Recent Developments in Magnetic Hyperthermia Therapy (MHT) and Magnetic Particle Imaging (MPI) in the Brain Tumor Field: A Scoping Review and Meta-Analysis
Abstract
1. Introduction
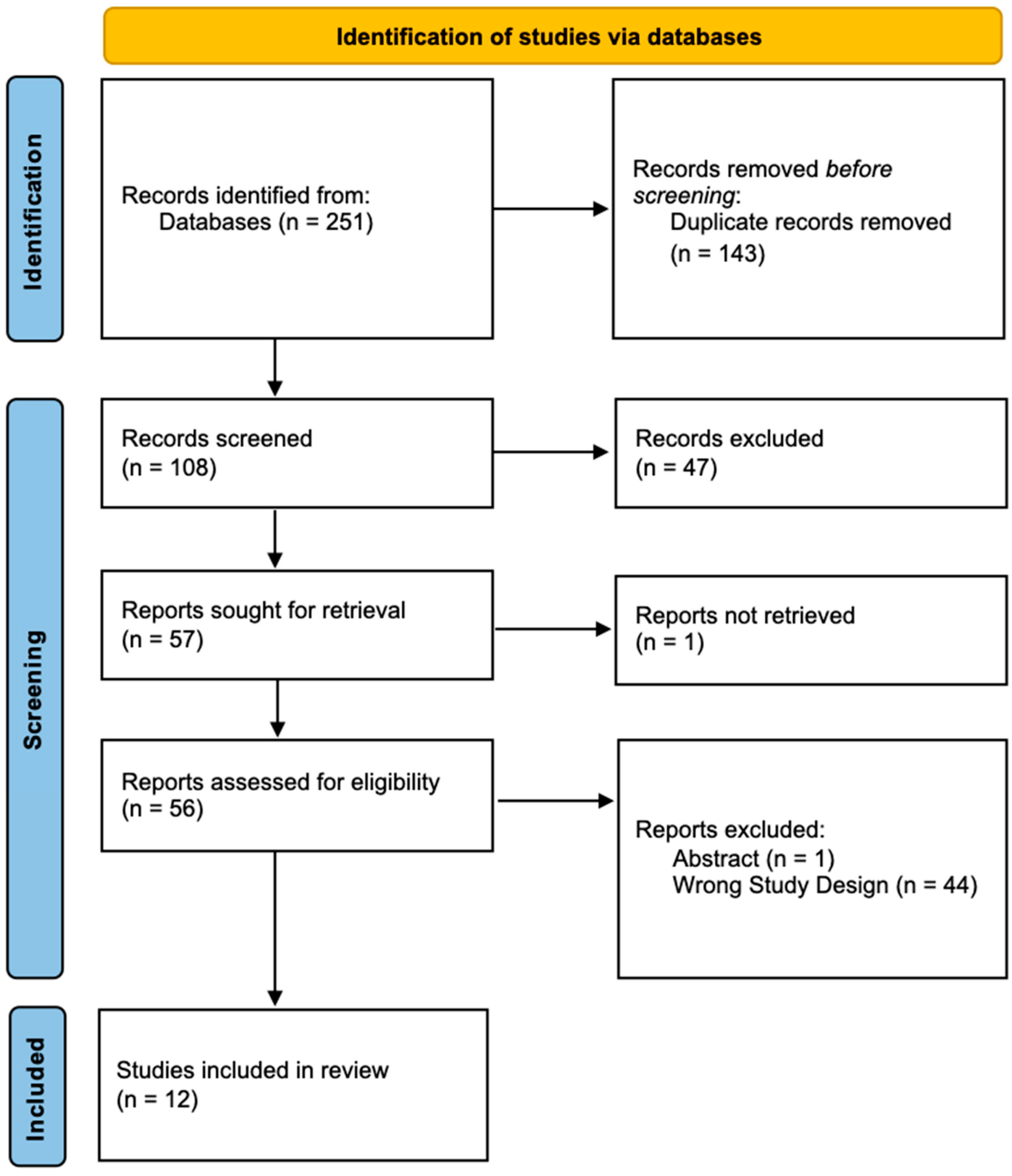
2. Methods
3. Results
3.1. Magnetic Hyperthermia
3.1.1. Nanoparticle Characteristics
3.1.2. Tabulated Experimental Parameters
3.2. Magnetic Particle Imaging
4. Discussion
Funding
Conflicts of Interest
References
- Mesfin, F.B.; Al-Dhahir, M.A. Gliomas. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Zeng, T.; Cui, D.; Gao, L. Glioma: An Overview of Current Classifications, Characteristics, Molecular Biology and Target Therapies. Front. Biosci. 2015, 20, 1104–1115. [Google Scholar]
- Weller, M.; Wick, W.; Aldape, K.; Brada, M.; Berger, M.; Pfister, S.M.; Nishikawa, R.; Rosenthal, M.; Wen, P.Y.; Stupp, R.; et al. Glioma. Nat. Rev. Dis. Primers 2015, 1, 15017. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.P.; Tirosh, I.; Trombetta, J.J.; Shalek, A.K.; Gillespie, S.M.; Wakimoto, H.; Cahill, D.P.; Nahed, B.V.; Curry, W.T.; Martuza, R.L.; et al. Single-Cell RNA-Seq Highlights Intratumoral Heterogeneity in Primary Glioblastoma. Science 2014, 344, 1396–1401. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.; Mittal, S.; Berens, M.E. Targeting Adaptive Glioblastoma: An Overview of Proliferation and Invasion. Neuro-Oncology 2014, 16, 1575–1584. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.; Paul, A.; Kellie, S.J.; O’Neill, G.M. Mesenchymal Migration as a Therapeutic Target in Glioblastoma. J. Oncol. 2010, 2010, 430142. [Google Scholar] [CrossRef] [PubMed]
