Elevating Supercapacitor Performance of Co3O4-g-C3N4 Nanocomposites Fabricated via the Hydrothermal Method
Abstract
:1. Introduction
2. Materials and Methods
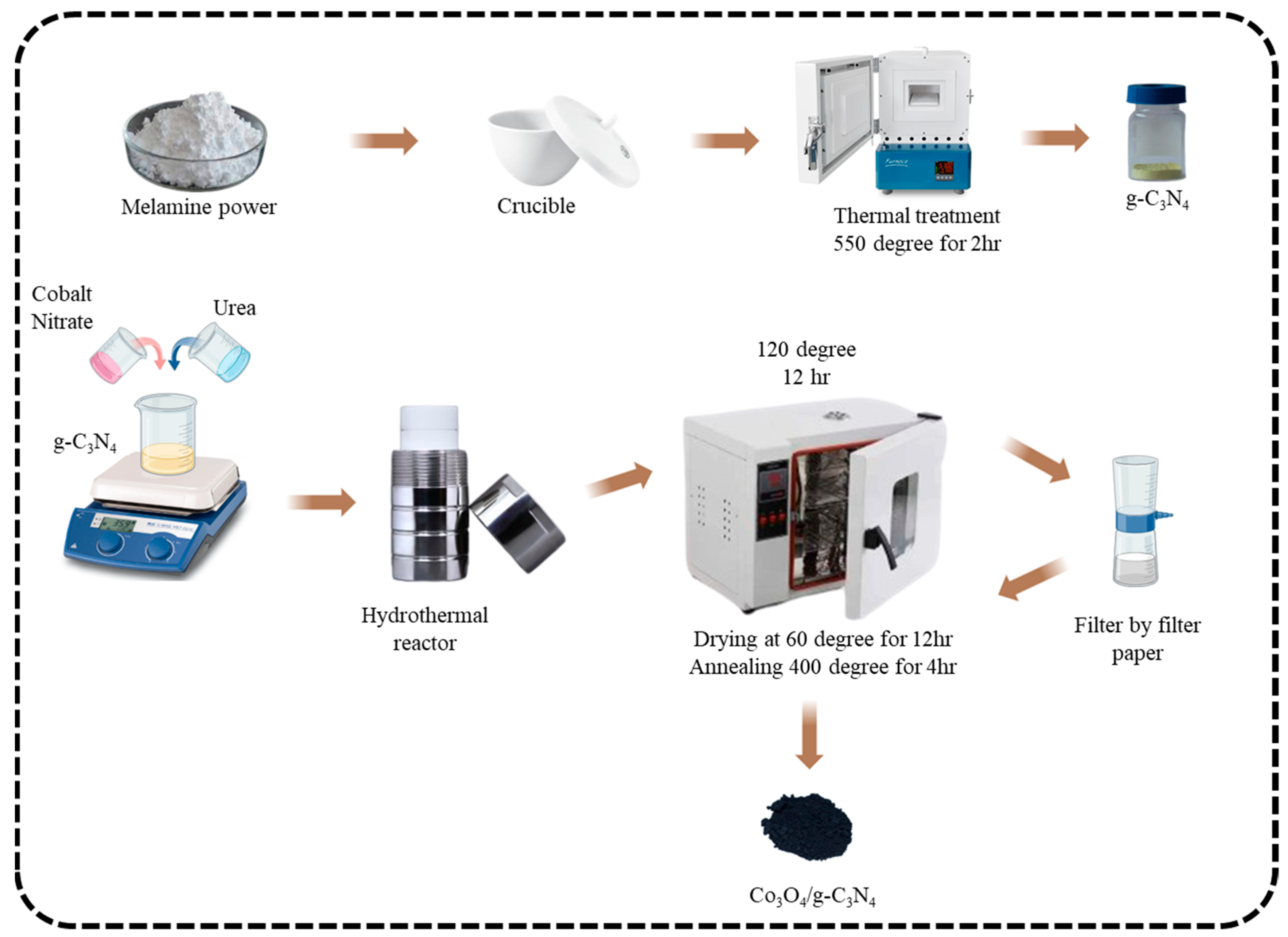
2.1. Preparation of Graphitic Carbon Nitride (g-C3N4)
2.2. Preparation of Cobalt Oxide-Graphitic Carbon Nitride (Co3O4-g-C3N4)
2.3. Preparation of Electrode
2.4. Equations
3. Results and Discussion
3.1. XRD Analysis
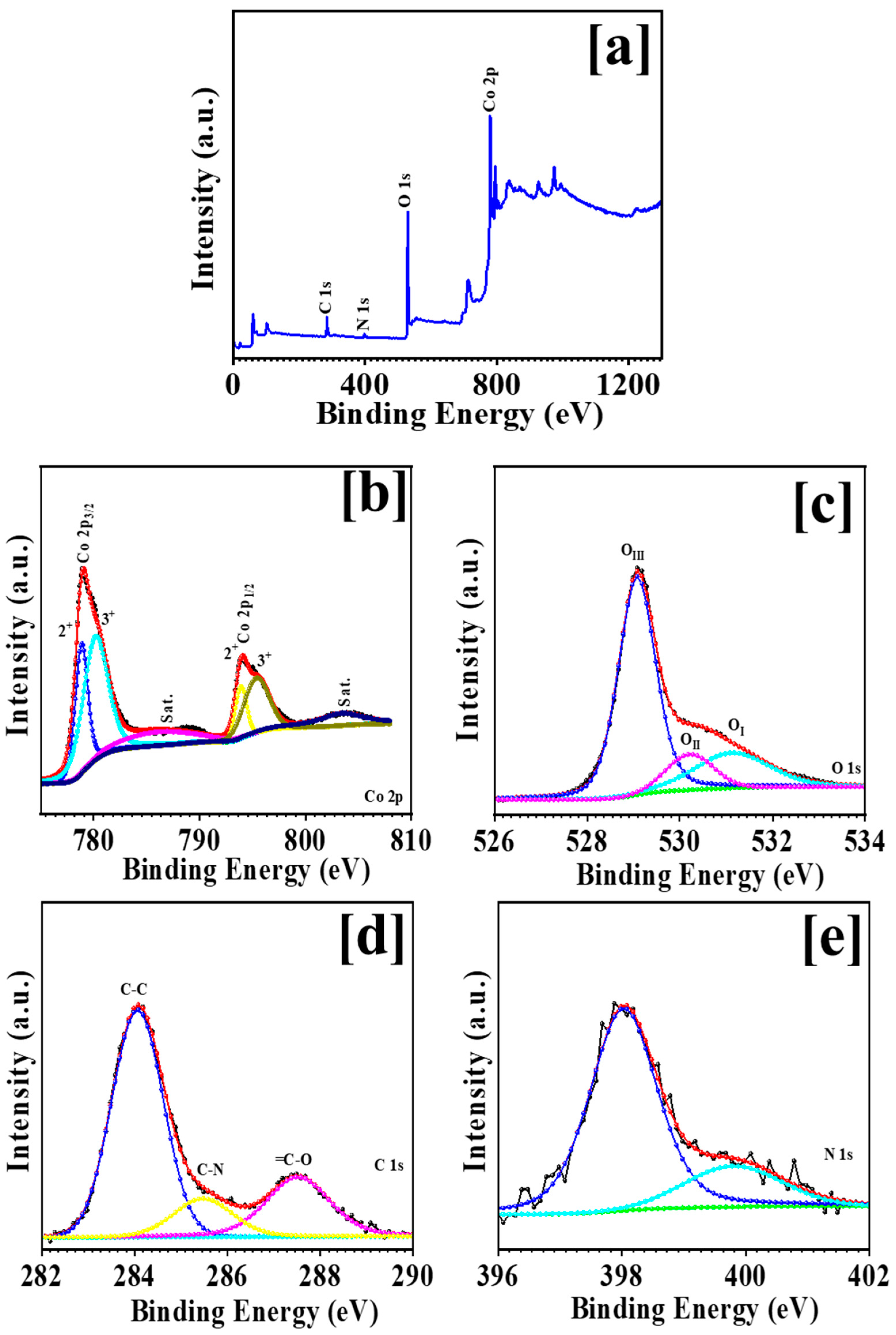
3.2. XPS Analysis
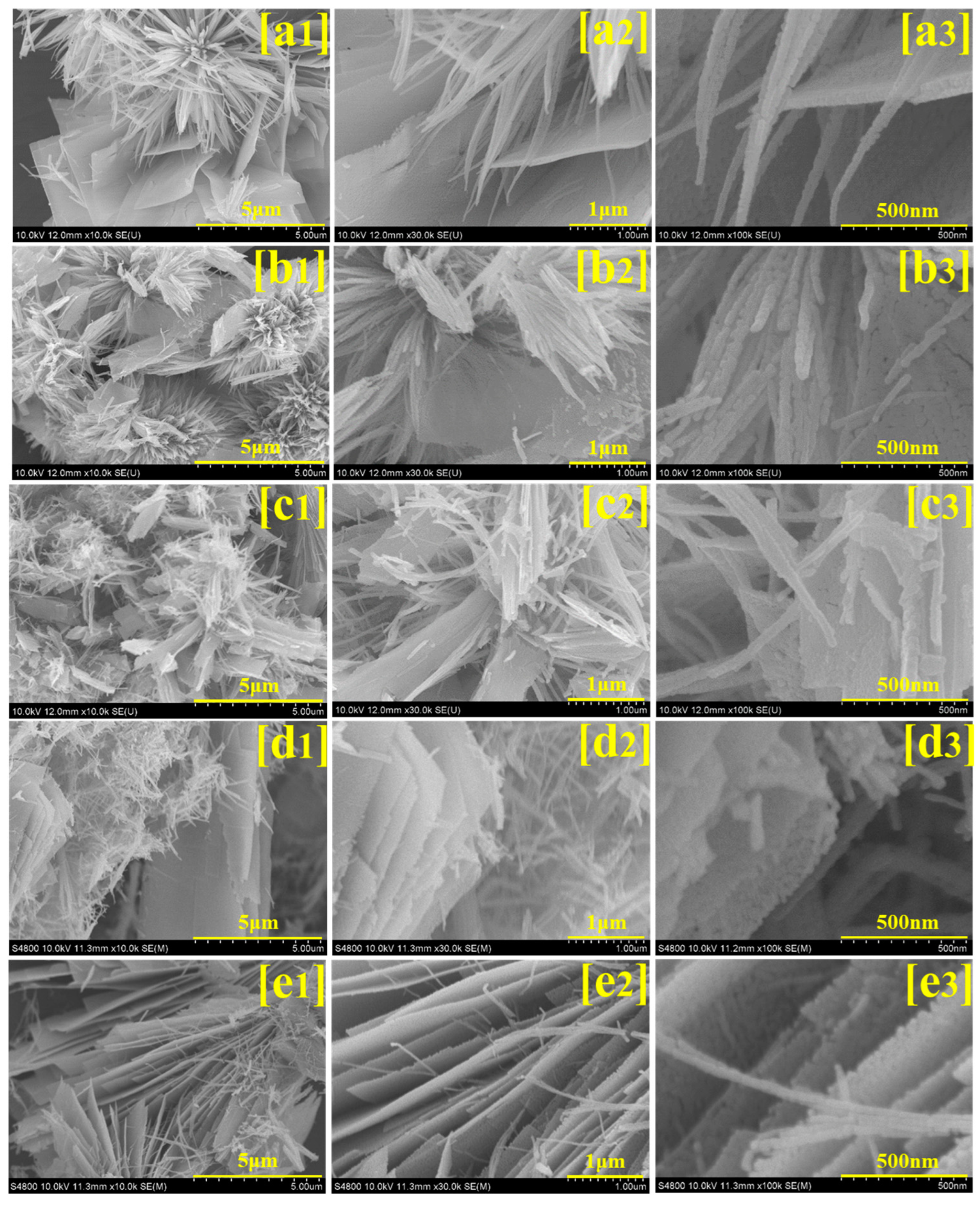
3.3. FESEM Analysis
3.4. Transmission Electron Microscopy (TEM) Analysis
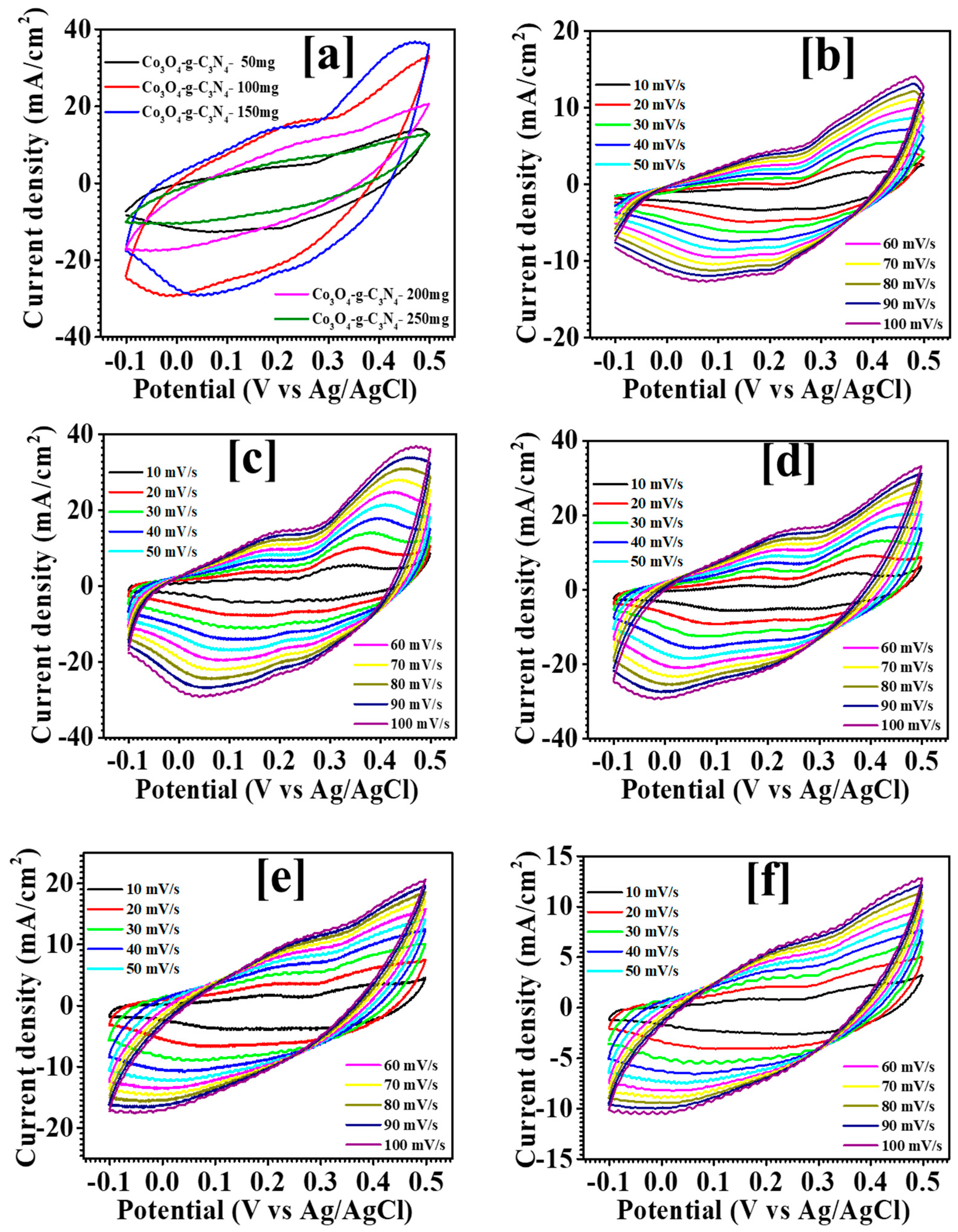
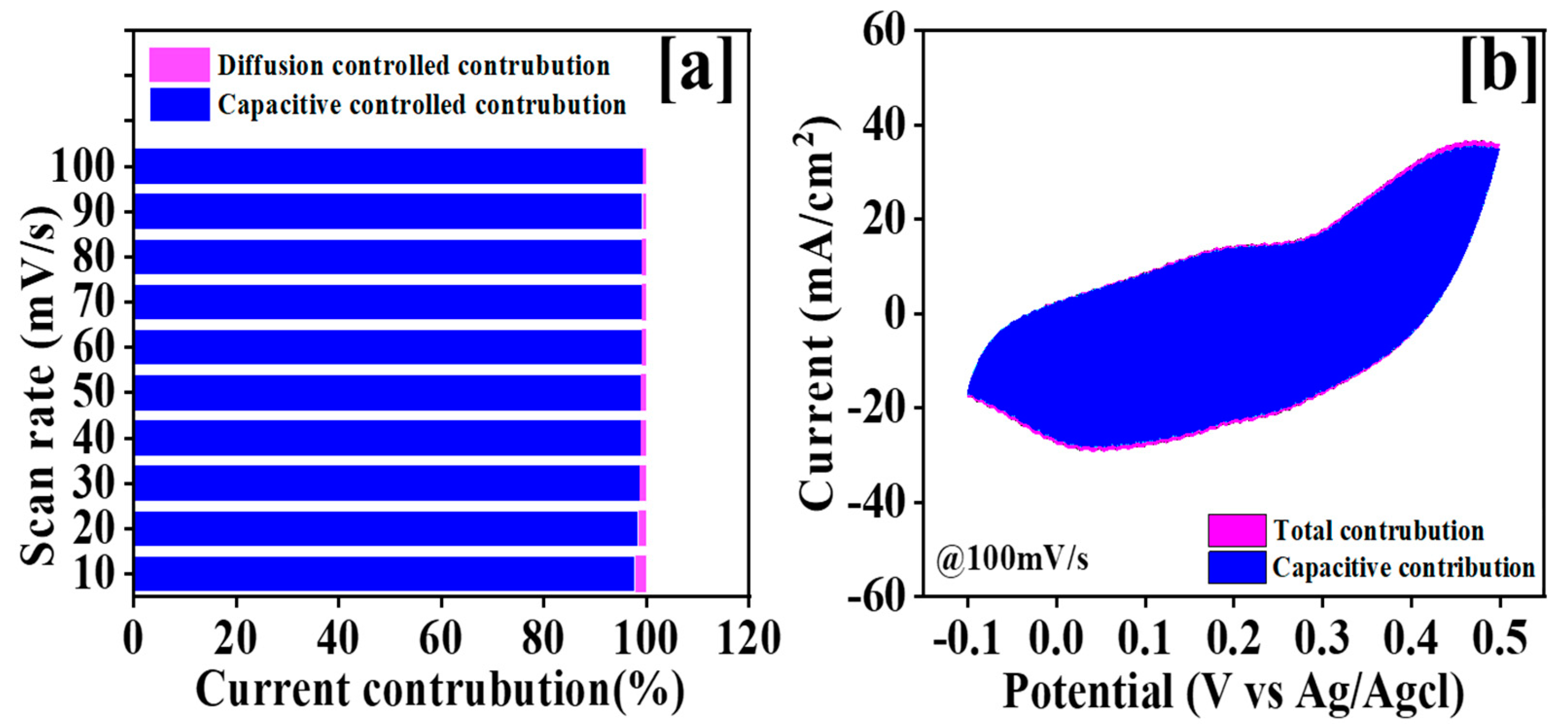
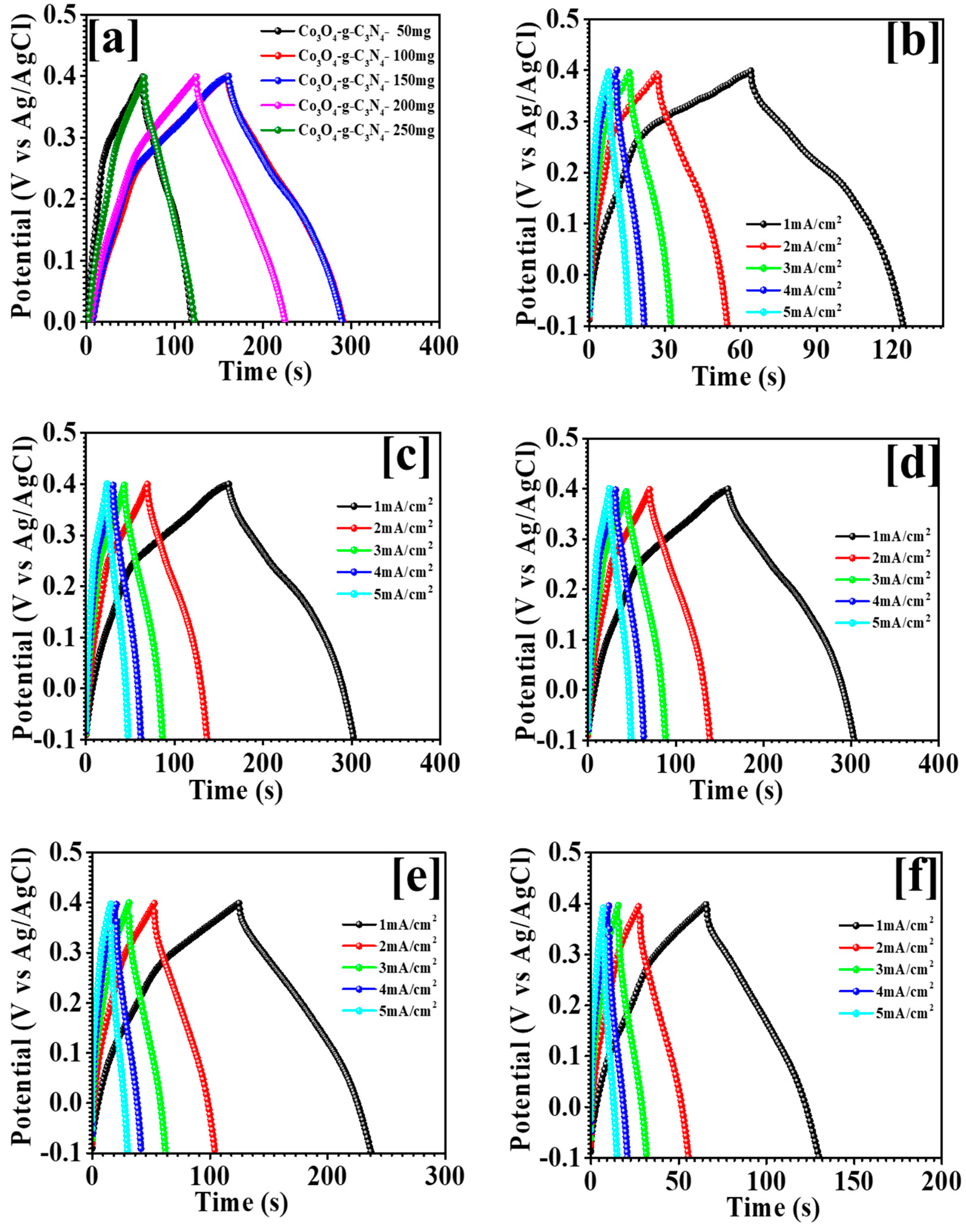
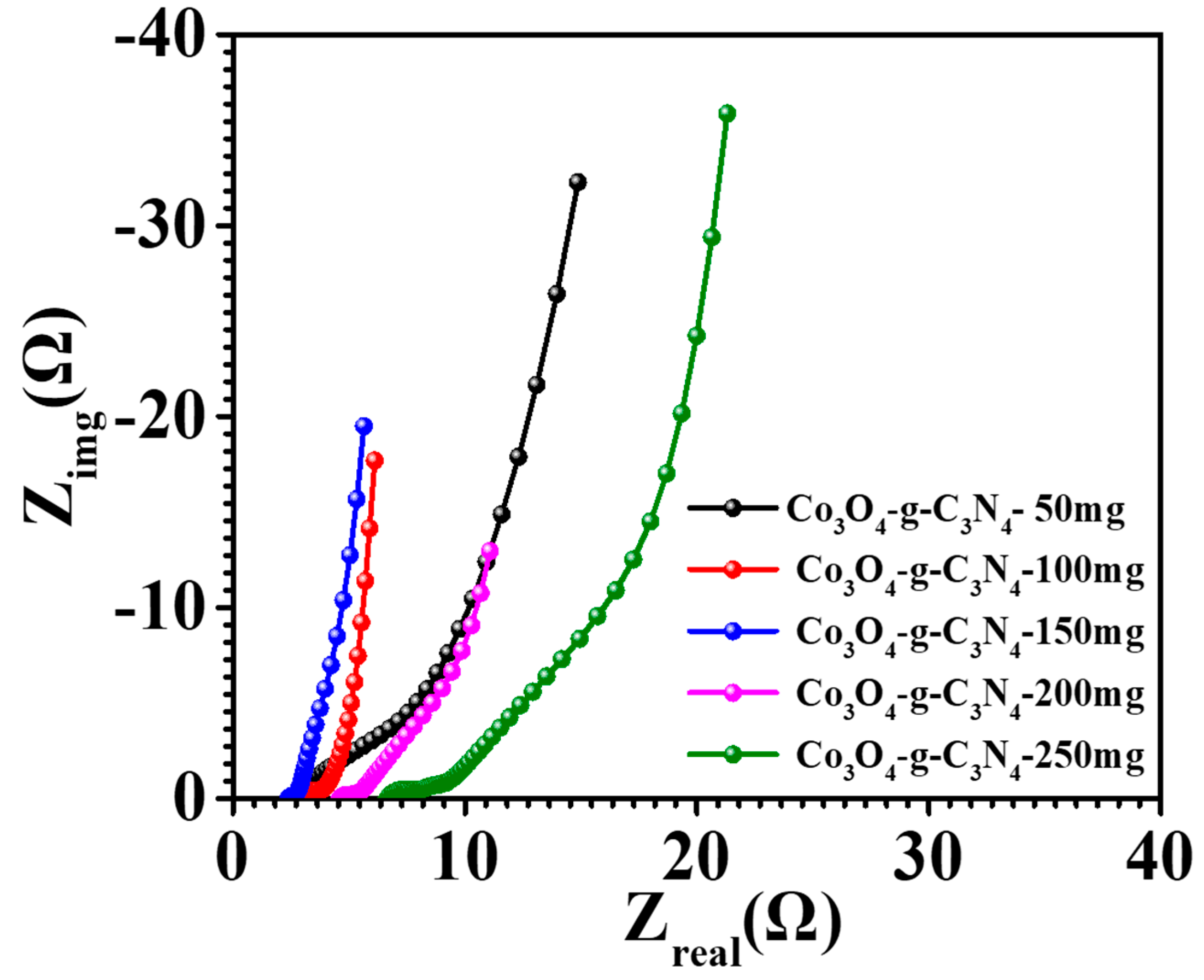
3.5. Electrochemical Study
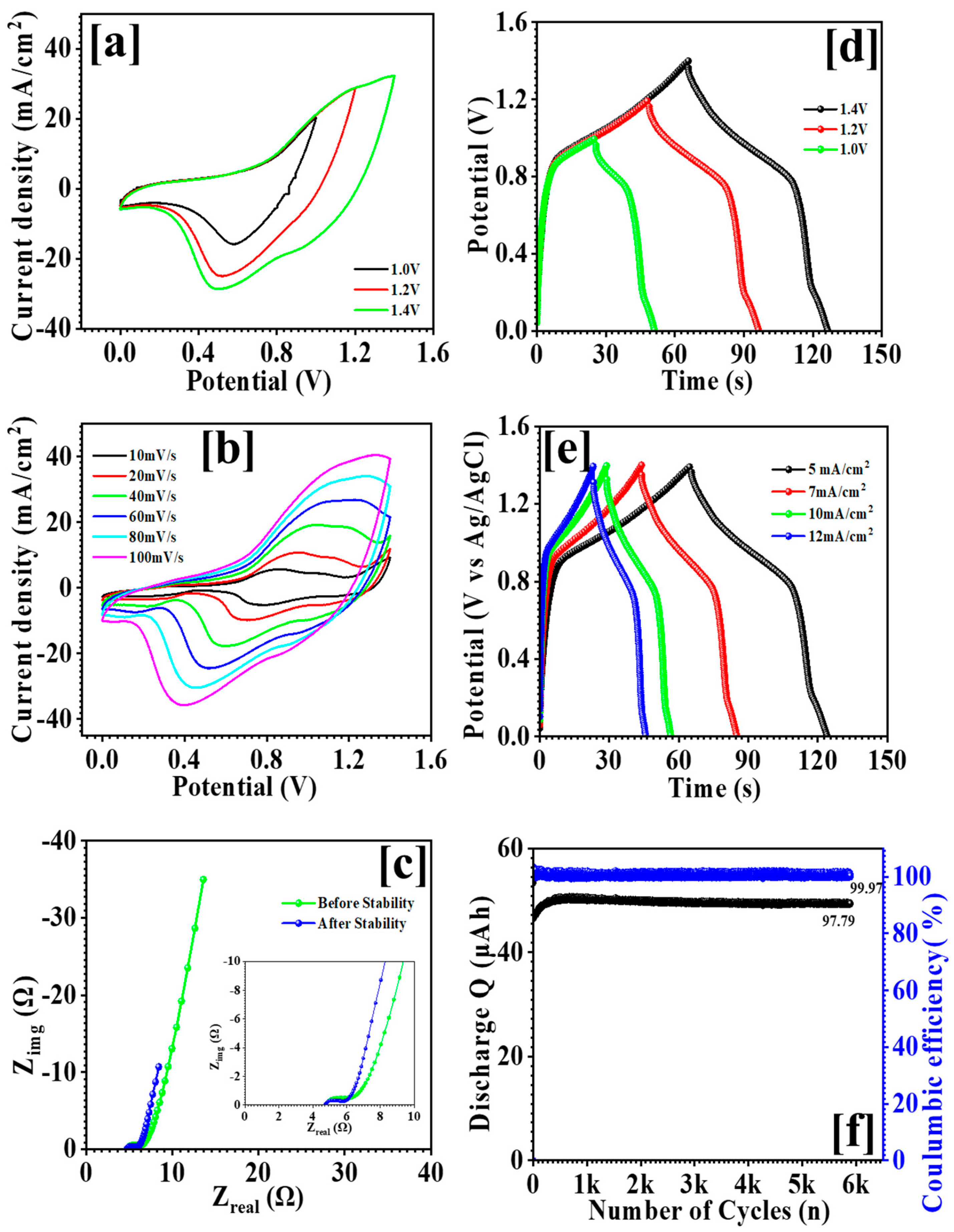
3.6. Electrochemical Study of an Asymmetric Supercapacitor
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Xie, L.-J.; Wu, J.-F.; Chen, C.-M.; Zhang, C.-M.; Wan, L.; Wang, J.-L.; Kong, Q.-Q.; Lv, C.-X.; Li, K.-X.; Sun, G.-H. A novel asymmetric supercapacitor with an activated carbon cathode and a reduced graphene oxide–cobalt oxide nanocomposite anode. J. Power Sources 2013, 242, 148–156. [Google Scholar]
- Sephra, P.J.; Baraneedharan, P.; Sivakumar, M.; Thangadurai, T.D.; Nehru, K. Size controlled synthesis of SnO2 and its electrostatic self-assembly over reduced graphene oxide for photocatalyst and supercapacitor application. Mater. Res. Bull. 2018, 106, 103–112. [Google Scholar]
- Simon, P.; Gogotsi, Y.; Dunn, B. Where Do Batteries End and Supercapacitors Begin? Science 2014, 343, 1210–1211. [Google Scholar]
