Micro/Nanorobotics in In Vitro Fertilization: A Paradigm Shift in Assisted Reproductive Technologies
Abstract
:1. Introduction
2. Discussion
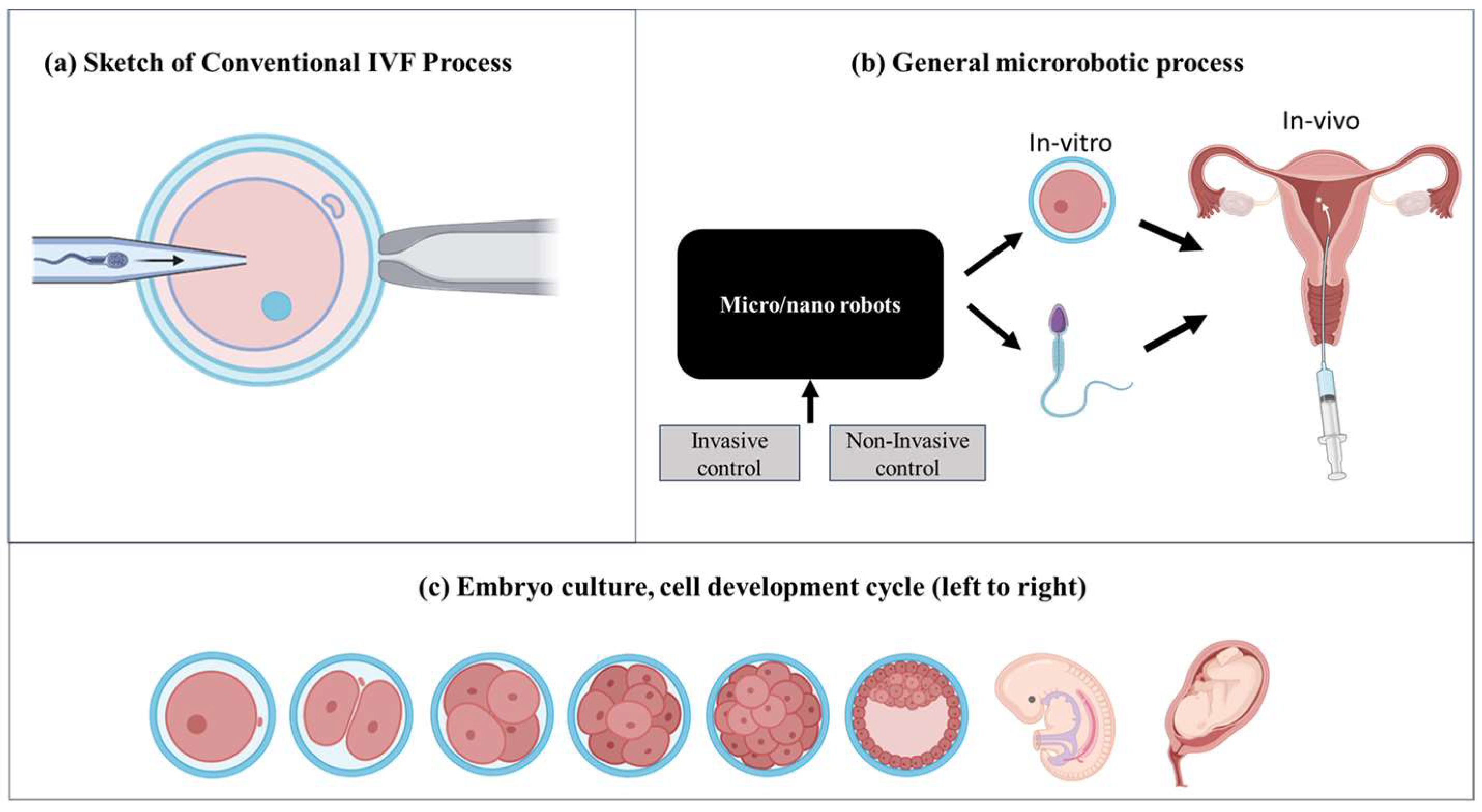
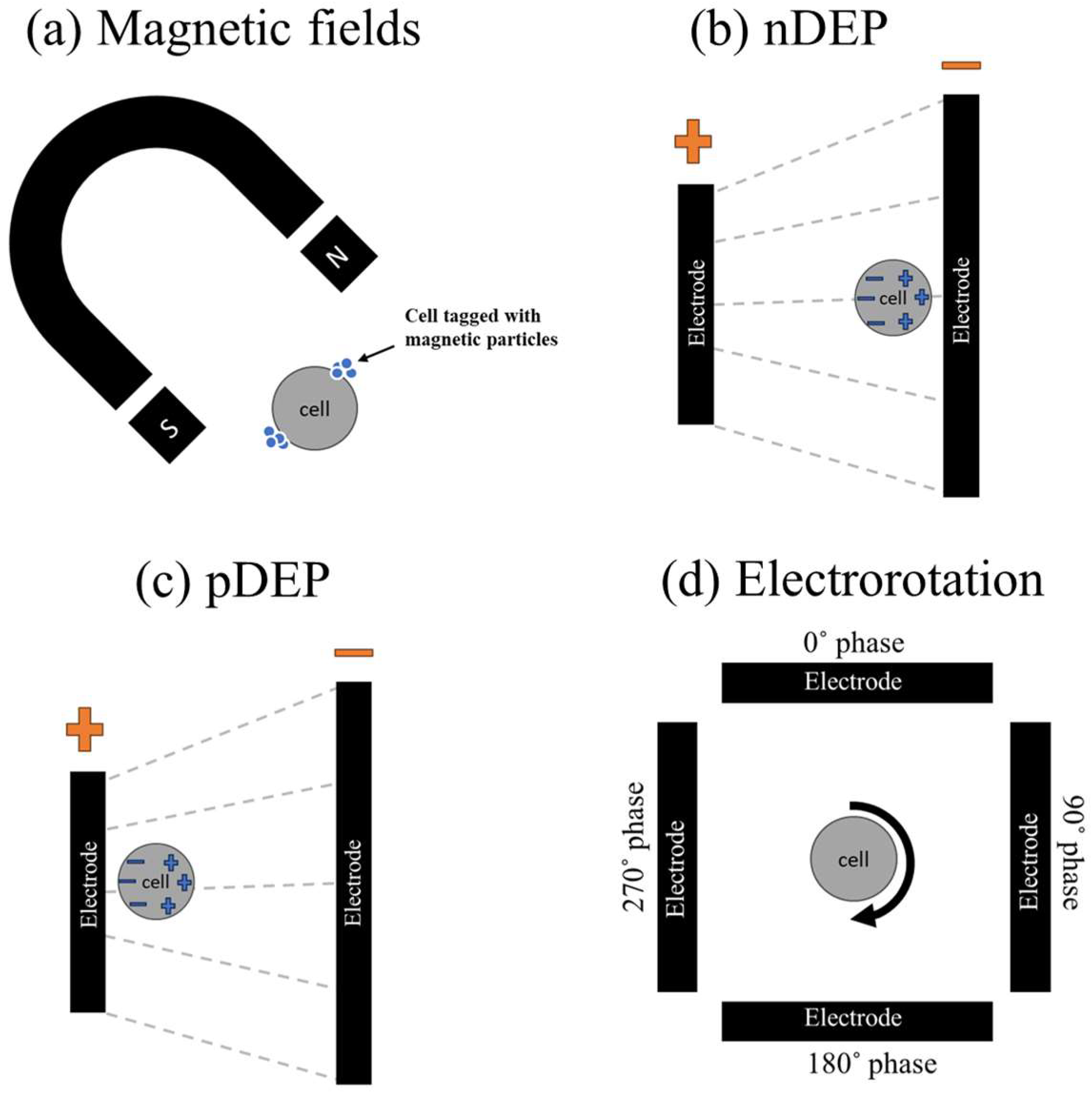
2.1. Principles of Micro/Nanorobotics in IVF
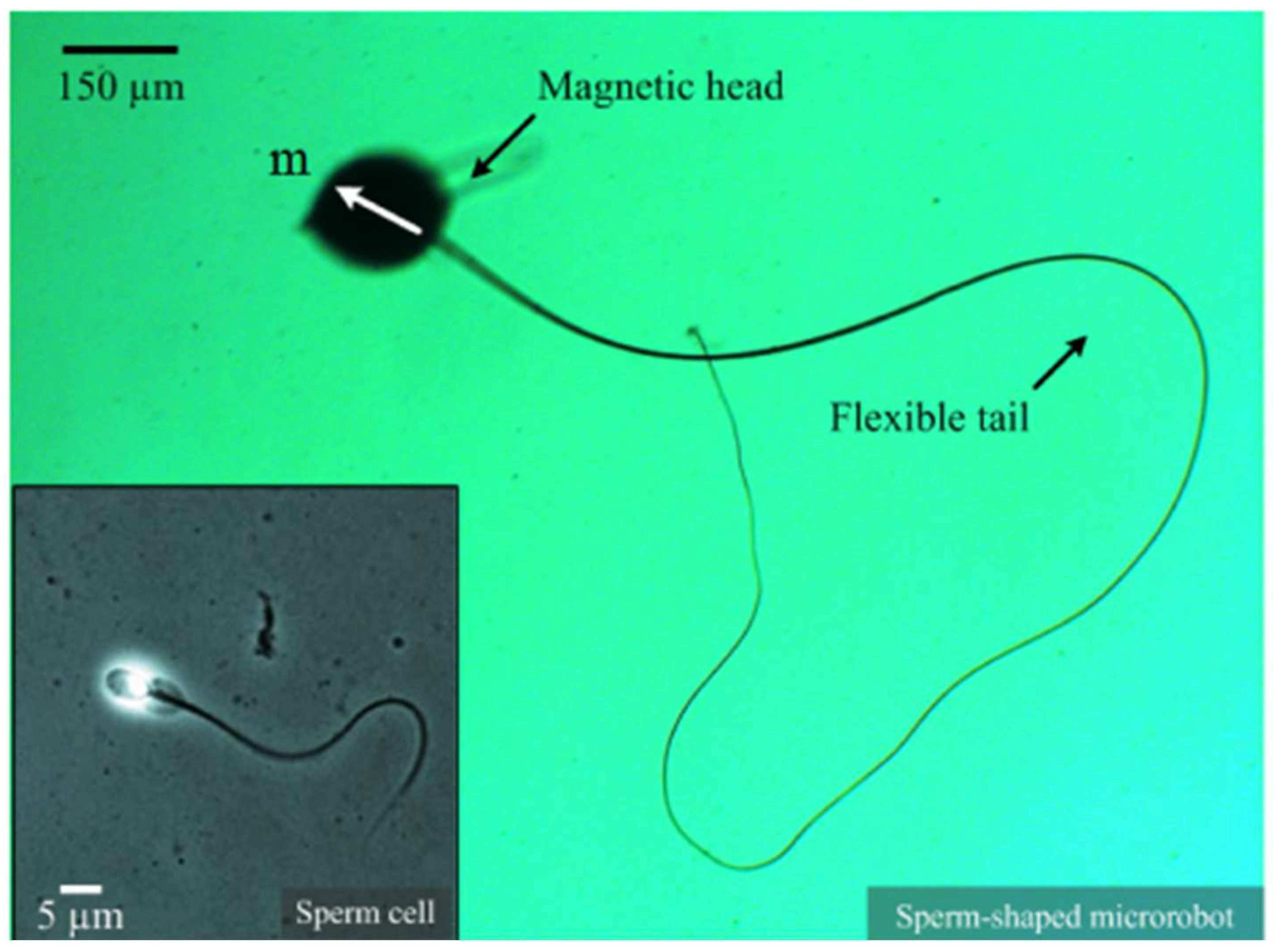
2.2. Advancements in Sperm Manipulation
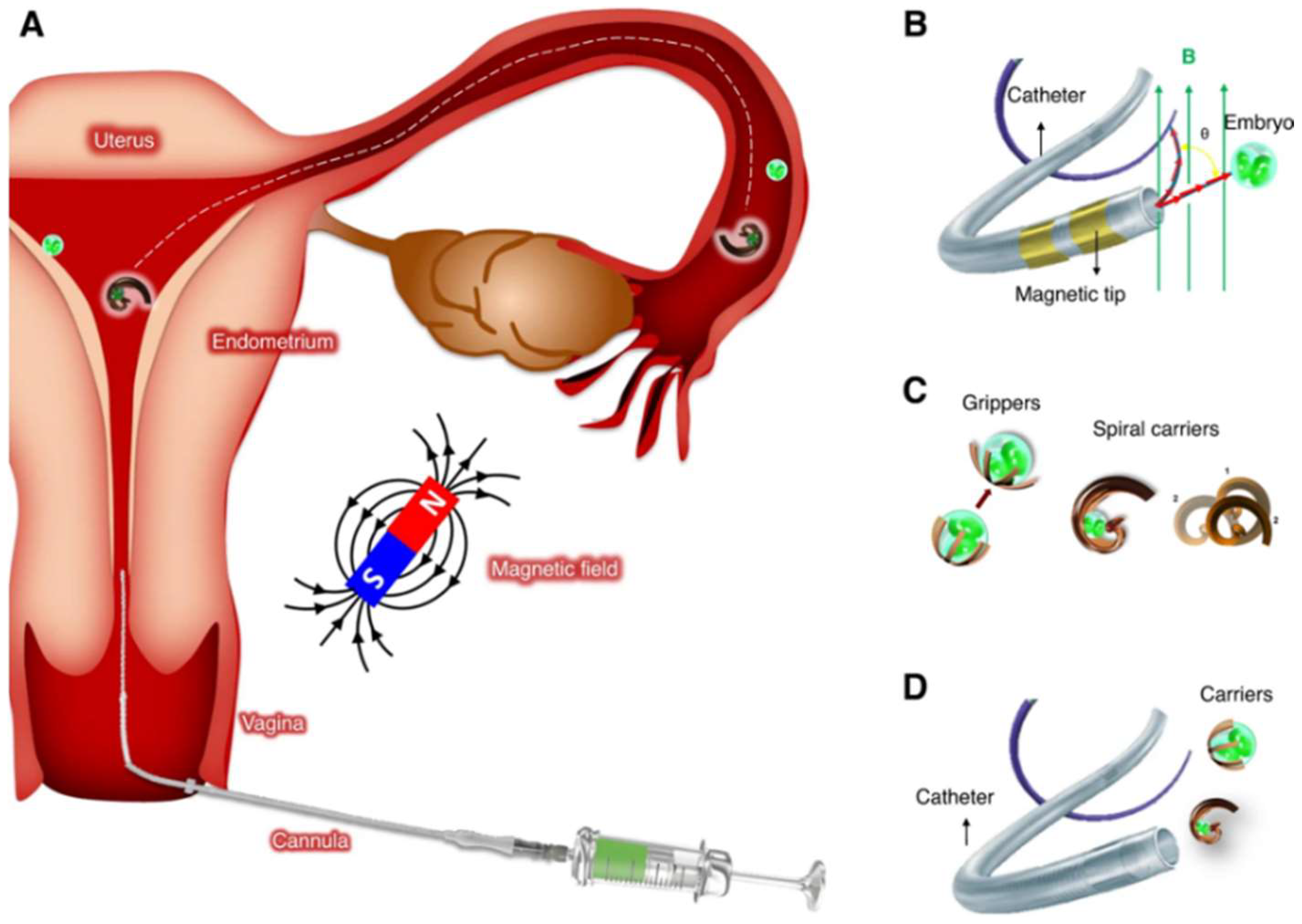
2.3. Innovations in Oocyte Retrieval
2.4. Advancements in Embryo Culture
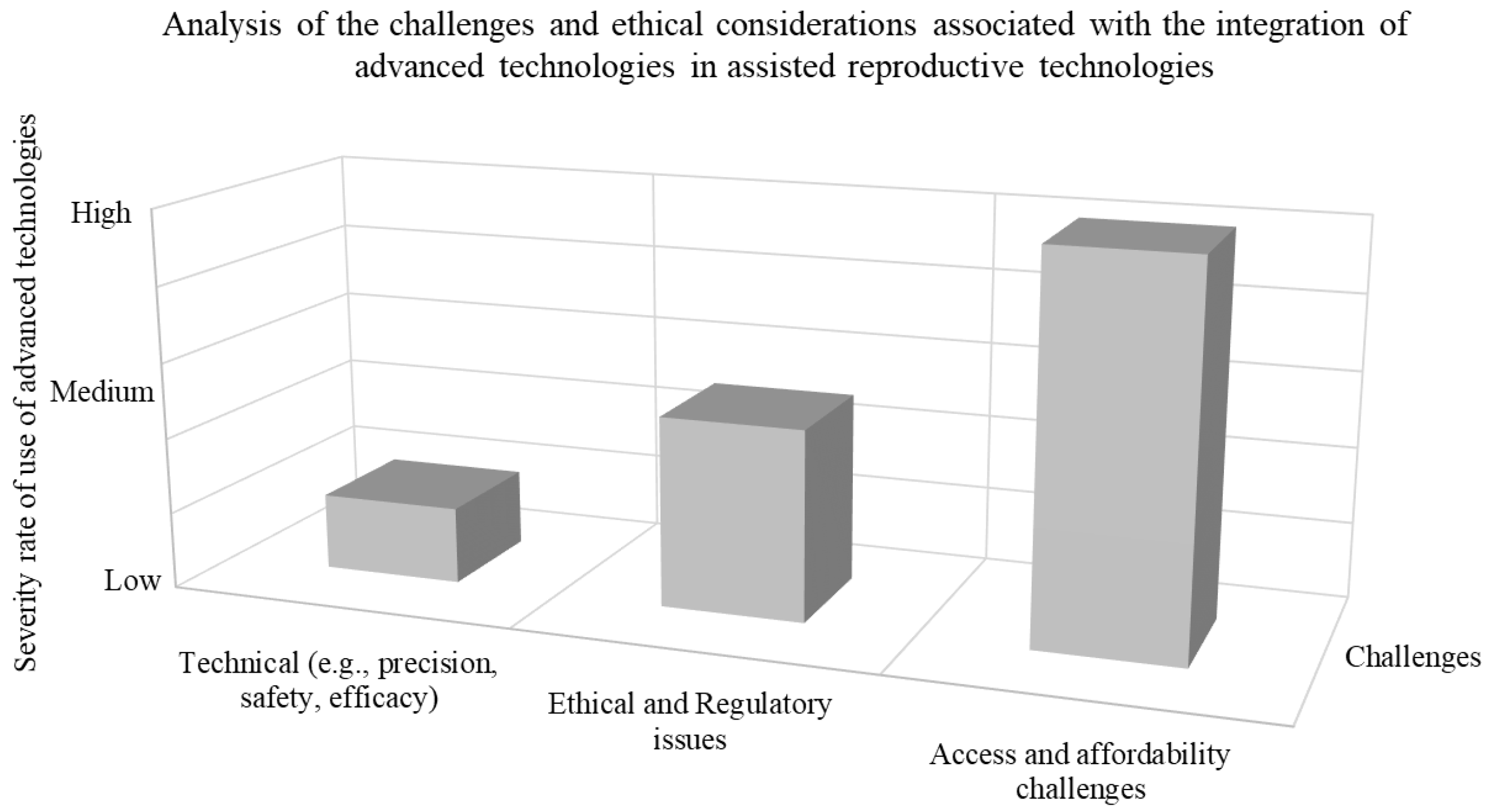
2.5. Benefits and Challenges
2.6. Advanced Micro/Nanorobotic Technology Regulatory Trials, Limitations, and Ethical Considerations
2.6.1. Technical Challenges
2.6.2. Ethical Challenges
2.6.3. Financial Challenges
2.7. Potential Risk and Safety Considerations Associated with the Use of Micro/Nanorobotics in IVF Procedures
2.7.1. Biocompatibility and Material Safety
2.7.2. Precision, Control, and Navigation
2.7.3. Sterility
2.7.4. Genetic and Epigenetic Considerations
2.7.5. Upholding Regulations and Ethics
2.7.6. Ensuring Continuous Monitoring
3. Future Directions
Summary of Our Current Research Pursuits
4. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Lazzari, E.; Potančoková, M.; Sobotka, T.; Gray, E.; Chambers, G.M. Projecting the Contribution of Assisted Reproductive Technology to Completed Cohort Fertility. Popul. Res. Policy Rev. 2023, 42, 6. [Google Scholar] [CrossRef] [PubMed]
- Chiware, T.M.; Vermeulen, N.; Blondeel, K.; Farquharson, R.; Kiarie, J.; Lundin, K.; Matsaseng, T.C.; Ombelet, W.; Toskin, I. IVF and other ART in low- and middle-income countries: A systematic landscape analysis. Hum. Reprod. Update 2021, 27, 213–228. [Google Scholar] [CrossRef] [PubMed]
- Chambers, G.M.; Sullivan, E.A.; Ishihara, O.; Chapman, M.G.; Adamson, G.D. The economic impact of assisted reproductive technology: A review of selected developed countries. Fertil. Steril. 2009, 91, 2281–2294. [Google Scholar] [CrossRef] [PubMed]
- Eskew, A.M.; Jungheim, E.S. A History of Developments to Improve in vitro Fertilization. Mo. Med. 2017, 114, 156–159. [Google Scholar] [PubMed]
- Silver, R.I.; Rodriguez, R.; Chang, T.S.; Gearhart, J.P. In vitro fertilization is associated with an increased risk of hypospadias. J. Urol. 1999, 161, 1954–1957. [Google Scholar] [CrossRef]
- Braude, P.; Johnson, M. Reflections on 40 years of IVF. BJOG Int. J. Obstet. Gynaecol. 2019, 126, 135–137. [Google Scholar] [CrossRef] [PubMed]
- Nauber, R.; Goudu, S.R.; Goeckenjan, M.; Bornhäuser, M.; Ribeiro, C.; Medina-Sánchez, M. Medical microrobots in reproductive medicine from the bench to the clinic. Nat. Commun. 2023, 14, 728. [Google Scholar] [CrossRef]
- Wang, J.; Sauer, M.V. In vitro fertilization (IVF): A review of 3 decades of clinical innovation and technological advancement. Ther. Clin. Risk Manag. 2006, 2, 355–364. [Google Scholar] [CrossRef]
- Asplund, K. Use of in vitro fertilization-ethical issues. Upsala J. Med. Sci. 2020, 125, 192–199. [Google Scholar] [CrossRef]
- Wheeler, M.B.; Walters, E.M.; Beebe, D.J. Toward culture of single gametes: The development of microfluidic platforms for assisted reproduction. Theriogenology 2007, 68, S178–S189. [Google Scholar] [CrossRef]