- Ostrom, Q.T.; Patil, N.; Cioffi, G.; Waite, K.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2013–2017. Neuro-Oncology 2020, 22, iv1–iv96. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.B.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus Concomitant and Adjuvant Temozolomide for Glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Sanai, N.; Polley, M.-Y.; McDermott, M.W.; Parsa, A.T.; Berger, M.S. An Extent of Resection Threshold for Newly Diagnosed Glioblastomas. J. Neurosurg. 2011, 115, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Vehlow, A.; Cordes, N. DDR1 (Discoidin Domain Receptor Tyrosine Kinase 1) Drives Glioblastoma Therapy Resistance by Modulating Autophagy. Autophagy 2019, 15, 1487–1488. [Google Scholar] [CrossRef] [PubMed]
- Shergalis, A.; Bankhead, A., 3rd; Luesakul, U.; Muangsin, N.; Neamati, N. Current Challenges and Opportunities in Treating Glioblastoma. Pharmacol. Rev. 2018, 70, 412–445. [Google Scholar] [CrossRef] [PubMed]
- Safari, M.; Khoshnevisan, A. Cancer Stem Cells and Chemoresistance in Glioblastoma Multiform: A Review Article. J. Stem Cells 2015, 10, 271–285. [Google Scholar] [PubMed]
- Gerard, C.S.; Straus, D.; Byrne, R.W. Surgical Management of Low-Grade Gliomas. Semin. Oncol. 2014, 41, 458–467. [Google Scholar] [CrossRef] [PubMed]
- AAoNS. Glioblastoma Multiforme. Available online: https://www.aans.org/Patients/Neurosurgical-Conditions-and-Treatments/Glioblastoma-Multiforme (accessed on 30 January 2024).
