An Intelligent Non-Invasive Blood Pressure Monitoring System Based on a Novel Polyvinylidene Fluoride Piezoelectric Thin Film
Abstract
:1. Introduction
2. Related Work
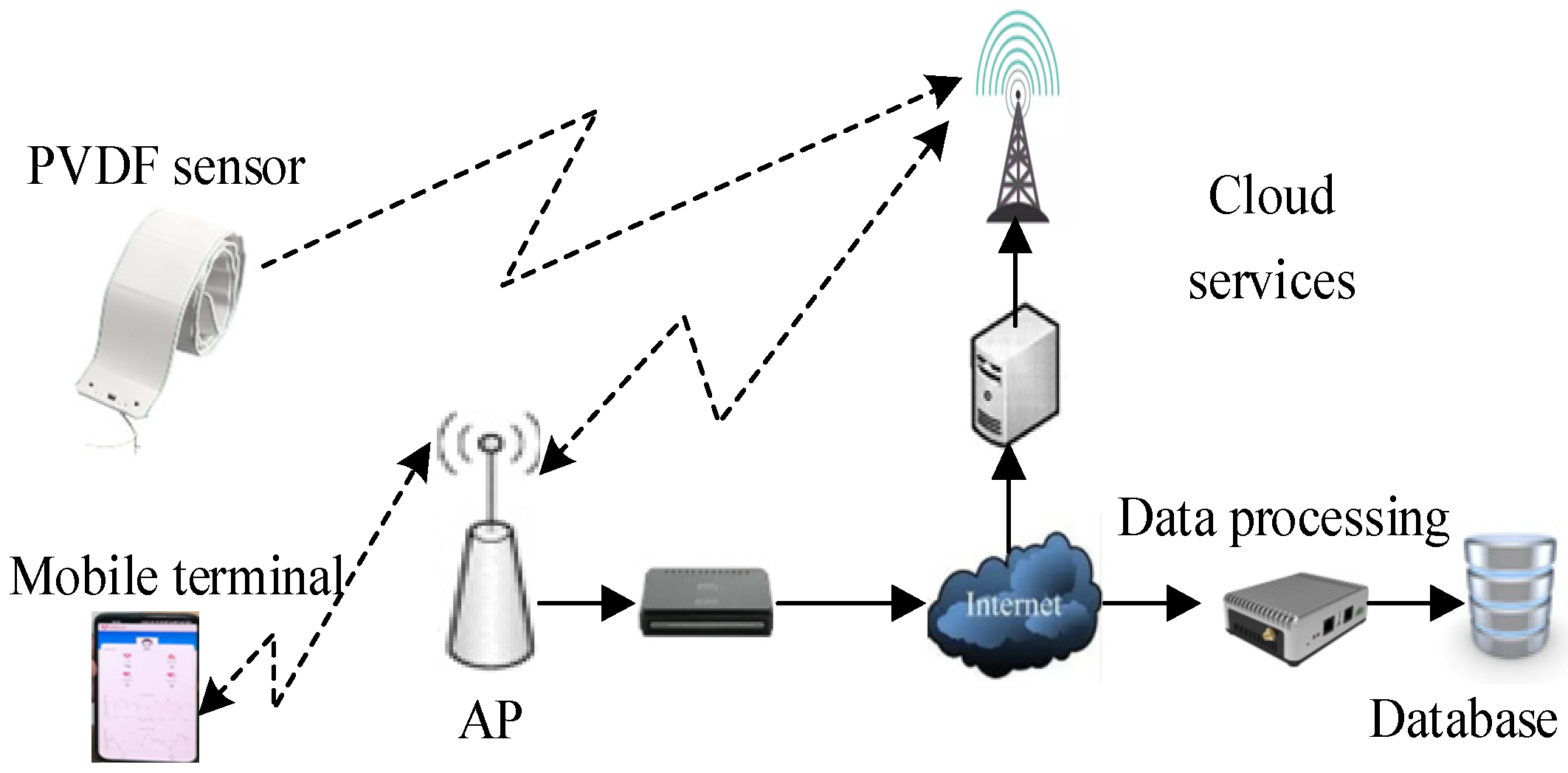
3. Design and Optimization of the Non-Invasive Blood Pressure Monitoring System
3.1. A Novel Highly Sensitive PVDF Sensor Technology
3.1.1. Innovative Modified Piezoelectric Polymer Materials
- (1)
- Add polyvinylidene fluoride, polyvinyl alcohol, and N,N-dimethylacetamide to a container in a weight ratio of (61–71):(19–22):(400–500). Stir the mixture uniformly at 60–80 °C for 2–4 h to ensure thorough mixing.
- (2)
- Maintain the temperature of the mixed solution, let it stand for 4–6 min to remove air bubbles, and obtain the PVDF solution.
- (1)
- Add polyvinylidene fluoride, polyvinyl alcohol, and N,N-dimethylacetamide to a container in a weight ratio of (61–71):(19–22):(400–500). Stir the mixture uniformly at 60–80 °C for 2–4 h to obtain a casting solution.
