calf thymus ds-DNA intercalation with pendimethalin herbicide at the surface of ZIF-8/Co/rGO/C3N4/ds-DNA/SPCE.; A bio-sensing approach for pendimethalin quantification confirmed by molecular docking study. Chemosphere 2023, 332, 138815. [Google Scholar] [CrossRef] [PubMed]
- Mehdizadeh, Z.; Shahidi, S.; Ghorbani-HasanSaraei, A.; Limooei, M.; Bijad, M. Monitoring of amaranth in drinking samples using voltammetric amplified electroanalytical sensor. Chem. Methodol. 2022, 6, 246–252. [Google Scholar]
- Roshanfekr, H. A simple specific dopamine aptasensor based on partially reduced graphene oxide–Au NPs composite. Prog. Chem. Biochem. Res. 2023, 6, 79–88. [Google Scholar]
- Cheraghi, S.; Taher, M.A.; Karimi-Maleh, H.; Karimi, F.; Shabani-Nooshabadi, M.; Alizadeh, M.; Al-Othman, A.; Erk, N.; Raman, P.K.Y.; Karaman, C. Novel enzymatic graphene oxide based biosensor for the detection of glutathione in biological body fluids. Chemosphere 2022, 287, 132187. [Google Scholar] [CrossRef] [PubMed]
- Arpitha, S.B.; Swamy, B.K.; Shashikumara, J.K. An efficient electrochemical sensor based on ZnO/Co3O4 nanocomposite modified carbon paste electrode for the sensitive detection of hydroquinone and resorcinol. Inorg. Chem. Commun. 2023, 152, 110656. [Google Scholar] [CrossRef]
- Hosseini Fakhrabad, A.; Sanavi Khoshnood, R.; Abedi, M.R.; Ebrahimi, M. Fabrication a composite carbon paste electrodes (CPEs) modified with Multi-Wall Carbon Nano-Tubes (MWCNTs/N, N-Bis (salicyliden)-1,3-propandiamine) for determination of lanthanum (III). Eurasian Chem. Commun. 2021, 3, 627–634. [Google Scholar]
- Li, Y.; Liu, J.; Zhang, Y.; Gu, M.; Wang, D.; Dang, Y.Y.; Ye, B.C.; Li, Y. A robust electrochemical sensing platform using carbon paste electrode modified with molecularly imprinted microsphere and its application on methyl parathion detection. Biosens. Bioelectron. 2018, 106, 71–77. [Google Scholar] [CrossRef]
- Wei, D.; Ivaska, A. Applications of ionic liquids in electrochemical sensors. Anal. Chim. Acta 2008, 607, 126–135. [Google Scholar] [CrossRef]
- Amer, W.A.; Wang, L.; Amin, A.M.; Ma, L.; Yu, H. Recent progress in the synthesis and applications of some ferrocene derivatives and ferrocene-based polymers. J. Inorg. Organomet. Polym. Mater. 2010, 20, 605–615. [Google Scholar] [CrossRef]
- Baig, N.; Sajid, M.; Saleh, T.A. Recent trends in nanomaterial-modified electrodes for electroanalytical applications. TrAC Trends Anal. Chem. 2019, 111, 47–61. [Google Scholar] [CrossRef]
- Srivastava, A.K.; Upadhyay, S.S.; Rawool, C.R.; Punde, N.S.; Rajpurohit, A.S. Voltammetric techniques for the analysis of drugs using nanomaterials based chemically modified electrodes. Curr. Anal. Chem. 2019, 15, 249–276. [Google Scholar] [CrossRef]
- Molahalli, V.; Bhat, V.S.; Shetty, A.; Hundekal, D.; Toghan, A.; Hegde, G. ZnO doped SnO2 nano flower decorated on graphene oxide/polypyrrole nanotubes for symmetric supercapacitor applications. J. Energy Storage 2023, 69, 107953. [Google Scholar] [CrossRef]
- Jayachandran, P.; Ilango, S.; Suseela, V.; Nirmaladevi, R.; Shaik, M.R.; Khan, M.; Shaik, B. Green synthesized silver nanoparticle-loaded liposome-based nanoarchitectonics for cancer management: In vitro drug release analysis. Biomedicines 2023, 11, 217. [Google Scholar] [CrossRef] [PubMed]
- Janitabar Darzi, S.; Bastami, H. Au decorated mesoporous TiO2 as a high performance photocatalyst towards crystal violet dye. Adv. J. Chem. A 2022, 5, 22–30. [Google Scholar]
- Sheikhshoaie, I.; Rezazadeh, A.; Ramezanpour, S. Removal of Pb (II) from aqueous solution by gel combustion of a new nano sized Co3O4/ZnO composite. Asian J. Nanosci. Mater. 2022, 5, 336–345. [Google Scholar]