- Lan, X.; Jörg, D.J.; Cavalli, F.M.G.; Richards, L.M.; Nguyen, L.V.; Vanner, R.J.; Guilhamon, P.; Lee, L.; Kushida, M.M.; Pellacani, D.; et al. Fate Mapping of Human Glioblastoma Reveals an Invariant Stem Cell Hierarchy. Nature 2017, 549, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Lathia, J.D.; Mack, S.C.; Mulkearns-Hubert, E.E.; Valentim, C.L.L.; Rich, J.N. Cancer Stem Cells in Glioblastoma. Genes. Dev. 2015, 29, 1203–1217. [Google Scholar] [CrossRef] [PubMed]
- Schupper, A.J.; Baron, R.B.; Cheung, W.; Rodriguez, J.; Kalkanis, S.N.; Chohan, M.O.; Andersen, B.J.; Chamoun, R.; Nahed, B.V.; Zacharia, B.E.; et al. 5-Aminolevulinic Acid for Enhanced Surgical Visualization of High-Grade Gliomas: A Prospective, Multicenter Study. J. Neurosurg. 2022, 136, 1525–1534. [Google Scholar] [CrossRef] [PubMed]
- Koshy, M.; Villano, J.L.; Dolecek, T.A.; Howard, A.; Mahmood, U.; Chmura, S.J.; Weichselbaum, R.R.; McCarthy, B.J. Improved Survival Time Trends for Glioblastoma Using the SEER 17 Population-Based Registries. J. Neuro-Oncol. 2012, 107, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Kalamida, D.; Karagounis, I.V.; Mitrakas, A.; Kalamida, S.; Giatromanolaki, A.; Koukourakis, M.I. Fever-Range Hyperthermia vs. Hypothermia Effect on Cancer Cell Viability, Proliferation and HSP90 Expression. PLoS ONE 2015, 10, e0116021. [Google Scholar] [CrossRef] [PubMed]
- Fajardo, L.F.; Egbert, B.; Marmor, J.; Hahn, G.M. Effects of Hyperthermia in a Malignant Tumor. Cancer 1980, 45, 613–623. [Google Scholar] [CrossRef]
- Van der Zee, J. Heating the Patient: A Promising Approach? Ann. Oncol. 2002, 13, 1173–1184. [Google Scholar] [CrossRef] [PubMed]
- Skandalakis, G.P.; Rivera, D.R.; Rizea, C.D.; Bouras, A.; Jesu Raj, J.G.; Bozec, D.; Hadjipanayis, C.G. Hyperthermia Treatment Advances for Brain Tumors. Int. J. Hyperth. 2020, 37, 3–19. [Google Scholar] [CrossRef] [PubMed]
- Dennis, C.L.; Ivkov, R. Physics of Heat Generation Using Magnetic Nanoparticles for Hyperthermia. Int. J. Hyperth. 2013, 29, 715–729. [Google Scholar] [CrossRef] [PubMed]
- Périgo, E.A.; Hemery, G.; Sandre, O.; Ortega, D.; Garaio, E.; Plazaola, F.; Teran, F.J. Fundamentals and Advances in Magnetic Hyperthermia. Appl. Phys. Rev. 2015, 2, 041302. [Google Scholar] [CrossRef]
- Soetaert, F.; Korangath, P.; Serantes, D.; Fiering, S.; Ivkov, R. Cancer Therapy with Iron Oxide Nanoparticles: Agents of Thermal and Immune Therapies. Adv. Drug Deliv. Rev. 2020, 163–164, 65–83. [Google Scholar] [CrossRef] [PubMed]
- Platt, S.; Nduom, E.; Kent, M.; Freeman, C.; Machaidze, R.; Kaluzova, M.; Wang, L.; Mao, H.; Hadjipanayis, C.G. Canine Model of Convection-Enhanced Delivery of Cetuximab-Conjugated Iron-Oxide Nanoparticles Monitored with Magnetic Resonance Imaging. Clin. Neurosurg. 2012, 59, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Kaluzova, M.; Bouras, A.; Machaidze, R.; Hadjipanayis, C.G. Targeted Therapy of Glioblastoma Stem-like Cells and Tumor Non-Stem Cells Using Cetuximab-Conjugated Iron-Oxide Nanoparticles. Oncotarget 2015, 6, 8788–8806. [Google Scholar] [CrossRef]
- Jordan, A.; Wust, P.; Fähling, H.; John, W.; Hinz, A.; Felix, R. Inductive Heating of Ferrimagnetic Particles and Magnetic Fluids: Physical Evaluation of Their Potential for Hyperthermia. Int. J. Hyperth. 1993, 9, 51–68. [Google Scholar] [CrossRef]
- Man, J.; Shoemake, J.D.; Ma, T.; Rizzo, A.E.; Godley, A.R.; Wu, Q.; Mohammadi, A.M.; Bao, S.; Rich, J.N.; Yu, J.S. Hyperthermia Sensitizes Glioma Stem-like Cells to Radiation by Inhibiting AKT Signaling. Cancer Res. 2015, 75, 1760–1769. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Ni, F.; Zhang, J.; Wang, C.; Lu, X.; Guo, Z.; Yao, S.; Shu, Y.; Xu, R. Thermal Analysis in the Rat Glioma Model during Directly Multipoint Injection Hyperthermia Incorporating Magnetic Nanoparticles. J. Nanosci. Nanotechnol. 2011, 11, 10333–10338. [Google Scholar] [CrossRef] [PubMed]