- Ferraz, M.; Ferronato, G.A. Opportunities involving microfluidics and 3D culture systems to the in vitro embryo production. Anim. Reprod. 2023, 20, e20230058. [Google Scholar] [CrossRef] [PubMed]
- Alias, A.B.; Huang, H.Y.; Yao, D.J. A Review on Microfluidics: An Aid to Assisted Reproductive Technology. Molecules 2021, 26, 4354. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Wu, T.; Zhang, J.; Gao, Y.; Wu, J.-M.; Wang, S. Revolutionizing the female reproductive system research using microfluidic chip platform. J. Nanobiotechnol. 2023, 21, 490. [Google Scholar] [CrossRef] [PubMed]
- Le Gac, S.; Nordhoff, V. Microfluidics for mammalian embryo culture and selection: Where do we stand now? Mol. Hum. Reprod. 2016, 23, 213–226. [Google Scholar] [CrossRef] [PubMed]
- Karcz, A.; Van Soom, A.; Smits, K.; Van Vlierberghe, S.; Verplancke, R.; Pascottini, O.B.; Van den Abbeel, E.; Vanfleteren, J. Development of a Microfluidic Chip Powered by EWOD for In Vitro Manipulation of Bovine Embryos. Biosensors 2023, 13, 419. [Google Scholar] [CrossRef] [PubMed]
- Karcz, A.; Van Soom, A.; Smits, K.; Verplancke, R.; Van Vlierberghe, S.; Vanfleteren, J. Electrically-driven handling of gametes and embryos: Taking a step towards the future of ARTs. Lab Chip 2022, 22, 1852–1875. [Google Scholar] [CrossRef] [PubMed]
- Ferraz, M.A.M.M.; Rho, H.S.; Hemerich, D.; Henning, H.H.W.; van Tol, H.T.A.; Hölker, M.; Besenfelder, U.; Mokry, M.; Vos, P.L.A.M.; Stout, T.A.E.; et al. An oviduct-on-a-chip provides an enhanced in vitro environment for zygote genome reprogramming. Nat. Commun. 2018, 9, 4934. [Google Scholar] [CrossRef] [PubMed]
- Kashaninejad, N.; Shiddiky, M.J.A.; Nguyen, N.-T. Advances in Microfluidics-Based Assisted Reproductive Technology: From Sperm Sorter to Reproductive System-on-a-Chip. Adv. Biosyst. 2018, 2, 1700197. [Google Scholar] [CrossRef]
- Singh, A.V.; Ansari, M.H.D.; Mahajan, M.; Srivastava, S.; Kashyap, S.; Dwivedi, P.; Pandit, V.; Katha, U. Sperm Cell Driven Microrobots—Emerging Opportunities and Challenges for Biologically Inspired Robotic Design. Micromachines 2020, 11, 448. [Google Scholar] [CrossRef]
- Ullrich, F.; Bergeles, C.; Pokki, J.; Ergeneman, O.; Erni, S.; Chatzipirpiridis, G.; Pané, S.; Framme, C.; Nelson, B.J. Mobility Experiments With Microrobots for Minimally Invasive Intraocular Surgery. Investig. Opthalmology Vis. Sci. 2013, 54, 2853–2863. [Google Scholar] [CrossRef]
- Durfey, C.L.; Swistek, S.E.; Liao, S.F.; Crenshaw, M.A.; Clemente, H.J.; Thirumalai, R.V.K.G.; Steadman, C.S.; Ryan, P.L.; Willard, S.T.; Feugang, J.M. Nanotechnology-based approach for safer enrichment of semen with best spermatozoa. J. Anim. Sci. Biotechnol. 2019, 10, 14. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Medina-Sánchez, M.; Magdanz, V.; Schwarz, L.; Hebenstreit, F.; Schmidt, O.G. Sperm-Hybrid Micromotor for Targeted Drug Delivery. ACS Nano 2018, 12, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Fan, X.; Sun, M.; Gao, C.; He, Q.; Xie, H. Magnetically Actuated Peanut Colloid Motors for Cell Manipulation and Patterning. ACS Nano 2018, 12, 2539–2545. [Google Scholar] [CrossRef] [PubMed]