- Zhang, Q.; Uchaker, E.; Candelaria, S.L.; Cao, G. Nanomaterials for energy conversion and storage. Chem. Soc. Rev. 2013, 42, 3127–3171. [Google Scholar] [CrossRef]
- Rabani, I.; Zafar, R.; Subalakshmi, K.; Kim, H.-S.; Bathula, C.; Seo, Y.-S. A facile mechanochemical preparation of Co3O4@g-C3N4 for application in supercapacitors and degradation of pollutants in water. J. Hazard. Mater. 2021, 407, 124360. [Google Scholar]
- Jow, T.R.; Zheng, J.P. Amorphous thin film ruthenium oxide as an electrode material for electrochemical capacitors. Mater. Res. Soc. Symp. Proc. 1995, 393, 433–438. [Google Scholar]
- Lokhande, C.D.; Dubal, D.P.; Joo, O.-S. Metal oxide thin film based supercapacitors. Curr. Appl. Phys. 2011, 11, 255–270. [Google Scholar]
- Farhadi, S.; Safabakhsh, J.; Zaringhadam, P. Synthesis, characterization, and investigation of optical and magnetic properties of cobalt oxide (Co3O4) nanoparticles. J. Nanostruct. Chem. 2013, 3, 69. [Google Scholar] [CrossRef]
- Ma, T.Y.; Dai, S.; Jaroniec, M.; Qiao, S.Z. Graphitic carbon nitride nanosheet-carbon nanotube three-dimensional porous composites as high-performance oxygen evolution electrocatalysts. Angew. Chem.—Int. Ed. 2014, 53, 7281–7285. [Google Scholar]
- Ding, Y.; Tang, Y.; Yang, L.; Zeng, Y.; Yuan, J.; Liu, T.; Zhang, S.; Liu, C.; Luo, S. Porous nitrogen-rich carbon materials from carbon self-repairing g-C3N4 assembled with graphene for high-performance supercapacitor. J. Mater. Chem. A 2016, 4, 14307–14315. [Google Scholar] [CrossRef]
- Tang, J.; Wang, T.; Salunkhe, R.R.; Alshehri, S.M.; Malgras, V.; Yamauchi, Y. Three-Dimensional Nitrogen-Doped Hierarchical Porous Carbon as an Electrode for High-Performance Supercapacitors. Chem.—Eur. J. 2015, 21, 17293–17298. [Google Scholar]