- Bouras, A.; Kaluzova, M.; Hadjipanayis, C.G. Radiosensitivity Enhancement of Radioresistant Glioblastoma by Epidermal Growth Factor Receptor Antibody-Conjugated Iron-Oxide Nanoparticles. J. Neuro-Oncol. 2015, 124, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Estelrich, J.; Sánchez-Martín, M.J.; Busquets, M.A. Nanoparticles in Magnetic Resonance Imaging: From Simple to Dual Contrast Agents. Int. J. Nanomed. 2015, 10, 1727–1741. [Google Scholar]
- Rivera-Rodriguez, A.; Hoang-Minh, L.B.; Chiu-Lam, A.; Sarna, N.; Marrero-Morales, L.; Mitchell, D.A.; Rinaldi-Ramos, C.M. Tracking Adoptive T Cell Immunotherapy Using Magnetic Particle Imaging. Nanotheranostics 2021, 5, 431–444. [Google Scholar] [CrossRef] [PubMed]
- Gleich, B.; Weizenecker, J. Tomographic Imaging Using the Nonlinear Response of Magnetic Particles. Nature 2005, 435, 1214–1217. [Google Scholar] [CrossRef] [PubMed]
- Hernando, D.; Levin, Y.S.; Sirlin, C.B.; Reeder, S.B. Quantification of Liver Iron with MRI: State of the Art and Remaining Challenges. J. Magn. Reson. Imaging 2014, 40, 1003–1021. [Google Scholar] [CrossRef] [PubMed]
- Healy, S.; Bakuzis, A.F.; Goodwill, P.W.; Attaluri, A.; Bulte, J.W.M.; Ivkov, R. Clinical Magnetic Hyperthermia Requires Integrated Magnetic Particle Imaging. WIREs Nanomed. Nanobiotechnol. 2022, 14, e1779. [Google Scholar] [CrossRef] [PubMed]
- Alphandéry, E.; Idbaih, A.; Adam, C.; Delattre, J.-Y.; Schmitt, C.; Guyot, F.; Chebbi, I. Chains of Magnetosomes with Controlled Endotoxin Release and Partial Tumor Occupation Induce Full Destruction of Intracranial U87-Luc Glioma in Mice under the Application of an Alternating Magnetic Field. J. Control. Release 2017, 262, 259–272. [Google Scholar] [CrossRef] [PubMed]
- Alphandéry, E.; Idbaih, A.; Adam, C.; Delattre, J.-Y.; Schmitt, C.; Guyot, F.; Chebbi, I. Development of Non-Pyrogenic Magnetosome Minerals Coated with Poly-l-Lysine Leading to Full Disappearance of Intracranial U87-Luc Glioblastoma in 100% of Treated Mice Using Magnetic Hyperthermia. Biomaterials 2017, 141, 210–222. [Google Scholar] [CrossRef] [PubMed]
- Alphandéry, E.; Idbaih, A.; Adam, C.; Delattre, J.-Y.; Schmitt, C.; Gazeau, F.; Guyot, F.; Chebbi, I. Biodegraded Magnetosomes with Reduced Size and Heating Power Maintain a Persistent Activity against Intracranial U87-Luc Mouse GBM Tumors. J. Nanobiotechnology 2019, 17, 126. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, A.; Midha, S.; Kumar, R.; Meena, R.; Singh, P.; Jha, S.K.; Kuanr, B.K. Rapid Tumor Inhibition via Magnetic Hyperthermia Regulated by Caspase 3 with Time-Dependent Clearance of Iron Oxide Nanoparticles. Biomater. Sci. 2021, 9, 2972–2990. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Muroski, M.E.; Petit, D.C.M.C.; Mansell, R.; Vemulkar, T.; Morshed, R.A.; Han, Y.; Balyasnikova, I.V.; Horbinski, C.M.; Huang, X.; et al. Rotating Magnetic Field Induced Oscillation of Magnetic Particles for in Vivo Mechanical Destruction of Malignant Glioma. J. Control. Release 2016, 223, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Wu, H.; Peng, B.; Zhang, S.; Ma, J.; Deng, G.; Zou, P.; Liu, J.; Chen, A.T.; Li, D.; et al. Vessel-Targeting Nanoclovers Enable Noninvasive Delivery of Magnetic Hyperthermia-Chemotherapy Combination for Brain Cancer Treatment. Nano Lett. 2021, 21, 8111–8118. [Google Scholar] [CrossRef]
- Rego, G.N.d.A.; Mamani, J.B.; Souza, T.K.F.; Nucci, M.P.; da Silva, H.R.; Gamarra, L.F. Therapeutic Evaluation of Magnetic Hyperthermia Using Fe3O4-Aminosilane-Coated Iron Oxide Nanoparticles in Glioblastoma Animal Model. Einstein 2019, 17, eAO4786. [Google Scholar] [CrossRef] [PubMed]
