An Overview of the Recent Advances in Pool Boiling Enhancement Materials, Structrure, and Devices
Abstract
:1. Introduction
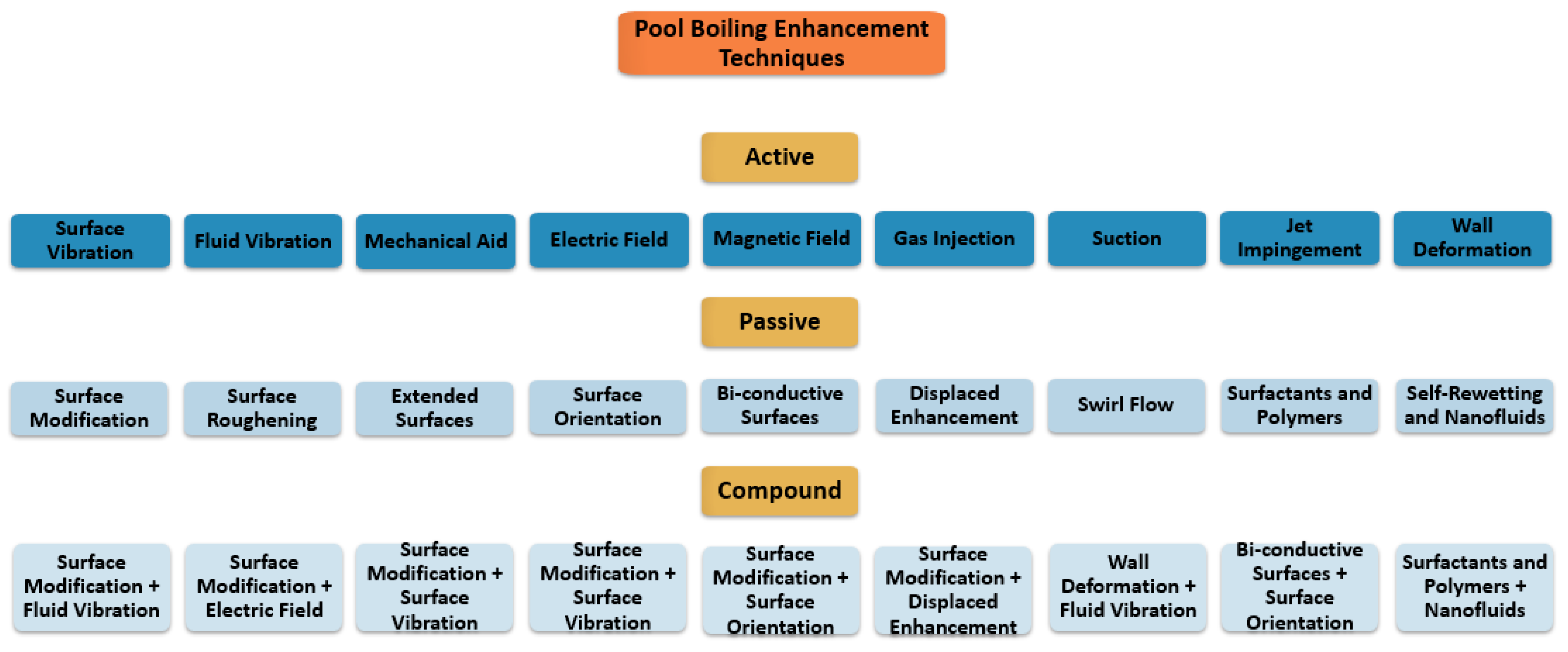
2. Pool Boiling Enhancement Techniques
2.1. Active Techniques
2.1.1. Surface Vibration
2.1.2. Fluid Vibration
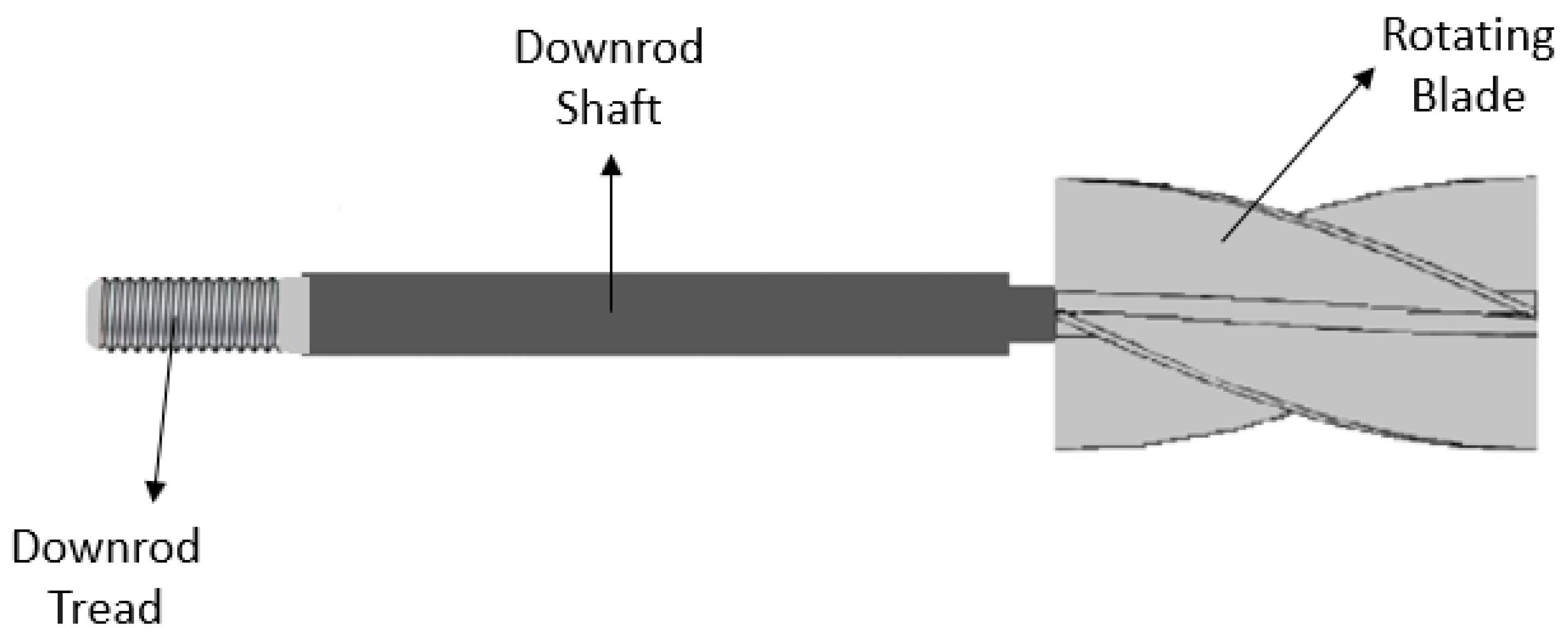
2.1.3. Mechanical Aid
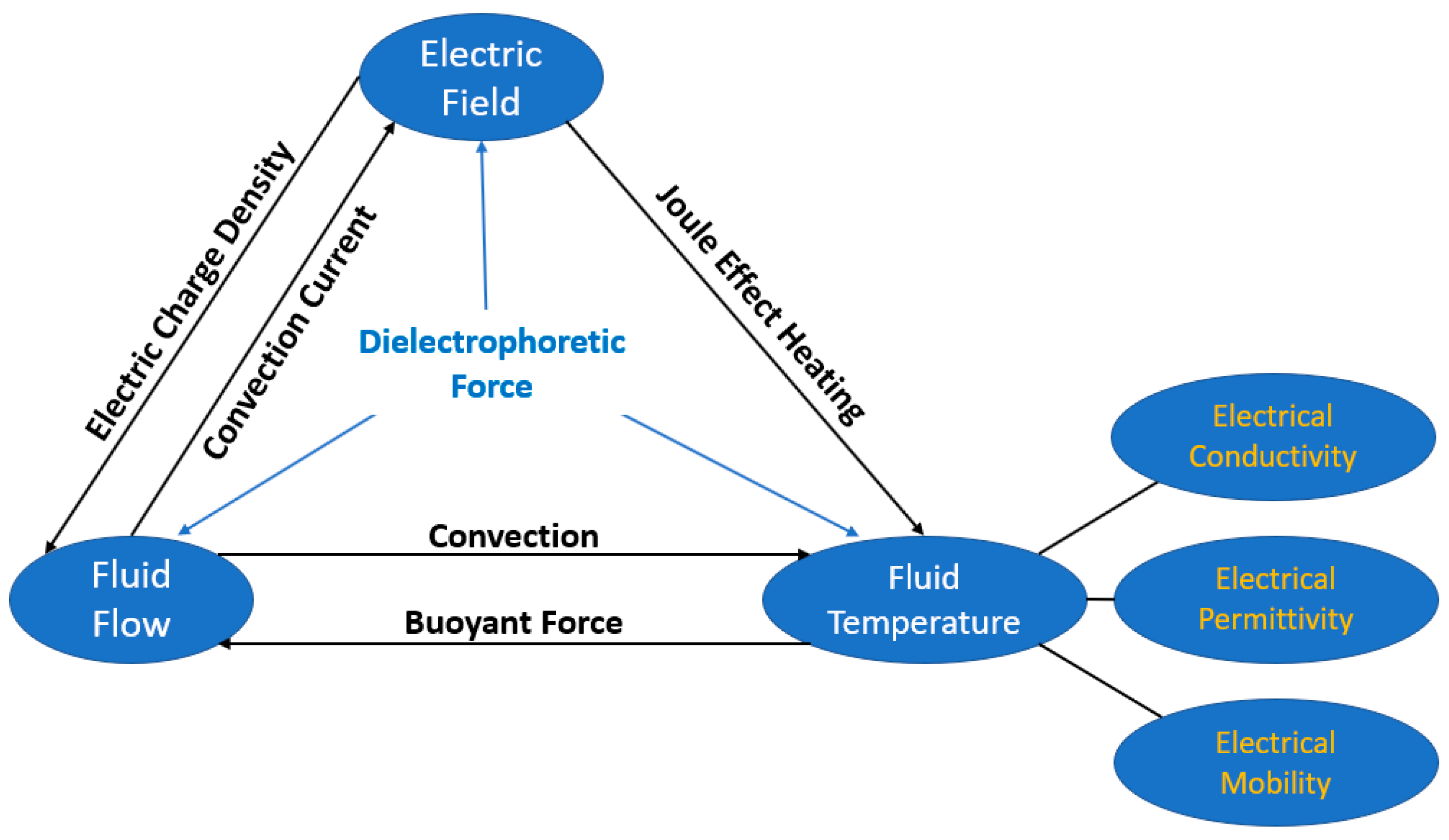
2.1.4. Electric Field
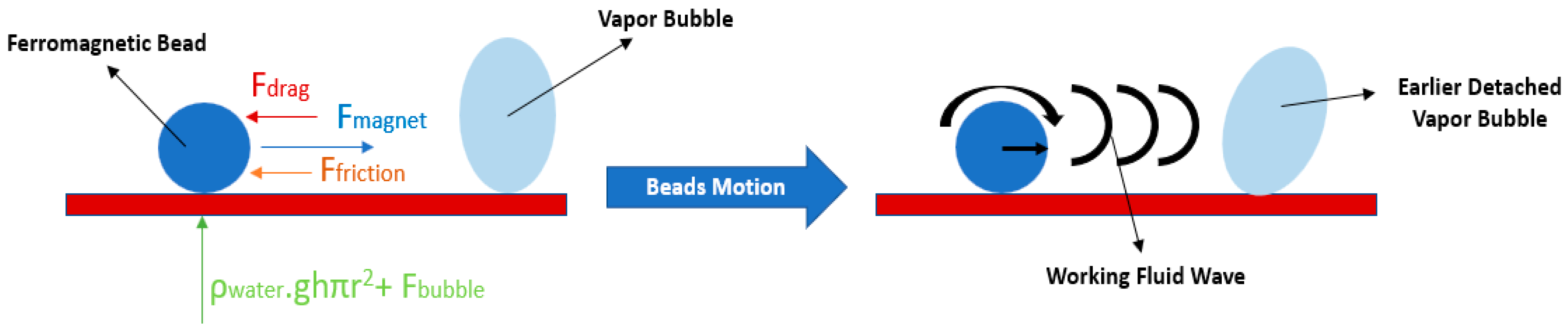
2.1.5. Magnetic Field
2.1.6. Gas Injection
2.1.7. Suction
2.1.8. Local Jet Impingement
2.1.9. Wall Deformation
2.2. Passive Techniques
2.2.1. Surface Modification
Nanoparticles
Nanotubes
Nanowires and Nanofibers
2.2.2. Surface Modification by Materials and Structures
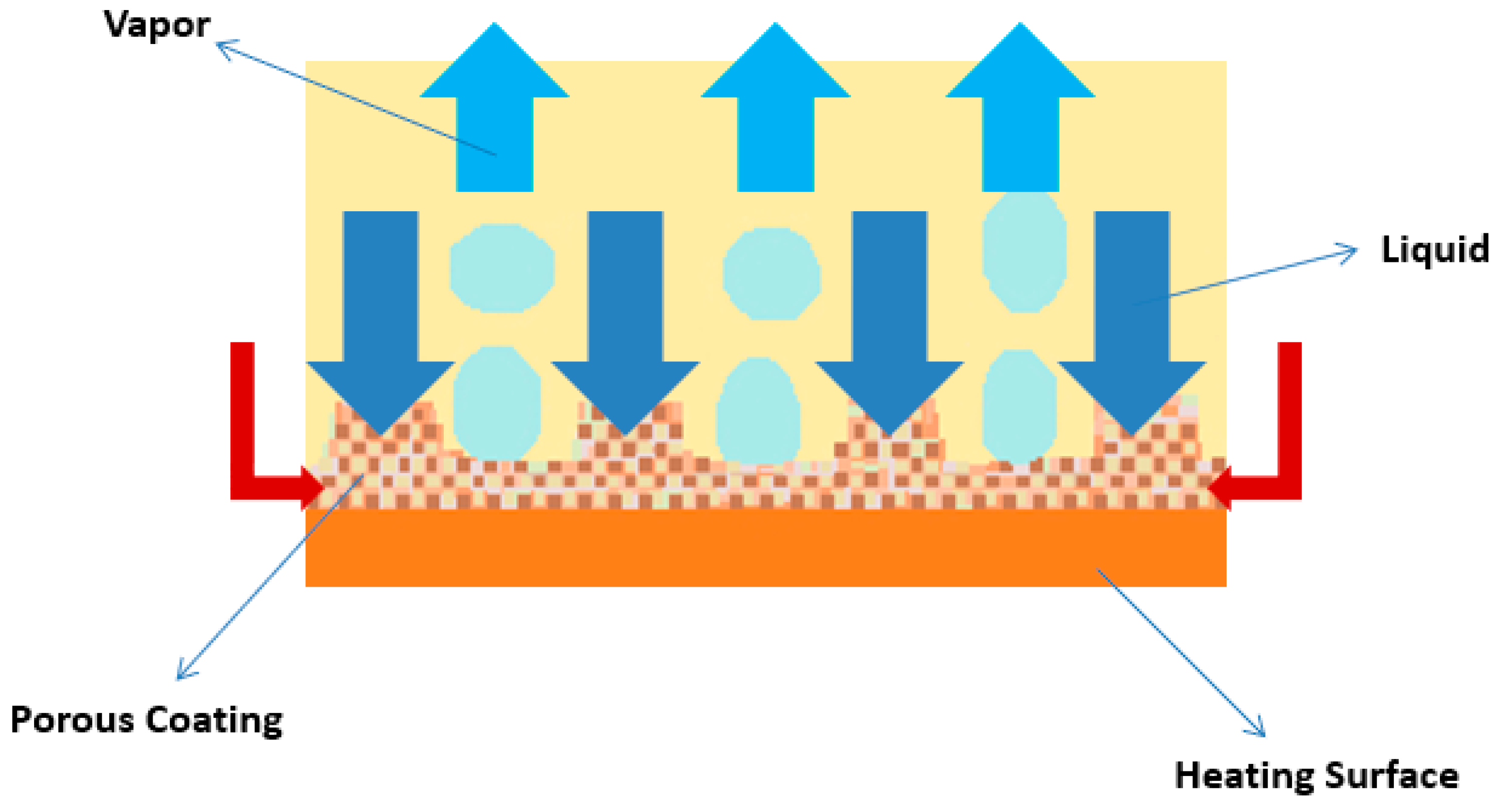
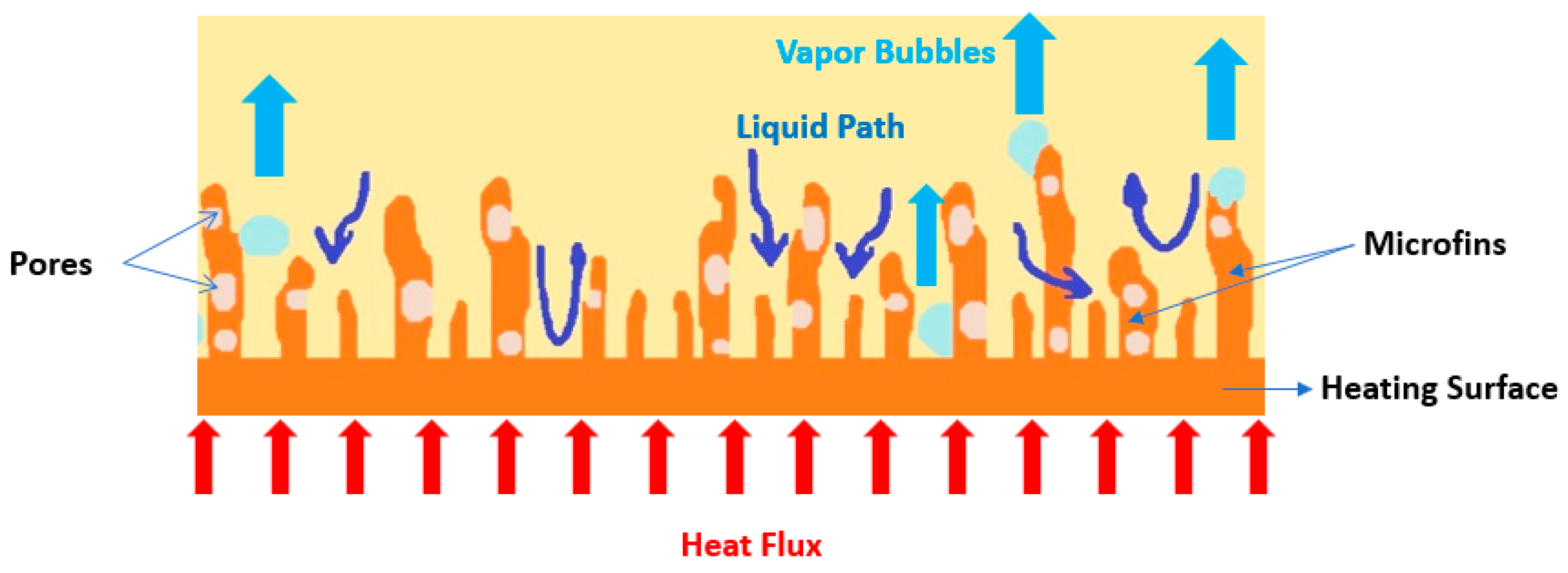
Porous Coatings and Structures
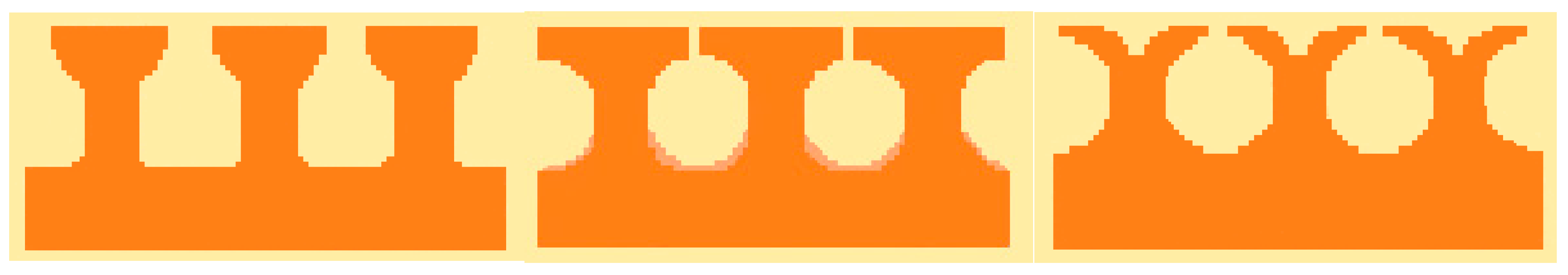
Tunnels and Reentrant Cavities
Wicking and Grooved Surfaces
Fins and Studs
