Evaluation of Phototoxicity of Short-Wavelength Laser Light Utilizing PCNA Accumulation
Abstract
:1. Introduction
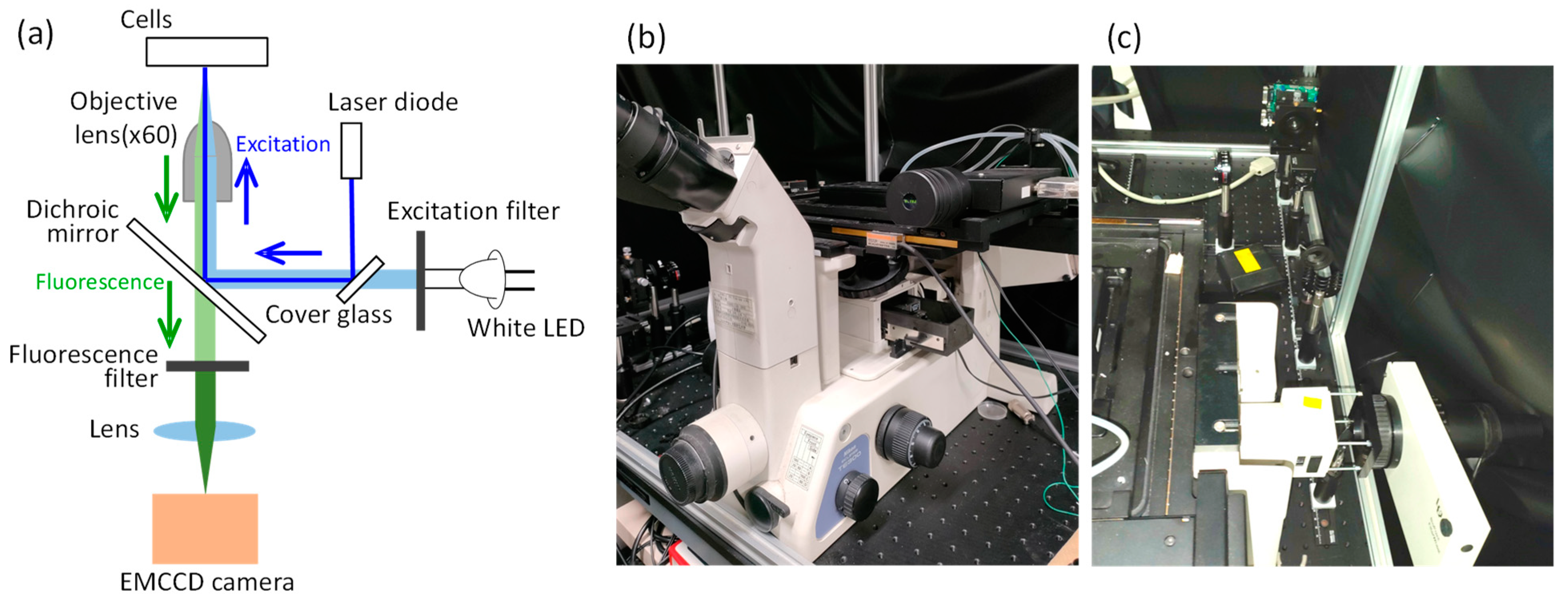
2. Methods
3. Results and Discussion
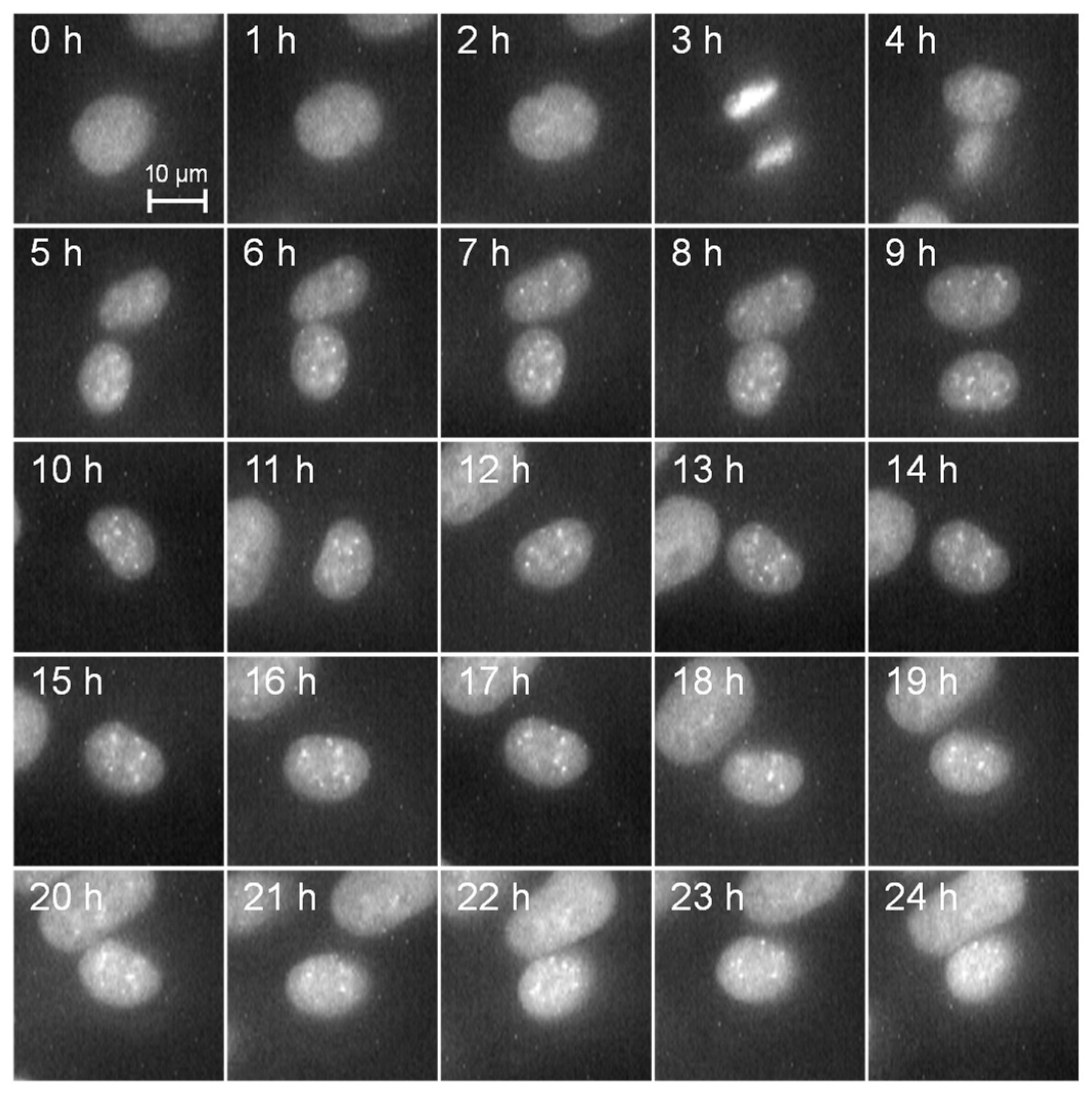
3.1. Cell Observation via Live Cell Imaging
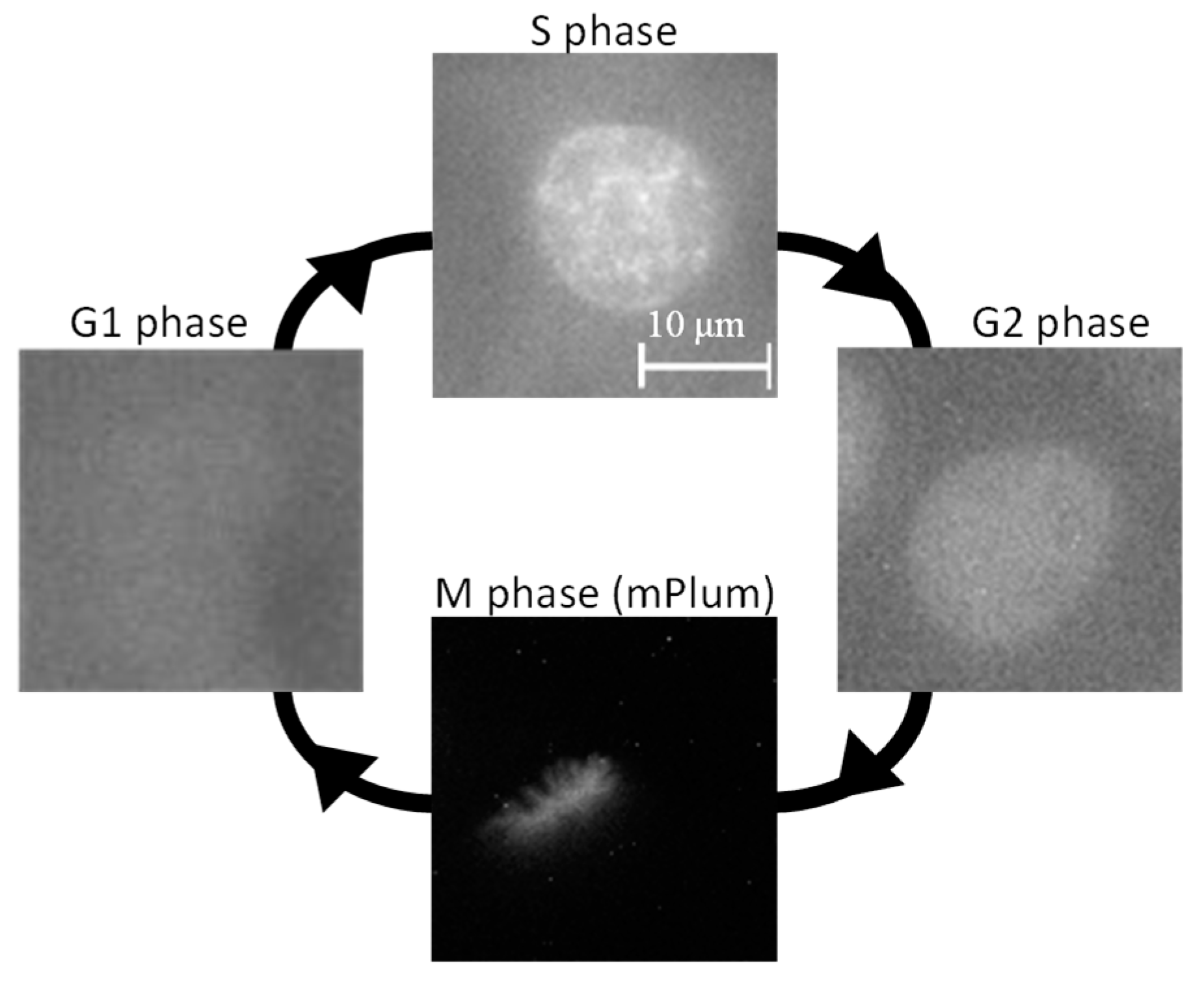
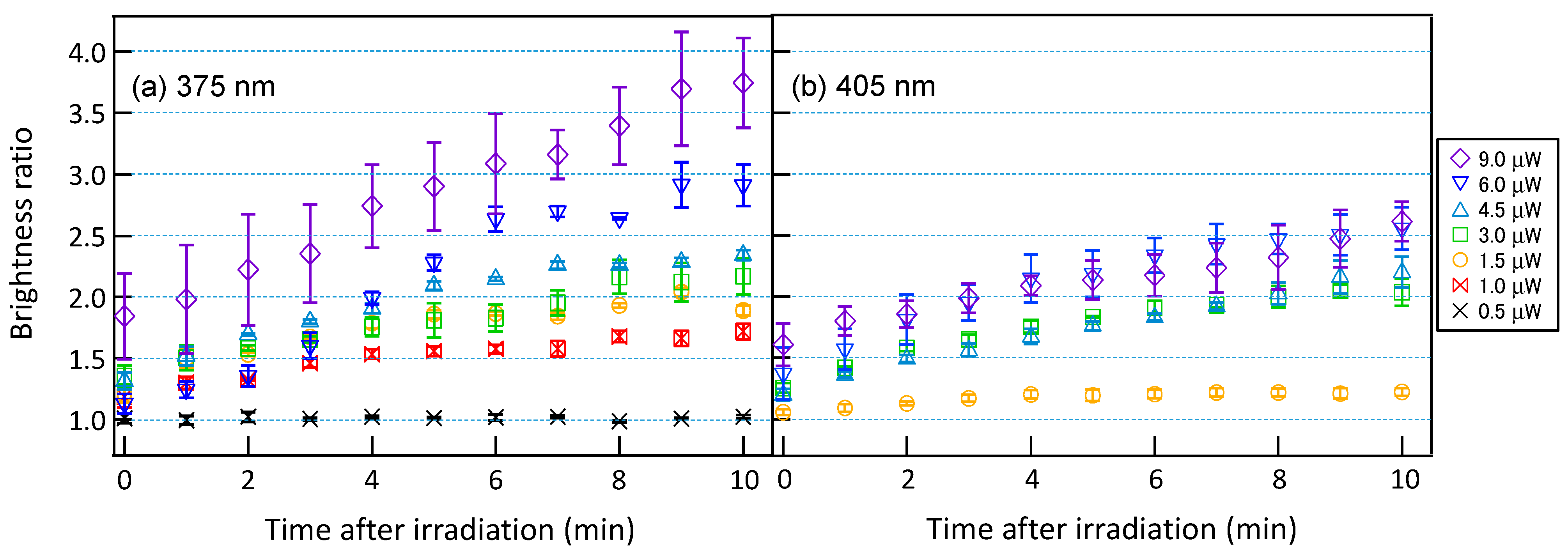
3.2. Accumulation of PCNA by Laser Irradiation
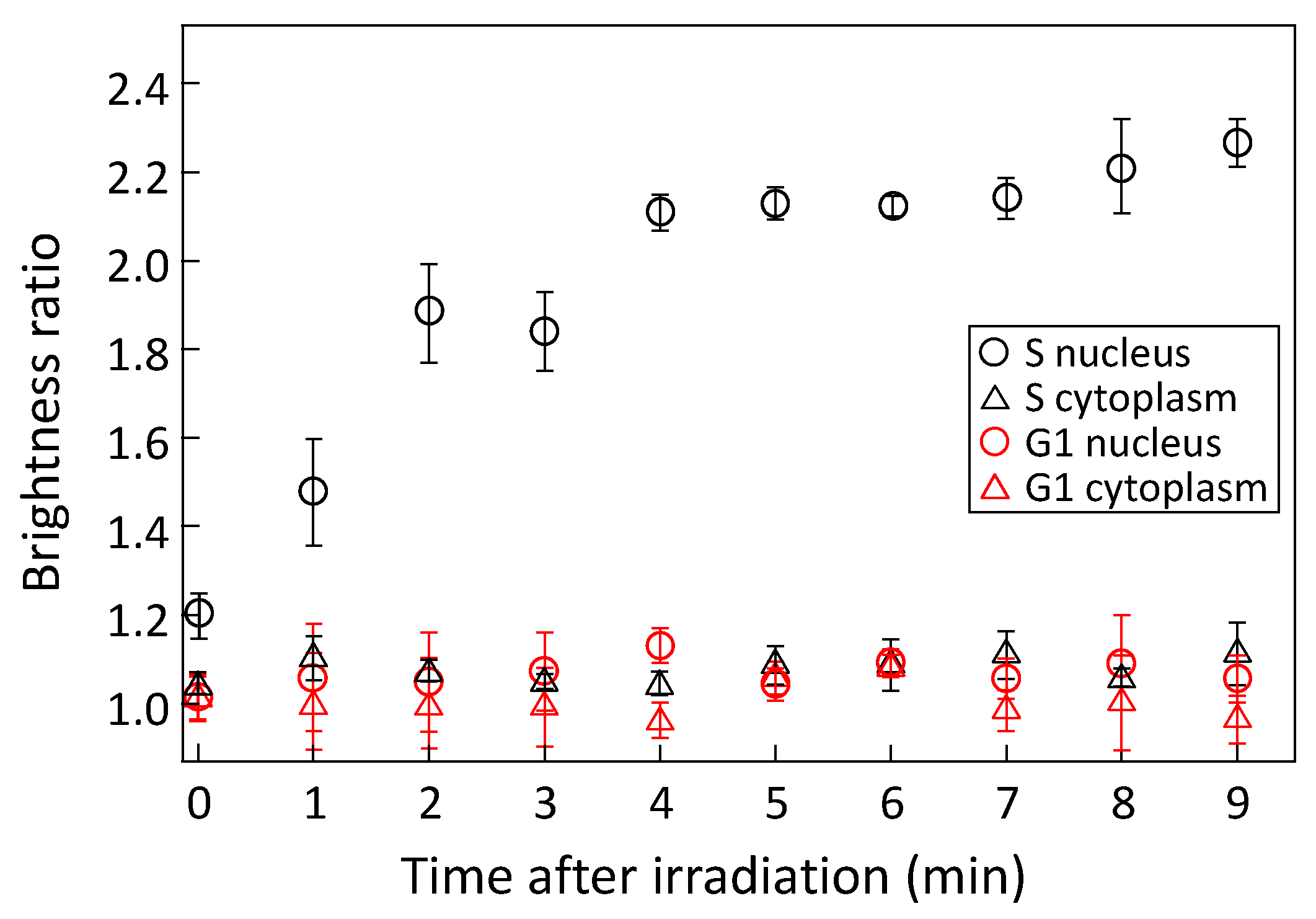
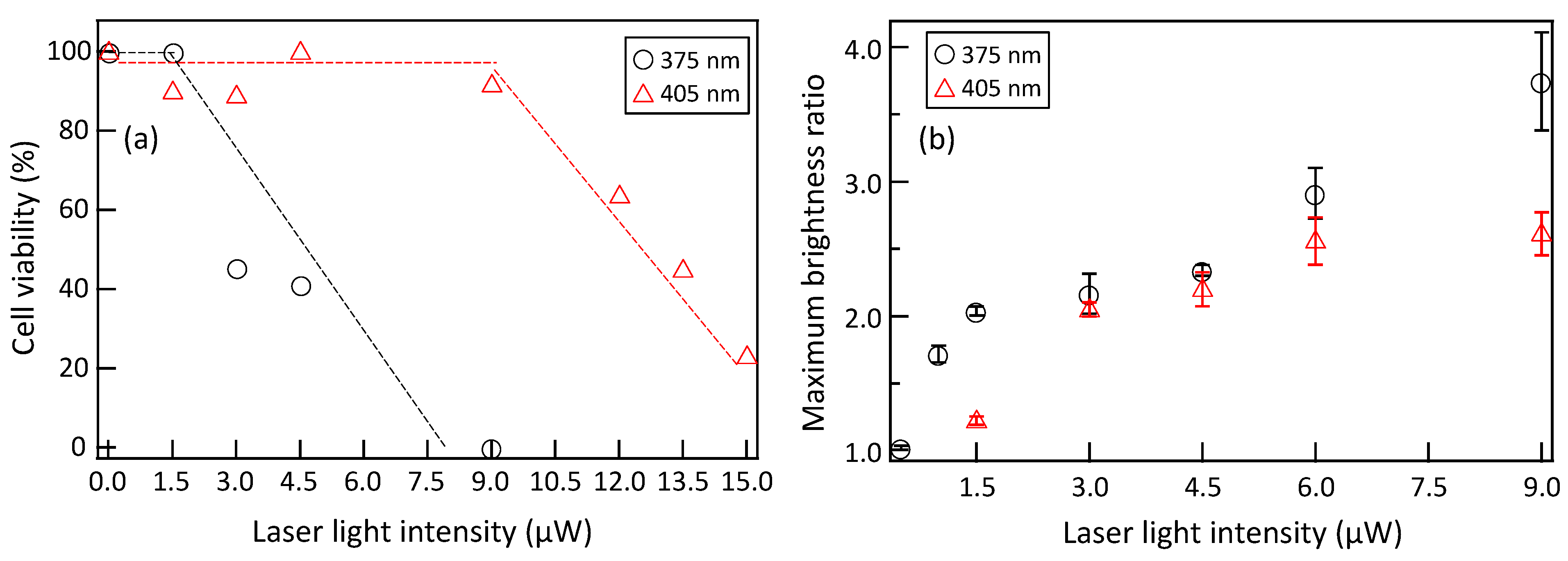
3.3. Comparison of PCNA Accumulation and Cell Survival Rate
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Brainard, G.C.; Hanifin, J.R.; Greeson, J.M.; Byrne, B.; Glickman, G.; Gerner, E.; Rollag, M.D. Action Spectrum for Melatonin Regulation in Humans: Evidence for a Novel Circadian Photoreceptor. J. Neurosci. 2001, 21, 6405. [Google Scholar] [CrossRef] [PubMed]
