β-Cyclodextrin-Stabilized Silver Nanoparticle Production Combined with Loop-Mediated Isothermal Amplification for the Visual Detection of Contagious Pathogens
Abstract
:1. Introduction
Principle
2. Experimental Section
2.1. Reagents
2.2. Instrumentation
2.3. Primer Design
2.4. Bacterial Cell Culture and DNA Extraction Using FTA Card
2.5. Loop-Mediated Isothermal Amplification Assay
2.6. Colorimetric Detection via β-CD-Stabilized Silver Nanoparticle Formation
2.7. Ultraviolet Absorbance Spectral Study of the β-CD-Stabilized Silver Nanoparticles
2.8. Agarose Gel Electrophoresis
2.9. Sensitivity and Specificity Tests
3. Results and Discussion
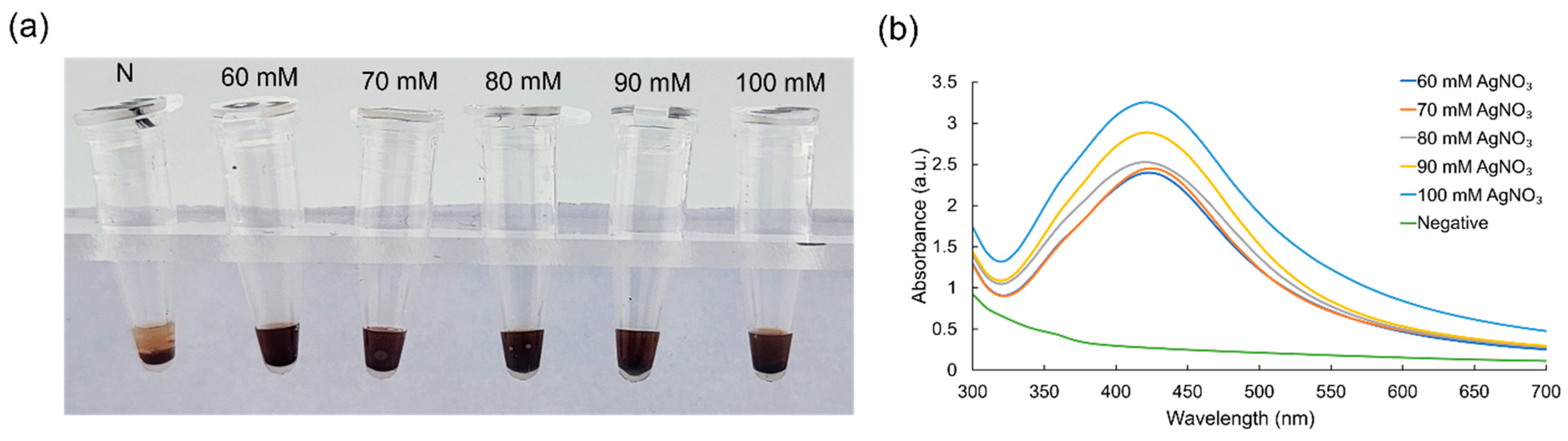
3.1. Optimization of Critical Experimental Conditions
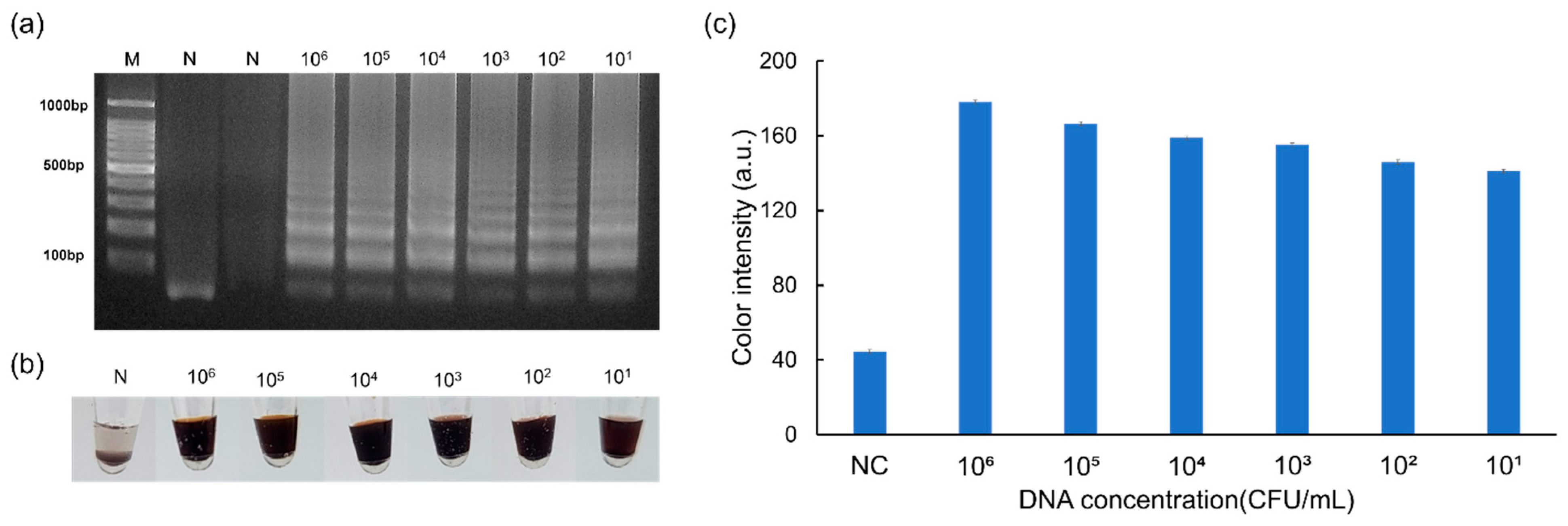
3.2. Results of the Sensitivity Assay
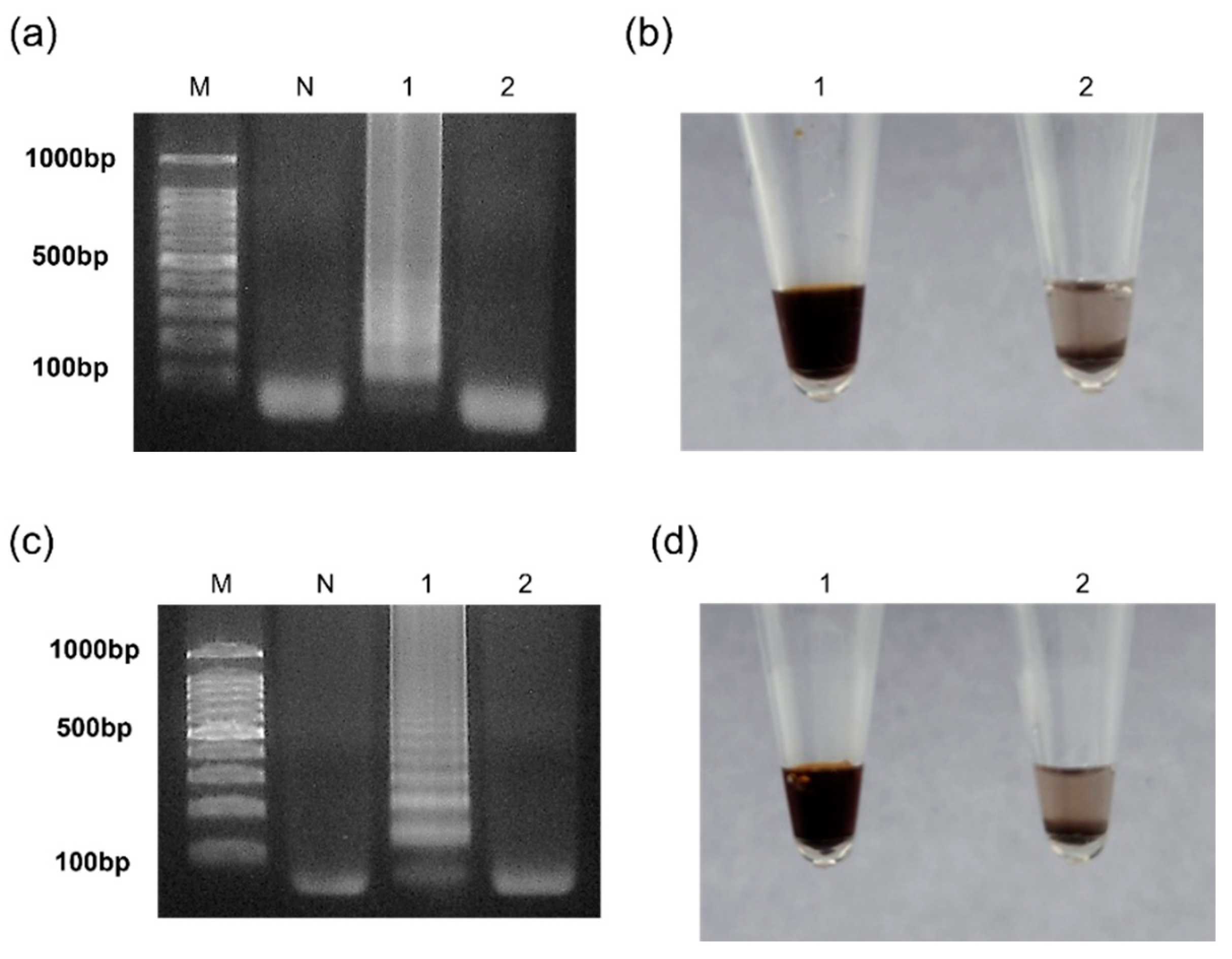
3.3. Results of the Selectivity Assay
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Vaidyanathan, G. Coronavirus variants are spreading in India–what scientists know so far. Nature 2021, 593, 321–322. [Google Scholar] [CrossRef]
- Hong, D.; Jo, E.J.; Jung, C.; Kim, M.G. Absorption-Modulated SiO2@ Au Core–Satellite Nanoparticles for Highly Sensitive Detection of SARS-CoV-2 Nucleocapsid Protein in Lateral Flow Immunosensors. ACS Appl. Mater. Interfaces 2022, 14, 45189–45200. [Google Scholar] [CrossRef]
- Popović, N.; Dinić, M.; Tolinački, M.; Mihajlović, S.; Terzić-Vidojević, A.; Bojić, S.; Djokić, J.; Golić, N.; Veljović, K. New insight into biofilm formation ability, the presence of virulence genes and probiotic potential of Enterococcus sp. dairy isolates. Front. Microbiol. 2018, 9, 78. [Google Scholar] [CrossRef]
- Anderson, A.C.; Jonas, D.; Huber, I.; Karygianni, L.; Wölber, J.; Hellwig, E.; Arweiler, N.; Vach, K.; Wittmer, A.; Al-Ahmad, A. Enterococcus faecalis from food, clinical specimens, and oral sites: Prevalence of virulence factors in association with biofilm formation. Front. Microbiol. 2016, 6, 1534. [Google Scholar] [CrossRef]