- Li, K.; Sun, Y.; Zhao, Z.; Zhu, T. Encapsulation of Co nanoparticles with single-atomic Co sites into nitrogen-doped carbon for electrosynthesis of hydrogen peroxide. Phys. Chem. Chem. Phys. 2024, 26, 3044–3050. [Google Scholar] [CrossRef]
- Suja, P.; John, J.; Rajan, T.P.D.; Anilkumar, G.M.; Yamaguchi, T.; Pillai, S.C.; Hareesh, U.S. Graphitic carbon nitride (g-C3N4) based heterogeneous single atom catalysts: Synthesis, characterisation and catalytic applications. J. Mater. Chem. A 2023, 11, 8599–8646. [Google Scholar]
- Zhu, F.; Sun, L.; Liu, Y.; Shi, W. Dual-defect site regulation on MOF-derived P-Co3O4@NC@Ov-NiMnLDH carbon arrays for high-performance supercapacitors. J. Mater. Chem. A 2022, 10, 21021–21030. [Google Scholar]
- Ren, Y.-F.; He, Z.-L.; Zhao, H.-Z.; Zhu, T. Fabrication of MOF-derived mixed metal oxides with carbon residues for pseudocapacitors with long cycle life. Rare Met. 2022, 41, 830–835. [Google Scholar]
- Catherine, H.N.; Chiu, W.L.; Chang, L.L.; Tung, K.L.; Hu, C. Gel-like Ag-Dicyandiamide Metal-Organic Supramolecular Network-Derived g-C3N4 for Photocatalytic Hydrogen Generation. ACS Sustain. Chem. Eng. 2022, 10, 8360–8369. [Google Scholar]
- Kavil, J.; Anjana, P.M.; Joshy, D.; Babu, A.; Raj, G.; Periyat, P.; Rakhi, R.B. G-C3N4/CuO and g-C3N4/Co3O4 nanohybrid structures as efficient electrode materials in symmetric supercapacitors. RSC Adv. 2019, 9, 38430–38437. [Google Scholar] [PubMed]
- Shi, F.; Chen, L.; Chen, M.; Jiang, D. A g-C3N4/nanocarbon/ZnIn2S4 nanocomposite: An artificial Z-scheme visible-light photocatalytic system using nanocarbon as the electron mediator. Chem. Commun. 2015, 51, 17144–17147. [Google Scholar]