- Hatori, M.; Gronfier, C.; Van Gelder, R.N.; Bernstein, P.S.; Carreras, J.; Panda, S.; Marks, F.; Sliney, D.; Hunt, C.E.; Hirota, T.; et al. Global rise of potential health hazards caused by blue light-induced circadian disruption in modern aging societies. NPJ Aging Mech Dis. 2017, 3, 9. [Google Scholar] [CrossRef] [PubMed]
- Beatty, S.; Koh, H.H.; Phil, M.; Henson, D.; Boulton, M. The role of oxidative stress in the pathogenesis of age-related macular degeneration. Surv. Ophthalmol. 2000, 45, 115. [Google Scholar] [CrossRef] [PubMed]
- Klein, R.; Klein, B.E.; Knudtson, M.D.; Meuer, S.M.; Swift, M.; Gangnon, R.E. Fifteen-year cumulative incidence of age-related macular degeneration: The Beaver Dam Eye Study. Ophthalmology 2007, 114, 253. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Tsujikawa, M.; Itobe, H.; Du, Z.J.; Xie, P.; Matsumura, N.; Fu, X.; Zhang, R.; Sonoda, K.; Egashira, K.; et al. Chronic photo-oxidative stress and subsequent MCP-1 activation as causative factors for age-related macular degeneration. J. Cell Sci. 2012, 125, 2407. [Google Scholar] [CrossRef] [PubMed]
- Organisciak, D.T.; Darrow, R.A.; Barsalou, L.; Darrow, R.M.; Lininger, L.A. Light-induced damage in the retina: Differential effects of dimethylthiourea on photoreceptor survival, apoptosis and DNA oxidation. Photochem. Photobiol. 1999, 70, 261. [Google Scholar]
- Aonuma, H.; Koide, K.; Masuda, K.; Watanabe, I. Retinal Light Damage: Protective Effect of α-Tocopherol. Jpn. J. Ophthalmol. 1997, 41, 160. [Google Scholar] [CrossRef]