- (2)
- Maintain the temperature of the casting solution and let it stand for 4–6 min to remove air bubbles. Pour the solution onto a glass plate to form a uniform coating with a thickness of 0.8–1.2 mm.
- (3)
- Immerse the glass plate with the coating into deionized water at 25–28 °C. Once the coating detaches from the glass plate, soak it in deionized water for 45–50 h to form the PVDF film. After removing the film, air-dry it at 25–28 °C.
3.1.2. Citric Acid-Guided Polyvinyl Alcohol Branching
3.2. Principle of Pulse Monitoring Based on Piezoelectric Polymers
3.2.1. Method of Connecting Piezoelectric Polymers
3.2.2. Blood Pressure Monitoring Principle Based on PVDF Sensors
4. Experimental Results and Analysis
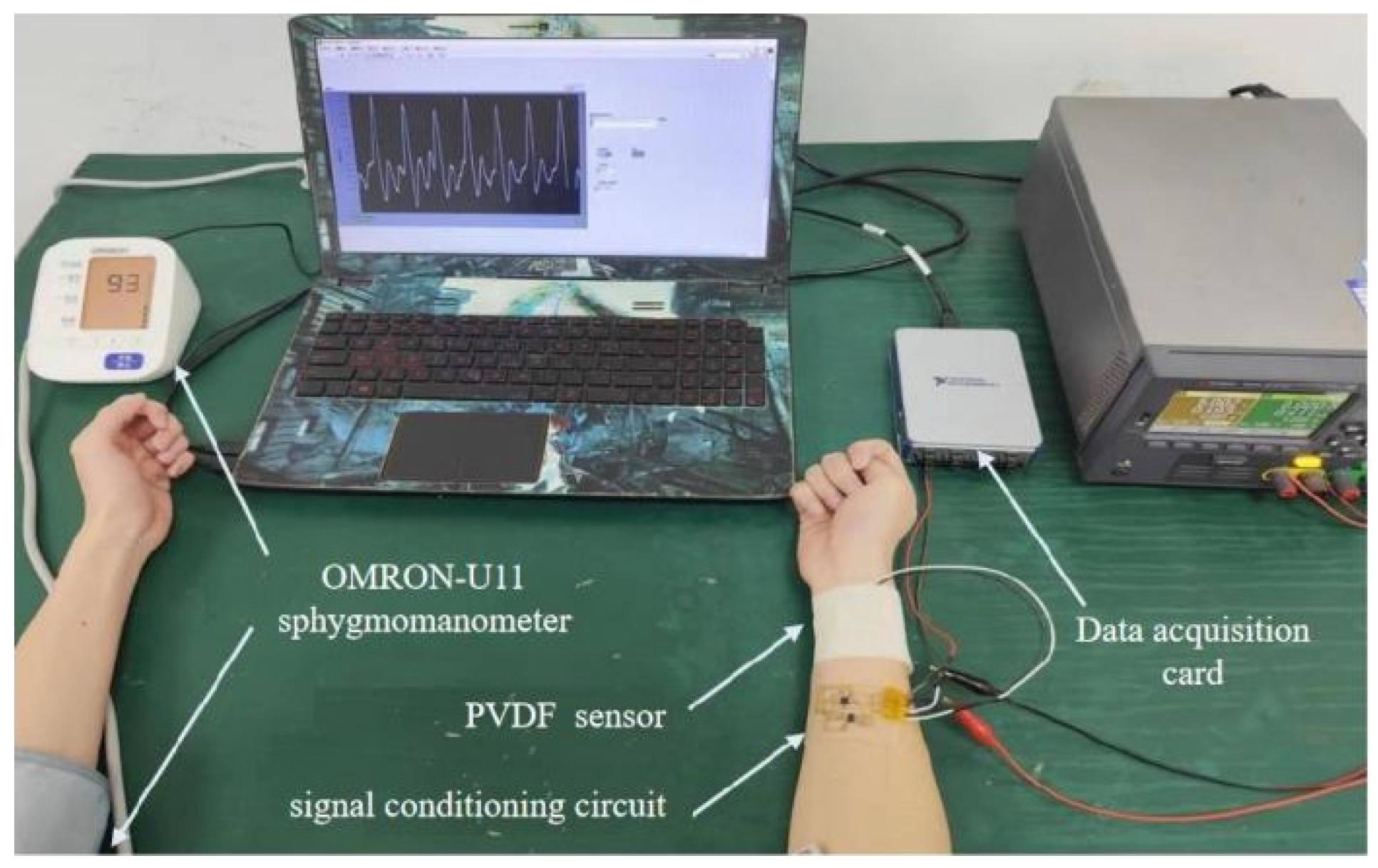
4.1. Experimental Design and Setup
- (1)
- Implementation Case 1
- (2)
- Implementation Case 2
- (3)
- Implementation Case 3
- (4)
- Implementation Case 4
- (5)
- Comparison Case 1
- (6)
- Comparison Case 2
- (7)
- Comparison Case 3
4.2. Interpretation of Experimental Data
4.2.1. Analysis of Piezoelectric Polymer Properties
4.2.2. Analysis of Intelligent Blood Pressure Monitoring Performance
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Madeddu, P. Cell therapy for the treatment of heart disease: Renovation work on the broken heart is still in progress. Free Radic. Biol. Med. 2021, 164, 206–222. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.L.; Zhang, Y.Q. Review of cardiovascular diseases risk assessment system of hypertension guidelines at home and abroad. Chin. J. Front. Med. Sci. (Electron. Version) 2020, 12, 33–40. [Google Scholar]