- Rego, G.N.A.; Nucci, M.P.; Mamani, J.B.; Oliveira, F.A.; Marti, L.C.; Filgueiras, I.S.; Ferreira, J.M.; Real, C.C.; Faria, D.d.P.; Espinha, P.L.; et al. Therapeutic Efficiency of Multiple Applications of Magnetic Hyperthermia Technique in Glioblastoma Using Aminosilane Coated Iron Oxide Nanoparticles: In Vitro and In Vivo Study. Int. J. Mol. Sci. 2020, 21, 958. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Liu, L.; Ma, M.; Zhang, Y. Modulation of Blood-Brain Tumor Barrier for Delivery of Magnetic Hyperthermia to Brain Cancer. J. Control. Release 2023, 355, 248–258. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Zhang, S.; Chen, P.; Liu, H.; Yin, H.; Li, H. Magnetotactic Bacteria, Magnetosomes and Their Application. Microbiol. Res. 2012, 167, 507–519. [Google Scholar] [CrossRef] [PubMed]
- Sarna, N.S.; Marrero-Morales, L.; DeGroff, R.; Rivera-Rodriguez, A.; Liu, S.; Chiu-Lam, A.; Rinaldi-Ramos, C.M. An Anatomically Correct 3d-Printed Mouse Phantom for Magnetic Particle Imaging Studies. Bioeng. Transl. Med. 2022, 7, e10299. [Google Scholar] [CrossRef] [PubMed]
- Graeser, M.; Thieben, F.; Szwargulski, P.; Werner, F.; Gdaniec, N.; Boberg, M.; Griese, F.; Möddel, M.; Ludewig, P.; van de Ven, D.; et al. Human-Sized Magnetic Particle Imaging for Brain Applications. Nat. Commun. 2019, 10, 1936. [Google Scholar] [CrossRef] [PubMed]
- Song, G.; Zheng, X.; Wang, Y.; Xia, X.; Chu, S.; Rao, J. A Magneto-Optical Nanoplatform for Multimodality Imaging of Tumors in Mice. ACS Nano 2019, 13, 7750–7758. [Google Scholar] [CrossRef] [PubMed]
- Buchholz, O.; Sajjamark, K.; Franke, J.; Wei, H.; Behrends, A.; Münkel, C.; Grüttner, C.; Levan, P.; von Elverfeldt, D.; Graeser, M.; et al. In Situ Theranostic Platform Combining Highly Localized Magnetic Fluid Hyperthermia, Magnetic Particle Imaging, and Thermometry in 3D. Theranostics 2024, 14, 324–340. [Google Scholar] [CrossRef] [PubMed]
- Arami, H.; Teeman, E.; Troksa, A.; Bradshaw, H.; Saatchi, K.; Tomitaka, A.; Gambhir, S.S.; Häfeli, U.O.; Liggitt, D.; Krishnan, K.M. Tomographic Magnetic Particle Imaging of Cancer Targeted Nanoparticles. Nanoscale 2017, 9, 18723–18730. [Google Scholar] [CrossRef] [PubMed]
- Meola, A.; Rao, J.; Chaudhary, N.; Song, G.; Zheng, X.; Chang, S.D. Magnetic Particle Imaging in Neurosurgery. World Neurosurg. 2019, 125, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Orendorff, R.; Peck, A.J.; Zheng, B.; Shirazi, S.N.; Matthew Ferguson, R.; Khandhar, A.P.; Kemp, S.J.; Goodwill, P.; Krishnan, K.M.; Brooks, G.A.; et al. First in Vivo Traumatic Brain Injury Imaging via Magnetic Particle Imaging. Phys. Med. Biol. 2017, 62, 3501–3509. [Google Scholar] [CrossRef] [PubMed]
- Maier-Hauff, K.; Ulrich, F.; Nestler, D.; Niehoff, H.; Wust, P.; Thiesen, B.; Orawa, H.; Budach, V.; Jordan, A. Efficacy and Safety of Intratumoral Thermotherapy Using Magnetic Iron-Oxide Nanoparticles Combined with External Beam Radiotherapy on Patients with Recurrent Glioblastoma Multiforme. J. Neuro-Oncol. 2011, 103, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Grauer, O.; Jaber, M.; Hess, K.; Weckesser, M.; Schwindt, W.; Maring, S.; Wölfer, J.; Stummer, W. Combined Intracavitary Thermotherapy with Iron Oxide Nanoparticles and Radiotherapy as Local Treatment Modality in Recurrent Glioblastoma Patients. J. Neuro-Oncol. 2019, 141, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Hegi, M.E.; Mason, W.P.; van den Bent, M.J.; Taphoorn, M.J.B.; Janzer, R.C.; Ludwin, S.K.; Allgeier, A.; Fisher, B.; Belanger, K.; et al. Effects of Radiotherapy with Concomitant and Adjuvant Temozolomide versus Radiotherapy Alone on Survival in Glioblastoma in a Randomised Phase III Study: 5-Year Analysis of the EORTC-NCIC Trial. Lancet Oncol. 2009, 10, 459–466. [Google Scholar] [CrossRef] [PubMed]
- NNI Supplement to the President’s 2023 Budget. Available online: https://www.nano.gov/2023BudgetSupplement (accessed on 20 December 2023).