- Sharma, M.; Yadav, S.; Srivastava, M.; Ganesh, N.; Srivastava, S. Promising anti-inflammatory bio-efficacy of saponin loaded silver nanoparticles prepared from the plant Madhuca longifolia. Asian J. Nanosci. Mater. 2022, 5, 313–326. [Google Scholar]
- Nareetsile, F.; Matshwele, J.T.; Odisitse, S. Metallo-drugs as promising antibacterial agents and their modes of action. J. Med. Chem. Sci. 2022, 5, 1109–1131. [Google Scholar]
- Zhang, Z.; Karimi-Maleh, H. In situ synthesis of label-free electrochemical aptasensor-based sandwich-like AuNPs/PPy/Ti3C2Tx for ultrasensitive detection of lead ions as hazardous pollutants in environmental fluids. Chemosphere 2023, 324, 138302. [Google Scholar] [CrossRef]
- Beitollahi, H.; Mahmoudi Moghaddam, H.; Tajik, S. Voltammetric determination of bisphenol A in water and juice using a lanthanum (III)-doped cobalt (II, III) nanocube modified carbon screen-printed electrode. Anal. Lett. 2019, 52, 1432–1444. [Google Scholar] [CrossRef]
- Azimi, S.; Amiri, M.; Imanzadeh, H.; Bezaatpour, A. Fe3O4@SiO2-NH2/CoSB modified carbon paste electrode for simultaneous detection of acetaminophen and chlorpheniramine. Adv. J. Chem A 2021, 4, 152–164. [Google Scholar]
- Ajoudanian, N.; Nezamzadeh-Ejhieh, A. Enhanced photocatalytic activity of nickel oxide supported on clinoptilolite nanoparticles for the photodegradation of aqueous cephalexin. Mater. Sci. Semicond. Process. 2015, 36, 162–169. [Google Scholar] [CrossRef]
- Atta, N.F.; Galal, A.; Hassan, S.H. Ultrasensitive determination of nalbuphine and tramadol narcotic analgesic drugs for postoperative pain relief using nano-cobalt oxide/ionic liquid crystal/carbon nanotubes-based electrochemical sensor. J. Electroanal. Chem. 2019, 839, 48–58. [Google Scholar] [CrossRef]
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Bijad, M.; Karimi-Maleh, H.; Farsi, M.; Shahidi, S.-A. An electrochemical-amplified-platform based on the nanostructure voltammetric sensor for the determination of carmoisine in the presence of tartrazine in dried fruit and soft drink samples. J. Food Meas. Charact. 2018, 12, 634–640. [Google Scholar] [CrossRef]
- Tekleab, D.; Czerw, R.; Carroll, D.L.; Ajayan, P.M. Electronic structure of kinked multiwalled carbon nanotubes. Appl. Phys. Lett. 2000, 76, 3594–3596. [Google Scholar] [CrossRef]
- Fayemi, O.E.; Adekunle, A.S.; Ebenso, E.E. Metal oxide nanoparticles/multi-walled carbon nanotube nanocomposite modified electrode for the detection of dopamine: Comparative electrochemical study. J. Biosens. Bioelectron. 2015, 6, 10–4172. [Google Scholar] [CrossRef]
- Kenarkob, M.; Pourghobadi, Z. Electrochemical sensor for acetaminophen based on a glassy carbon electrode modified with ZnO/Au nanoparticles on functionalized multi-walled carbon nano-tubes. Microchem. J. 2019, 146, 1019–1025. [Google Scholar] [CrossRef]
- Zhao, G.C.; Xu, M.Q.; Zhang, Q. A novel hydrogen peroxide sensor based on the redox of ferrocene on room temperature ionic liquid film. Electrochem. Commun. 2008, 10, 1924–1926. [Google Scholar] [CrossRef]
- Zhilei, W.; Zaijun, L.; Xiulan, S.; Yinjun, F.; Junkang, L. Synergistic contributions of fullerene, ferrocene, chitosan and ionic liquid towards improved performance for a glucose sensor. Biosens. Bioelectron. 2010, 25, 1434–1438. [Google Scholar] [CrossRef]
- Choosang, J.; Khumngern, S.; Thavarungkul, P.; Kanatharana, P.; Numnuam, A. An ultrasensitive label-free electrochemical immunosensor based on 3D porous chitosan–graphene–ionic liquid–ferrocene nanocomposite cryogel decorated with gold nanoparticles for prostate-specific antigen. Talanta 2021, 224, 121787. [Google Scholar] [CrossRef]