- Shen, X.; He, W.; Sun, J.; Zhang, Z.; Li, Q.; Zhang, H.; Long, M. Development and validation of a nomogram to predict the future risk of cardiovascular disease. Rev. Cardiovasc. Med. 2023, 24, 35–42. [Google Scholar] [CrossRef]
- Gomadam, P.; Shah, A.; Qureshi, W.; Yeboah, P.N.; Freedman, B.I.; Bowden, D.; Soliman, E.Z.; Yeboah, J. Blood pressure indices and cardiovascular disease mortality in persons with or without diabetes mellitus. J. Hypertens. 2018, 36, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Wang, F.; Mao, J.; Huang, Y.; Zhou, P.; Luo, J. A protocol for digitalized collection of Traditional Chinese Medicine(TCM) pulse information using bionic pulse diagnosis equipment. Phenomics 2023, 3, 519–534. [Google Scholar] [CrossRef] [PubMed]
- Sony, S.; Laventure, S.; Sadhu, A. A literature review of next-generation smart sensing technology in structural health monitoring. Struct. Control Health Monit. 2019, 26, e2321. [Google Scholar] [CrossRef]
- Hammock, M.L.; Chortos, A.; Tee, B.C.K.; Tok, J.B.H.; Bao, Z. 25th anniversary article: The evolution of electronic skin (e-skin): A brief history, design considerations, and recent progress. Adv. Mater. 2013, 25, 5997–6038. [Google Scholar] [CrossRef]
- Yang, T.; Xie, D.; Li, Z.; Zhu, H. Recent advances in wearable tactile sensors: Materials, sensing mechanisms, and device performance. Mater. Sci. Eng. R Rep. 2017, 115, 1–37. [Google Scholar] [CrossRef]