- Soto, F.; Chrostowski, R. Frontiers of Medical Micro/Nanorobotics: In Vivo Applications and Commercialization Perspectives toward Clinical Uses. Front. Bioeng. Biotechnol. 2018, 6, 170. [Google Scholar] [CrossRef] [PubMed]
- Beladi-Mousavi, S.M.; Khezri, B.; Krejčová, L.; Heger, Z.; Sofer, Z.; Fisher, A.C.; Pumera, M. Recoverable Bismuth-Based Microrobots: Capture, Transport, and On-Demand Release of Heavy Metals and an Anticancer Drug in Confined Spaces. ACS Appl. Mater. Interfaces 2019, 11, 13359–13369. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wang, K.; Hou, C.; Li, C.; Zhang, F.; Ren, W.; Dong, L.; Zhao, J. Self-sensing intelligent microrobots for noninvasive and wireless monitoring systems. Microsyst. Nanoeng. 2023, 9, 102. [Google Scholar] [CrossRef] [PubMed]
- Yesin, K.B.; Vollmers, K.; Nelson, B.J. Analysis and design of wireless magnetically guided microrobots in body fluids. In Proceedings of the IEEE International Conference on Robotics and Automation, New Orleans, LA, USA, 26 April–1 May 2004; Proceedings, ICRA ‘04. 2004. Volume 1332, pp. 1333–1338. [Google Scholar]
- Sitti, M.; Ceylan, H.; Hu, W.; Giltinan, J.; Turan, M.; Yim, S.; Diller, E. Biomedical Applications of Untethered Mobile Milli/Microrobots. Proc. IEEE 2015, 103, 205–224. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Wang, H.; Zhong, S.; Qiu, Y.; Shi, Q.; Sun, T.; Huang, Q.; Fukuda, T. Design and Control of a Surface-Dimple-Optimized Helical Microdrill for Motions in High-Viscosity Fluids. IEEE/ASME Trans. Mechatron. 2023, 28, 429–439. [Google Scholar] [CrossRef]
- Celi, N.; Gong, D.; Cai, J. Artificial flexible sperm-like nanorobot based on self-assembly and its bidirectional propulsion in precessing magnetic fields. Sci. Rep. 2021, 11, 21728. [Google Scholar] [CrossRef]
- Feng, L.; Liang, S.; Zhou, X.; Yang, J.; Jiang, Y.; Zhang, D.; Arai, F. On-chip microfluid induced by oscillation of microrobot for noncontact cell transportation. Appl. Phys. Lett. 2017, 111, 203703. [Google Scholar] [CrossRef]
- Fang, Y.; Wu, R.; Lee, J.M.; Chan, L.H.M.; Chan, K.Y.J. Microfluidic in-vitro fertilization technologies: Transforming the future of human reproduction. TrAC Trends Anal. Chem. 2023, 160, 116959. [Google Scholar] [CrossRef]
- Magdanz, V.; Khalil, I.S.M.; Simmchen, J.; Furtado, G.P.; Mohanty, S.; Gebauer, J.; Xu, H.; Klingner, A.; Aziz, A.; Medina-Sánchez, M.; et al. IRONSperm: Sperm-templated soft magnetic microrobots. Sci. Adv. 2020, 6, eaba5855. [Google Scholar] [CrossRef]
- Liu, D.; Guo, R.; Wang, B.; Hu, J.; Lu, Y. Magnetic Micro/Nanorobots: A New Age in Biomedicines. Adv. Intell. Syst. 2022, 4, 2200208. [Google Scholar] [CrossRef]
- Fernandez, E.I.; Ferreira, A.S.; Cecílio, M.H.M.; Chéles, D.S.; de Souza, R.C.M.; Nogueira, M.F.G.; Rocha, J.C. Artificial intelligence in the IVF laboratory: Overview through the application of different types of algorithms for the classification of reproductive data. J. Assist. Reprod. Genet. 2020, 37, 2359–2376. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Sun, M.; Fan, X.; Lin, Z.; Chen, W.; Wang, L.; Dong, L.; He, Q. Reconfigurable magnetic microrobot swarm: Multimode transformation, locomotion, and manipulation. Sci. Robot. 