- Marek, V.; Mélik-Parsadaniantz, S.; Villette, T.; Montoya, F.; Baudouin, C.; Brignole-Baudouin, F.; Denoyer, A. Blue light phototoxicity toward human corneal and conjunctival epithelial cells in basal and hyperosmolar conditions. Free. Radic. Biol. Med. 2018, 126, 27. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.; Yun, J.; Yoon, Y.D.; Park, S.; Seo, Y.; Park, W.; Chu, H.Y.; Park, K.H.; Lee, M.Y.; Lee, C.W.; et al. Blue light effect on retinal pigment epithelial cells by display devices. Integr. Biol. 2017, 9, 436. [Google Scholar] [CrossRef]
- Nakashima, Y.; Ohta, S.; Wolf, A.M. Blue light-induced oxidative stress in live skin. Free Radic. Biol. Med. 2017, 108, 300. [Google Scholar]
- Lee, J.B.; Kim, S.H.; Lee, S.C.; Kim, H.G.; Ahn, H.G.; Li, Z.; Yoon, K.C. Blue Light–Induced Oxidative Stress in Human Corneal Epithelial Cells: Protective Effects of Ethanol Extracts of Various Medicinal Plant Mixtures. Investig. Ophthalmol. Vis. Sci. 2014, 55, 4119. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Gorman, A.; Zhou, X.; Sandra, C.; Chen, E. Involvement of Caspase-3 in Photoreceptor Cell Apoptosis Induced by In Vivo Blue Light Exposure. Investig. Ophthalmol. Vis. Sci. 2002, 43, 3349. [Google Scholar]
- Wu, J.; Seregard, S.; Spångberg, B.; Oskarsson, M.; Chen, E. Blue light induced apoptosis in rat retina. Eye 1999, 13, 577. [Google Scholar] [CrossRef] [PubMed]
- Del Olmo-Aguado, S.; Núñez-Álvarez, C.; Osborne, N.N. Blue Light Action on Mitochondria Leads to Cell Death by Necroptosis. Neurochem. Res. 2016, 41, 2324. [Google Scholar] [CrossRef] [PubMed]
- Maclean, M.; MacGregor, S.J.; Anderson, J.G.; Woolsey, G. Inactivation of bacterial pathogens following exposure to light from a 405-nanometer light-emitting diode array. Appl. Environ. Microbiol. 2009, 75, 1932. [Google Scholar] [CrossRef] [PubMed]
- Quandt, B.M.; Pfister, M.S.; Lübben, J.F.; Spano, F.; Rossi, R.M.; Bona, G.; Boesel, L.F. POF-yarn weaves: Controlling the light out-coupling of wearable phototherapy devices. Biomed. Opt. Express 2017, 8, 4316. [Google Scholar] [CrossRef]
- Zhuang, J.; Xia, L.; Zou, Z.; Yin, J. Blue light induces ROS mediated apoptosis and degradation of AML1-ETO oncoprotein in Kasumi-1 cells. Med. Oncol. 2022, 39, 52. [Google Scholar] [CrossRef]
- Cabral, J.; Gonçalves, R.A. Blue Light Disinfection in Hospital Infection Control: Advantages, Drawbacks, and Pitfalls. Antibiotics 2019, 8, 58. [Google Scholar] [CrossRef]
- ACGIH Ultaraviolet Radiation. Available online: https://www.acgih.org/ultraviolet-radiation/ (accessed on 1 March 2024).
