Coaxial 3D Bioprinting Process Research and Performance Tests on Vascular Scaffolds
Abstract
:1. Introduction
2. Materials and Methods
2.1. Selection and Preparation of Materials
- Weigh the solid reagent particles using an electronic balance (LICHEN, Changsha, China) before use;
- Pour the sodium alginate particles into batches and dissolve them in deionized water. The constant temperature magnetic stirrer (Shanghai Sile Instrument Co., Ltd., Shanghai, China) was used to stir the mixture, ensuring complete dissolution;
- Dissolve the solid calcium chloride particles in deionized water by stirring with a glass rod for 5 min until fully dissolved;
- Sterilize the prepared sodium alginate solution first, and then inject the HUVEC (Shenggong Biotechnology (Shanghai) Co., Ltd., Shanghai, China) suspension into the prepared sodium alginate solution to complete the preparation of the bioink. Finally, keep it at room temperature before use.
2.2. Modeling the Fluid Behavior of Bioink
2.3. Theoretical Model Analysis and Numerical Simulation of Coaxial Jet Process
- At constant density, the material is considered incompressible: conservation of mass translates into conservation of volume;
- The fluid is assumed to be peristaltic, and inertial effects are negligible compared to viscous forces;
- Bioink materials are modeled using non-Newtonian mechanical behavior, where the viscosity of the deposited fibers can be defined in terms of a power law exponent;