2019, 4, eaav8006. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Jiang, Y.; Lin, M.; Zhang, J.; Guo, H.; Yang, F.; Leung, W.; Xu, C. Ultrasound-Responsive Materials for Drug/Gene Delivery. Front. Pharmacol. 2019, 10, 1650. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Esteban-Fernández de Ávila, B.; Gao, W.; Zhang, L.; Wang, J. Micro/Nanorobots for Biomedicine: Delivery, Surgery, Sensing, and Detoxification. Sci. Robot. 2017, 2, eaam6431. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Yang, Z.; Ferreira, A.; Zhang, L. Control and Autonomy of Microrobots: Recent Progress and Perspective. Adv. Intell. Syst. 2022, 4, 2100279. [Google Scholar] [CrossRef]
- Sun, H.C.M.; Liao, P.; Wei, T.; Zhang, L.; Sun, D. Magnetically Powered Biodegradable Microswimmers. Micromachines 2020, 11, 404. [Google Scholar] [CrossRef]
- Huang, L.; Pan, Y.; Wang, M.; Ren, L. Driving modes and characteristics of biomedical micro-robots. Eng. Regen. 2023, 4, 411–426. [Google Scholar] [CrossRef]
- Wehner, M.; Truby, R.L.; Fitzgerald, D.J.; Mosadegh, B.; Whitesides, G.M.; Lewis, J.A.; Wood, R.J. An integrated design and fabrication strategy for entirely soft, autonomous robots. Nature 2016, 536, 451–455. [Google Scholar] [CrossRef] [PubMed]
- Khalil, I.S.M.; Tabak, A.F.; Hosney, A.; Mohamed, A.; Klingner, A.; Ghoneima, M.; Sitti, M. Sperm-shaped magnetic microrobots: Fabrication using electrospinning, modeling, and characterization. In Proceedings of the 2016 IEEE International Conference on Robotics and Automation (ICRA), Stockholm, Sweden, 16–21 May 2016; pp. 1939–1944. [Google Scholar]
- Jiang, V.S.; Bormann, C.L. Artificial intelligence in the in vitro fertilization laboratory: A review of advancements over the last decade. Fertil. Steril. 2023, 120, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, M.; Shi, H.; Yi, S.; Li, Q.; Su, Y.; Guo, Y.; Hu, L.; Sun, J.; Sun, Y.P. Causes and Effects of Oocyte Retrieval Difficulties: A Retrospective Study of 10,624 Cycles. Front. Endocrinol. 2021, 12, 564344. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Mayorga-Martinez, C.C.; Pané, S.; Zhang, L.; Pumera, M. Magnetically Driven Micro and Nanorobots. Chem. Rev. 2021, 121, 4999–5041. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Zhou, Q.; Vincent, M.; Deng, Y.; Yu, J.; Xu, J.; Xu, T.; Tang, T.; Bian, L.; Wang, Y.-X.J.; et al. Multifunctional biohybrid magnetite microrobots for imaging-guided therapy. Sci. Robot. 2017, 2, eaaq1155. [Google Scholar] [CrossRef] [PubMed]
- Alapan, Y.; Yasa, O.; Yigit, B.; Yasa, I.C.; Erkoc, P.; Sitti, M. Microrobotics and Microorganisms: Biohybrid Autonomous Cellular Robots. Annu. Rev. Control Robot. Auton. Syst. 2019, 2, 205–230. [Google Scholar] [CrossRef]
- Rose, B.I. Approaches to oocyte retrieval for advanced reproductive technology cycles planning to utilize in vitro maturation: A review of the many choices to be made. J. Assist. Reprod. Genet. 2014, 31, 1409–1419. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Townsend, A.D.; Hayter, E.A.; Birk, H.M.; Sell, S.A.; Martin, R.S. Insert-based microfluidics for 3D cell culture with analysis. Anal. Bioanal. Chem. 2018, 410, 3025–3035. [Google Scholar] [CrossRef]