- Xu, F.; Li, X.; Shi, Y.; Li, L.; Wang, W.; He, L.; Liu, R. Recent developments for flexible pressure sensors: A review. Micromachines 2018, 9, 580. [Google Scholar] [CrossRef]
- Doshi, S.M.; Thostenson, E.T. Thin and flexible carbon nanotube-based pressure sensors with ultrawide sensing range. ACS Sens. 2018, 3, 1276–1282. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Jin, X.; Zheng, Y.; Chang, X.; Wang, W.; Lin, T.; Zheng, F.; Onyilagha, O.; Zhu, Z. A porous and air gap elastomeric dielectric layer for wearable capacitive pressure sensor with high sensitivity and a wide detection range. J. Mater. Chem. C 2020, 8, 11468–11476. [Google Scholar] [CrossRef]
- Zhuo, B.; Chen, S.; Zhao, M.; Guo, X. High sensitivity flexible capacitive pressure sensor using polydimethylsiloxane elastomer dielectric layer micro-structured by 3-D printed mold. IEEE J. Electron Devices Soc. 2017, 5, 219–223. [Google Scholar] [CrossRef]
- Zhu, B.; Niu, Z.; Wang, H.; Leow, W.R.; Wang, H.; Li, Y.; Zheng, L.; Wei, J.; Huo, F.; Chen, X. Micro structured graphene arrays for highly sensitive flexible tactile sensors. Small 2014, 10, 3625–3631. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Gu, Y.; Xiong, Z.; Cui, Z.; Zhang, T. Silk-molded flexible, ultrasensitive, and highly stable electronic skin for monitoring human physiological signals. Adv. Mater. 2014, 26, 1336–1342. [Google Scholar] [CrossRef] [PubMed]