- Yewale, M.A.; Jadhvar, A.A.; Kharade, R.B.; Kadam, R.A.; Kumar, V.; Nakate, U.T.; Shelke, P.B.; Bobade, D.H.; Teli, A.M.; Dhas, S.D.; et al. Hydrothermally synthesized Ni3V2O8 nanoparticles with horny surfaces for HER and supercapacitor application. Mater. Lett. 2023, 338, 134033. [Google Scholar]
- Guo, M.; Balamurugan, J.; Thanh, T.D.; Kim, N.H.; Lee, J.H. Facile fabrication of Co2CuS4 nanoparticle anchored N-doped graphene for high-performance asymmetric supercapacitors. J. Mater. Chem. A 2016, 4, 17560–17571. [Google Scholar] [CrossRef]
- Jiang, D.; Xu, Q.; Meng, S.; Xia, C.; Chen, M. Construction of cobalt sulfide/graphitic carbon nitride hybrid nanosheet composites for high performance supercapacitor electrodes. J. Alloys Compd. 2017, 706, 41–47. [Google Scholar]
- Zhang, L.; Hu, X.; Wang, Z.; Sun, F.; Dorrell, D.G. Experimental impedance investigation of an ultracapacitor at different conditions for electric vehicle applications. J. Power Sources 2015, 287, 129–138. [Google Scholar] [CrossRef]
- Yewale, M.A.; Kadam, R.A.; Nakate, U.T.; Teli, A.M.; Kumar, V.; Beknalkar, S.A.; Jadhavar, A.A.; Kadam, S.L.; Shelke, N.T.; Shin, D.K. Sphere-shaped CuCo2O4 nanostructures battery type electrode for supercapacitor via hydrothermal synthesis approach. Colloids Surf. A Physicochem. Eng. Asp. 2023, 679, 132541. [Google Scholar]
- Yewale, M.A.; Kadam, R.A.; Kaushik, N.K.; Linh, N.N.; Teli, A.M.; Shin, J.C.; Lingamdinne, L.P.; Koduru, J.R.; Shin, D.K. Mesoporous hexagonal nanorods of NiCo2O4 nanoparticles via hydrothermal route for supercapacitor application. Chem. Phys. Lett. 2022, 800, 139654. [Google Scholar]