- Li, J.; Li, X.; Luo, T.; Wang, R.; Liu, C.; Chen, S.; Li, D.; Yue, J.; Cheng, S.H.; Sun, D. Development of a magnetic microrobot for carrying and delivering targeted cells. Sci. Robot. 2018, 3, eaat8829. [Google Scholar] [CrossRef]
- Wang, W.; He, Y.; Liu, H.; Guo, Q.; Ge, Z.; Yang, W. Bubble-based microrobot: Recent progress and future perspective. Sens. Actuators A Phys. 2023, 360, 114567. [Google Scholar] [CrossRef]
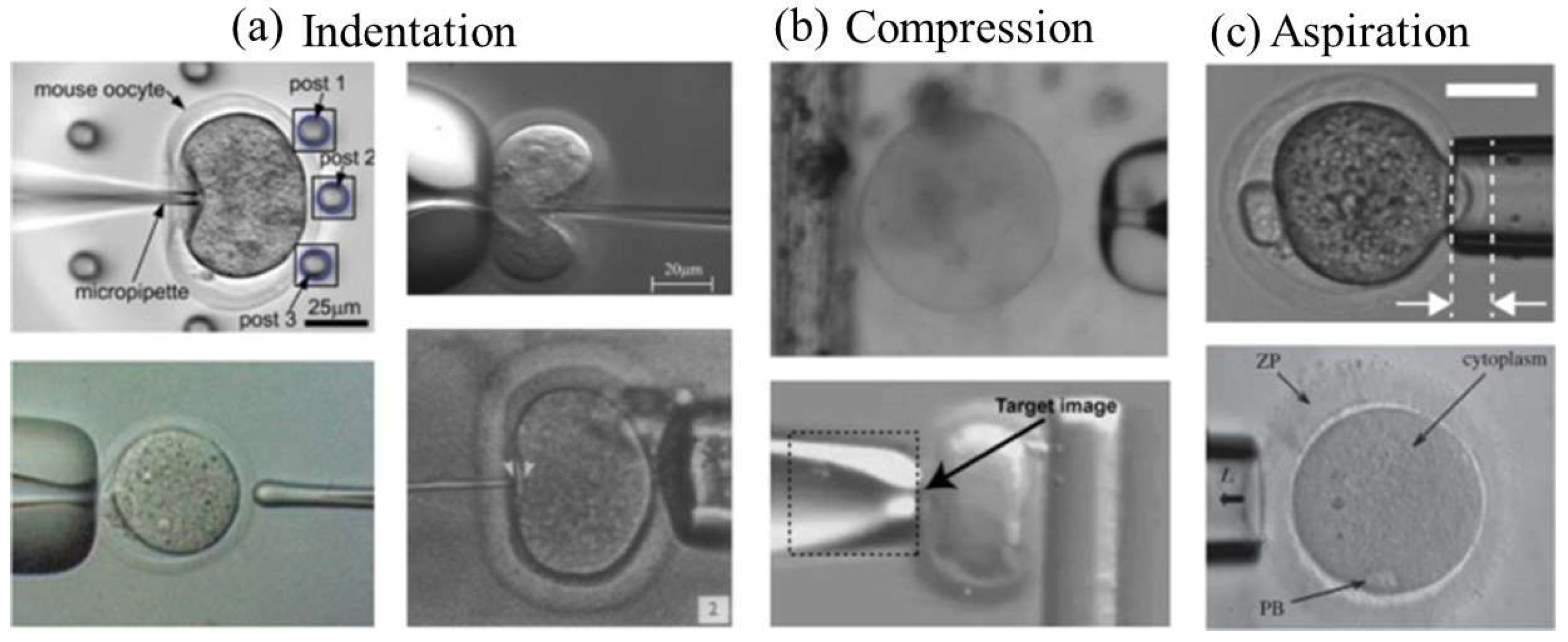
- Yanez, L.Z.; Camarillo, D.B. Microfluidic analysis of oocyte and embryo biomechanical properties to improve outcomes in assisted reproductive technologies. Mol. Hum. Reprod. 2017, 23, 235–247. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Shi, J.; Zong, Z.; Wan, K.T.; Sun, Y. Elastic and viscoelastic characterization of mouse oocytes using micropipette indentation. Ann. Biomed. Eng. 2012, 40, 2122–2130. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Wan, K.T.; Roberts, K.P.; Bischof, J.C.; Nelson, B.J. Mechanical property characterization of mouse zona pellucida. IEEE Trans. NanoBioscience 2003, 2, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Green, D.P. Mammalian sperm cannot penetrate the zona pellucida solely by force. Exp. Cell Res. 1987, 169, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Murayama, Y.; Constantinou, C.E.; Omata, S. Micro-mechanical sensing platform for the characterization of the elastic properties of the ovum via uniaxial measurement. J. Biomech. 2004, 37, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Abadie, J.; Roux, C.; Piat, E.; Filiatre, C.; Amiot, C. Experimental measurement of human oocyte mechanical properties on a micro and nanoforce sensing platform based on magnetic springs. Sens. Actuators B Chem. 2014, 190, 429–438. [Google Scholar] [CrossRef]
- Wacogne, B.; Pieralli, C.; Roux, C.; Gharbi, T. Measuring the mechanical behaviour of human oocytes with a very simple SU-8 micro-tool. Biomed Microdevices 2008, 10, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Yanez, L.Z.; Han, J.; Behr, B.B.; Pera, R.A.R.; Camarillo, D.B. Human oocyte developmental potential is predicted by mechanical properties within hours after fertilization. Nat. Commun. 2016, 7, 10809. [Google Scholar] [CrossRef] [PubMed]
- Khalilian, M.; Navidbakhsh, M.; Valojerdi, M.R.; Chizari, M.; Yazdi, P.E. Estimating Young’s modulus of zona pellucida by micropipette aspiration in combination with theoretical models of ovum. J. R. Soc. Interface 2010, 7, 687–694. [Google Scholar] [CrossRef]
- Abdullah, K.A.L.; Atazhanova, T.; Chavez-Badiola, A.; Shivhare, S.B. Automation in ART: Paving the Way for the Future of Infertility Treatment. Reprod. Sci. 2023, 30, 1006–1016. [Google Scholar] [CrossRef]
- Wang, R.; Pan, W.; Jin, L.; Li, Y.; Geng, Y.; Gao, C.; Chen, G.; Wang, H.; Ma, D.; Liao, S. Artificial intelligence in reproductive medicine. Reproduction 2019, 158, R139–R154. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, S.A.; Ahmed, A.; Noh, S.; Gharamaleki, N.L.; Kim, S.; Chowdhury, A.M.M.B.; Kim, J.-Y.; Pané, S.; Nelson, B.J.; Choi, H. Autonomous 3D positional control of a magnetic microrobot using reinforcement learning. Nat. Mach. Intell. 2024, 6, 92–105. [Google Scholar] [CrossRef]