- Fan, D.; Cao, Y.; Cao, M.; Wang, Y.; Cao, Y.; Gong, T. Nanomedicine in Cancer Therapy. Signal Transduct. Target. Ther. 2023, 8, 293. [Google Scholar] [CrossRef] [PubMed]
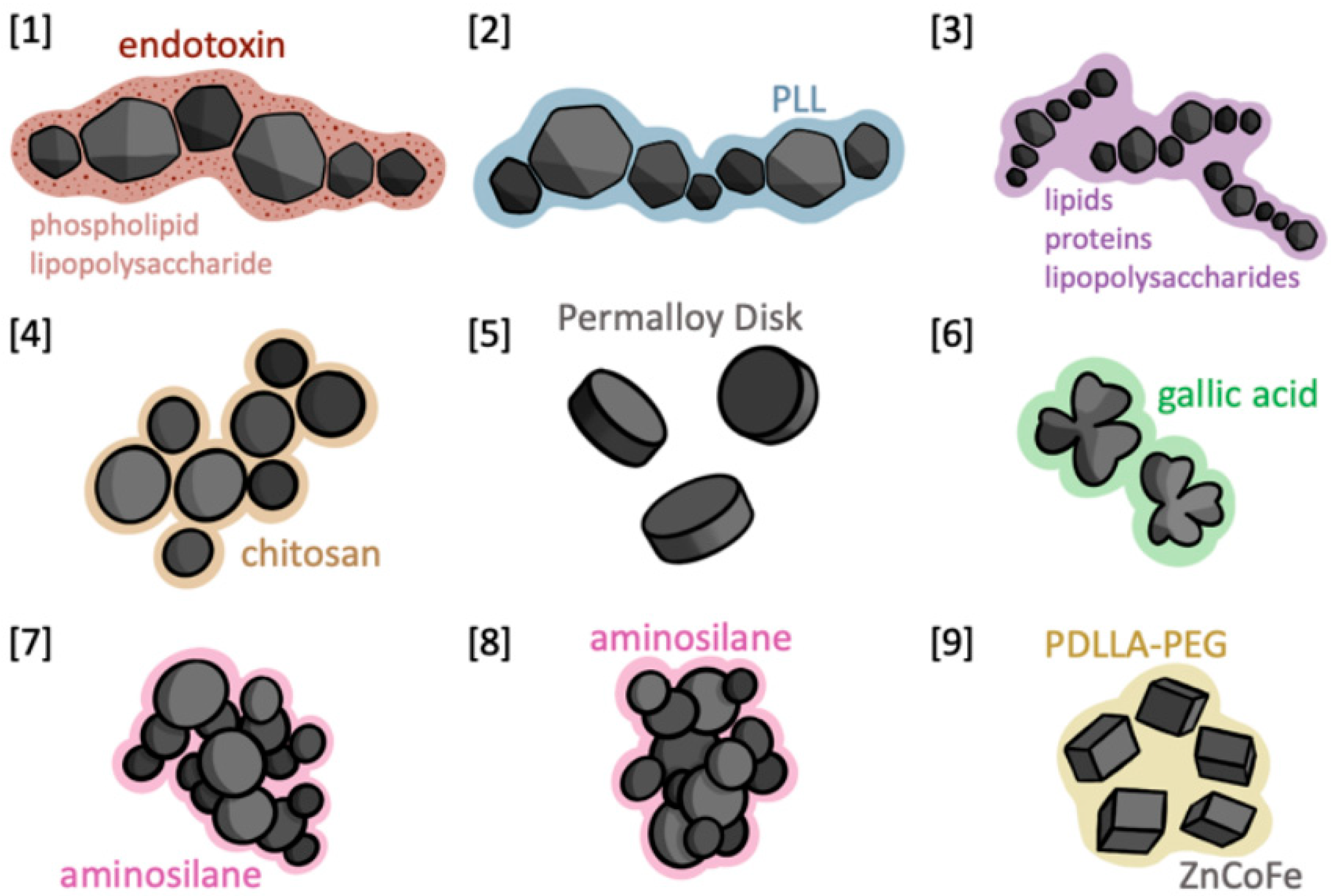
| First Author | Year | Journal | Organism | Tumor Line | Nanoparticle |
|---|---|---|---|---|---|
| Alphandéry [37] | 2017 | Journal of Controlled Release | Mouse | U87-Luc | Magnetosomes |
| Alphandéry [38] | 2017 | Biomaterials | Mouse | U87-Luc | Magnetosomes coated with poly-l-lysine/iron-oxide nanoparticles (IONPs) |
| Alphandéry [39] | 2019 | Journal of Nanobiotechnology | Mouse | U87-Luc | Magnetosome chains |
| Chauhan [40] | 2021 | Biomaterials Science | Rat | C6 | Chitosan-coated Fe3O4 |
| Cheng [41] | 2016 | Journal of Controlled Release | Mouse | U87-Fluc-green fluorescent protein (GFP) | Disk-shaped permalloy magnetic particles |
| Liu [42] | 2021 | Nano Letters | Mouse | GL-261 | Gallic acid-coated magnetic nanoclovers |
| Rego [43] | 2019 | Einstein (Sao Paolo) | Rat | C6 | Aminosilane-coated superparamagnetic iron oxide nanoparticles (SPIONa) |
| Rego [44] | 2020 | International Journal of Molecular Sciences | Rat | C6 | SPIONa |
| Wu [45] | 2023 | Journal of Controlled Release | Mouse | GL-261 and U87 | Zinc- and cobalt-doped cubic IONPs |
| Particle | Mean Size | H_C | M_S | M_r/M_S | Mass (µg) | Volume (μL) | SAR (W/gFe) | Δt (°C) |
|---|---|---|---|---|---|---|---|---|
| CM [1] | ~45 nm | ~200–300 Oe | – | ~0.35 | 40 | 2 | 4 | ~4 |
| IONP [1] | 17–20 nm | ~120 Oe | – | ~0.15 | 40 | 2 | 0 | |
| M-PLL [2] | ~45 nm core, 4–17 nm organic layer | ~5 mT | – | ~0.19 | 500–700 | 2 | 1.3 | 17.5 |
| IONP [2] | 17–20 nm | ~11 mT | – | ~0.15 | 500 | 2 | 0.2 | 8.5 °C |
| CM [3] | 37.5 ± 5.2 nm (11 in vitro) | 20 mT | – | 0.3 | 40 | 2 | 4.7 ± 1.5 | 4 ± 1 |
| Chitosan-coated Fe3O4 [4] | 37 nm | – | 71.5 emu/g | – | 600–1000 | – | 460 | 7 (first), 9 (second) |
| Disk-shaped permalloy [5] | 2 μm diameter | 250 Oe | – | – | 10 | – | 0.005 | 0 |