- Zhang, W.; Xu, J.; Wang, H.; Yao, S. CNT anchored by NiCo2O4 nanoparticles with hybrid structure for ultrahigh-performance supercapacitor. J. Mater. Sci. Mater. Electron. 2020, 31, 5948–5957. [Google Scholar]
- Chang, W.; Xue, W.; Liu, E.; Fan, J.; Zhao, B. Highly efficient H2 production over NiCo2O4 decorated g-C3N4 by photocatalytic water reduction. Chem. Eng. J. 2019, 362, 392–401. [Google Scholar]
- Yewale, M.A.; Kadam, R.A.; Kaushik, N.K.; Vattikuti, S.V.P.; Lingamdinne, L.P.; Koduru, J.R.; Shin, D.K. Hydrothermally synthesized microrods and microballs of NiCo2O4 for supercapacitor application. Ceram. Int. 2022, 48, 22037–22046. [Google Scholar]
- Xue, Y.; Chen, T.; Song, S.; Kim, P.; Bae, J. DNA-directed fabrication of NiCo2O4 nanoparticles on carbon nanotubes as electrodes for high-performance battery-like electrochemical capacitive energy storage device. Nano Energy 2019, 56, 751–758. [Google Scholar] [CrossRef]
- Yewale, M.A.; Kadam, R.A.; Kaushik, N.K.; Nguyen, L.N.; Nakate, U.T.; Lingamdinne, L.P.; Koduru, J.R.; Auti, P.S.; Vattikuti, S.V.P.; Shin, D.K. Electrochemical supercapacitor performance of NiCo2O4 nanoballs structured electrodes prepared via hydrothermal route with varying reaction time. Colloids Surf. A Physicochem. Eng. Asp. 2022, 653, 129901. [Google Scholar]
- Abouali, S.; Garakani, M.A.; Xu, Z.L.; Kim, J.K. NiCo2O4/CNT nanocomposites as bi-functional electrodes for Li ion batteries and supercapacitors. Carbon 2016, 102, 262–272. [Google Scholar]
- Li, Y.; Zheng, L.; Wang, W.; Wen, Y. Controllable Synthesis of NiCo2O4/CNT Composites for Supercapacitor Electrode Materials. Int. J. Electrochem. Sci. 2020, 15, 11567–11583. [Google Scholar] [CrossRef]