- Lei, K.F.; Hsieh, Y.Z.; Chiu, Y.Y.; Wu, M.-H. The structure design of piezoelectric poly (vinylidene fluoride)(PVDF) polymer-based sensor patch for the respiration monitoring under dynamic walking conditions. Sensors 2015, 15, 18801–18812. [Google Scholar] [CrossRef] [PubMed]
- Sekine, T.; Sugano, R.; Tashiro, T.; Sato, J.; Takeda, Y.; Matsui, H.; Kumaki, D.; Dos Santos, F.D.; Miyabo, A.; Tokito, S. Fully printed wearable vital sensor for human pulse rate monitoring using ferroelectric polymer. Sci. Rep. 2018, 8, 4442. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Liu, T.; Sun, X.C.; Li, P.; Xu, Y.-S.; Hua, J.-G.; Yu, Y.-H.; Li, S.-X.; Dai, Y.-Z.; Song, X.-Y.; et al. Flexible pressure sensor based on PVDF nanofiber. Sens. Actuators A Phys. 2018, 280, 319–325. [Google Scholar] [CrossRef]
- Ling, Z.B.; Qi, X.H.; Li, W.N.; Cai, J. The research of breathing signal detection belt basedon PVDF. Piezoelectrics Acoustooptics 2014, 36, 72–75. [Google Scholar]
- Xu, Z.J.; Han, G.Q. Design of respiratory signal detection system with PVDF piezoelectric film sensor. Mach. Build. Autom. 2017, 46, 185–187. [Google Scholar]
- Chen, C.; Zhang, R.J.; Li, Q. A simple design for physiological signal monitoring circuit. Sci. Technol. Innov. 2018, 8, 47–49. [Google Scholar]
- Xin, Y.; Xu, Y.; Guo, C.; Sun, H.; Zhu, J.; Liu, T. Study on sleep monitoring pillow based on piezoelectric film sensors. Piezoelectrics Acoustooptics 2018, 40, 283–287. [Google Scholar]