| Gallic acid-coated magnetic nanoclovers [6] | 20.7 nm | ~700 Oe | ~110 emu/g | – | 25 mg/kg | ~7000 | To 48.4 | |
| SPIONs_Amin [7] | 110 ± 5 nm | – | 790.93 A/m | – | 50 | 10 | 194.917 | To 42 |
| SPION_Amin [8] | 100 nm | – | – | – | – | 40 @ 4 coords | 286 | To 43 |
| Zinc- and cobalt-doped cubic iron oxide nanoparticles [9] | 52 nm | 571 Oe | 125 emu/g | – | 50 | – | 3890 | To 46 |
| Particle | Number of Animals | Age | Sex | Strain | MNP Injection PTI | Field Strength (mT) | Field Frequency (kHz) | Number of MS | AMF Duration (min) | Delivery Method | Survival |
|---|---|---|---|---|---|---|---|---|---|---|---|
| CM [1] | 70 (10/group) | 7 weeks | female | Nude mice ~20 g | D8 | 30 | 198 | 12–15 | 30 | Intratumoral Injection | – |
| IONP [1] | |||||||||||
| M-PLL [2] | 54 (9/group) | 5 weeks | female | Athymic nude mice ~18 g | D5 | 27 | 202 | 23–27 | 30 | Intratumoral Injection | 100% day 350 MSD > 350 (last mouse lived to 140 without heat vs. 50 for ctrl) |
| IONP [2] | MSD 57 | ||||||||||
| CM [3] | 60 (10/group) | 6 weeks | female | Nude mice ~20 g | D8 | 27 | 198 | 3–15 | 30 | Intratumoral Injection | 50% survival at day 250 w/15 MS, 0 for 3 MS or ctrl MSD > 250 |
| Chitosan-coated Fe3O4 [4] | 18 (6/group) | 10–12 weeks | male | Wistar Rats | D12–14 | 14 kA/m | 335 | 2 | 20 | Intratumoral Injection | Complete tumor inhibition in 32 days; no recurrence in 5 months post-mht |
| Disk-shaped permalloy magnetic particles [5] | 18 (5/group, one group of 3) | 6 weeks | male | Athymic nude mice 18–22 g | incubation or D3 | 1000 | 0.02 | 7 | 30 | Incubation/Intratumoral Injection | MSD 63 treatment vs. 56 control |
| Gallic acid-coated magnetic nanoclovers [6] | 70 (7/group) | 5–6 weeks | female | C57BL6 (GL261 glioma), Balb/c (flank) | D14 | 27 ka/m | 371 | 1 | 10 | Tail vein/CED | 43% survival at D60, 0% in control group after D42 |
| SPIONs_Amin [7] | 10 | 2 months | male | Wistar Rats 290–350 g | D22 | 200 G | 874 | 1 | 40 | Intratumoral Injection | – |