Fins and Porous Structures
Fins and Particles or Multiscale Structures
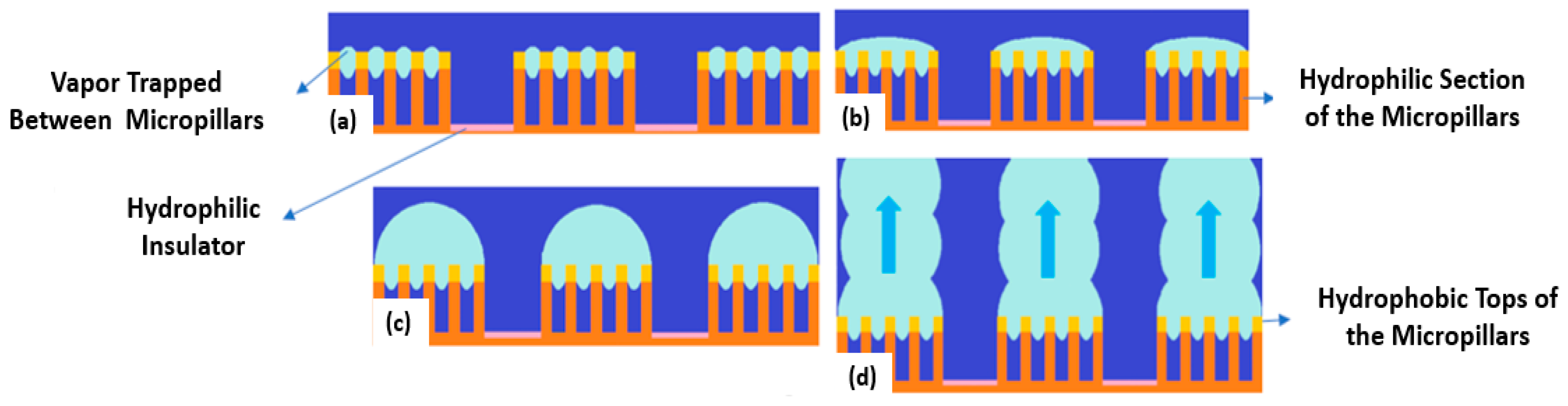
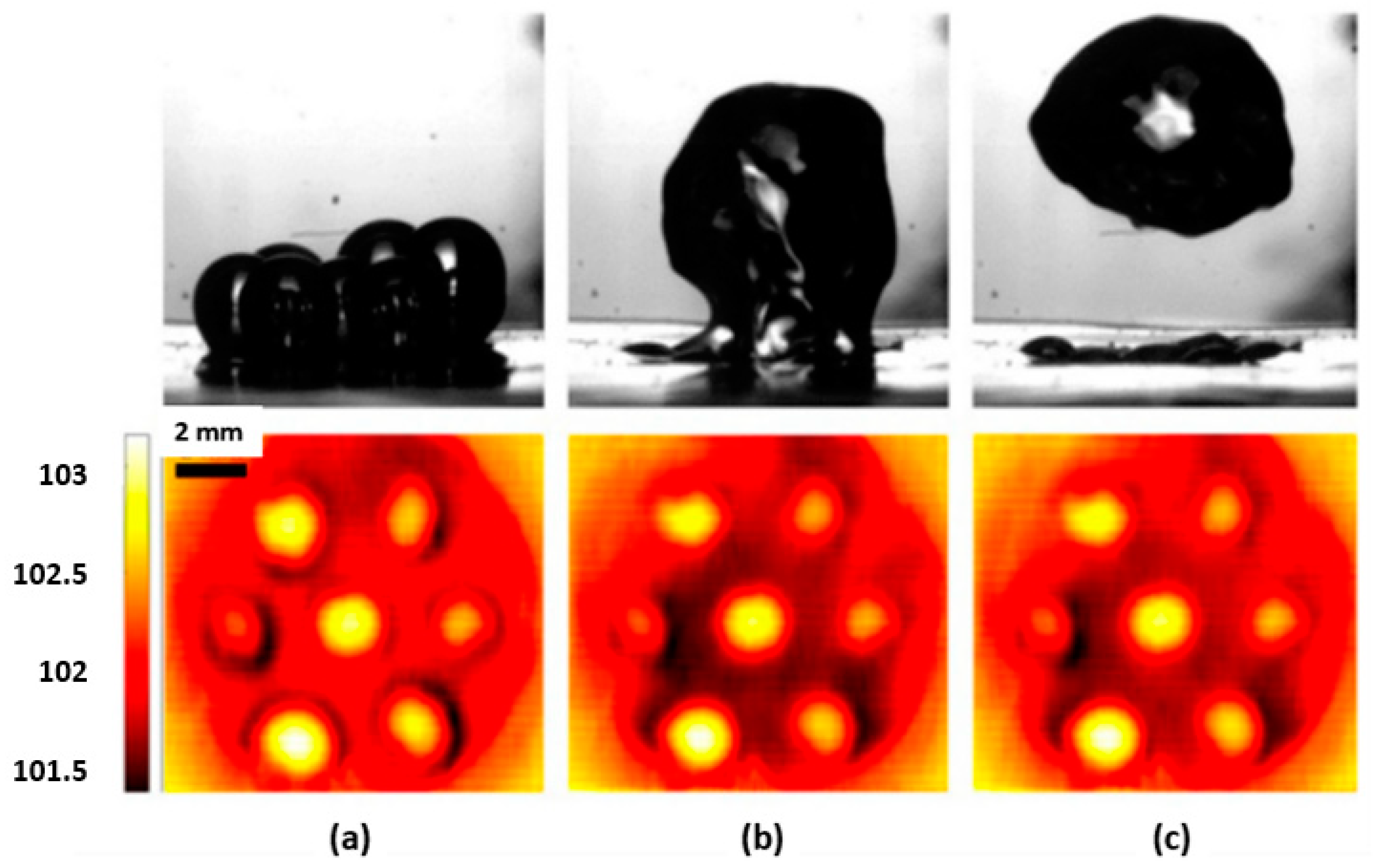
Hydrophilic, Hydrophobic, and Biphilic Surfaces
Surface Roughening
Extended Surface
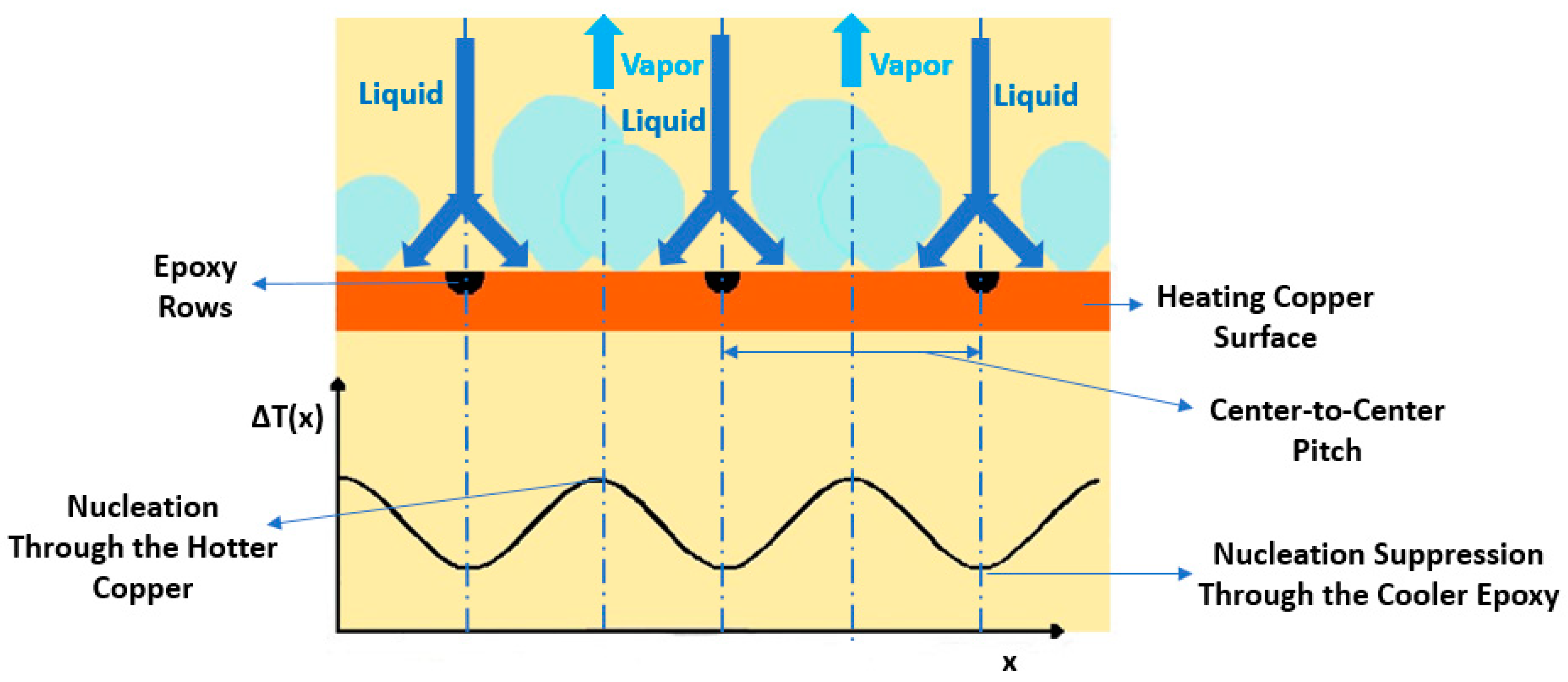
Bi-Conductive Surfaces
2.2.3. Fluid Flow Enhancement
Displaced Enhancement
Swirl Flow
Surface Orientation and Gap Width
2.3. Compound Enhancement
2.3.1. Wall Deformation and Fluid Vibration
2.3.2. Fluid Vibration and Surface Enhancement
2.3.3. Electric Field and Surface Enhancement
2.3.4. Local Jet Impingement and Surface Enhancement
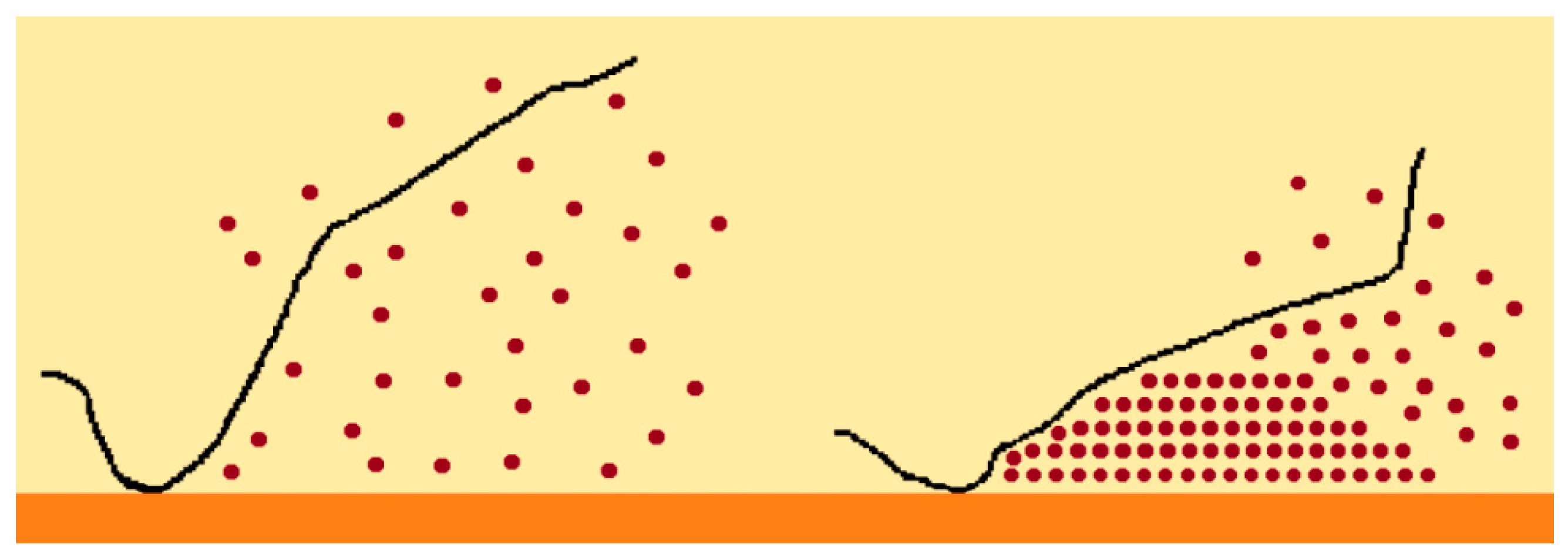
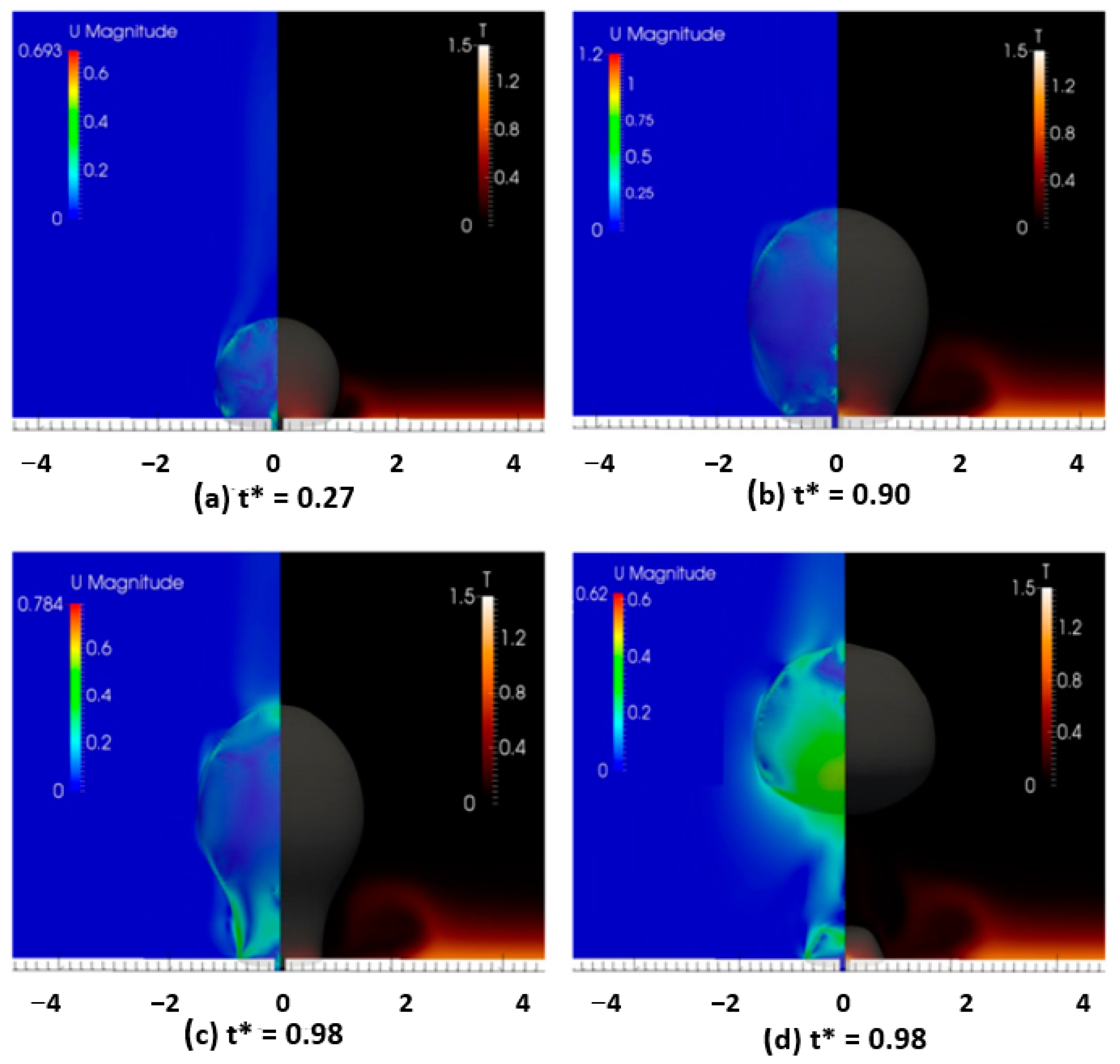
3. Influence of the Liquid Pool Height on the Boiling Heat Transfer Enhancement
4. Limitations, Challenges, and Recommendations for Further Research
- (i)
- Despite the verified progress in the nucleate pool boiling heat transfer performance employing innovative coating materials and techniques, the large-scale applications of these concepts remain hindered or, in some cases, even totally unpractical. To overcome such limitations, emphasis should be given to the durability and cost-effectiveness of the coatings, which are the main factors that still limit their large-scale implementation in applications like water harvesting, self-cleaning, aerospace, and thermal management based on the use of heat exchangers, heat tubes, thermosyphons, and other devices. In this direction, innovative fabrication methodologies are required to be implemented to ensure the cost-effectiveness, reliability, and longevity of the enhanced boiling surfaces. Although there have already been reports of effective enhancing methodologies, there is still a lack of comprehensive techno-economic analysis for these enhanced surfaces to be applied in practical nucleate boiling heat transfer situations.