- Kim, M.G.; Choi, Y.-H. Electrocatalytic Properties of Co3O4 Prepared on Carbon Fibers by Thermal Metal–Organic Deposition for the Oxygen Evolution Reaction in Alkaline Water Electrolysis. Nanomaterials 2023, 13, 1021. [Google Scholar]
- Xiao, M.; Meng, Y.; Duan, C.; Hu, Q.; Li, R.; Zhu, F.; Zhang, Y. Preparation of Co3O4/nitrogen-doped carbon composite by in situ solvothermal with ionic liquid and its electrochemical performance as lithium-ion battery anode. Ionics 2019, 25, 475–482. [Google Scholar]
- Feng, C.; Zhang, J.; He, Y.; Zhong, C.; Hu, W.; Liu, L.; Deng, Y. Sub-3 nm Co3O4 Nano films with Enhanced Supercapacitor Properties. ACS Nano 2015, 9, 1730–1739. [Google Scholar]
- Liao, Q.; Li, N.; Jin, S.; Yang, G.; Wang, C. All-Solid-State Symmetric Supercapacitor Based on Co3O4 Nanoparticles on Vertically Aligned Graphene. ACS Nano 2015, 9, 5310–5317. [Google Scholar] [PubMed]
- Kadam, R.A.; Yewale, M.A.; Teli, A.M.; Nakate, U.T.; Kumar, V.; Kadam, S.L.; Shin, D.K. Bimetallic Co3V2O8 microstructure: A versatile bifunctional electrode for supercapacitor and electrocatalysis applications. Surf. Interfaces 2023, 41, 103267. [Google Scholar]
- Beknalkar, S.A.; Teli, A.M.; Khot, A.C.; Dongale, T.D.; Yewale, M.A.; Nirmal, K.A.; Shin, J.C. A new path to high-performance supercapacitors: Utilizing Ag-embedded CoFe-phosphate and Ti3C2 MXene as hybrid electrodes. J. Energy Storage 2023, 72, 108272. [Google Scholar]
- Yewale, M.A.; Kumar, V.; Kadam, R.A.; Kharade, R.B.; Teli, A.M.; Beknalkar, S.A.; Dhas, S.D.; Nakate, U.T.; Shin, D.K. Wrapped nanochain microstructures of Ni3V2O8 nanoparticles for supercapacitor applications using the hydrothermal method. J. Energy Storage 2023, 73, 109005. [Google Scholar]