- Douki, T.; Court, M.; Sauvaigo, S.; Odin, F.; Cadet, J. Formation of the main UV-induced thymine dimeric lesions within isolated and cellular DNA as measured by high performance liquid chromatography-tandem mass spectrometry. J. Biol. Chem. 2000, 275, 11678. [Google Scholar] [CrossRef]
- Pfeifer, G.P.; Besaratinia, A. UV wavelength-dependent DNA damage and human non-melanoma and melanoma skin cancer. Photochem. Photobiol. Sci. 2012, 11, 90. [Google Scholar] [CrossRef]
- Besaratinia, A.; Synold, T.W.; Chen, H.H.; Chang, C.; Xi, B.; Riggs, A.D.; Pfeifer, G.P. DNA lesions induced by UV A1 and B radiation in human cells: Comparative analyses in the overall genome and in the p53 tumor suppressor gene. Proc. Natl. Acad. Sci. USA 2005, 102, 10058. [Google Scholar] [CrossRef] [PubMed]
- McMillan, T.J.; Leatherman, E.; Ridley, A.; Shorrocks, J.; Tobi, S.E.; Whiteside, J.R. Cellular effects of long wavelength UV light (UVA) in mammalian cells. J. Pharm. Pharmacol. 2008, 60, 969. [Google Scholar] [CrossRef] [PubMed]
- Kuse, Y.; Ogawa, K.; Tsuruma, K.; Shimazawa, M.; Hara, H. Damage of photoreceptor-derived cells in culture induced by light emitting diode-derived blue light. Sci. Rep. 2014, 4, 5223. [Google Scholar] [CrossRef] [PubMed]
- Godley, B.F.; Shamsi, F.A.; Liang, F.Q.; Jarrett, S.G.; Davies, S.; Boultion, M. Blue light induces mitochondrial DNA damage and free radical production in epithelial cells. J. Biol. Chem. 2005, 280, 21061. [Google Scholar] [CrossRef] [PubMed]
- Grangeteau, C.; Lepinois, F.; Winckler, P.; Perrier-Cornet, J.M.; Dupont, S.; Beney, L. Cell Death Mechanisms Induced by Photo-Oxidation Studied at the Cell Scale in the Yeast Saccharomyces cerevisiae. Front. Microbiol. 2018, 9, 264. [Google Scholar] [CrossRef]
- Osaka, N.; Matsuyama, T.; Wada, K.; Okamoto, K.; Kawakita, A.; Murata, K.; Sugimoto, K. Quantitative Evaluation of Phototoxicity Induced by Blue Laser Irradiation Using Live Cell Imaging. Rev. Laser Eng. 2023, 51, 393. (In Japanese) [Google Scholar]
- van Roessel, P.; Brand, A.H. Imaging into the future: Visualizing gene expression and protein interactions with fluorescent proteins. Nature Cell Biol. 2002, 4, E15. [Google Scholar] [CrossRef]
- Sugimoto, K.; Senda-Murata, K.; Oka, S. Construction of three quadruple-fluorescent MDA435 cell lines that enable monitoring of the whole chromosome segregation process in the living state. Mut. Res. 2008, 657, 56. [Google Scholar] [CrossRef] [PubMed]
- Michalet, X.; Pinaud, F.F.; Bentolila, L.A.; Tsay, J.M.; Doose, S.; Li, J.J.; Sundaresan, G.; Wu, A.M.; Gambhir, S.S.; Weiss, S. Quantum Dots for Live Cells, in Vivo Imaging, and Diagnostics. Science 2005, 307, 538. [Google Scholar] [CrossRef]