- Chen, W.; Kobayashi, T.; Ichikawa, S.; Takeuchi, Y.; Togawa, T. Continuous estimation of systolic blood pressure using the pulse arrival time and intermittent calibration. Med. Biol. Eng. Comput. 2000, 38, 569–574. [Google Scholar] [CrossRef] [PubMed]






| Implementation 1 Sample 1 | Implementation 2 Sample 2 | Implementation 3 Sample3 | Implementation 4 Sample 4 | Comparison 1 Sample 5 | Comparison 2 Sample 6 | Comparison 3 Sample 7 | |
|---|---|---|---|---|---|---|---|
| PVDF | 61 g | 66 g | 71 g | --- | 61 g | 61 g | --- |
| citric acid-PVA | --- | --- | --- | 61 g | --- | --- | 61 g |
| polyvinyl alcohol | 19 g | 21 g | 22 g | 19 g | --- | 18 g | --- |
| succinic acid–polyvinyl alcohol | --- | --- | --- | --- | --- | --- | 19 g |
| N,N-dimethylacetamide | 420 g | 450 g | 500 g | 420 g | 420 g | 420 g | 420 g |
| stirring temperature | 60 °C | 70 °C | 80 °C | 60 °C | 60 °C | 60 °C | 60 °C |
| stirring time | 4 h | 3 h | 2 h | 4 h | 4 h | 4 h | 4 h |
| deionization temperature | 26 °C | 27 °C | 28 °C | 26 °C | 26 °C | 26 °C | 26 °C |
| deionization time | 50 h | 48 h | 45 h | 50 h | 50 h | 50 h | 50 h |
| characteristics | dense and smooth surface | more biocompatible |
| Sample Type | Piezoelectric Coefficient d33 (pC/N) | Dielectric Constant (Relative to Vacuum) |
|---|---|---|
| Sample 1 | −41.2 | 13.4 |
| Sample 2 | −40.3 | 13.1 |
| Sample 3 | −38.8 | 12.8 |
| Sample 4 | −40.9 | 14.2 |
| Sample 5 | −33.0 | 6.7 |
| Sample 6 | −34.2 | 7.1 |
| Sample 7 | −32.1 | 7.0 |