- (ii)
- Durability tests should be implemented on the coatings for performance inferring and to make a regular comparison of the employed methodologies and materials, allowing for further lifespan determination for the coatings. In addition, future studies should be conducted based on the analysis of the corrosion and erosion resistances, and adhesion strengths of the enhanced surfaces to be applied to nucleate pool boiling heat transfer enhancement ends.
- (iii)
- The compound or hybrid enhancement route should be further investigated to better understand the possibilities of combining more than one enhancement technique searching for enhanced heat transfer capability, such as the inclusion of nanoparticles on microstructured porous coatings, the inclusion of nanotubes on microchannels, and the combined usage of microporous and hydrophilic or hydrophobic coatings.
- (iv)
- Limiting values for CHF should be established using each type of enhanced heat transfer boiling surface, which should be achieved together with the durability testing results. Values for the CHF limits should be obtained for newly produced surfaces and for aged surfaces according to a well-defined start-up/cool-down cycle.
- (v)
- A standard definition for a smooth heating surface should be adopted, which should be taken as the reference boiling surface in each heat transfer enhancement route, and its preparation method should be unified to properly address the enhancement level in the HTC and CHF and the respective inter-laboratorial comparison.
- (vi)
- A ranking criterion should be assumed for structured surface enhancements in the nucleate HTC and CHF values under pool boiling scenarios. A minimum percentage compared to a bare, plain heating surface should be assumed, in which the enhancement qualifies the surface as possessing superior nucleate HTC and CHF. It would also be useful to classify the heat transfer enhancement techniques according to the experimental obtained results obtained by standardized methods under the same operating conditions.
- (vii)
- The pool boiling regime offers a harsh environment for heat transfer surfaces. The existing high surface temperatures, together with the large interfacial fluid flow velocities, are adverse to the durability of surface features. Hence, the durability testing for each new enhanced structured surface should be performed with intermittent start-up and shut-down cycles. For instance, at least 10 start-up/shutdown cycles should be conducted, with each cycle lasting a few hours, followed by a cool-down for 24 h.
- (viii)
- The published results often demonstrate the beneficial features of biphilic heating surfaces over conventional hydrophilic and hydrophobic ones. Nonetheless, there are still many aspects closely linked to this technological area that should be better known. For example, the sustainability of the hydrophobic regions should be investigated by calculating the heating wall shear stress to assess whether such surfaces can withstand the continuous bubble generation and detachment from the surface over time. Also, the shape, size, and pitch of the wettability patterns need to be optimized for heat transfer enhancement purposes.
- (ix)
- The criteria for inferring CHF should be unified among the research community because it is clearly subjective. Most investigators detect CHF from the sudden rise of the heat transfer surface temperature but do not agree on its value. For instance, the authors Ahn et al. [169] recorded the CHF at a very high 86 K superheat, and the authors Kong et al. [170] recorded the CHF at only an 8.2 K superheat. This lack of uniformity in the experimental procedures will lead to a wider variation in the reliability of the actual CHF predictive equations and models and an inaccurate comparison of the nucleate pool boiling heat transfer enhancement results from different elements of the research community.
5. Conclusions
- (i)
- Pool boiling enhancement techniques usually achieve higher HTC and CHF values when compared with those provided through nucleate pool boiling alone. The most suitable pool boiling enhancement techniques should be selected considering the boiling space, the achievable nucleate boiling heat transfer performance, the investment cost, geometrical factors like the size and shape of the boiling surface, and the application field. The implementation of structured enhanced surfaces under pool boiling scenarios involves concerns intertwined from many aspects. On the one hand, the structures introduce surface irregularities and increase the number of active nucleation sites on the heating surface, which may improve the heat transfer performance. On the other hand, based on the Cassie–Baxter theory, the structures alter the contact angle and wettability, which control the interfacial hydrodynamic behavior of the vapor bubbles, promoting the initiation of the CHF. The pool boiling HTC and CHF increase with a nanostructured surface compared with an untreated surface. This increase can be appreciable, up to 100% or even more, depending on the material, thickness, and structure of the coating. Nonetheless, despite the large number of published works addressing pool boiling heat transfer enhancement, there is still a noticeable scarcity of sufficiently large available databases of surface enhancement methods regarding the fluid type, material, dimensions, and orientation of the heating surface as well as the enhancement methodology pattern and operating pressure. This evidence has led to less-than-adequate available findings for the design of enhanced surfaces for thermal management purposes.
- (ii)
- The coating of the heating surface with nanoparticles, nanotubes, nanowires, nanofibers, nanoporous, and nanofilm layers, among other forms, has the advantage of nucleate boiling heat transfer enhancement by means of capillary wicking within the nanostructures. The taller nanotubes often give better nucleate HTC and CHF values. The underlying mechanisms for such results are not yet completely understood, but the availability of vapor embryos at low heat fluxes and pathways for liquid flow appears to be one of the fundamental reasons. Nonetheless, the topography of these nanostructures may lead to localized flow obstruction, hindering in any long-term enhancement. The coating of the boiling surfaces is mostly based on chemical synthesis or self-assembly, which is easy, scalable, cost-effective, and can be applied with different metals and metal oxides. However, the broadened distribution of possible shapes, sizes, and spacings in the nanotubes and nanoparticles is still challenging. Additionally, the interfacial strength between the nanoparticles and the substrate may not be sufficient to sustain the harsh environment provoked by the fluid or bubbles at high temperature under pool boiling conditions, causing film delamination, particle damage, or even detachment from the surface. The surface roughening and the incorporation of fins, microchannels, tunnels, reentrant cavities, and microporous structures, among others, have their main beneficial feature in the increased active nucleation site density that increases the nucleation boiling HTC. Also, the use of microporous structures is particularly noteworthy given their capability to enhance CHF values through the separation of the vapor and fluid pathways. The use of extended surfaces, porous mesh, and foam often provide different enhancements in the nucleate HTC and CHF values, together with the elimination of the incipience superheat. Also, the combined usage of extended surfaces with fluid subcooling is particularly effective for achieving nucleate boiling heat transfer benefits. Nonetheless, the attachment of an extended surface to a temperature-sensitive device may result in a contact resistance that will increase the device temperature, which may induce strong thermal stresses. Moreover, it can be concluded that the separation of the vapor and liquid pathways can be achieved by different procedures, for example, the following ones: (i) Mixed wettability, which enhances the liquid supply to the surface and thus increasing the CHF. This can be achieved by coating a hydrophilic surface with hydrophobic spots, where the bubbles nucleate and prevent the nucleation from the surrounding hydrophilic areas caused by the localized lateral cooling induced by the nucleation at the hydrophobic spots. The triple contact line does not spread beyond the hydrophobic spots, and thus lateral bubble coalescence diminishes, which creates space for the liquid to replenish the heating surface. (ii) The heating surface is coated with a porous layer over which pin fins are created. The bubbles nucleate from the flat porous areas, whereas liquid replenishment occurs through the vertical porous by capillary wicking. The bubbles nucleate over the pin fins, and the capillary liquid supply occurs through the non-finned areas. (iii) Using parallel microchannels with coated fins, the vapor bubbles will nucleate from the porous fin tip, and the liquid falls and impinges on the channel bottom, resulting in heat transfer enhancement.
- (iii)
- The compound or hybrid enhancement techniques include the use of one active technique together with a passive technique and two or more active techniques, like the combined usage of space confinement wall deformation and ultrasound waves. These combined techniques often exhibit promising nucleate pool boiling heat transfer enhancement trends. Considering safe CHF enhancement as a factor of chief importance, the integration of advanced approaches, such as ultrasound-assisted methods, magnetic fields, and other techniques, through innovative preparation methods and materials is highly recommended. Nonetheless, the hybrid surface enhancements that involve the use of nanocoatings are susceptible to obstruction and deterioration over time. Also, the integration of hybrid and hierarchical structures makes the accurate evaluation of the impact due to the isolated role of the surface features on CHF amelioration difficult.
- (iv)
- The selection of the fabrication process plays a relevant role according to cost, durability, reliability, and effectiveness standpoints. For instance, lithography can define surface geometric features and spacing with very high resolution. Such features allow the study of the impact of the surface geometric features on the boiling phenomena. Additionally, lithography is compatible with the silicon IC/MEMS processes, allowing their applications to be integrated with silicon devices. On the other hand, techniques using nanoporous materials, which can result in nanostructures with controllable dimensions and density, are found to be cost-effective and scalable. All the enhanced surface fabrication methodologies offer a suitable strategy to produce structured surfaces and control their wettability, but all of them also have their beneficial and disadvantageous features regarding geometry; size; density; and uniformity of the surface nanostructures, equipment, material reliability and durability, and boiling enhanced heat transfer application. Also, the selection of the material of the surface structures is relevant because they must present enough robustness to endure high temperatures and harsh environments during the pool boiling process. Considering the cost, thermophysical properties, and manufacturability, the choice of the most suitable material should be made. Silicon has the highest material cost per unit area, but it is often selected due to its excellent mechanical strength and thermal conductivity. The metals also possess superior mechanical strength and thermophysical properties, which allow them to be applied for many boiling heat transfer purposes.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kerlin, T.W.; Upadhyaya, B.R. Chapter 13—Boiling water reactors. In Dynamics and Control of Nuclear Reactors; Academic Press: Cambridge, MA, USA, 2019; pp. 167–189. [Google Scholar] [CrossRef]