- Kiruthiga, A.; Padmavathy, K.; Shabana, P.; Naveenkumar, V.; Gnanadesikan, S.; Malaiyan, J. Improved detection of esp, hyl, asa1, gelE, cylA virulence genes among clinical isolates of Enterococci. BMC Res. Notes 2020, 13, 170. [Google Scholar] [CrossRef] [PubMed]
- Saito, N.; Kitazawa, J.; Horiuchi, H.; Yamamoto, T.; Kimura, M.; Inoue, F.; Matsui, M.; Minakawa, S.; Itoga, M.; Tsuchiya, J.; et al. Interhospital transmission of vancomycin-resistant Enterococcus faecium in Aomori, Japan. Antimicrob. Resist. Infect. Control 2022, 11, 99. [Google Scholar] [CrossRef]
- Gorrie, C.; Higgs, C.; Carter, G.; Stinear, T.P.; Howden, B. Genomics of vancomycin-resistant Enterococcus faecium. Microb. Genom. 2019, 5, e000283. [Google Scholar] [CrossRef]
- Dinh, V.P.; Lee, N.Y. Dual-mode visual detection strategies of viable pathogens for point-of-care testing. Biosens. Bioelectron. 2023, 221, 114904. [Google Scholar] [CrossRef]
- Sivakumar, R.; Dinh, V.P.; Lee, N.Y. Copper Sulfate-Induced Diphenylamine for Rapid Colorimetric Point-of-Care Detection of Contagious Pathogens Combined with Loop-Mediated Isothermal Amplification. ACS Sustain. Chem. Eng. 2023, 11, 2079–2088. [Google Scholar] [CrossRef]
- Lagier, J.C.; Edouard, S.; Pagnier, I.; Mediannikov, O.; Drancourt, M.; Raoult, D. Current and past strategies for bacterial culture in clinical microbiology. Clin. Microbiol. Rev. 2015, 28, 208–236. [Google Scholar] [CrossRef]
- Kim, S.K.; Sung, H.; Hwang, S.H.; Kim, M.N. A new quantum dot-based lateral flow immunoassay for the rapid detection of influenza viruses. BioChip J. 2022, 16, 175–182. [Google Scholar] [CrossRef]
- Trinh, K.T.L.; Lee, N.Y. Fabrication of wearable PDMS device for rapid detection of nucleic acids via recombinase polymerase amplification operated by human body heat. Biosensors 2022, 12, 72. [Google Scholar] [CrossRef]
- Jiang, L.; Lan, X.; Ren, L.; Yang, M.; Wei, B.; Wang, Y. Design of a Digital LAMP Detection Platform Based on Droplet Microfluidic Technology. Micromachines 2023, 14, 1077. [Google Scholar] [CrossRef]