| Number of Measurements | 1 | 2 | 3 | 4 | 5 | Mean Value | Standard Deviation |
|---|---|---|---|---|---|---|---|
| Electronic Blood Pressure Monitor | 77/119 | 73/113 | 71/114 | 81/123 | 80/115 | 76/117 | 4.3/4.2 |
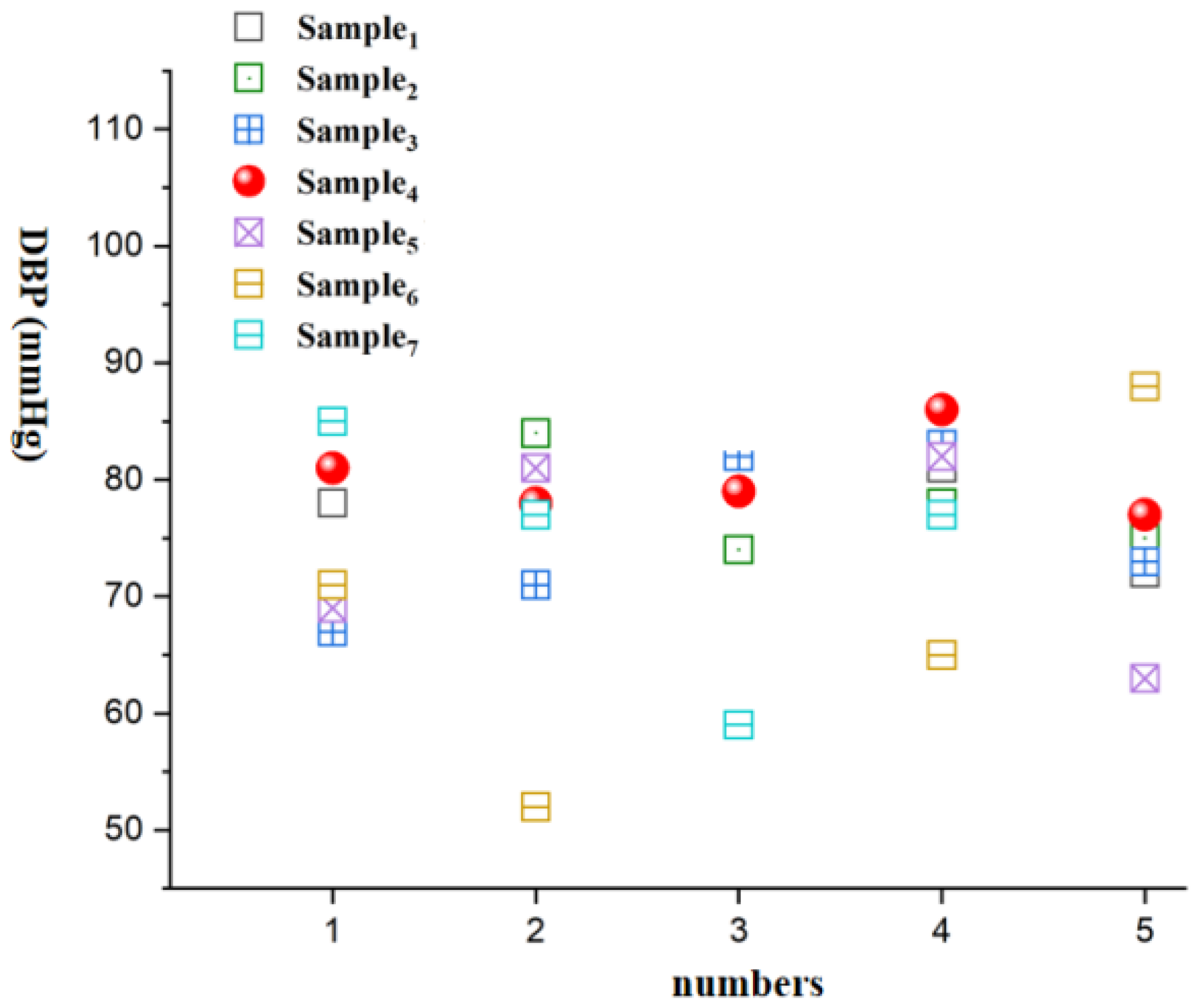
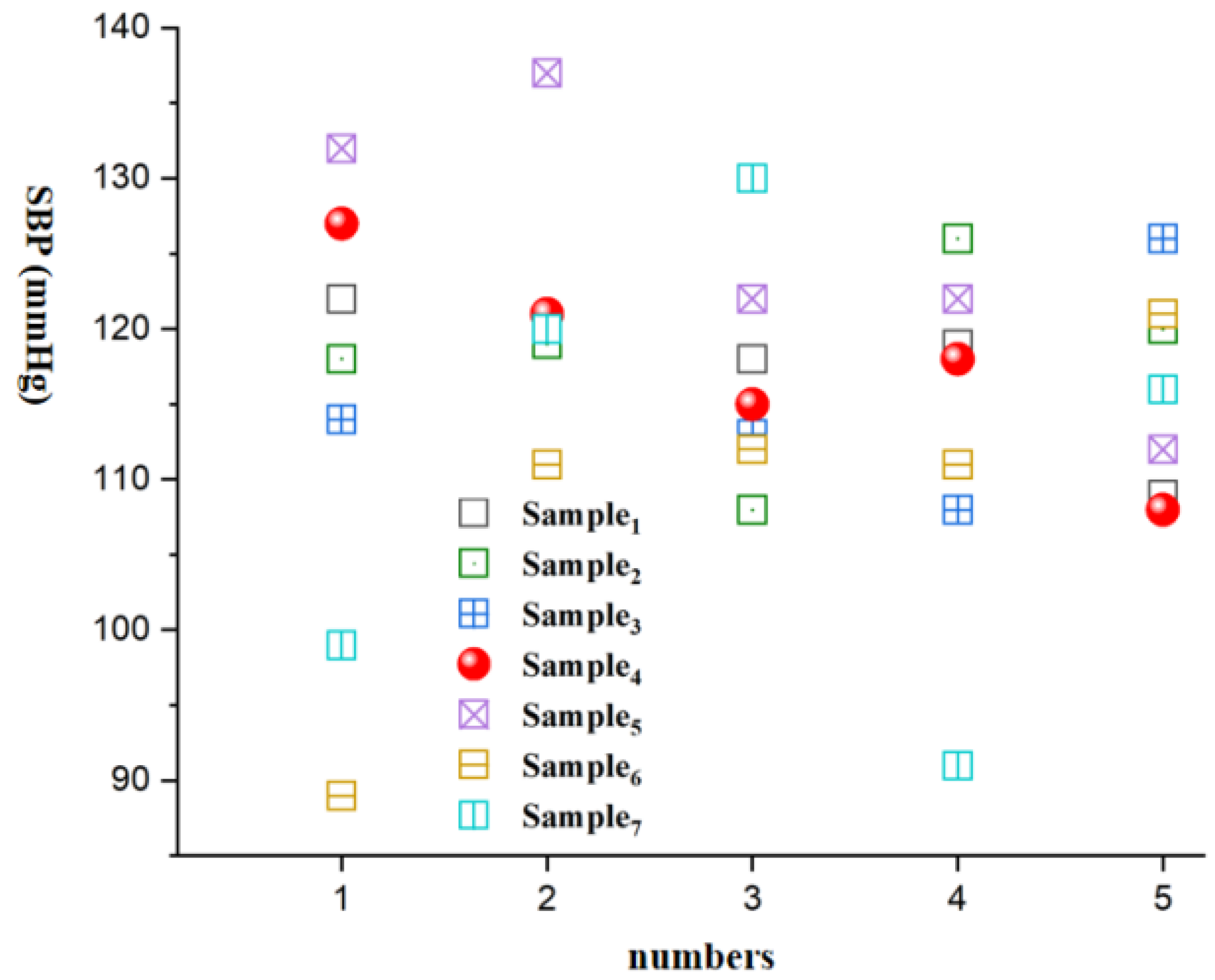
| Number of Measurements | Sample 1 | Sample2 | Sample 3 | Sample 4 | Sample 5 | Sample 6 | Sample 7 |
|---|---|---|---|---|---|---|---|
| 1 | 78/122 | 69/118 | 67/114 | 81/127 | 69/132 | 71/89 | 85/99 |
| 2 | 71/119 | 84/119 | 71/120 | 78/121 | 81/137 | 52/111 | 77/120 |
| 3 | 74/118 | 74/108 | 82/113 | 79/115 | 90/122 | 92/112 | 59/130 |
| 4 | 81/119 | 78/126 | 83/108 | 86/118 | 82/122 | 65/111 | 77/91 |
| 5 | 72/109 | 75/120 | 73/126 | 77/108 | 63/112 | 88/121 | 110/116 |
| Mean Value | 75/117 | 76/118 | 75/116 | 79/118 | 77/125 | 74/109 | 82/111 |
| Standard Deviation | 4.2/4.9 | 5.5/6.5 | 7.0/6.9 | 1.6/7.1 | 10.8/9.8 | 16.5/11.8 | 18.5/15.9 |
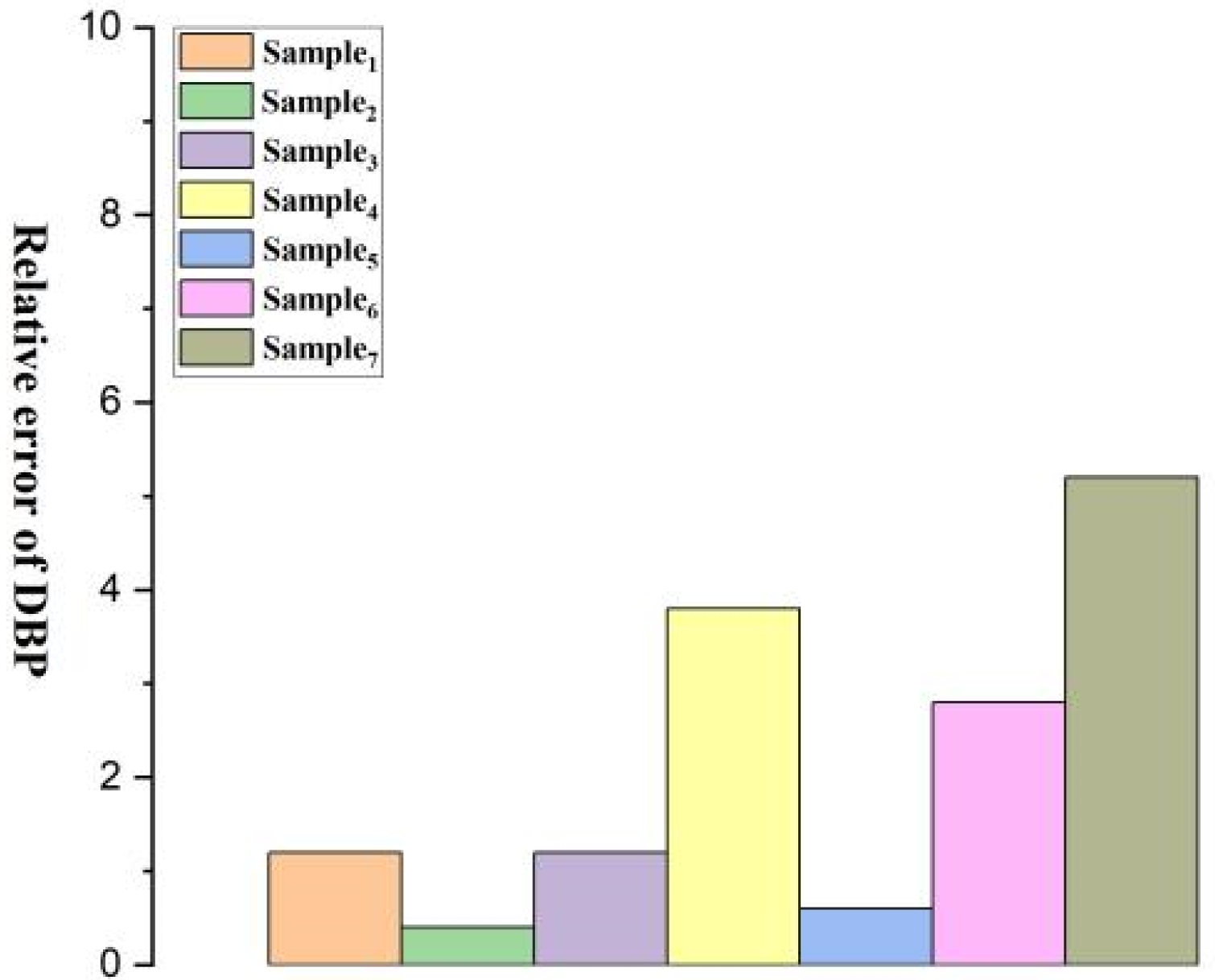
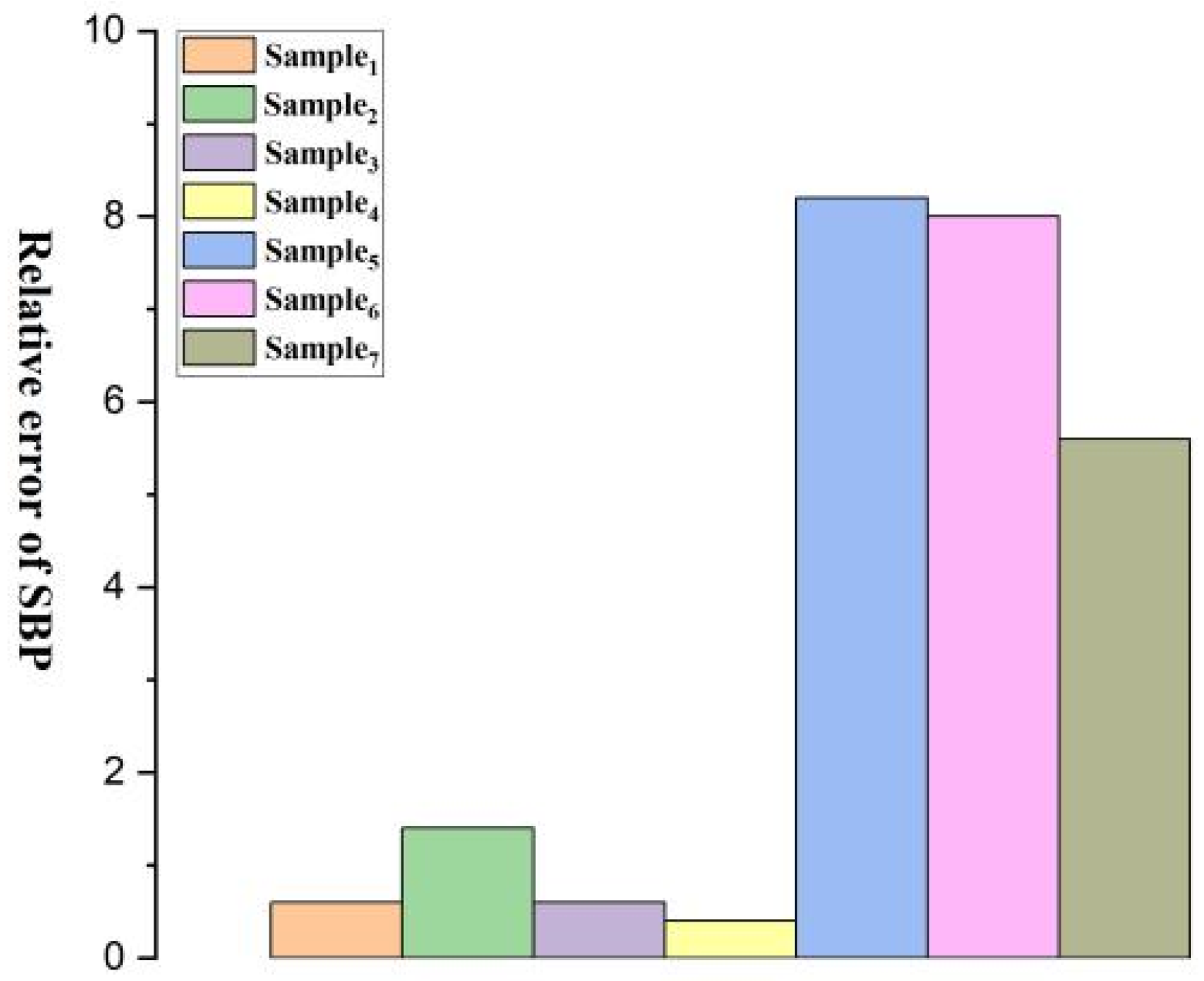
| Relative Error | 1.2/0.6 | 0.4/1.4 | 1.2/0.6 | 3.8/0.4 | 0.6/8.2 | 2.8/8.0 | 5.2/5.6 |
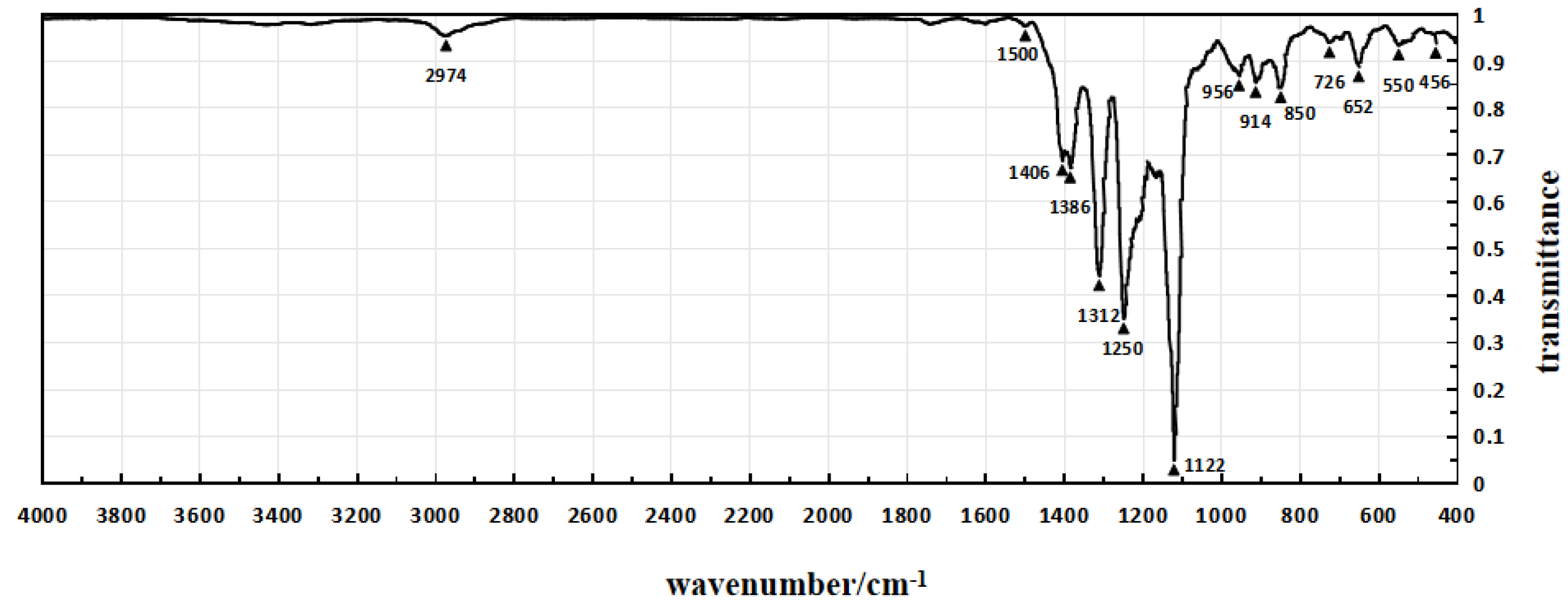
| Sample 1 | Sample 4 | Sample 5 | |||
|---|---|---|---|---|---|
| Peak Wavenumber (cm−1) | Transmittance | Peak Wavenumber (cm−1) | Transmittance | Peak Wavenumber (cm−1) | Transmittance |
| 560 | 82% | 560 | 81% | 550 | 93% |
| 652 | 83% | 652 | 82% | 652 | 89% |
| 856 | 81% | 856 | 81% | 700 | 95% |