- Singh, T.; Atieh, M.A.; Al-Ansari, T.; Mohammad, A.W.; Mckay, G. The Role of Nanofluids and Renewable Energy in the Development of Sustainable Desalination Systems: A Review. Water 2020, 12, 2002. [Google Scholar] [CrossRef]
- Moita, A.; Moreira, A.; Pereira, J. Nanofluids for the Next Generation Thermal Management of Electronics: A Review. Symmetry 2021, 13, 1362. [Google Scholar] [CrossRef]
- Sureshkumar, R.; Mohideen, S.T.; Nethaji, N. Heat transfer characteristics of nanofluids in heat pipes: A review. Renew. Sustain. Energy Rev. 2013, 20, 397–410. [Google Scholar] [CrossRef]
- Dixit, T.; Ghosh, I. Review of micro- and mini-channel heat sinks and heat exchangers for single phase fluids. Renew. Sustain. Energy Rev. 2015, 41, 1298–1311. [Google Scholar] [CrossRef]
- Zhang, K.-I.; Liu, Z.-H.; Zheng, B.-C. A new 3D chip cooling technology using micro-channels thermosyphon with super-moist fluids and nanofluids. Energy Convers. Manag. 2016, 128, 44–56. [Google Scholar] [CrossRef]
- Bergman, T.L.; Incropera, F.P.; DeWitt, D.P.; Lavine, A.S. Fundamentals of Heat and Mass Transfer; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Khan, S.A.; Atieh, M.A.; Koç, M. Micro-Nano Scale Surface Coating for Nucleate Boiling Heat Transfer: A Critical Review. Energies 2018, 11, 3189. [Google Scholar] [CrossRef]
- Hsu, C.-C.; Chen, P.-H. Surface wettability effects on critical heat flux of boiling heat transfer using nanoparticle coatings. Int. J. Heat Mass Transf. 2012, 55, 3713–3719. [Google Scholar] [CrossRef]
- Motezakker, A.R.; Sadaghiani, A.K.; Çelik, S.; Larsen, T.; Villanueva, L.G.; Kosar, A. Optimum ratio of hydrophobic to hydrophilic areas of biphilic surfaces in thermal fluid systems involving boiling. Int. J. Heat Mass Transf. 2019, 135, 164–174. [Google Scholar] [CrossRef]
- Shen, B.; Mine, T.; Iwata, N.; Hidaka, S.; Takahashi, K.; Takata, Y. Deterioration of boiling heat transfer on biphilic surfaces under very low pressures. Exp. Therm. Fluid Sci. 2020, 113, 110026. [Google Scholar] [CrossRef]
- Kim, D.E.; Yu, D.I.; Jerng, D.W.; Kim, M.H.; Ahn, H.S. Review of boiling heat transfer enhancement on micro/nanostructured surfaces. Exp. Therm. Fluid Sci. 2015, 66, 173–196. [Google Scholar] [CrossRef]
- Kalita, S.; Sen, P.; Sen, D.; Das, S.; Das, A.K.; Saha, B.B. Experimental study of nucleate pool boiling heat transfer on microporous structured by chemical etching method. Therm. Sci. Eng. Prog. 2021, 26, 101114. [Google Scholar] [CrossRef]
- Mudhafar, M.A.H.; Zheng-hao, W. Optimization of Pool Boiling Heat Transfer on microporous metal coating surfaces with FC-72 as a working fluid. Heat Mass Transf. 2022, 58, 1963–1977. [Google Scholar] [CrossRef]
- Moghadasi, H.; Malekian, N.; Saffari, H.; Gheitaghy, A.M.; Zhang, G.Q. Recent Advances in the Critical Heat Flux Amelioration of Pool Boiling Surfaces Using Metal Oxide Nanoparticle Deposition. Energies 2020, 13, 4026. [Google Scholar] [CrossRef]
- Zhao, H.; Dash, S.; Dhillon, N.S.; Kim, S.; Lettiere, B.; Varanasi, K.K.; Hart, A.J. Microstructured Ceramic-Coated Carbon Nanotube Surfaces for High Heat Flux Pool Boiling. ACS Appl. Nano Mater. 2019, 2, 5538–5545. [Google Scholar] [CrossRef]
- Lee, S.; Seo, G.H.; Lee, S.; Jeong, U.; Lee, S.J.; Kim, S.J.; Choi, W. Layer-by-layer carbon nanotube coatings for enhanced pool boiling heat transfer on metal surfaces. Carbon 2016, 107, 607–618. [Google Scholar] [CrossRef]
- Mao, L.; Zhou, W.; Hu, X.; He, Y.; Zhang, G.; Zhang, L.; Fu, R. Pool boiling performance and bubble dynamics on graphene oxide nanocoating surface. Int. J. Therm. Sci. 2020, 147, 106514. [Google Scholar] [CrossRef]
- Liang, G.; Mudawar, I. Review of pool boiling enhancement by surface modification. Int. J. Heat Mass Transf. 2019, 128, 892–933. [Google Scholar] [CrossRef]
- Cho, H.J.; Wang, E.N. Bubble nucleation, growth, and departure: A new, dynamic understanding. Int. J. Heat Mass Transf. 2019, 145, 118803. [Google Scholar] [CrossRef]
- Liu, J.; Orejon, D.; Zhang, N.; Terry, J.G.; Walton, A.J.; Sefiane, K. Bubble Coalescence During Pool Boiling with Different Surface Characteristics. Heat Transf. Eng. 2024, 45, 360–380. [Google Scholar] [CrossRef]
- Wang, X.; Fadda, D.; Godinez, J.; Lee, J.; You, S.M. Effect of wettability on pool boiling heat transfer with copper microporous coated surface. Int. J. Heat Mass Transf. 2022, 194, 123059. [Google Scholar] [CrossRef]
- Xu, N.; Liu, Z.; Yu, X.; Gao, J.; Chu, H. Processes, models and the influencing factors for enhanced boiling heat transfer in porous structures. Renew. Sustain. Energy Rev. 2024, 192, 114244. [Google Scholar] [CrossRef]
- Bergles, A.E. The Influence of Heated-Surface Vibration on Pool Boiling. J. Heat Transfer. 1969, 91, 152–154. [Google Scholar] [CrossRef]
- Hosseinian, A.; Isfahani, A.H.M.; Shirani, E. Experimental investigation of surface vibration effects on increasing the stability and heat transfer coefficient of MWCNTs-water nanofluid in a flexible double pipe heat exchanger. Exp. Therm. Fluid Sci. 2018, 90, 275–285. [Google Scholar] [CrossRef]
- Unno, N.; Yuki, K.; Taniguchi, J.; Satake, S. Boiling heat transfer enhancement by self-excited vibration. Int. J. Heat Mass Transf. 2020, 153, 119588. [Google Scholar] [CrossRef]
- Zhang, B.; Gong, S.; Dong, S.; Xiong, Z.; Guo, Q. Experimental investigation on vibration characteristics of subcooled and saturated pool boiling. Appl. Therm. Eng. 2023, 218, 119297. [Google Scholar] [CrossRef]
- Prisnyakov, V.F.; Navruzov, Y.V.; Mamotov, P.V.; Stoichev, A.V. Characteristics of heat emission from a vibrating heat source in a vessel with liquid. Teplofiz. Vysok. Temp. 1992, 30, 105–110. [Google Scholar]
- Zitko, V.; Afgan, N. Boiling Heat Transfer from Oscillating Surface. J. Enhanc. Heat Transf. 1994, 1, 191–196. [Google Scholar] [CrossRef]
- Atashi, H.; Alaei, A.; Kafshgari, M.H.; Aeinehvand, R.; Rahimi, S.K. New Pool Boiling Heat Transfer in the Presence of Low-Frequency Vibrations Into a Vertical Cylindrical Heat Source. Exp. Heat Transf. 2014, 27, 428–437. [Google Scholar] [CrossRef]
- Sathyabhama, A.; Prashanth, S.P. Enhancement of Boiling Heat Transfer Using Surface Vibration. Heat Transf. 2017, 46, 49–60. [Google Scholar] [CrossRef]
- Abadi, S.M.A.N.R.; Ahmadpour, A.; Meyer, J.P. Effects of vibration on pool boiling heat transfer from a vertically aligned array of heated tubes. Int. J. Multiph. Flow 2019, 118, 97–112. [Google Scholar] [CrossRef]
- Alangar, S. Effect of boiling surface vibration on heat transfer. Heat Mass Transf. 2017, 53, 73–79. [Google Scholar] [CrossRef]
- Alimoradi, H.; Zaboli, S.; Shams, M. Numerical simulation of surface vibration effects on improvement of pool boiling heat transfer characteristics of nanofluid. Korean J. Chem. Eng. 2022, 39, 69–85. [Google Scholar] [CrossRef]
- Brennen, C.E. Cavitation and Bubble Dynamics; Cambridge University Press: New York, NY, USA, 2014. [Google Scholar]
- Quintana-Buil, G.; González-Cinca, R. Acoustic effects on heat transfer on the ground and in microgravity conditions. Int. J. Heat Mass Transf. 2021, 178, 121627. [Google Scholar] [CrossRef]
- Baffigi, F.; Bartoli, C. Heat transfer enhancement from a circular cylinder to distilled water by ultrasonic waves in subcooled boiling conditions. In Interdisciplinary Transport Phenomena VI: Fluid, Thermal, Biological, Materials and Space Sciences; Wiley: Volterra, Italy, 2009. [Google Scholar]
- Tang, J.; Sun, L.; Wu, D.; Du, M.; Xie, G.; Yang, K. Effects of ultrasonic waves on subcooled pool boiling on a small plain heating surface. Chem. Eng. Sci. 2019, 201, 274–287. [Google Scholar] [CrossRef]
- Bartoli, C.; Baffigi, F. Use of ultrasonic waves in sub-cooled boiling. Appl. Therm. Eng. 2012, 47, 95–110. [Google Scholar] [CrossRef]
- Legay, M.; Gondrexon, N.; Le Person, S.; Boldo, P.; Bontemps, A. Enhancement of Heat Transfer by Ultrasound: Review and Recent Advances. Int. J. Chem. Eng. 2011, 2011, 670108. [Google Scholar] [CrossRef]
- Hetsroni, G.; Moldavsky, L.; Fichman, M.; Pogrebnyak, E.; Mosyak, A. Ultrasonic enhancement of subcooled pool boiling of freely oscillated wires. Int. J. Multiph. Flow 2014, 67, 13–21. [Google Scholar] [CrossRef]
- Sitter, J.S.; Snyder, T.J.; Chung, J.N.; Marston, P.L. Acoustic field interaction with a boiling system under terrestrial gravity and microgravity. J. Acoust. Soc. Am. 1998, 104, 2561–2569. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-Y.; Kim, Y.G.; Kang, B.H. Enhancement of natural convection and pool boiling heat transfer via ultrasonic vibration. Int. J. Heat Mass Transf. 2004, 47, 2831–2840. [Google Scholar] [CrossRef]
- Mondal, K.; Bhattacharya, A. Pool boiling enhancement through induced vibrations in the liquid pool due to moving solid bodies—A numerical study using the lattice Boltzmann method (LBM). Phys. Fluids 2021, 33, 093310. [Google Scholar] [CrossRef]
- Suriyawong, A.; Saisorn, S.; Wongwises, S. Pool boiling heat transfer enhancement of distilled water with passive rotating blades installed above the heating surface. Exp. Therm. Fluid Sci. 2017, 87, 109–116. [Google Scholar] [CrossRef]
- Ashouri, M.; Rahmati, P.; Hakkaki-Fard, A. On the effect of corrugated conical frustum on pool boiling heat transfer. Exp. Therm. Fluid Sci. 2022, 130, 110494. [Google Scholar] [CrossRef]
- Zaghdoudi, C.; Lallemand, M. Pool Boiling Heat Transfer Enhancement by Means of High DC Electric Field. Arab. J. Sci. Eng. 2005, 30, 189–212. [Google Scholar]
- Hristov, Y.; Zhao, D.; Kenning, D.B.R.; Sefiane, K.; Karayiannis, T.G. A study of nucleate boiling and critical heat flux with EHD enhancement. Heat Mass Transf. 2009, 45, 999–1017. [Google Scholar] [CrossRef]
- Di Marco, P.; Grassi, W. Effects of external electric field on pool boiling: Comparison of terrestrial and microgravity data in the ARIEL experiment. Exp. Therm. Fluid Sci. 2011, 35, 780–787. [Google Scholar] [CrossRef]
- Darabi, J.; Ekula, K. Development of a chip-integrated micro cooling device. Microelectron. J. 2003, 34, 1067–1074. [Google Scholar] [CrossRef]
- Nguyen, T. Electro-Hydro-Dynamics- Enhancement of Multi-Phase Heat Transfer, Faculty of Engineering (Mechanical), University of Technology, Sydney, Australia. Available online: https://www.slideserve.com/lucky/electro-hydro-dynamics-enhancement-of-multi-phase-heat-transfer-powerpoint-ppt-presentation (accessed on 1 February 2024).