- Egorov, E.; Pieters, C.; Korach-Rechtman, H.; Shklover, J.; Schroeder, A. Robotics, microfluidics, nanotechnology and AI in the synthesis and evaluation of liposomes and polymeric drug delivery systems. Drug Deliv. Transl. Res. 2021, 11, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Chen, J.; Zhang, J.; Zhao, Z.; Zhao, Y.; Sun, J.; Chang, H. Application of micro/nanorobot in medicine. Front. Bioeng. Biotechnol. 2024, 12, 1347312. [Google Scholar] [CrossRef] [PubMed]
- Hanassab, S.; Abbara, A.; Yeung, A.C.; Voliotis, M.; Tsaneva-Atanasova, K.; Kelsey, T.W.; Trew, G.H.; Nelson, S.M.; Heinis, T.; Dhillo, W.S. The prospect of artificial intelligence to personalize assisted reproductive technology. NPJ Digit. Med. 2024, 7, 55. [Google Scholar] [CrossRef] [PubMed]
- Elnaggar, A.; Kang, S.; Tian, M.; Han, B.; Keshavarz, M. State of the Art in Actuation of Micro/Nanorobots for Biomedical Applications. Small Sci. 2024, 4, 2300211. [Google Scholar] [CrossRef]
- Jiang, V.S.; Pavlovic, Z.J.; Hariton, E. The Role of Artificial Intelligence and Machine Learning in Assisted Reproductive Technologies. Obstet. Gynecol. Clin. N. Am. 2023, 50, 747–762. [Google Scholar] [CrossRef] [PubMed]
- Medenica, S.; Zivanovic, D.; Batkoska, L.; Marinelli, S.; Basile, G.; Perino, A.; Cucinella, G.; Gullo, G.; Zaami, S. The Future Is Coming: Artificial Intelligence in the Treatment of Infertility Could Improve Assisted Reproduction Outcomes—The Value of Regulatory Frameworks. Diagnostics 2022, 12, 2979. [Google Scholar] [CrossRef]
- Singh, A.V.; Chandrasekar, V.; Janapareddy, P.; Mathews, D.E.; Laux, P.; Luch, A.; Yang, Y.; Garcia-Canibano, B.; Balakrishnan, S.; Abinahed, J.; et al. Emerging Application of Nanorobotics and Artificial Intelligence To Cross the BBB: Advances in Design, Controlled Maneuvering, and Targeting of the Barriers. ACS Chem. Neurosci. 2021, 12, 1835–1853. [Google Scholar] [CrossRef]
- Xu, K.; Liu, B. Recent progress in actuation technologies of micro/nanorobots. Beilstein J. Nanotechnol. 2021, 12, 756–765. [Google Scholar] [CrossRef]
- Aziz, A.; Pane, S.; Iacovacci, V.; Koukourakis, N.; Czarske, J.; Menciassi, A.; Medina-Sánchez, M.; Schmidt, O.G. Medical Imaging of Microrobots: Toward In Vivo Applications. ACS Nano 2020, 14, 10865–10893. [Google Scholar] [CrossRef] [PubMed]
- Yan, W. Toward Better Treatment for Women’s Reproductive Health: New Devices, Nanoparticles, and Even Robotic Sperm May Hold the Key to Preventing a Range of Health Conditions. IEEE Pulse 2018, 9, 21–24. [Google Scholar] [CrossRef] [PubMed]
- Medina-Sánchez, M.; Schmidt, O.G. Medical microbots need better imaging and control. Nature 2017, 545, 406–408. [Google Scholar] [CrossRef] [PubMed]
- Sequeira, R.C.; Criswell, T.; Atala, A.; Yoo, J.J. Microfluidic Systems for Assisted Reproductive Technologies: Advantages and Potential Applications. Tissue Eng. Regen. Med. 2020, 17, 787–800. [Google Scholar] [CrossRef] [PubMed]