- Teli, A.M.; Bhat, T.S.; Beknalkar, S.A.; Mane, S.M.; Chaudhary, L.S.; Patil, D.S.; Pawar, S.A.; Efstathiadis, H.; Shin, J.C. Bismuth manganese oxide based electrodes for asymmetric coin cell supercapacitor. Chem. Eng. J. 2022, 430, 133138. [Google Scholar]
- Teli, A.M.; Beknalkar, S.A.; Amte, R.U.; Morankar, P.J.; Yewale, M.A.; Burungale, V.V.; Jeon, C.W.; Efstathiadis, H.; Shin, J.C. Investigating into the intricacies of charge storage kinetics in NbMn-oxide composite electrodes for asymmetric supercapacitor and HER applications. J. Alloys Compd. 2023, 965, 171305. [Google Scholar]
- Patil, A.M.; Wang, J.; Li, S.; Hao, X.; Du, X.; Wang, Z.; Hao, X.; Abudula, A.; Guan, G. Bilateral growth of monoclinic WO3 and 2D Ti3C2Tx on 3D free-standing hollow graphene foam for all-solid-state supercapacitor. Chem. Eng. J. 2021, 421, 127883. [Google Scholar]
- Patil, A.M.; Moon, S.; Jadhav, A.A.; Hong, J.; Kang, K.; Jun, S.C. Modifying Electronic Structure of Cation-Exchanged Bimetallic Sulfide/Metal Oxide Heterostructure through In Situ Inclusion of Silver (Ag) Nanoparticles for Extrinsic Pseudocapacitor. Adv. Funct. Mater. 2023, 33, 2305264. [Google Scholar]
- Shinde, P.A.; Lokhande, A.C.; Chodankar, N.R.; Patil, A.M.; Kim, J.H.; Lokhande, C.D. Temperature dependent surface morphological modifications of hexagonal WO3 thin films for high performance supercapacitor application. Electrochim. Acta 2017, 224, 397–404. [Google Scholar]
- Dubal, D.P.; Chodankar, N.R.; Holze, R.; Kim, D.H.; Gomez-Romero, P. Ultrathin Mesoporous RuCo2O4 Nanoflakes: An Advanced Electrode for High-Performance Asymmetric Supercapacitors. ChemSusChem 2017, 10, 1771–1782. [Google Scholar] [PubMed]