- Kim, T.Y.; Kim, S.; Jung, J.H.; Woo, M.A. Paper-based Radial Flow Assay Integrated to Portable Isothermal Amplification Chip Platform for Colorimetric Detection of Target DNA. Biochip J. 2023, 17, 263–273. [Google Scholar] [CrossRef]
- Huang, Y.; Gao, Z.; Ma, C.; Sun, Y.; Huang, Y.; Jia, C.; Zhao, J.; Feng, S. An integrated microfluidic chip for nucleic acid extraction and continued cdPCR detection of pathogens. Analyst 2023, 148, 2758–2766. [Google Scholar] [CrossRef]
- Safavieh, M.; Kanakasabapathy, M.K.; Tarlan, F.; Ahmed, M.U.; Zourob, M.; Asghar, W.; Shafiee, H. Emerging loop-mediated isothermal amplification-based microchip and microdevice technologies for nucleic acid detection. ACS Biomater. Sci. Eng. 2016, 2, 278–294. [Google Scholar] [CrossRef]
- Srimongkol, G.; Ditmangklo, B.; Choopara, I.; Thaniyavarn, J.; Dean, D.; Kokpol, S.; Vilaivan, T.; Somboonna, N. Rapid colorimetric loop-mediated isothermal amplification for hypersensitive point-of-care Staphylococcus aureus enterotoxin A gene detection in milk and pork products. Sci. Rep. 2020, 10, 7768. [Google Scholar] [CrossRef]
- Lee, J.E.; Mun, H.; Kim, S.R.; Kim, M.G.; Chang, J.Y.; Shim, W.B. A colorimetric Loop-mediated isothermal amplification (LAMP) assay based on HRP-mimicking molecular beacon for the rapid detection of Vibrio parahaemolyticus. Biosens. Bioelectron. 2020, 151, 111968. [Google Scholar] [CrossRef]
- Wang, Y.; Dai, J.; Liu, Y.; Yang, J.; Hou, Q.; Ou, Y.; Ding, Y.; Ma, B.; Chen, H.; Li, M.; et al. Development of a potential penside colorimetric LAMP assay using neutral red for detection of African swine fever virus. Front. Microbiol. 2021, 12, 609821. [Google Scholar] [CrossRef] [PubMed]
- Urrutia-Cabrera, D.; Liou, R.H.C.; Wang, J.H.; Chan, J.; Hung, S.S.C.; Hewitt, A.W.; Martin, K.R.; Edwards, T.L.; Kwan, P.; Wong, R.C.B. Comparative analysis of loop-mediated isothermal amplification (LAMP)-based assays for rapid detection of SARS-CoV-2 genes. Sci. Rep. 2021, 11, 22493. [Google Scholar] [CrossRef] [PubMed]
- Tanner, N.A.; Zhang, Y.; Evans, T.C., Jr. Visual detection of isothermal nucleic acid amplification using pH-sensitive dyes. Biotechniques 2015, 58, 59–68. [Google Scholar] [CrossRef]