- Sun, W.; Zhai, Z.Q.; Wang, D.D.; Liu, S.F.; Jiao, K. Electrochemistry of hemoglobin entrapped in a Nafion/nano-ZnO film on carbon ionic liquid electrode. Bioelectrochemistry 2009, 74, 295–300. [Google Scholar] [CrossRef]
- Mohammadnavaz, A.; Beitollahi, H.; Modiri, S. Electro-catalytic determination of l-Cysteine using multi walled carbon nanotubes-Co3O4 nanocomposite/benzoylferrocene/ionic liquid modified carbon paste electrode. Inorganica Chim. Acta 2023, 548, 121340. [Google Scholar] [CrossRef]
- Baghbanpoor, P.; Shishehbore, M.R.; Beitollahi, H.; Sheibani, A. The application of ferrocene derivative and CeO–ZnO nanocomposite-modified carbon paste electrode for simultaneous detection of penicillamine and tryptophan. Russ. J. Electrochem. 2022, 58, 235–247. [Google Scholar] [CrossRef]


| Electrochemical Sensor | Electrochemical Method | Linear Range | Limit of Detection | Ref. |
|---|---|---|---|---|
| Polydiphenylamine@electrochemically reduced graphene oxide/glassy carbon electrode | Amperometry | 1.4 μM–541 µM | 0.10 µM | [5] |
| Ni3S4/NiS2/MoOx composite/glassy carbon electrode | Amperometry | 5 μM–796 μM | 0.26 µM | [16] |
| Au nanoparticle-reduced graphene oxide/glassy carbon electrode | DPV | 5 μM–110 μM | 3.9 µM | [17] |
| Multi-walled carbon nanotubes/CPE in the presence of methyldopa as a mediator | Square wave voltammetry | 0.2 µM–250.0 µM | 0.1 µM | [18] |
| Potassium iodide (mediator)/glassy carbon electrode | DPV | 9 µM–120 µM | 3.5 µM | [19] |
| CV | 30 µM–1500 µM | 30 µM | ||
| Catechol (electrochemical indicator)/CPE | DPV | 70 µM–1000 µM | 50 µM | [20] |
| CV | 100 µM–1000 µM | 58 µM | ||
| Ferrocene carboxylic acid/CPE | DPV | 6.5 µM–100 µM | 6.15 µM | [21] |
| CV | 75 µM–1000 µM | 60.4 µM | ||
| ZnIn2S4 nanoparticles/CPE | Square wave voltammetry | 0.5 μM–80.0 μM | 0.3 µM | [22] |
| Cobalt salophen Schiff base complex/CPE | Square wave voltammetry | 0.1 µM–100.0 µM | 0.1 µM | [23] |
| Multi-walled carbon nanotubes paste electrode in the presence of chlorpromazine as a mediator | Linear sweep voltammetry | 0.5 μM–500 μM | 0.2 µM | [24] |
| MWCNT-Co3O4/BF/ILCPE | DPV | 0.05–100.0 μM | 0.015 µM | This work |
| Sample | Spiked Concentration | Found Concentration | Recovery | R.S.D. (%) |
|---|---|---|---|---|
| D-PA capsules | 0 | 3.7 μM | - | 3.4% |
| 1.0 μM | 4.6 μM | 97.9% | 1.9% | |
| 2.0 μM | 5.9 μM | 103.5% | 2.4% | |
| 3.0 μM | 6.8 μM | 101.5% | 3.0% | |
| 4.0 μM | 7.6 μM | 98.7% | 2.7% | |
| Urine | 0 | - | - | - |
| 5.0 μM | 5.1 μM | 102.0% | 1.8% | |
| 6.0 μM | 5.8 μM | 96.7% | 3.4% | |
| 7.0 μM | 7.3 μM | 104.3% | 2.3% | |
| 8.0 μM | 7.9 μM | 98.7% | 2.1% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohammadnavaz, A.; Beitollahi, H.; Modiri, S. Construction and Application of an Electrochemical Sensor for Determination of D-Penicillamine Based on Modified Carbon Paste Electrode. Micromachines 2024, 15, 220. https://doi.org/10.3390/mi15020220
Mohammadnavaz A, Beitollahi H, Modiri S. Construction and Application of an Electrochemical Sensor for Determination of D-Penicillamine Based on Modified Carbon Paste Electrode. Micromachines. 2024; 15(2):220. https://doi.org/10.3390/mi15020220
Chicago/Turabian StyleMohammadnavaz, Arefeh, Hadi Beitollahi, and Sina Modiri. 2024. "Construction and Application of an Electrochemical Sensor for Determination of D-Penicillamine Based on Modified Carbon Paste Electrode" Micromachines 15, no. 2: 220. https://doi.org/10.3390/mi15020220
APA StyleMohammadnavaz, A., Beitollahi, H., & Modiri, S. (2024). Construction and Application of an Electrochemical Sensor for Determination of D-Penicillamine Based on Modified Carbon Paste Electrode. Micromachines, 15(2), 220. https://doi.org/10.3390/mi15020220