- Extrusion-based bioprinting is temperature-independent, and fiber deposition is considered isothermal.
2.3.1. Theoretical Analysis of Coaxial Nozzle Fluid Model
2.3.2. Theoretical Analysis of Cross-linking Processes
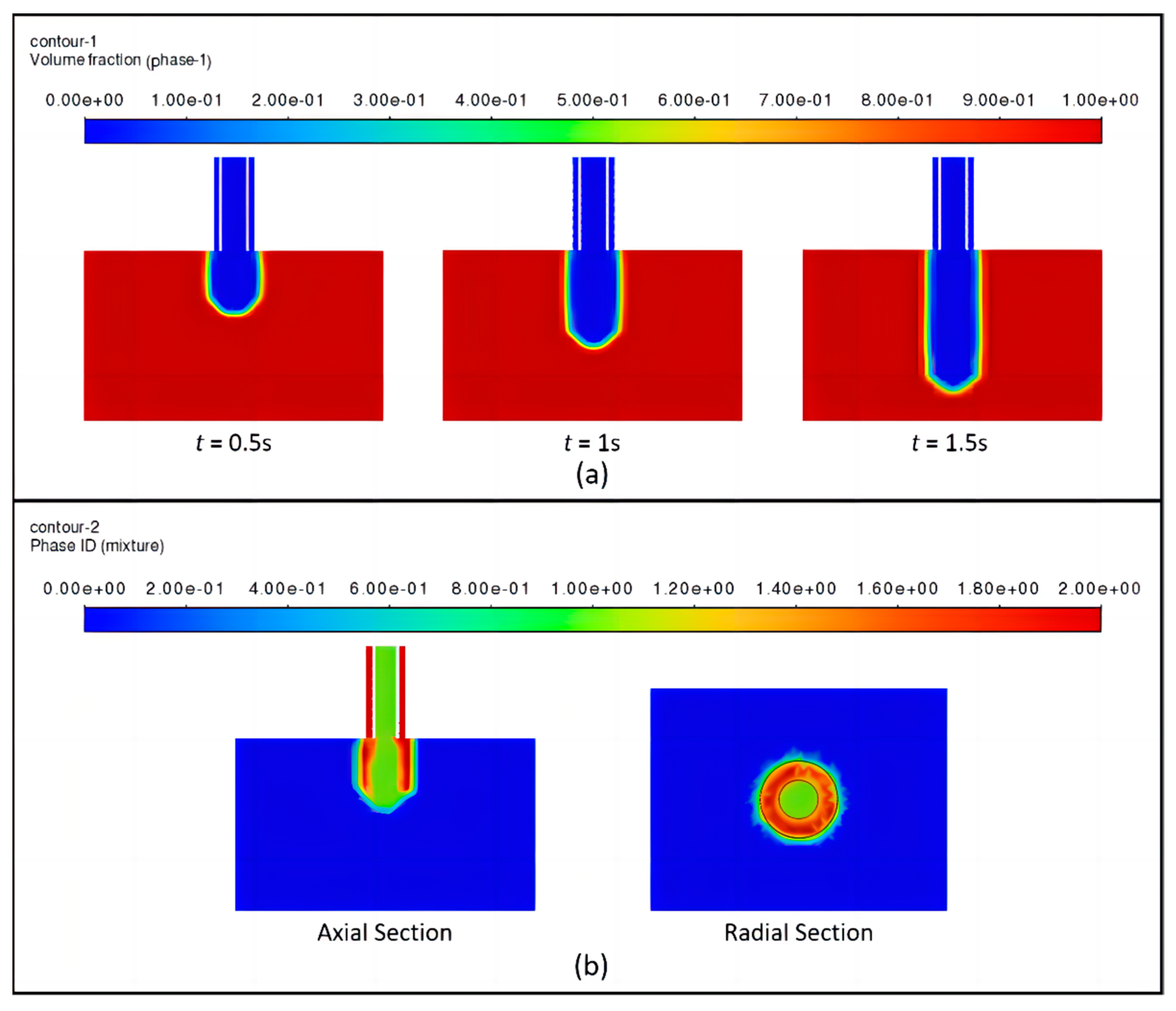
2.3.3. Theoretical Analysis of Coaxial Jet Molding Process
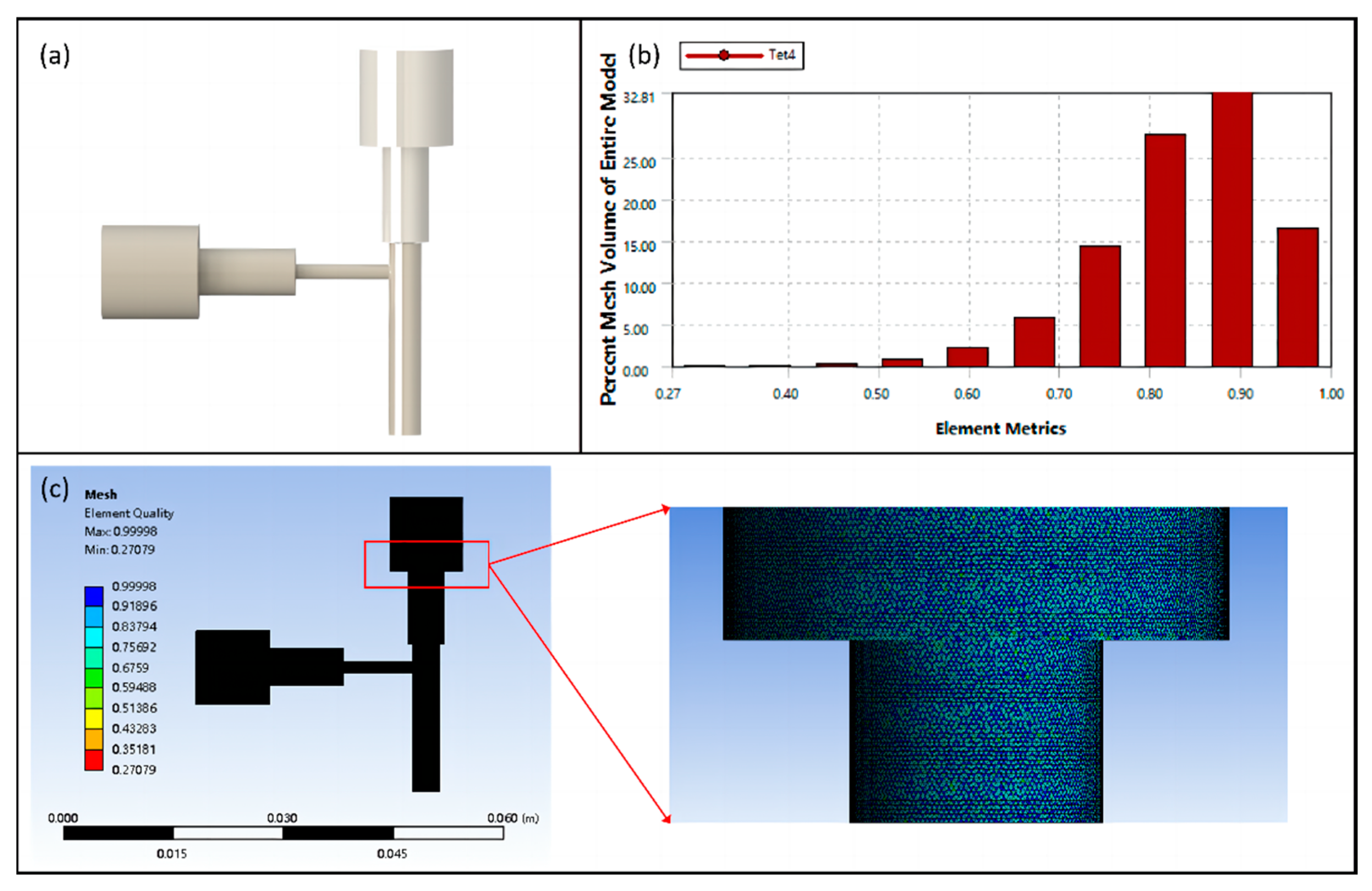
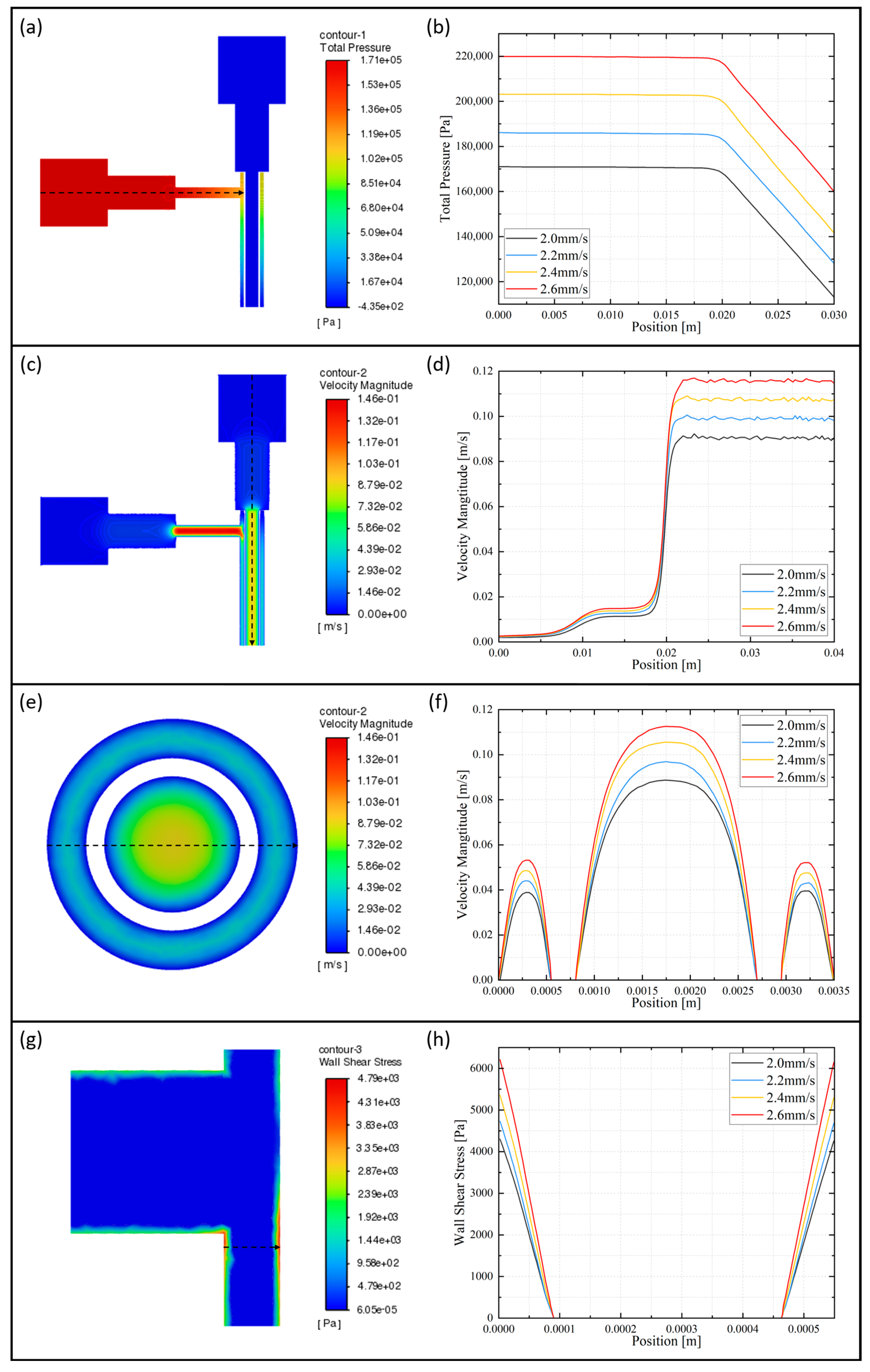
2.3.4. Numerical Simulation of Coaxial Jet
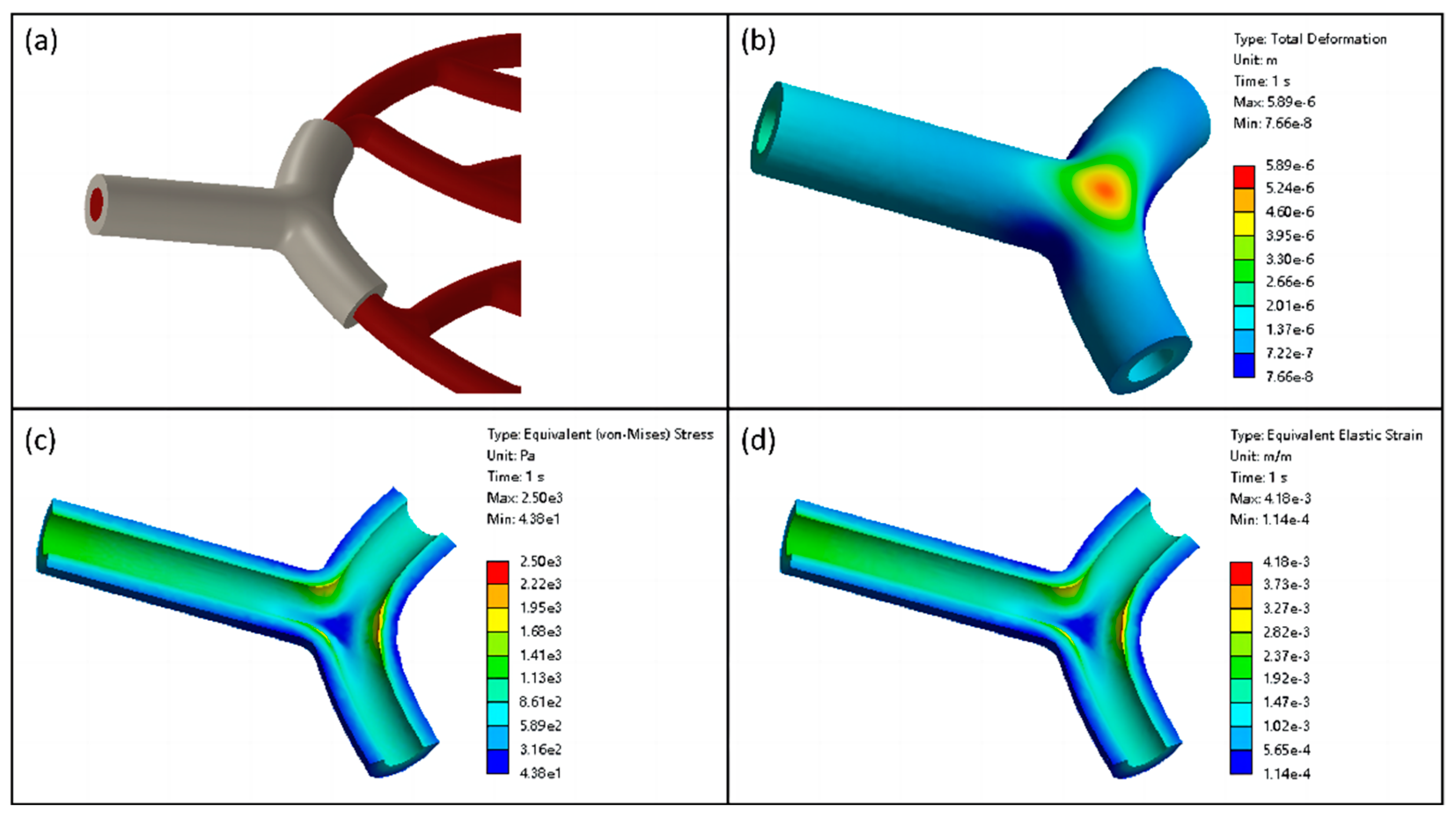
2.4. Theoretical Analysis and Numerical Simulation of Vascular Scaffolds
- It is assumed that the blood flow velocity remains constant in the blood vessels;
- It is assumed that the deformation of the vessel wall is minimal as blood flows through the vessel;
- It is assumed that blood is an incompressible Newtonian fluid.