- Grammatikos, S.; Svoliantopoulos, I.; Gizeli, E. Naked-eye Detection of LAMP-Produced Nucleic Acids in Saliva using Chitosan-capped AuNPs in a Single-Tube Assay. Anal. Chem. 2023, 95, 18514–18521. [Google Scholar] [CrossRef]
- Sivakumar, R.; Park, S.Y.; Lee, N.Y. Quercetin-Mediated Silver Nanoparticle Formation for the Colorimetric Detection of Infectious Pathogens Coupled with Loop-Mediated Isothermal Amplification. ACS Sens. 2023, 8, 1422–1430. [Google Scholar] [CrossRef]
- Kumvongpin, R.; Jearanaikool, P.; Wilailuckana, C.; Sae-Ung, N.; Prasongdee, P.; Daduang, S.; Wongsena, M.; Boonsiri, P.; Kiatpathomchai, W.; Swangvaree, S.S.; et al. High sensitivity, loop-mediated isothermal amplification combined with colorimetric gold-nanoparticle probes for visual detection of high risk human papillomavirus genotypes 16 and 18. J. Virol. Methods 2016, 234, 90–95. [Google Scholar] [CrossRef]
- Wong, J.K.; Yip, S.P.; Lee, M.H.T. Ultrasensitive and closed-tube colorimetric loop-mediated isothermal amplification assay using carboxyl-modified gold nanoparticles. Small 2014, 10, 1495–1499. [Google Scholar] [CrossRef]
- Ma, X.; Miao, P. Silver nanoparticle@ DNA tetrahedron-based colorimetric detection of HIV-related DNA with cascade strand displacement amplification. J. Mater. Chem. B 2019, 7, 2608–2612. [Google Scholar] [CrossRef]
- Sadowski, Z.; Maliszewska, I.H.; Grochowalska, B.; Polowczyk, I.; Kozlecki, T. Synthesis of silver nanoparticles using microorganisms. Mater. Sci.-Pol. 2008, 26, 419–424. [Google Scholar]
- Rajamanikandan, R.; Ilanchelian, M. β-cyclodextrin functionalised silver nanoparticles as a duel colorimetric probe for ultrasensitive detection of Hg2+ and S2− ions in environmental water samples. Mater. Today Commun. 2018, 15, 61–69. [Google Scholar] [CrossRef]
- Syafiuddin, A.; Salmiati; Salim, M.R.; Beng Hong Kueh, A.; Hadibarata, T.; Nur, H. A review of silver nanoparticles: Research trends, global consumption, synthesis, properties, and future challenges. J. Chin. Chem. Soc. 2017, 64, 732–756. [Google Scholar] [CrossRef]