- von Schondorf-Gleicher, A.; Mochizuki, L.; Orvieto, R.; Patrizio, P.; Caplan, A.S.; Gleicher, N. Revisiting selected ethical aspects of current clinical in vitro fertilization (IVF) practice. J. Assist. Reprod. Genet. 2022, 39, 591–604. [Google Scholar] [CrossRef] [PubMed]
- Beers, B.C.v. The Ethics of IVF. Bioeth. Faith Pract. 2019, 4, 3. [Google Scholar] [CrossRef]
- Lv, Y.; Pu, R.; Tao, Y.; Yang, X.; Mu, H.; Wang, H.; Sun, W. Applications and Future Prospects of Micro/Nanorobots Utilizing Diverse Biological Carriers. Micromachines 2023, 14, 983. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Ge, X.; Chen, X.; Mao, W.; Qian, X.; Yuan, W.E. Micro/Nanorobot: A Promising Targeted Drug Delivery System. Pharmaceutics 2020, 12, 665. [Google Scholar] [CrossRef] [PubMed]
- Giri, G.; Maddahi, Y.; Zareinia, K. A Brief Review on Challenges in Design and Development of Nanorobots for Medical Applications. Appl. Sci. 2021, 11, 10385. [Google Scholar] [CrossRef]
- Cobb, L.N.; Ke, R.W. Ethical considerations in the field of assisted reproductive technology. Minerva Endocrinol. 2018, 43, 80–86. [Google Scholar] [CrossRef]
- Advena-Regnery, B.; Dederer, H.G.; Enghofer, F.; Cantz, T.; Heinemann, T. Framing the ethical and legal issues of human artificial gametes in research, therapy, and assisted reproduction: A German perspective. Bioethics 2018, 32, 314–326. [Google Scholar] [CrossRef]
- Gooßens, D. The use of human artificial gametes and the limits of reproductive freedom. Bioethics 2021, 35, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Hendriks, S.; Dondorp, W.; de Wert, G.; Hamer, G.; Repping, S.; Dancet, E.A.F. Potential consequences of clinical application of artificial gametes: A systematic review of stakeholder views. Hum. Reprod. Update 2015, 21, 297–309. [Google Scholar] [CrossRef] [PubMed]
- Dimitriadis, I.; Zaninovic, N.; Badiola, A.C.; Bormann, C.L. Artificial intelligence in the embryology laboratory: A review. Reprod. Biomed. Online 2022, 44, 435–448. [Google Scholar] [CrossRef]
- Chow, D.J.X.; Wijesinghe, P.; Dholakia, K.; Dunning, K.R. Does artificial intelligence have a role in the IVF clinic? Reprod. Fertil. 2021, 2, C29–C34. [Google Scholar] [CrossRef]
- Sadeghi, M.R. Will Artificial Intelligence Change the Future of IVF? J. Reprod. Infertil. 2022, 23, 139–140. [Google Scholar] [CrossRef] [PubMed]
- Letterie, G. Artificial intelligence and assisted reproductive technologies: 2023. Ready for prime time? Or not. Fertil. Steril. 2023, 120, 32–37. [Google Scholar] [CrossRef]
- Benhal, P.; Chase, J.G.; Gaynor, P.; Oback, B.; Wang, W. AC electric field induced dipole-based on-chip 3D cell rotation. Lab Chip 2014, 14, 2717–2727. [Google Scholar] [CrossRef] [PubMed]
- Benhal, P.; Broda, A.; Najafali, D.; Malik, P.; Mohammed, A.; Ramaswamy, B.; Depireux, D.A.; Shimoji, M.; Shukoor, M.; Shapiro, B. On-chip testing of the speed of magnetic nano- and micro-particles under a calibrated magnetic gradient. J. Magn. Magn. Mater. 2019, 474, 187–198. [Google Scholar] [CrossRef]
- Benhal, P.; Quashie, D., Jr.; Cheang, U.K.; Ali, J. Propulsion kinematics of achiral microswimmers in viscous fluids. Appl. Phys. Lett. 2021, 118, 204103. [Google Scholar] [CrossRef]