2.4.1. Theoretical Analysis of Vascular Scaffolds
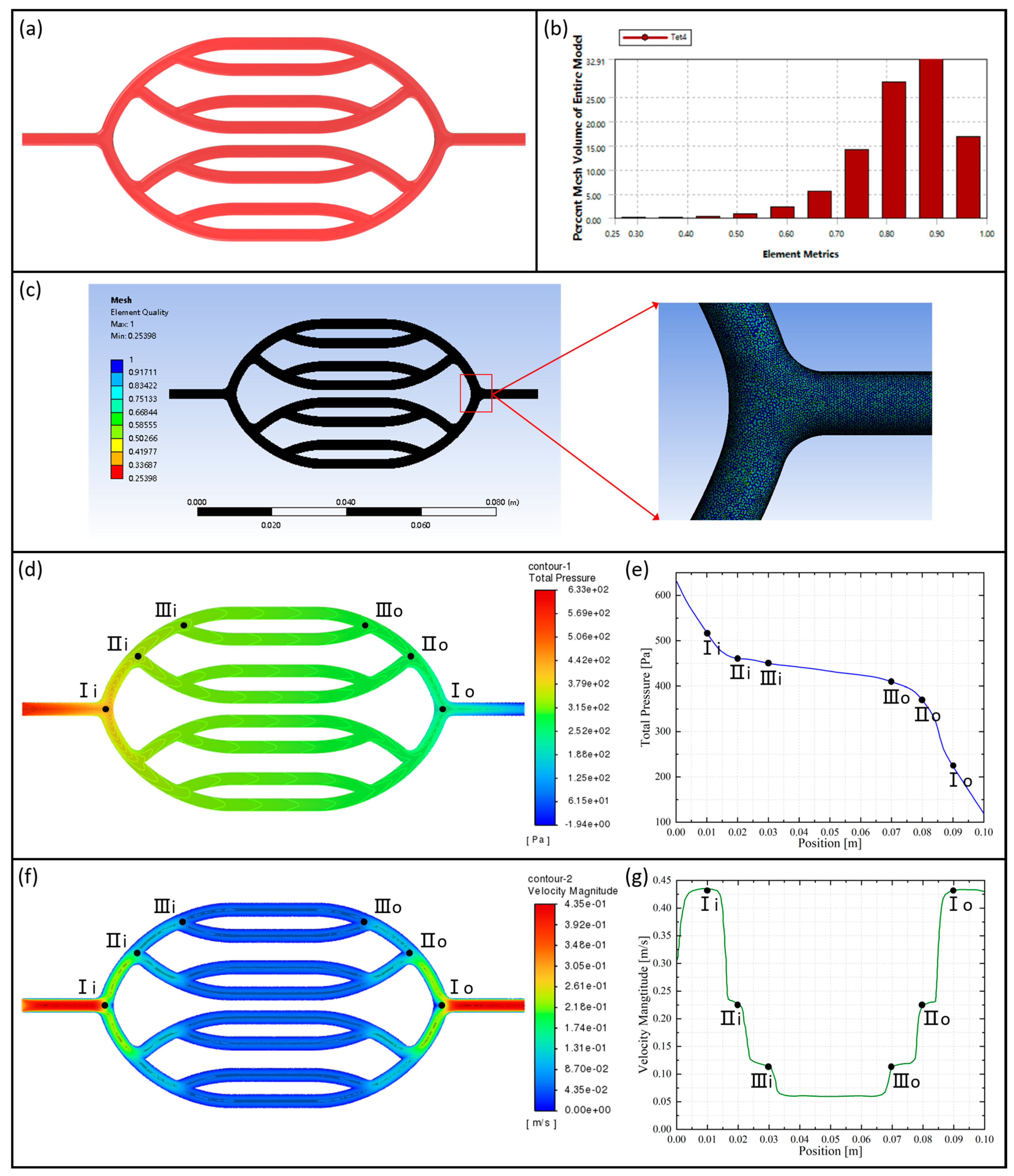
2.4.2. Numerical Simulation of Vascular Scaffolds
2.5. Fabrication Methods
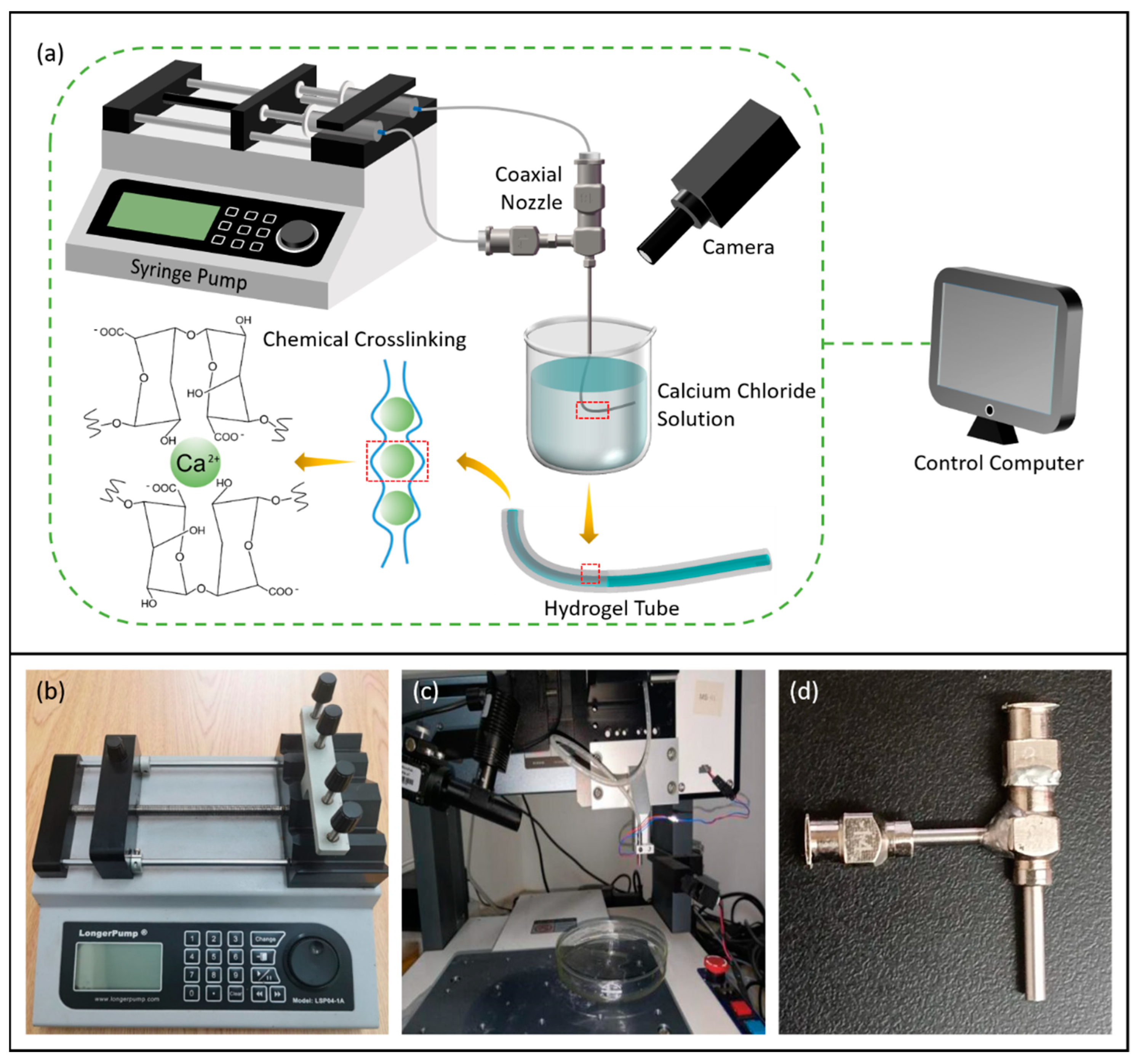
2.5.1. 3D Bioprinting System Based on Coaxial Jet
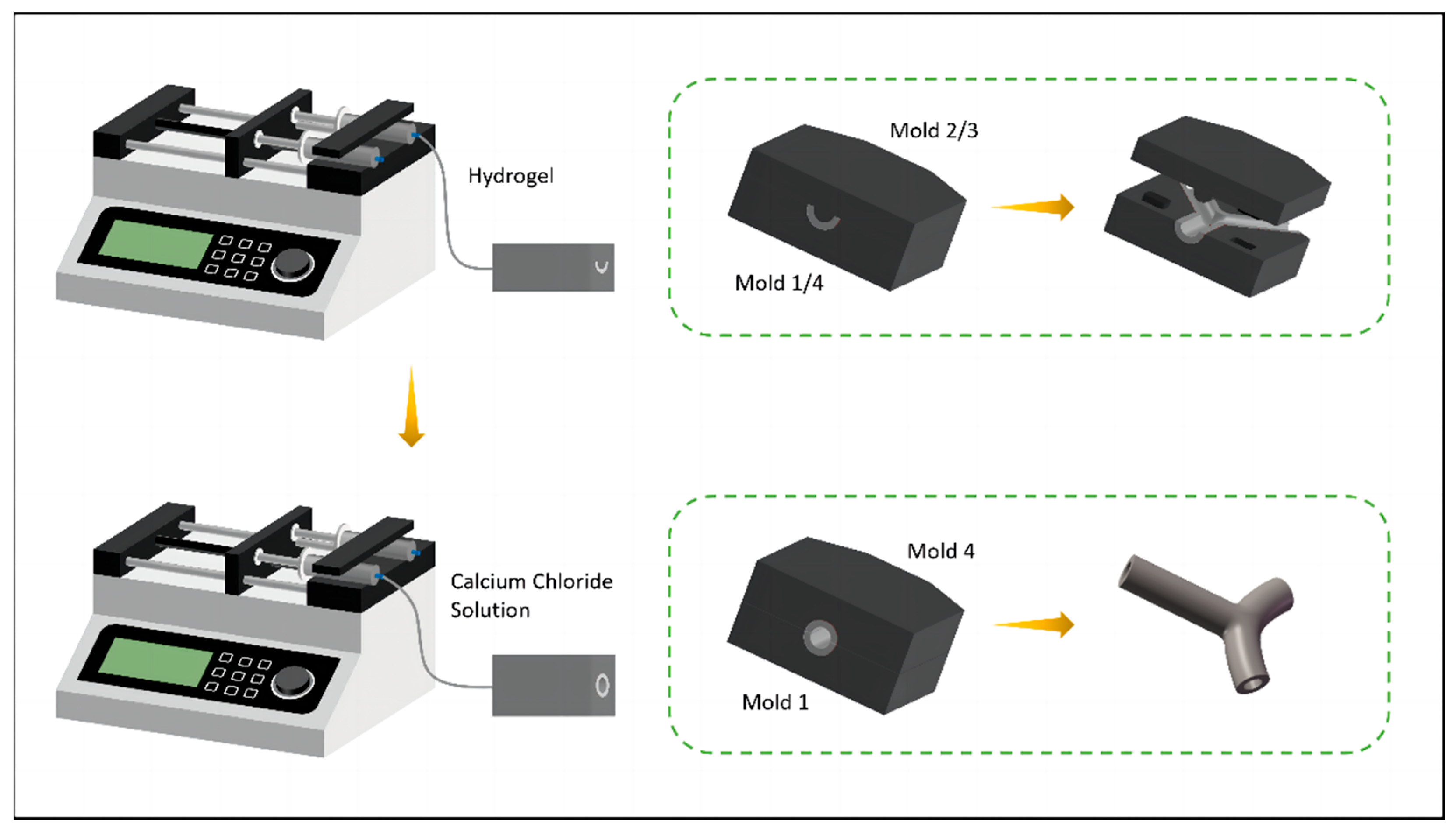
2.5.2. Mold System
2.6. Experimental Verification
2.6.1. Degradability Test
2.6.2. Water Absorption Test
2.6.3. Mechanical Properties Test
2.6.4. Biocompatibility Test
3. Results and Discussion
3.1. Experimental Analysis of Fabrication Process
3.1.1. Printability of Vascular Scaffolds and Optimization of Process Parameters
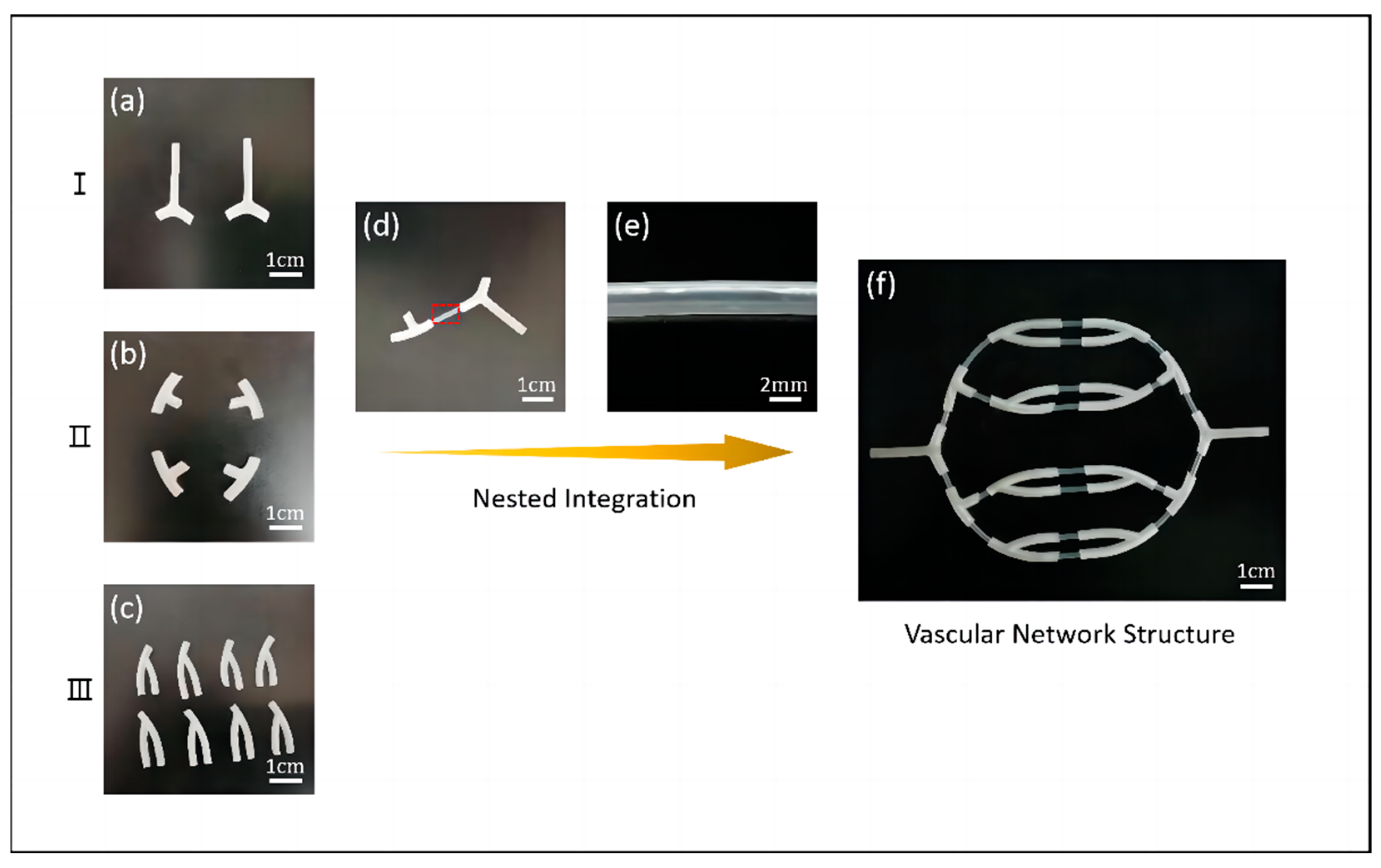
3.1.2. Research of Vascular Network Scaffolds
3.2. Characterization of Vascular Scaffolds
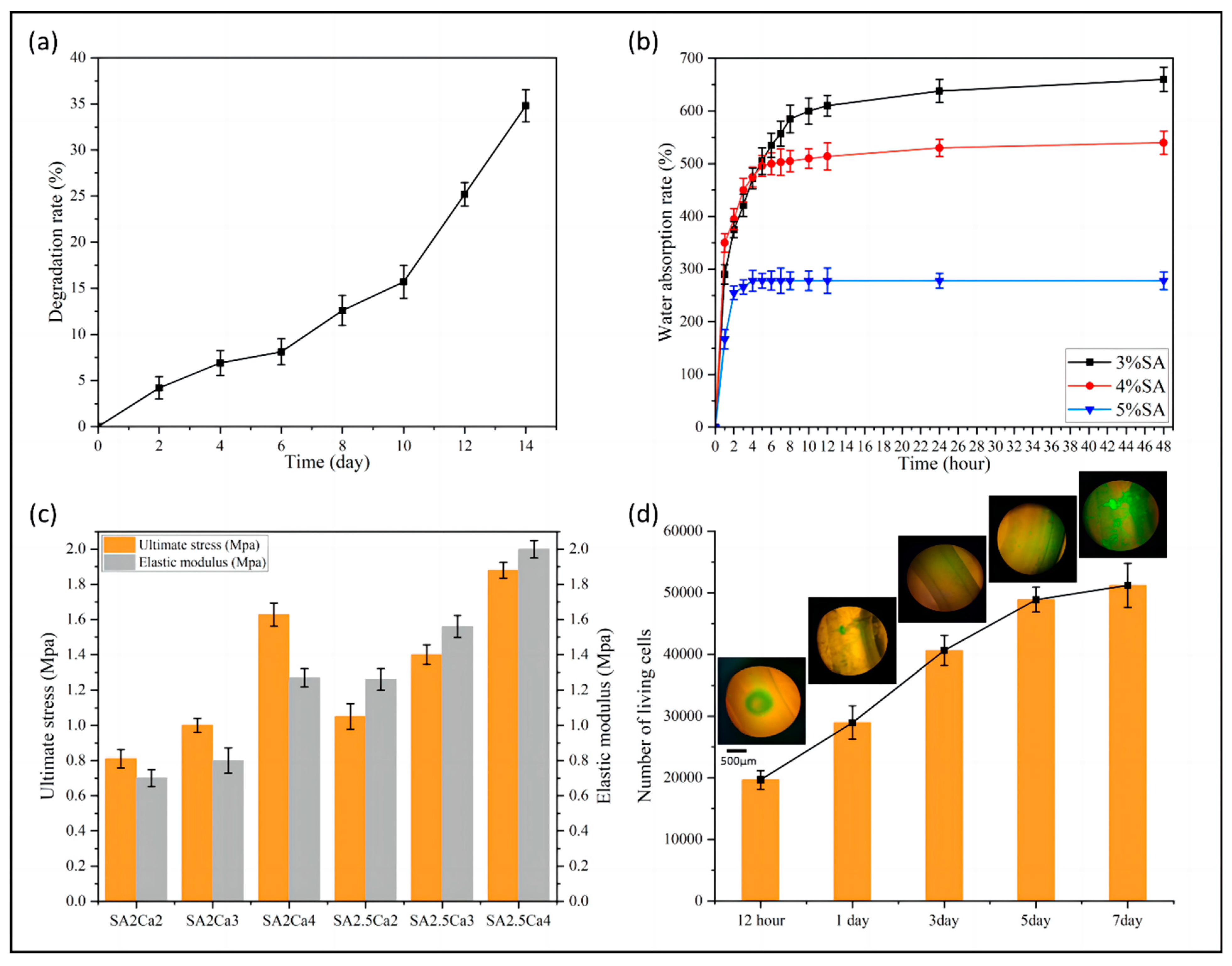
3.2.1. Degradability of Vascular Scaffolds
3.2.2. Water Absorption of Vascular Scaffolds
3.2.3. Mechanical Properties of Vascular Scaffolds
3.2.4. Biocompatibility of Vascular Scaffolds
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Goldstein, L.B. Introduction for Focused Updates in Cerebrovascular Disease. Stroke 2020, 51, 708–710. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.A.G.; White, C.J. Progress in peripheral arterial disease. Prog. Cardiovasc. Dis. 2021, 65, 1. [Google Scholar] [CrossRef] [PubMed]
- Henry, J.J.D.; Yu, J.; Wang, A.; Lee, R.; Fang, J.; Li, S. Engineering the mechanical and biological properties of nanofibrous vascular grafts for in situ vascular tissue engineering. Biofabrication 2017, 9, 035007. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Li, X.; Xu, T.; Zhang, L. Acellular Small-Diameter Tissue-Engineered Vascular Grafts. Appl. Sci. 2019, 9, 2864. [Google Scholar] [CrossRef]