| SPION_Amin [8] | 45 (9/group) | – | male | Wistar Rats 250–350 g | D14 | 300 G | 309 | 1–3 | 30 | Intratumoral Injection | – |
| Zinc- and cobalt-doped cubic iron oxide nanoparticles [9] | 56 (7/group) | 6–7 weeks | female | C57BL/6 and nude mice | D8 | 27 kA/m | 410 | – | 10 | Tail vein | Median survival of 60 days for experimental group |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rentzeperis, F.; Rivera, D.; Zhang, J.Y.; Brown, C.; Young, T.; Rodriguez, B.; Schupper, A.; Price, G.; Gomberg, J.; Williams, T.; et al. Recent Developments in Magnetic Hyperthermia Therapy (MHT) and Magnetic Particle Imaging (MPI) in the Brain Tumor Field: A Scoping Review and Meta-Analysis. Micromachines 2024, 15, 559. https://doi.org/10.3390/mi15050559
Rentzeperis F, Rivera D, Zhang JY, Brown C, Young T, Rodriguez B, Schupper A, Price G, Gomberg J, Williams T, et al. Recent Developments in Magnetic Hyperthermia Therapy (MHT) and Magnetic Particle Imaging (MPI) in the Brain Tumor Field: A Scoping Review and Meta-Analysis. Micromachines. 2024; 15(5):559. https://doi.org/10.3390/mi15050559
Chicago/Turabian StyleRentzeperis, Frederika, Daniel Rivera, Jack Y. Zhang, Cole Brown, Tirone Young, Benjamin Rodriguez, Alexander Schupper, Gabrielle Price, Jack Gomberg, Tyree Williams, and et al. 2024. "Recent Developments in Magnetic Hyperthermia Therapy (MHT) and Magnetic Particle Imaging (MPI) in the Brain Tumor Field: A Scoping Review and Meta-Analysis" Micromachines 15, no. 5: 559. https://doi.org/10.3390/mi15050559
APA StyleRentzeperis, F., Rivera, D., Zhang, J. Y., Brown, C., Young, T., Rodriguez, B., Schupper, A., Price, G., Gomberg, J., Williams, T., Bouras, A., & Hadjipanayis, C. (2024). Recent Developments in Magnetic Hyperthermia Therapy (MHT) and Magnetic Particle Imaging (MPI) in the Brain Tumor Field: A Scoping Review and Meta-Analysis. Micromachines, 15(5), 559. https://doi.org/10.3390/mi15050559