- Rajamanikandan, R.; Ilanchelian, M. Naked eye and optical biosensing of cysteine over the other amino acids using β-cyclodextrin decorated silver nanoparticles as a nanoprobe. New J. Chem. 2018, 42, 9193–9199. [Google Scholar] [CrossRef]
- Saha, S.K.; Chowdhury, P.; Saini, P.; Babu, S.P.S. Ultrasound assisted green synthesis of poly (vinyl alcohol) capped silver nanoparticles for the study of its antifilarial efficacy. Appl. Surf. Sci. 2014, 288, 625–632. [Google Scholar] [CrossRef]
- Poulson, B.G.; Alsulami, Q.A.; Sharfalddin, A.; El Agammy, E.F.; Mouffouk, F.; Emwas, A.H.; Jaremko, L.; Jaremko, M. Cyclodextrins: Structural, chemical, and physical properties, and applications. Polysaccharides 2021, 3, 1–31. [Google Scholar] [CrossRef]
- Yang, M.; Zhu, L.; Yang, W.; Xu, W. Nucleic acid-templated silver nanoclusters: A review of structures, properties, and biosensing applications. Coord. Chem. Rev. 2023, 491, 215247. [Google Scholar] [CrossRef]
- Volkov, I.L.; Smirnova, A.; Makarova, A.A.; Reveguk, Z.V.; Ramazanov, R.R.; Usachov, D.Y.; Adamchuk, V.K.; Kononov, A.I. DNA with ionic, atomic, and clustered silver: An XPS study. J. Phys. Chem. B 2017, 121, 2400–2406. [Google Scholar] [CrossRef]
- New, S.Y.; Lee, S.T.; Su, X.D. DNA-templated silver nanoclusters: Structural correlation and fluorescence modulation. Nanoscale 2016, 8, 17729–17746. [Google Scholar] [CrossRef] [PubMed]
- Andrade, P.F.; de Faria, A.F.; da Silva, D.S.; Bonacin, J.A.; do Carmo Gonçalves, M. Structural and morphological investigations of β-cyclodextrin-coated silver nanoparticles. Colloids Surf. B Biointerfaces 2014, 118, 289–297. [Google Scholar] [CrossRef]
- Suárez-Cerda, J.; Nuñez, G.A.; Espinoza-Gómez, H.; Flores-López, L.Z. A comparative study of the effect of α-, β-, and γ-cyclodextrins as stabilizing agents in the synthesis of silver nanoparticles using a green chemistry method. Mater. Sci. Eng. C 2014, 43, 21–26. [Google Scholar] [CrossRef]