- O’Doherty, M.G.; Cairns, K.; O’Neill, V.; Lamrock, F.; Jørgensen, T.; Brenner, H.; Schöttker, B.; Wilsgaard, T.; Siganos, G.; Kuulasmaa, K.; et al. Effect of major lifestyle risk factors, independent and jointly, on life expectancy with and without cardiovascular disease: Results from the Consortium on Health and Ageing Network of Cohorts in Europe and the United States (CHANCES). Eur. J. Epidemiol. 2016, 31, 455–468. [Google Scholar] [CrossRef] [PubMed]
- Moore, M.J.; Tan, R.P.; Yang, N.; Rnjak-Kovacina, J.; Wise, S.G. Bioengineering artificial blood vessels from natural materials. Trends Biotechnol. 2022, 40, 693–707. [Google Scholar] [CrossRef] [PubMed]
- Vedadghavami, A.; Minooei, F.; Mohammadi, M.H.; Khetani, S.; Rezaei, A.; Mashayekhan, S.; Sanati-Nezhad, A. Manufacturing of hydrogel biomaterials with controlled mechanical properties for tissue engineering applications. Acta Biomater. 2017, 62, 42–62. [Google Scholar] [CrossRef] [PubMed]
- Tresoldi, C.; Bianchi, E.; Pellegata, A.F.; Dubini, G.; Mantero, S. Estimation of the physiological mechanical conditioning in vascular tissue engineering by a predictive fluid-structure interaction approach. Comput. Methods Biomech. Biomed. Engine 2017, 20, 1077–1088. [Google Scholar] [CrossRef] [PubMed]
- Rosellini, E.; Barbani, N.; Lazzeri, L.; Cascone, M.G. Biomimetic and Bioactive Small Diameter Tubular Scaffolds for Vascular Tissue Engineering. Biomimetics 2022, 7, 199. [Google Scholar] [CrossRef]
- Liang, B.; Shi, Q.; Xu, J.; Chai, Y.M.; Xu, J.G. Poly (Glycerol Sebacate)-Based Bio-Artificial Multiporous Matrix for Bone Regeneration. Front. Chem. 2020, 8, 603577. [Google Scholar] [CrossRef]
- Chen, C.; Kieffer, R.; Ebert, H.; Prehm, M.; Zhang, R.B.; Zeng, X.; Liu, F.; Ungar, G.; Tschierske, C. Chirality Induction through Nano-Phase Separation: Alternating Network Gyroid Phase by Thermotropic Self-Assembly of X-Shaped Bolapolyphiles. Angew. Chem. Int. Ed. 2020, 59, 2725–2729. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Guo, L.; Zhang, X.; Hao, J. Environmentally stable, photochromic and thermotropic organohydrogels for low cost on-demand optical devices. J. Colloid. Interface Sci. 2020, 578, 315–325. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.R.; Yu, G.; Potter, T.D.; Walker, M.; Gray, S.J.; Li, J.; Boyd, N.J. Molecular simulation approaches to the study of thermotropic and lyotropic liquid crystals. Crystals 2022, 12, 685. [Google Scholar] [CrossRef]
- Sivaramakrishna, D.; Choudhury, S.K.; Cheppali, S.K.; Swamy, M.J. Structure, thermotropic phase behavior and membrane interaction of N-acyl-β-alaninols. Homologs of stress-combating N-acylethanolamines. Chem. Phys. Lipids. 2021, 236, 105056. [Google Scholar] [CrossRef] [PubMed]
- Tao, H.; Rigoni, C.; Li, H.; Koistinen, A.; Timonen, J.V.I.; Zhou, J.; Kontturi, E.; Rojas, O.J.; Chu, G. Thermodynamically controlled multiphase separation of heterogeneous liquid crystal colloids. Nat. Commun. 2023, 14, 5277. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Mu, L.; Zhang, F.; Mo, Z.; Jin, C.; Qi, W. Manufacture and property research of heparin grafted electrospinning PCU artificial vascular scaffolds. Mater. Sci. Eng. C-Mater. 2017, 78, 854–861. [Google Scholar] [CrossRef] [PubMed]
- Rychter, M.; Baranowska, K.A.; Lulek, J. Progress and perspectives in bioactive agent delivery via electrospun vascular grafts. RSC Adv. 2017, 7, 32164–32184. [Google Scholar] [CrossRef]
- Elsayed, Y.; Lekakou, C.; Labeed, F.; Tomlins, P. Fabrication and characterisation of biomimetic, electrospun gelatin fibre scaffolds for tunica media-equivalent, tissue engineered vascular grafts. Mater. Sci. Eng. C Mater. 2016, 61, 473–483. [Google Scholar] [CrossRef]