- Nakagawa, C.; Senda-Murata, K.; Kawakita-Yamaguchi, A.; Fukada, T.; Sugimoto, K. Molecular Behavior of CENP-A and PCNA throughout the Cell Cycle in Living Human HT-1080 Cells. Mol. Biol. 2016, 5, 1000160. [Google Scholar]
- Waga, S.; Hannon, G.J.; Beach, D.; Stillman, B. The p21 inhibitor of cyclin-dependent kinases controls DNA replication by interaction with PCNA. Nature 1994, 369, 574. [Google Scholar] [CrossRef] [PubMed]
- Maga, G.; Hübscher, U. Proliferating cell nuclear antigen (PCNA): A dancer with many partners. J. Cell Sci. 2003, 116, 3051. [Google Scholar] [CrossRef] [PubMed]
- Bravo, R.; Macdonald-Bravo, H. Existence of two populations of cyclin/proliferating cell nuclear antigen during the cell cycle: Association with DNA replication sites. J. Cell Biol. 1987, 105, 1549. [Google Scholar] [CrossRef] [PubMed]
- Essers, J.; Theil, A.F.; Baldeyron, C.; van Cappellen, W.A.; Houtsmuller, A.B.; Kanaar, R.; Vermeulen, W. Nuclear Dynamics of PCNA in DNA Replication and Repair. Mol. Cell. Biol. 2005, 25, 9350. [Google Scholar] [CrossRef] [PubMed]
- Ersoy, I.; Bunyak, F.; Chagin, V.; Cardoso, M.C.; Palaniappan, K. Segmentation and Classification of Cell Cycle Phases in Fluorescence Imaging. Lect Notes Comput Sci. 2009, 5762, 617. [Google Scholar]
- Kroemer, G.; Galluzzi, L.; Vandenabeele, P.; Abrams, J.; Alnemri, E.S.; Baehrecke, E.H.; Blagosklonny, M.V.; El-Deiry, W.S.; Golstein, P.; Green, D.R.; et al. Classification of cell death: Recommendations of the Nomenclature Committee on Cell Death 2009. Cell Death Differ. 2009, 16, 3. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsuyama, T.; Osaka, N.; Yamaguchi, M.; Kanamaru, N.; Wada, K.; Kawakita, A.; Murata, K.; Sugimoto, K.; Okamoto, K. Evaluation of Phototoxicity of Short-Wavelength Laser Light Utilizing PCNA Accumulation. Micromachines 2024, 15, 646. https://doi.org/10.3390/mi15050646
Matsuyama T, Osaka N, Yamaguchi M, Kanamaru N, Wada K, Kawakita A, Murata K, Sugimoto K, Okamoto K. Evaluation of Phototoxicity of Short-Wavelength Laser Light Utilizing PCNA Accumulation. Micromachines. 2024; 15(5):646. https://doi.org/10.3390/mi15050646
Chicago/Turabian StyleMatsuyama, Tetsuya, Noboru Osaka, Mikiya Yamaguchi, Naohiro Kanamaru, Kenji Wada, Ai Kawakita, Kaori Murata, Kenji Sugimoto, and Koichi Okamoto. 2024. "Evaluation of Phototoxicity of Short-Wavelength Laser Light Utilizing PCNA Accumulation" Micromachines 15, no. 5: 646. https://doi.org/10.3390/mi15050646
APA StyleMatsuyama, T., Osaka, N., Yamaguchi, M., Kanamaru, N., Wada, K., Kawakita, A., Murata, K., Sugimoto, K., & Okamoto, K. (2024). Evaluation of Phototoxicity of Short-Wavelength Laser Light Utilizing PCNA Accumulation. Micromachines, 15(5), 646. https://doi.org/10.3390/mi15050646