- Selva Sharma, A.; SasiKumar, T.; Ilanchelian, M. A rapid and sensitive colorimetric sensor for detection of silver ions based on the non-aggregation of gold nanoparticles in the presence of ascorbic acid. J. Clust. Sci. 2018, 29, 655–662. [Google Scholar] [CrossRef]
- Zhang, C.; Zheng, T.; Wang, H.; Chen, W.; Huang, X.; Liang, J.; Tan, W. Rapid one-pot detection of SARS-CoV-2 based on a lateral flow assay in clinical samples. Anal. Chem. 2021, 93, 3325–3330. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, M.; Liu, C.; Chen, J.; Luo, X.; Xue, Y.; Srimongkol, J. Sensitive and rapid on-site detection of SARS-CoV-2 using a gold nanoparticle-based high-throughput platform coupled with CRISPR/Cas12-assisted RT-LAMP. Sens. Actuator B Chem. 2021, 345, 130411. [Google Scholar] [CrossRef]
- Chan, S.K.; Du, P.; Ignacio, C.; Mehta, S.; Newton, I.G.; Steinmetz, N.F. Virus-like particles as positive controls for COVID-19 RT-LAMP diagnostic assays. Biomacromolecules 2021, 22, 1231–1243. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; El-Tholoth, M.; Li, Y.; Graham-Wooten, J.; Liang, Y.; Li, J.; Li, W.; Weiss, S.R.; Collman, R.G.; Bau, H.H. Single-and two-stage, closed-tube, point-of-care, molecular detection of SARS-CoV-2. Anal. Chem. 2021, 93, 13063–13071. [Google Scholar] [CrossRef] [PubMed]
- Ge, A.; Liu, F.; Teng, X.; Cui, C.; Wu, F.; Liu, W.; Ma, B. A Palm Germ-Radar (PaGeR) for rapid and simple COVID-19 detection by reverse transcription loop-mediated isothermal amplification (RT-LAMP). Biosens. Bioelectron. 2022, 200, 113925. [Google Scholar] [CrossRef]
- Lee, S.; Khoo, V.S.L.; Medriano, C.A.D.; Lee, T.; Park, S.Y.; Bae, S. Rapid and in-situ detection of fecal indicator bacteria in water using simple DNA extraction and portable loop-mediated isothermal amplification (LAMP) PCR methods. Water Res. 2019, 160, 371–379. [Google Scholar] [CrossRef]
- Park, B.H.; Oh, S.J.; Jung, J.H.; Choi, G.; Seo, J.H.; Lee, E.Y.; Seo, T.S. An integrated rotary microfluidic system with DNA extraction, loop-mediated isothermal amplification, and lateral flow strip based detection for point-of-care pathogen diagnostics. Biosens. Bioelectron. 2017, 91, 334–340. [Google Scholar] [CrossRef]