- Raymond, L.; Hua, W.; Valentin, N.; Coulter, R.; Bandala, E.; Leong, K.; Okaikoi, J.; Jin, Y. Coaxial Nozzle-Assisted Embedded 3D Printing of Single-Layered Channels Within a Yield-Stress Matrix Bath. J. Manuf. Sci. Eng. 2024, 146, 011010. [Google Scholar] [CrossRef]
- Hamed, R.; Zhou, L.Y.; Shao, L.; He, Y. Coaxial 3D bioprinting of organ prototyps from nutrients delivery to vascularization. J. Zhejiang Univ. Sci. A 2020, 21, 859–875. [Google Scholar]
- Christensen, K.; Xu, C.; Chai, W.; Zhang, Z.; Fu, J.; Huang, Y. Freeform inkjet printing of cellular structures with bifurcations. Biotechnol. Bioeng. 2015, 112, 1047–1055. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Hu, Y.; Liu, C.; Yao, H.; Liu, B.; Mi, S. A Novel Strategy for Creating Tissue-Engineered Biomimetic Blood Vessels Using 3D Bioprinting Technology. Materials 2018, 11, 1581. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Kim, M.G.; Kim, J.; Jeon, J.S.; Park, J.; Yi, H.G. Bioprinting methods for fabricating in vitro tubular blood vessel models. Cyborg Bionic Syst. 2023, 4, 0043. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, Y.; Yu, Y.; Ozbolat, I.T. 3D coaxial bioprinting of vasculature. Methods Mol. Biol. 2020, 2140, 171–181. [Google Scholar] [PubMed]
- Miller, J.S. The billion cell construct: Will three-dimensional printing get us there? PLoS Bio. 2014, 12, e1001882. [Google Scholar] [CrossRef]
- Zhu, W.; Ma, X.; Gou, M.; Mei, D.; Zhang, K.; Chen, S. 3D printing of functional biomaterials for tissue engineering. Curr. Opin. Biotechnol. 2016, 40, 103–112. [Google Scholar] [CrossRef]
- Axpe, E.; Oyen, M.L. Applications of Alginate-Based Bioinks in 3D Bioprinting. Int. J. Mol. Sci. 2016, 17, 1976. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ao, Q.; Tian, X.; Fan, J.; Tong, H.; Hou, W.; Bai, S. Gelatin-Based Hydrogels for Organ 3D Bioprinting. Polymers 2017, 9, 401. [Google Scholar] [CrossRef]