| Sample Code | b Value | Transfer Coefficient (α) | Diffusion Coefficient (D) (cm/S) × 10−8 |
|---|---|---|---|
| Co3O4-g-C3N4-50 mg | 0.58 | 0.31 | 1.16 |
| Co3O4-g-C3N4-100 mg | 0.72 | 0.33 | 6.16 |
| Co3O4-g-C3N4-150 mg | 0.84 | 0.48 | 7.37 |
| Co3O4-g-C3N4-200 mg | 0.56 | 0.46 | 0.55 |
| Co3O4-g-C3N4-250 mg | 0.80 | 0.40 | 0.23 |
| Sample Code | Specific Capacitance (Cs) F/g | Energy Density (ED) Wh/kg | Power Density (PD) W/g | ESR (Rs) (Ωcm2) |
|---|---|---|---|---|
| Co3O4-g-C3N4-50 mg | 93 | 3.25 | 192 | 2.86 |
| Co3O4-g-C3N4-100 mg | 189 | 6.57 | 166 | 2.82 |
| Co3O4-g-C3N4-150 mg | 198 | 6.85 | 166 | 2.34 |
| Co3O4-g-C3N4-200 mg | 152 | 5.27 | 166 | 4.54 |
| Co3O4-g-C3N4-250 mg | 86 | 3.01 | 166 | 6.59 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yewale, M.A.; Kumar, V.; Teli, A.M.; Beknalkar, S.A.; Nakate, U.T.; Shin, D.-K. Elevating Supercapacitor Performance of Co3O4-g-C3N4 Nanocomposites Fabricated via the Hydrothermal Method. Micromachines 2024, 15, 414. https://doi.org/10.3390/mi15030414
Yewale MA, Kumar V, Teli AM, Beknalkar SA, Nakate UT, Shin D-K. Elevating Supercapacitor Performance of Co3O4-g-C3N4 Nanocomposites Fabricated via the Hydrothermal Method. Micromachines. 2024; 15(3):414. https://doi.org/10.3390/mi15030414
Chicago/Turabian StyleYewale, Manesh A., Vineet Kumar, Aviraj M. Teli, Sonali A. Beknalkar, Umesh T. Nakate, and Dong-Kil Shin. 2024. "Elevating Supercapacitor Performance of Co3O4-g-C3N4 Nanocomposites Fabricated via the Hydrothermal Method" Micromachines 15, no. 3: 414. https://doi.org/10.3390/mi15030414