- Ozdemir, M.R.; Sadaghiani, A.K.; Motezakker, A.R.; Parapari, S.S.; Park, H.S.; Acar, H.Y.; Kosar, A. Experimental studies on ferrofluid pool boiling in the presence of external magnetic force. Appl. Therm. Eng. 2018, 139, 598–608. [Google Scholar] [CrossRef]
- Rhamati, P.; Ebrahimi-Dehshali, M.; Hakkaki-Fard, A. Enhancement of pool boiling heat transfer using ferromagnetic beads in a variable magnetic field. Appl. Therm. Eng. 2020, 164, 114439. [Google Scholar] [CrossRef]
- O’Connor, J.P.; You, S.M.; Chang, J.Y. Gas-Saturated Pool Boiling Heat Transfer from Smooth and Microporous Surfaces in FC-72. J. Heat Transf. 1996, 118, 662–667. [Google Scholar] [CrossRef]
- You, S.M.; Simon, T.W.; Bar-Cohen, A. Experiments on nucleate boiling heat transfer with a highly-wetting dielectric fluid. In Effects of Pressure, Subcooling and Dissolved Gas Content; American Society of Mechanical Engineers, Heat Transfer Division: Minnesota, MN, USA, 1990; pp. 45–142. [Google Scholar]
- Sarafraz, M.M.; Peyghambarzadeh, S.M.; Fazel, S.A.A. Enhancement of the pool boiling heat transfer coefficient using the gas injection into the water. Pol. J. Chem. Technol. 2012, 14, 100–109. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, W.; Liu, B.; Yu, X.; Wei, J. Experimental Study of Enhanced Boiling Heat Transfer with Suction. Microgravity Sci. Technol. 2021, 33, 39. [Google Scholar] [CrossRef]
- Mitsutake, Y.; Monde, M. Ultra High Critical Heat Flux During Forced Flow Boiling Heat Transfer with an Impingement Jet. ASME J. Heat Transf. 2003, 125, 1038–1045. [Google Scholar] [CrossRef]
- Ma, C.-F.; Bergles, A.E. Jet impingement nucleate boiling. Int. J. Heat Mass Transf. 1986, 29, 1095–1101. [Google Scholar] [CrossRef]
- Wolf, D.H.; Incropera, F.P.; Viskanta, R. Local jet impingement boiling heat transfer. Int. J. Heat Mass Transf. 1996, 39, 1395–1406. [Google Scholar] [CrossRef]
- Ndao, S.; Peles, Y.; Jensen, M.K. Experimental investigation of flow boiling heat transfer of jet impingement on smooth and micro structured surfaces. Int. J. Heat Mass Transf. 2012, 55, 5093–5101. [Google Scholar] [CrossRef]
- Leal, L.; Lavieille, P.; Miscevic, M.; Pigache, F.; Tadrist, L. Control of pool boiling incipience in confined space: Dynamic morphing of the wall effect. In Proceedings of the 3rd Micro and Nano Flows Conference, Thessaloniki, Greece, 22–24 August 2011. [Google Scholar]
- Jun, S.; Kim, J.; You, S.M.; Kim, H.Y. Effect of heater orientation on pool boiling heat transfer from sintered copper microporous coating in saturated water. Int. J. Heat Mass Transf. 2016, 103, 277–284. [Google Scholar] [CrossRef]
- An, S.; Kim, D.-Y.; Lee, J.-G.; Jo, H.S.; Kim, M.-W.; Al-Deyab, S.S.; Choi, J.; Yoon, S.S. Supersonically sprayed reduced graphene oxide film to enhance critical heat flux in pool boiling. Int. J. Heat Mass Transf. 2016, 98, 124–130. [Google Scholar] [CrossRef]
- Ho, J.Y.; Wong, K.K.; Leong, K.C.; Yang, C. Enhanced Nucleate Pool Boiling from Microstructured Surfaces Fabricated by Selective Laser Melting. In Proceedings of the ASME 2016 5th International Conference on Micro/Nanoscale Heat and Mass Transfer, Biopolis, Singapore, 4–6 January 2016. [Google Scholar]
- Chen, S.-W.; Hsieh, J.-C.; Chou, C.-T.; Lin, H.-H.; Shen, S.-C.; Tsai, M.-J. Experimental investigation and visualization on capillary and boiling limits of micro-grooves made by different processes. Sens. Actuators A Phys. 2007, 139, 78–87. [Google Scholar] [CrossRef]
- Koizumi, Y.; Ohtake, H.; Sato, T. Pool Boiling Characteristics of Heat Transfer Surface with Micro Structures Created by Using MEMS Technology. In Proceedings of the 14th International Heat Transfer Conference, Washington, DC, USA, 8–13 August 2010. [Google Scholar]
- Watanabe, Y.; Enoki, K.; Okawa, T. Nanoparticle layer detachment and its influence on the heat transfer characteristics in saturated pool boiling of nanofluids. Int. J. Heat Mass Transf. 2018, 125, 171–178. [Google Scholar] [CrossRef]
- Wu, W.; Bostanci, H.; Chow, L.C.; Hong, Y.; Su, M.; Kizito, J.P. Nucleate boiling heat transfer enhancement for water and FC-72 on titanium oxide and silicon oxide surfaces. Int. J. Heat Mass Transf. 2010, 53, 1773–1777. [Google Scholar] [CrossRef]
- Forrest, E.; Williamson, E.; Buongiorno, J.; Hu, L.-W.; Rubner, M.; Cohen, R. Augmentation of nucleate boiling heat transfer and critical heat flux using nanoparticle thin-film coatings. Int. J. Heat Mass Transf. 2010, 53, 58–67. [Google Scholar] [CrossRef]
- Chen, M.-H.; Chuang, Y.-J.; Tseng, F.-G. Self-masked high-aspect-ratio polymer nanopillars. Nanotechnology 2008, 19, 505301. [Google Scholar] [CrossRef]
- White, S.B.; Shih, A.J.; Pipe, K.P. Boiling surface enhancement by electrophoretic deposition of particles from a nanofluid. Int. J. Heat Mass Transf. 2011, 54, 4370–4375. [Google Scholar] [CrossRef]
- Yeom, H.; Sridharan, K.; Corradini, M. Bubble Dynamics in Pool Boiling on Nanoparticle-coated Surfaces. Heat Transf. Eng. 2015, 36, 1013–1027. [Google Scholar] [CrossRef]
- Souza, R.R.; Gonçalves, I.M.; Rodrigues, R.O.; Minas, G.; Miranda, J.M.; Moreira, A.L.N.; Lima, R.; Coutinho, G.; Pereira, J.E.; Moita, A.S. Recent advances on the thermal properties and applications of nanofluids: From nanomedicine to renewable energies. Appl. Therm. Eng. 2022, 201, 117725. [Google Scholar] [CrossRef]
- Freitas, E.; Pontes, P.; Cautela, R.; Bahadur, V.; Miranda, J.; Ribeiro, A.P.C.; Souza, R.R.; Oliveira, J.D.; Copetti, J.B.; Lima, R.; et al. Pool Boiling of Nanofluids on Biphilic Surfaces: An Experimental and Numerical Study. Nanomaterials 2021, 11, 125. [Google Scholar] [CrossRef] [PubMed]
- Kiyomura, I.S.; Manetti, L.L.; Cunha, A.P.; Ribatski, G.; Cardoso, E.M. An analysis of the effects of nanoparticles deposition on characteristics of the heating surface and on pool boiling of water. Int. J. Heat Mass Transf. 2017, 106, 666–674. [Google Scholar] [CrossRef]
- Souza, R.R.; Passos, J.P.; Cardoso, E.M. Influence of nanoparticle size and gap size on nucleate boiling using HFE7100. Exp. Therm. Fluid Sci. 2014, 59, 195–201. [Google Scholar] [CrossRef]
- Shah, K.A.; Tali, B.A. Synthesis of carbon nanotubes by catalytic chemical vapor deposition: A review on carbon sources, catalysts and substrates. Mater. Sci. Semicond. Process. 2016, 41, 67–82. [Google Scholar] [CrossRef]
- Ahn, H.S.; Sinha, N.; Zhang, M.; Banerjee, D.; Fang, S.; Baughman, R.H. Pool Boiling Experiments on Multiwalled Carbon Nanotube (MWCNT) Forests. J. Heat Transf. 2006, 128, 1335–1342. [Google Scholar] [CrossRef]
- Ujereh, S.; Fisher, T.; Mudawar, I. Effects of carbon nanotube arrays on nucleate pool boiling. Int. J. Heat Mass Transf. 2007, 50, 4023–4038. [Google Scholar] [CrossRef]
- Jaikumar, A.; Gupta, A.; Kandlikar, S.G.; Yang, C.-Y.; Su, C.-Y. Scale effects of graphene and graphene oxide coatings on pool boiling enhancement mechanisms. Int. J. Heat Mass Transf. 2007, 109, 357–366. [Google Scholar] [CrossRef]
- Kumar, G.U.; Soni, K.; Suresh, S.; Ghosh, K.; Thansekhar, M.R.; Babu, P.D. Modified surfaces using seamless graphene/carbon nanotubes based nanostructures for enhancing pool boiling heat transfer. Exp. Therm. Fluid Sci. 2018, 96, 493–506. [Google Scholar] [CrossRef]
- Wang, W.; Varghese, O.K.; Paulose, M.; Grimes, G.A.; Wang, Q.; Dickey, E.C. A study on the growth and structure of titania nanotubes. J. Mater. Res. 2004, 19, 417–422. [Google Scholar] [CrossRef]
- Chen, Y.; Mo, D.; Zhao, H.; Ding, N. Pool boiling on the superhydrophilic surface with TiO2 nanotube arrays. Sci. China Ser. E Technol. Sci. 2009, 52, 1596–1600. [Google Scholar] [CrossRef]
- Yao, Z.; Lu, Y.-W.; Kandlikar, S.G. Direct growth of copper nanowires on a substrate for boiling application. Micro Nano Lett. 2011, 6, 563–566. [Google Scholar] [CrossRef]
- Kumar, G.U.; Suresh, S.; Thansekhar, M.R.; Babu, P.D. Effect of diameter of metal nanowires on pool boiling heat transfer with FC-72. Appl. Surf. Sci. 2017, 423, 509–520. [Google Scholar] [CrossRef]
- Wen, R.; Li, Q.; Wang, W.; Latour, B.; Li, C.H.; Li, C.; Lee, Y.-C.; Yang, R. Enhanced bubble nucleation and liquid rewetting for highly efficient boiling heat transfer on two-level hierarchical surfaces with patterned copper nanowire arrays. Nano Energy 2017, 38, 59–65. [Google Scholar] [CrossRef]
- Ray, M.; Deb, S.; Bhaumik, S. Pool boiling heat transfer of refrigerant R-134a on TiO2 nano wire arrays surface. Appl. Therm. Eng. 2016, 107, 1294–1303. [Google Scholar] [CrossRef]
- Jun, S.; Ray, S.S.; Yarin, A.L. Pool boiling on nano-textured surfaces. Int. J. Heat Mass Transf. 2013, 62, 99–111. [Google Scholar] [CrossRef]
- Choi, C.-H.; Krishnan, S.; TeGrotenhuis, W.; Chang, C.-H. Capillary Rise of Nanostructured Microwicks. Micromachines 2018, 9, 153. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, M.M.; Karayiannis, T.G. Pool boiling review: Part II—Heat transfer enhancement. Therm. Sci. Eng. Prog. 2021, 25, 101023. [Google Scholar] [CrossRef]
- Patil, C.M.; Kandlikar, S.G. Review of the manufacturing techniques for porous surfaces used in enhanced pool boiling. Heat Transf. Eng. 2014, 35, 887–902. [Google Scholar] [CrossRef]
- Jun, S.; Wi, H.; Gurung, A.; Amaya, M.; You, S.M. Pool Boiling Heat Transfer Enhancement of Water Using Brazed Copper Microporous Coatings. J. Heat Transf. 2016, 138, 071502. [Google Scholar] [CrossRef]
- Seo, H.; Chu, J.H.; Kwon, S.-Y.; Bang, I.C. Pool boiling CHF of reduced graphene oxide, graphene, and SiC-coated surfaces under highly wettable FC-72. Int. J. Heat Mass Transf. 2015, 82, 490–502. [Google Scholar] [CrossRef]
- Sarangi, S.; Weibel, J.A.; Garimella, S.V. Quantitative Evaluation of the Dependence of Pool Boiling Heat Transfer Enhancement on Sintered Particle Coating Characteristics. J. Heat Transf. 2017, 139, 021502. [Google Scholar] [CrossRef]
- Nishikawa, K.; Ito, T. Augmentation of nucleate boiling heat transfer by prepared surfaces. In Heat Transfer in Energy Problems; Hemisphere: Washington, DC, USA, 1980; pp. 111–118. [Google Scholar]
- O’Neill, P.S.; Gottzmann, C.F.; Terbot, J.W. Novel Heat Exchangers Increases Cascade Cycle Efficiency for Natural Gas Liquefaction. In Advances in Cryogenic Engineering; Springer: Berlin/Heidelberg, Germany, 1972; pp. 420–437. [Google Scholar] [CrossRef]
- Jaikumar, A.; Rishi, A.; Gupta, A.; Kandlikar, S.G. Microscale morphology effects of copper-graphene oxide coatings on pool boiling characteristics. J. Heat Transf. 2017, 139, 111509. [Google Scholar] [CrossRef]
- Protich, Z.; Santhanam, K.S.V.; Jaikumar, A.; Kandlikar, S.G.; Wong, P. Electrochemical Deposition of Copper in Graphene Quantum Dot Bath: Pool Boiling Enhancement. J. Electrochem. Soc. 2016, 163, 166–172. [Google Scholar] [CrossRef]
- Li, C.; Peterson, G.P. Parametric Study of Pool Boiling on Horizontal Highly Conductive Microporous Coated Surfaces. J. Heat Transf. 2007, 129, 1465–1475. [Google Scholar] [CrossRef]
- Tang, Y.; Tang, B.; Li, Q.; Qing, J. Pool-boiling enhancement by novel metallic nanoporous surface. Exp. Therm. Fluid Sci. 2013, 44, 194–198. [Google Scholar] [CrossRef]
- Lu, L.; Fu, T.; Tang, Y.; Tang, T.; Tang, B.; Wan, Z. A novel in-situ nanostructure forming route and its application in pool-boiling enhancement. Exp. Therm. Fluid Sci. 2016, 72, 140–148. [Google Scholar] [CrossRef]
- Hendricks, T.J.; Krishnan, S.; Choi, C.; Chang, C.-H. Enhancement of Pool Boiling Heat Transfer Using Nanostructured Surfaces on Aluminum and Copper. Int. J. Heat Mass Transf. 2010, 53, 3357–3365. [Google Scholar] [CrossRef]
- Baig, N.; Kammakakam, I.; Falath, W. Nanomaterials: A review of synthesis methods, properties, recent progress, and challenges. Mater. Adv. 2021, 2, 1821–1871. [Google Scholar] [CrossRef]
- Nakayama, W.; Daikoku, T.; Kuwahara, H.; Nakajima, T. Dynamic Model of Enhanced Boiling Heat Transfer on Porous Surfaces—Part I: Experimental Investigation. J. Heat Transf. 1980, 102, 445–450. [Google Scholar] [CrossRef]
- Pastuzko, R. Pool boiling for extended surfaces with narrow tunnels—Visualization and a simplified model. Exp. Therm. Fluid Sci. 2012, 38, 149–164. [Google Scholar] [CrossRef]
- Halon, T.; Zajaczkowski, B.; Michaei, S.; Rulliere, R.; Bonjour, J. Experimental study of low pressure pool boiling of water from narrow tunnel surfaces. Int. J. Therm. Sci. 2017, 121, 348–357. [Google Scholar] [CrossRef]
- Moita, A.S.; Teodori, E.; Moreira, A.L.N. Influence of surface topography in the boiling mechanisms. Int. J. Heat Fluid Flow 2015, 52, 50–63. [Google Scholar] [CrossRef]
- Teodori, E.; Moita, A.S.; Moreira, A.L.N. Evaluation of pool boiling heat transfer over micro-structured surfaces by combining high-speed visualization and PIV measurements. In Proceedings of the 10th International Symposium on Particle Image Velocimetry—PIV13, Delft, The Netherlands, 1–3 July 2013. [Google Scholar]
- Ji, W.-T.; Zhao, C.-Y.; Zhang, D.-C.; Zhao, P.-F.; Li, Z.-Y.; He, Y.-L.; Tao, W.-Q. Pool boiling heat transfer of R134a outside reentrant cavity tubes at higher heat flux. Appl. Therm. Eng. 2017, 127, 1364–1371. [Google Scholar] [CrossRef]
- Bergles, A.E. Augmentation of Heat Transfer, Two-Phase. Thermopedia—A-To-Z Guide to Thermodynamics, Heat & Mass Transfer, and Fluids Engineering. 2011. Available online: https://doi.org/10.1615/AtoZ.a.augmentation_of_heat_transfer_two-phase (accessed on 1 February 2024).