- Wang, F.; Jin, P.; Feng, Y.; Fu, J.; Wang, P.; Liu, X.; Zhang, Y.; Ma, Y.; Yang, Y.; Yang, A.; et al. Flexible Doppler ultrasound device for the monitoring of blood flow velocity. Sci. Adv. 2021, 7, 44. [Google Scholar] [CrossRef]
- Ruediger, S.L.; Koep, J.L.; Keating, S.E.; Pizzey, F.K.; Coombes, J.S.; Bailey, T.G. Effect of menopause on cerebral artery blood flow velocity and cerebrovascular reactivity: Systematic review and meta-analysis. Maturitas 2021, 148, 24–32. [Google Scholar] [CrossRef]
- Ye, F.; Yin, S.; Li, M.; Li, Y.; Zhong, J. In-vivo full-field measurement of microcirculatory blood flow velocity based on intelligent object identification. J. Biomed. Opt. 2020, 25, 016003. [Google Scholar] [CrossRef] [PubMed]


| Model | Inner Diameter (mm) | Outer Diameter (mm) | Wall Thickness (mm) |
|---|---|---|---|
| 14G | 1.60 | 2.10 | 0.2500 |
| 18G | 0.90 | 1.30 | 0.2000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, J.; Gong, Y.; Xu, M.; Chen, H.; Shao, H.; Zhou, R. Coaxial 3D Bioprinting Process Research and Performance Tests on Vascular Scaffolds. Micromachines 2024, 15, 463. https://doi.org/10.3390/mi15040463
Sun J, Gong Y, Xu M, Chen H, Shao H, Zhou R. Coaxial 3D Bioprinting Process Research and Performance Tests on Vascular Scaffolds. Micromachines. 2024; 15(4):463. https://doi.org/10.3390/mi15040463
Chicago/Turabian StyleSun, Jiarun, Youping Gong, Manli Xu, Huipeng Chen, Huifeng Shao, and Rougang Zhou. 2024. "Coaxial 3D Bioprinting Process Research and Performance Tests on Vascular Scaffolds" Micromachines 15, no. 4: 463. https://doi.org/10.3390/mi15040463
APA StyleSun, J., Gong, Y., Xu, M., Chen, H., Shao, H., & Zhou, R. (2024). Coaxial 3D Bioprinting Process Research and Performance Tests on Vascular Scaffolds. Micromachines, 15(4), 463. https://doi.org/10.3390/mi15040463