- Li, M.; Luan, Z.; Liu, Y.; Yang, C.; Wang, Y.; Ma, C.; Shi, C. Ultrafast bacterial cell lysis using a handheld corona treater and loop-mediated isothermal amplification for rapid detection of foodborne pathogens. Food Control 2021, 128, 108178. [Google Scholar] [CrossRef]
- Li, C.; Chen, X.; Wen, R.; Ma, P.; Gu, K.; Li, C.; Zhou, C.; Lei, C.; Tang, Y.; Wang, H. Immunocapture magnetic beads enhanced the LAMP-CRISPR/Cas12a method for the sensitive, specific, and visual detection of Campylobacter jejuni. Biosensors 2022, 12, 154. [Google Scholar] [CrossRef]
- He, Y.; Xie, T.; Tong, Y. Rapid and highly sensitive one-tube colorimetric RT-LAMP assay for visual detection of SARS-CoV-2 RNA. Biosens. Bioelectron. 2021, 187, 113330. [Google Scholar] [CrossRef]
- Zhou, Y.; Xiao, J.; Ma, X.; Wang, Q.; Zhang, Y. An effective established biosensor of bifunctional probes-labeled AuNPs combined with LAMP for detection of fish pathogen Streptococcus iniae. Appl. Microbiol. Biotechnol. 2018, 102, 5299–5308. [Google Scholar] [CrossRef]
- Hui, J.; Gu, Y.; Zhu, Y.; Chen, Y.; Guo, S.J.; Tao, S.C.; Zhang, Y.; Liu, P. Multiplex sample-to-answer detection of bacteria using a pipette-actuated capillary array comb with integrated DNA extraction, isothermal amplification, and smartphone detection. Lab Chip 2018, 18, 2854–2864. [Google Scholar] [CrossRef]
- Zhang, L.; Tian, F.; Liu, C.; Feng, Q.; Ma, T.; Zhao, Z.; Li, T.; Jiang, X.; Sun, J. Hand-powered centrifugal microfluidic platform inspired by the spinning top for sample-to-answer diagnostics of nucleic acids. Lab Chip 2018, 18, 610–619. [Google Scholar] [CrossRef]
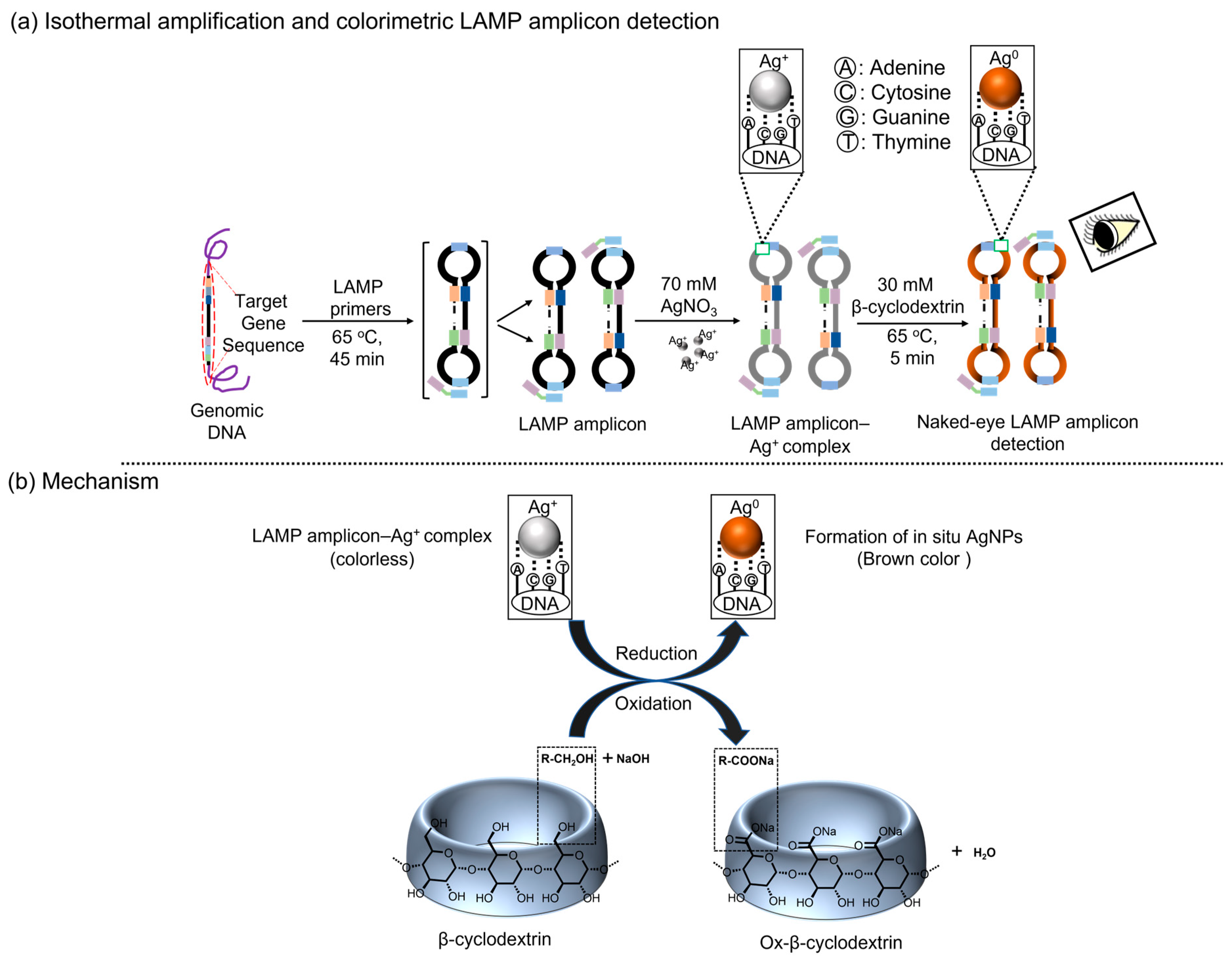


| Target Gene | Primer | Primer Sequences (5′-3′) |
|---|---|---|
| esp gene (E. faecium) | LB | TGATGTTGACACAACAGTTAAGGG |
| F3 | CCAGAACACTTATGGAACAG | |
| B3 | GTTGGGCTTTGTGACCTG | |
| FIP | CGTGTCTCCGCTCTCTTCTTTTTATTTGCAAGATATTGATGGTG | |
| BIP | ATCGGGAAACCTGAATTAGAAGAAGAACTCGTGGATGAATACTTTC | |
| E gene (SARS-CoV-2) | LF | AGGAACACCACGAAGGCC |