- Hwang, G.S.; Fleming, E.; Carne, B.; Sharratt, S.; Nam, Y.; Dussinger, P.; Ju, Y.S.; Kaviany, M. Multi-artery heat pipe spreader: Lateral liquid supply. Int. J. Heat Mass Transf. 2011, 54, 2334–2340. [Google Scholar] [CrossRef]
- Nasersharifi, Y.; Kaviany, M.; Hwang, G. Pool-boiling enhancement using multilevel modulated wick. Appl. Therm. Eng. 2018, 137, 268–276. [Google Scholar] [CrossRef]
- Raghupathi, P.A.; Kandlikar, S.G. Pool boiling enhancement through contact line augmentation. Appl. Phys. Lett. 2017, 110, 204101. [Google Scholar] [CrossRef]
- Deghani-Ashkezari, E.; Salimpour, M.R. Effect of groove geometry on pool boiling heat transfer of water-titanium oxide nanofluid. Heat Mass Transf. 2018, 54, 3473–3481. [Google Scholar] [CrossRef]
- Tang, K.; Bai, J.; Chen, S.; Zhang, S.; Li, J.; Sun, Y.; Chen, G. Pool Boiling Performance of Multilayer Micromeshes for Commercial High-Power Cooling. Micromachines 2021, 12, 980. [Google Scholar] [CrossRef] [PubMed]
- Mudawar, I.; Anderson, T.M. Optimization of Enhanced Surfaces for High Flux Chip Cooling by Pool Boiling. J. Electron. Packag. 1993, 115, 89–100. [Google Scholar] [CrossRef]
- Rainey, K.N.; You, S.M. Pool boiling heat transfer from plain and microporous square pin-finned surfaces in saturated FC-72. J. Heat Transf. 2000, 122, 509–516. [Google Scholar] [CrossRef]
- Chang, J.Y.; You, S.M. Enhanced Boiling Heat Transfer from Micro-Porous Cylindrical Surfaces in Saturated FC-87 and R-123. J. Heat Transf. 1997, 119, 319–325. [Google Scholar] [CrossRef]
- Rioux, R.P.; Nolan, E.C.; Li, C.H. A systematic study of pool boiling heat transfer on structured porous surfaces: From nanoscale through microscale to macroscale. AIP Adv. 2014, 4, 117133. [Google Scholar] [CrossRef]
- Li, C.H.; Rioux, R.P. Independent and collective roles of surface structures at different length scales on pool boiling heat transfer. Sci. Rep. 2016, 6, 37044. [Google Scholar] [CrossRef] [PubMed]
- Chu, K.-H.; Joung, Y.S.; Enright, R.; Buie, C.R.; Wang, E.N. Hierarchically structured surfaces for boiling critical heat flux enhancement. Appl. Phys. Lett. 2013, 102, 151602. [Google Scholar] [CrossRef]
- Rahman, M.M.; Olçeroglu, E.; McCarthy, M. Role of Wickability on the Critical Heat Flux of Structured Superhydrophilic Surfaces. Langmuir 2014, 30, 11225–11234. [Google Scholar] [CrossRef]
- Zuber, N. On the Stability of Boiling Heat Transfer. Trans. Am. Soc. Mech. Eng. 1958, 80, 711–714. [Google Scholar] [CrossRef]
- Rahman, M.M.; McCarthy, M. Effect of length scales on the boiling enhancement of structured copper surfaces. J. Heat Transf. 2017, 139, 111508. [Google Scholar] [CrossRef]
- Betz, A.R.; Jenkins, J.; Kim, C.-J.; Attinger, D. Boiling heat transfer on superhydrophilic, superhydrophobic, and superbiphilic surfaces. Int. J. Heat Mass Transf. 2013, 57, 733–741. [Google Scholar] [CrossRef]
- Phan, H.T.; Caney, N.; Marty, P.; Colasson, S.; Gavillet, J. Surface wettability control by nanocoating: The effects on pool boiling heat transfer and nucleation mechanism. Int. J. Heat Mass Transf. 2009, 52, 5459–5471. [Google Scholar] [CrossRef]
- Kim, J.M.; Yu, D.I.; Park, H.S.; Moriyama, K.; Kim, M.H. Smart surface in pool boiling: Thermally-induced wetting transition. Int. J. Heat Mass Transf. 2017, 109, 231–241. [Google Scholar] [CrossRef]
- Jo, H.J.; Kim, S.H.; Kim, H.; Kim, J.; Kim, M.H. Nucleate boiling performance on nano/microstructures with different wetting surfaces. Nanoscale Res. Lett. 2012, 7, 242. [Google Scholar] [CrossRef] [PubMed]
- Betz, A.R.; Xu, J.; Qiu, H.; Attinger, D. Do surfaces with mixed hydrophilic and hydrophobic areas enhance pool boiling? Appl. Phys. Lett. 2010, 97, 141909. [Google Scholar] [CrossRef]
- Choi, C.-H.; David, M.; Gao, Z.; Chang, A.; Allen, M.; Wang, H.; Chang, C.-H. Large-scale Generation of Patterned Bubble Arrays on Printed Bi-functional Boiling Surfaces. Sci. Rep. 2016, 6, 23760. [Google Scholar] [CrossRef] [PubMed]
- Bertossi, R.; Caney, N.; Gruss, J.A.; Poncelet, O. Pool boiling enhancement using switchable polymers coating. Appl. Therm. Eng. 2015, 77, 121–126. [Google Scholar] [CrossRef]
- Azarkish, H. Pool boiling enhancement on biphilic micropillar arrays: Control on the thin film evaporator and rewetting flow. Numer. Heat Transf. Part A Appl. 2020, 78, 60–72. [Google Scholar] [CrossRef]
- Zhang, J.; Zou, Z.; Fu, C. A Review of the Complex Flow and Heat Transfer Characteristics in Microchannels. Micromachines 2023, 14, 1451. [Google Scholar] [CrossRef]
- Kim, J.; Jun, S.; Laksnarain, R.; You, S.M. Effect of surface roughness on pool boiling heat transfer at a heated surface having moderate wettability. Int. J. Heat Mass Transf. 2016, 101, 992–1002. [Google Scholar] [CrossRef]
- Chaudhri, I.H.; McDougall, I.R. Ageing studies in nucleate pool boiling of isopropyl acetate and perchloroethylene. Int. J. Heat Mass Transf. 1969, 12, 681–688. [Google Scholar] [CrossRef]
- Kim, J.; Jun, S.; Lee, J.; Godinez, J.; You, S.M. Effect of Surface Roughness on Pool Boiling Heat Transfer of Water on a Superhydrophilic Aluminum Surface. J. Heat Transf. 2017, 139, 101501. [Google Scholar] [CrossRef]
- Kim, J.S.; Girard, A.; Jun, S.; Lee, J.; You, S.M. Effect of surface roughness on pool boiling heat transfer of water on hydrophobic surfaces. Int. J. Heat Mass Transf. 2018, 118, 802–811. [Google Scholar] [CrossRef]
- Ferjancic, K.; Golobic, I. Surface effects on pool boiling CHF. Exp. Therm. Fluid Sci. 2002, 25, 565–571. [Google Scholar] [CrossRef]
- Fan, S.; Jiao, L.; Wang, K.; Duan, F. Pool boiling heat transfer of saturated water on rough surfaces with the effect of roughening techniques. Int. J. Heat Mass Transf. 2020, 159, 120054. [Google Scholar] [CrossRef]
- Chu, K.-H.; Enright, R.; Wang, E.N. Structured surfaces for enhanced pool boiling heat transfer. Appl. Phys. Lett. 2012, 100, 241603. [Google Scholar] [CrossRef]
- Kandlikar, S.G. A Theoretical Model to Predict Pool Boiling CHF Incorporating Effects of Contact Angle and Orientation. J. Heat Transf. 2001, 123, 1071–1079. [Google Scholar] [CrossRef]
- Kandlikar, S.G. Controlling bubble motion over heated surface through evaporation momentum force to enhance pool boiling heat transfer. Appl. Phys. Lett. 2013, 102, 051611. [Google Scholar] [CrossRef]