| Title of Article | Journal Name | Publication Year |
|---|---|---|
| Autonomous 3D positional control of a magnetic microrobot using reinforcement learning [64]. | Nature Machine Intelligence | 2024 |
| Application of micro/nanorobot in medicine [66]. | Frontiers in bioengineering and biotechnology | 2024 |
| The prospect of artificial intelligence to personalize assisted reproductive technology [67]. | Digital Medicine | 2024 |
| State of the Art in Actuation of Micro/Nanorobots for Biomedical Applications [68]. | Small Science | 2024 |
| The Role of Artificial Intelligence and Machine Learning in Assisted Reproductive Technologies [69]. | Obstetrics and Gynecology Clinics of North America | 2023 |
| Medical microrobots in reproductive medicine from the bench to the clinic [7]. | Nature Communications | 2023 |
| Control and Autonomy of Microrobots: Recent Progress and Perspective [39]. | Advanced Intelligent Systems | 2022 |
| Robotics, microfluidics, nanotechnology, and AI in the synthesis and evaluation of liposomes and polymeric drug delivery systems [65]. | Drug delivery and translational research | 2022 |
| The Future Is Coming: Artificial Intelligence in the Treatment of Infertility Could Improve Assisted Reproduction Outcomes—The Value of Regulatory Frameworks [70]. | Diagnostics | 2021 |
| Emerging Application of Nanorobotics and Artificial Intelligence To Cross the BBB: Advances in Design, Controlled Maneuvering, and Targeting of the Barriers [71]. | ACS Chemical Neuroscience | 2021 |
| Recent progress in actuation technologies of micro/nanorobots [72]. | Beilstein journal of nanotechnology | 2021 |
| Medical imaging of microrobots: toward in vivo applications [73]. | Medical Imaging of Microrobots: Toward In Vivo Applications | 2020 |
| Sperm Cell Driven Microrobots—Emerging Opportunities and Challenges for Biologically Inspired Robotic Design [19] | Micromachines | 2020 |
| Machine learning for active matter [19]. | Nature Machine Intelligence | 2019 |
| Artificial intelligence in reproductive medicine [63]. | Reproduction | 2019 |
| Toward Better Treatment for Women’s Reproductive Health: New Devices, Nanoparticles and Even Robotic Sperm May Hold the Key to Preventing a Range of Health Conditions [74]. | IEEE pulse | 2018 |
| Medical microbots need better imaging and control [75]. | Nature | 2017 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benhal, P. Micro/Nanorobotics in In Vitro Fertilization: A Paradigm Shift in Assisted Reproductive Technologies. Micromachines 2024, 15, 510. https://doi.org/10.3390/mi15040510
Benhal P. Micro/Nanorobotics in In Vitro Fertilization: A Paradigm Shift in Assisted Reproductive Technologies. Micromachines. 2024; 15(4):510. https://doi.org/10.3390/mi15040510
Chicago/Turabian StyleBenhal, Prateek. 2024. "Micro/Nanorobotics in In Vitro Fertilization: A Paradigm Shift in Assisted Reproductive Technologies" Micromachines 15, no. 4: 510. https://doi.org/10.3390/mi15040510
APA StyleBenhal, P. (2024). Micro/Nanorobotics in In Vitro Fertilization: A Paradigm Shift in Assisted Reproductive Technologies. Micromachines, 15(4), 510. https://doi.org/10.3390/mi15040510