| LB | ACTGCTGCAACATCGTGAAC | |
| F3 | GAAACCGGCACCCTGATC | |
| B3 | GGAGCTGTTCAGGTTCTTCA | |
| FIP | TCAGGATAGCCAGGGTCACCAGTGAACTCCGTGCTGCTCT | |
| BIP | CGCTCTGAGACTGTGCGCTTCGCGGCTGTACACGTAGA |
| Target | Sensing Strategies | Sensitivity | Total Assay Time | Ref. |
|---|---|---|---|---|
| E. faecalis | Colorimetry | 10 pg | 60 min | [44] |
| S. Typhimurium | Colorimetry | 50 CFU | 80 min | [45] |
| S. aureus | Colorimetry | 104 CFU g−1 | 65 min | [46] |
| C. jejuni | Colorimetry | 8 CFU mL−1 | 120 min | [47] |
| SARS-CoV-2 | Colorimetry | 200 copies mL−1 | 60 min | [48] |
| S. iniae | SPR | 102 CFU | 120 min | [49] |
| S. aureus | SPR | 10 CFU g−1 | 60 min | [17] |
| E. coli | Fluorescence | 200 copies | 85 min | [50] |
| V. parahaemolyticus | Fluorescence | 104 copies μL−1 | 90 min | [51] |
| SARS-CoV-2 | Colorimetry | 10 fg µL−1 | 50 min | This work |
| E. faecium | Colorimetry | 101 CFU mL−1 | 50 min | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sivakumar, R.; Byun, J.Y.; Lee, N.Y. β-Cyclodextrin-Stabilized Silver Nanoparticle Production Combined with Loop-Mediated Isothermal Amplification for the Visual Detection of Contagious Pathogens. Micromachines 2024, 15, 378. https://doi.org/10.3390/mi15030378
Sivakumar R, Byun JY, Lee NY. β-Cyclodextrin-Stabilized Silver Nanoparticle Production Combined with Loop-Mediated Isothermal Amplification for the Visual Detection of Contagious Pathogens. Micromachines. 2024; 15(3):378. https://doi.org/10.3390/mi15030378
Chicago/Turabian StyleSivakumar, Rajamanickam, Jae Yoon Byun, and Nae Yoon Lee. 2024. "β-Cyclodextrin-Stabilized Silver Nanoparticle Production Combined with Loop-Mediated Isothermal Amplification for the Visual Detection of Contagious Pathogens" Micromachines 15, no. 3: 378. https://doi.org/10.3390/mi15030378
APA StyleSivakumar, R., Byun, J. Y., & Lee, N. Y. (2024). β-Cyclodextrin-Stabilized Silver Nanoparticle Production Combined with Loop-Mediated Isothermal Amplification for the Visual Detection of Contagious Pathogens. Micromachines, 15(3), 378. https://doi.org/10.3390/mi15030378