| 946 | 80% | 910 | 80% | 726 | 94% |
| 1122 | 5% | 946 | 77% | 850 | 84% |
| 1244 | 43% | 1122 | 5% | 914 | 85% |
| 1316 | 36% | 1244 | 23% | 956 | 87% |
| 1382 | 51% | 1316 | 30% | 1122 | 5% |
| 1414 | 48% | 1406 | 40% | 1168 | 65% |
| 1626 | 95% | 1636 | 91% | 1250 | 35% |
| 1716 | 94% | 1720 | 72% | 1312 | 44% |
| 2934 | 74% | 2934 | 72% | 1386 | 67% |
| 2974 | 73% | 2974 | 69% | 1406 | 69% |
| 3390 | 44% | 3394 | 41% | 2974 | 95% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Zhou, T.; Liu, M.; Zhao, Q.; Liu, Y. An Intelligent Non-Invasive Blood Pressure Monitoring System Based on a Novel Polyvinylidene Fluoride Piezoelectric Thin Film. Micromachines 2024, 15, 659. https://doi.org/10.3390/mi15050659
Li S, Zhou T, Liu M, Zhao Q, Liu Y. An Intelligent Non-Invasive Blood Pressure Monitoring System Based on a Novel Polyvinylidene Fluoride Piezoelectric Thin Film. Micromachines. 2024; 15(5):659. https://doi.org/10.3390/mi15050659
Chicago/Turabian StyleLi, Shilin, Taoyun Zhou, Muzhou Liu, Qiaomei Zhao, and Yi Liu. 2024. "An Intelligent Non-Invasive Blood Pressure Monitoring System Based on a Novel Polyvinylidene Fluoride Piezoelectric Thin Film" Micromachines 15, no. 5: 659. https://doi.org/10.3390/mi15050659
APA StyleLi, S., Zhou, T., Liu, M., Zhao, Q., & Liu, Y. (2024). An Intelligent Non-Invasive Blood Pressure Monitoring System Based on a Novel Polyvinylidene Fluoride Piezoelectric Thin Film. Micromachines, 15(5), 659. https://doi.org/10.3390/mi15050659