- McNeil, D.A.; Raeisi, A.H.; Kew, P.A.; Hamed, R.S. The effect of substrate conduction on boiling data on pin-fin heat sinks. Appl. Therm. Eng. 2015, 88, 102–117. [Google Scholar] [CrossRef]
- Deng, Z.; Liu, X.; Wu, S.; Zhang, C. Pool boiling heat transfer enhancement by bi-conductive surfaces. Int. J. Therm. Sci. 2021, 167, 107041. [Google Scholar] [CrossRef]
- Heidary, A.; Moghadasi, H.; Saffari, H. Impact of dimensional characteristics of low-conductive channels on the enhancement of pool boiling: An experimental analysis. Int. J. Mech. Sci. 2021, 209, 106710. [Google Scholar] [CrossRef]
- Rahman, M.M.; Pollack, J.; McCarthy, M. Increasing Boiling Heat Transfer using Low Conductivity Materials. Sci. Rep. 2015, 5, 13145. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Ji, X.; Zhang, W.; Liu, G. Pool boiling heat transfer of ultra-light copper foam with open cells. Int. J. Multiph. Flow 2008, 34, 1008–1022. [Google Scholar] [CrossRef]
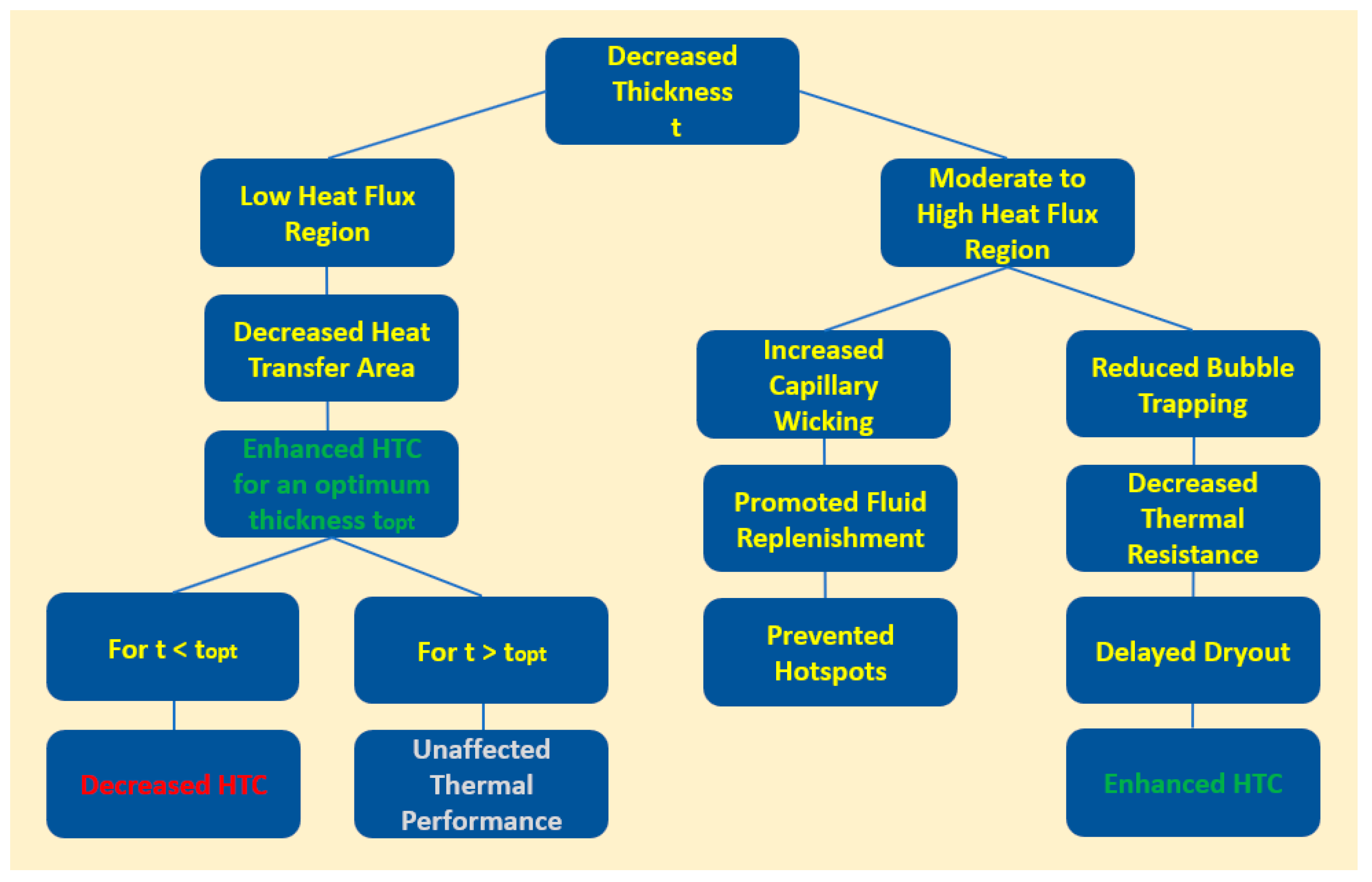
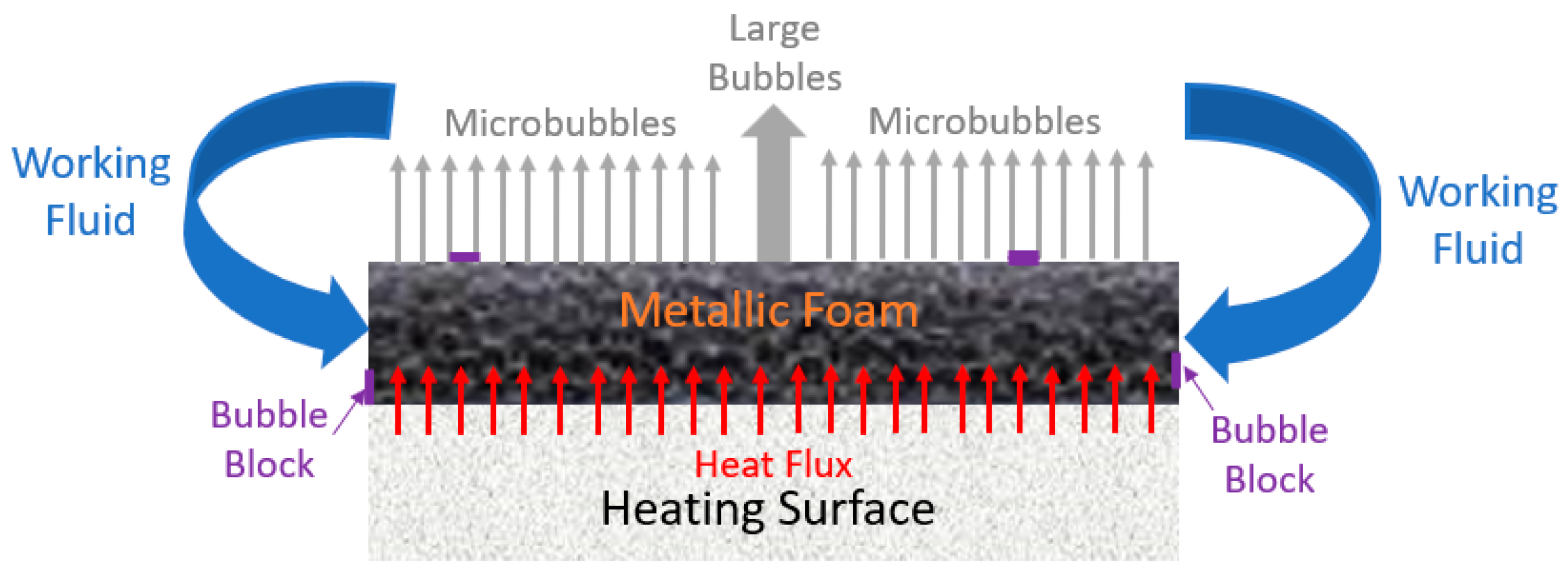
- Manetti, L.L.; Moita, A.S.O.H.; Souza, R.R.; Cardoso, E.M. Effect of copper foam thickness on pool boiling heat transfer of HFE-7100. Int. J. Heat Mass Transf. 2020, 152, 119547. [Google Scholar] [CrossRef]
- Yang, Y.; Ji, X.; Xu, J. Pool boiling heat transfer on copper foam covers with water as working fluid. Int. J. Therm. Sci. 2010, 49, 1227–1237. [Google Scholar] [CrossRef]
- Xu, Z.G.; Zhao, C.Y. Experimental study on pool boiling heat transfer in gradient metal foams. Int. J. Heat Mass Transf. 2015, 85, 824–829. [Google Scholar] [CrossRef]
- Shi, J.; Jia, X.; Feng, D.; Chen, Z.; Dang, C. Wettability effect on pool boiling heat transfer using a multiscale copper foam surface. Int. J. Heat Mass Transf. 2020, 146, 118726. [Google Scholar] [CrossRef]
- Hayes, A.; Raghupathi, P.A.; Emery, T.S.; Kandlikar, S.G. Regulating flow of vapor to enhance pool boiling. Appl. Therm. Eng. 2019, 149, 1044–1051. [Google Scholar] [CrossRef]
- Chauhan, A.; Kandlikar, S.G. Characterization of a Dual Taper Thermosiphon Loop for CPU Cooling in Data Centers. Appl. Therm. Eng. 2019, 146, 450–458. [Google Scholar] [CrossRef]
- Chauhan, A. High Heat Flux Dissipation Using Innovative Dual Tapered Manifold in Pool Boiling, and Thermosiphon Loop for CPU Cooling in Data Centers. Ph.D. Thesis, Kate Gleason College of Engineering, Rochester Institute of Technology, Rochester, NY, USA, 2021. [Google Scholar]
- Chauhan, A.; Kandlikar, S.G. Geometrical effects on heat transfer mechanisms during pool boiling in Dual Tapered Microgap with HFE7000. Int. J. Heat Mass Transf. 2022, 183, 122165. [Google Scholar] [CrossRef]
- Mokkapati, V.; Lin, C.-H. Numerical Study of a Exhaust Heat Recovery System Using Corrugated Tube Heat Exchanger with Modified Twisted Tape Inserts. Int. J. Energy Eng. 2019, 9, 12–24. [Google Scholar]
- Nanan, K.; Thianpong, C.; Promvonge, P.; Eiamsa-Ard, S. Investigation of heat transfer enhancement by perforated helical twisted-tapes. Int. Commun. Heat Mass Transf. 2014, 52, 106–112. [Google Scholar] [CrossRef]
- Ebrahimi-Dehshali, M.; Najm-Barzanji, S.Z.; Hakkaki-Fard, A. Pool boiling heat transfer enhancement by twisted-tape fins. Appl. Therm. Eng. 2018, 135, 170–177. [Google Scholar] [CrossRef]
- Geisler, K.J.L.; Bar-Cohen, A. Confinement effects on nucleate boiling and critical heat flux in buoyancy-driven microchannels. Int. J. Heat Mass Transf. 2009, 52, 2427–2436. [Google Scholar] [CrossRef]
- Mukherjee, S.; Mudawar, I. Smart Pumpless Loop fo Micro-Channel Electronic Cooling Using Flat and Enhanced Surfaces. IEEE Trans. Compon. Packag. Technol. 2003, 26, 99–109. [Google Scholar] [CrossRef]
- Cardoso, E.M.; Kannengieser, O.; Stutz, B.; Passos, J.C. FC72 and FC87 nucleate boiling inside a narrow horizontal space. Exp. Therm. Fluid Sci. 2011, 35, 1038–1045. [Google Scholar] [CrossRef]
- Boziuk, T.R.; Smith, M.K.; Glezer, A. Enhanced boiling heat transfer on plain and featured surfaces using acoustic actuation. Int. J. Heat Mass Transf. 2017, 108, 181–190. [Google Scholar] [CrossRef]
- Quan, X.; Gao, M.; Cheng, P.; Li, J. An experimental investigation of pool boiling heat transfer on smooth/rib surfaces under an electric field. Int. J. Heat Mass Transf. 2015, 85, 595–608. [Google Scholar] [CrossRef]
- Liu, B.; Garivalis, A.I.; Cao, Z.; Zhang, Y.; Wei, J.; Di Marco, P. Effects of electric field on pool boiling heat transfer over microstructured surfaces under different liquid subcoolings. Int. J. Heat Mass Transf. 2022, 183, 122154. [Google Scholar] [CrossRef]
- Rau, M.; Garimella, S.V. Confined Jet Impingement with Boiling on a Variety of Enhanced Surfaces. ASME J. Heat Transf. 2014, 136, 101503. [Google Scholar] [CrossRef]
- Nishikawa, K.; Kusuda, H.; Yamasaki, K.; Tanaka, K. Nucleate Boiling at Low Liquid Levels. Bull. JSME 1969, 10, 328–338. [Google Scholar] [CrossRef]
- Shukla, M.Y.; Kandlikar, S.G. Influence of Liquid Height on Bubble Coalescence, Vapor Venting, Liquid Return, and Heat Transfer in Pool Boiling. Int. J. Heat Mass Transf. 2021, 173, 121261. [Google Scholar] [CrossRef]
- Ahn, H.S.; Sathyamurthi, V.; Banerjee, D. Pool Boiling Experiments on a Nano-Structured Surface. IEEE Trans. Compon. Packag. Technol. 2009, 32, 156–165. [Google Scholar] [CrossRef]
- Kong, X.; Zhang, Y.; Wei, J. Experimental study of pool boiling heat transfer on novel bistructured surfaces based on micro-pin-finned surface. Exp. Therm. Fluid Sci. 2018, 91, 9–19. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira, J.; Souza, R.; Lima, R.; Moreira, A.; Moita, A. An Overview of the Recent Advances in Pool Boiling Enhancement Materials, Structrure, and Devices. Micromachines 2024, 15, 281. https://doi.org/10.3390/mi15020281
Pereira J, Souza R, Lima R, Moreira A, Moita A. An Overview of the Recent Advances in Pool Boiling Enhancement Materials, Structrure, and Devices. Micromachines. 2024; 15(2):281. https://doi.org/10.3390/mi15020281
Chicago/Turabian StylePereira, José, Reinaldo Souza, Rui Lima, António Moreira, and Ana Moita. 2024. "An Overview of the Recent Advances in Pool Boiling Enhancement Materials, Structrure, and Devices" Micromachines 15, no. 2: 281. https://doi.org/10.3390/mi15020281







