Micro- and Nanofluidic pH Sensors Based on Electrodiffusioosmosis
Abstract
:1. Introduction
2. Materials and Methods
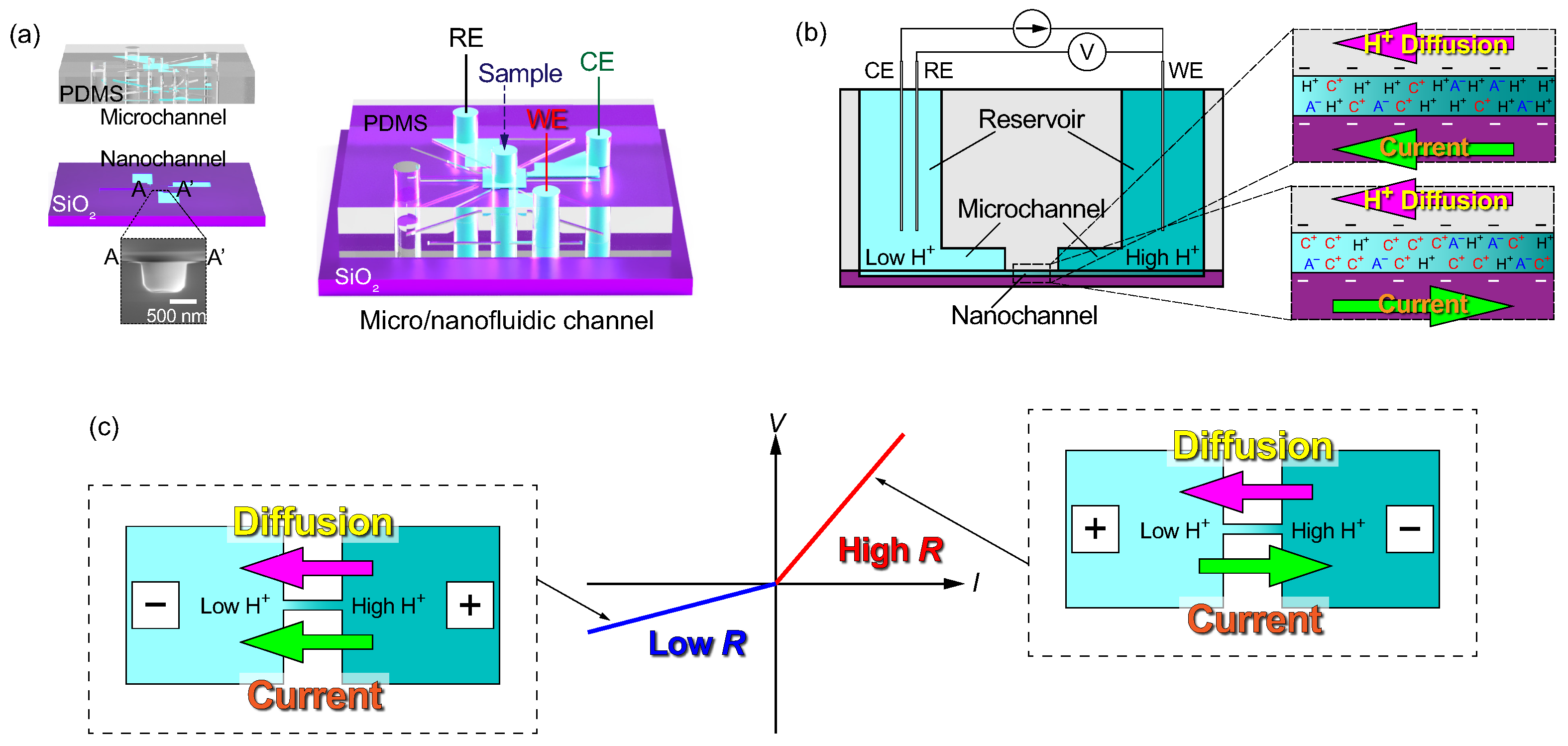
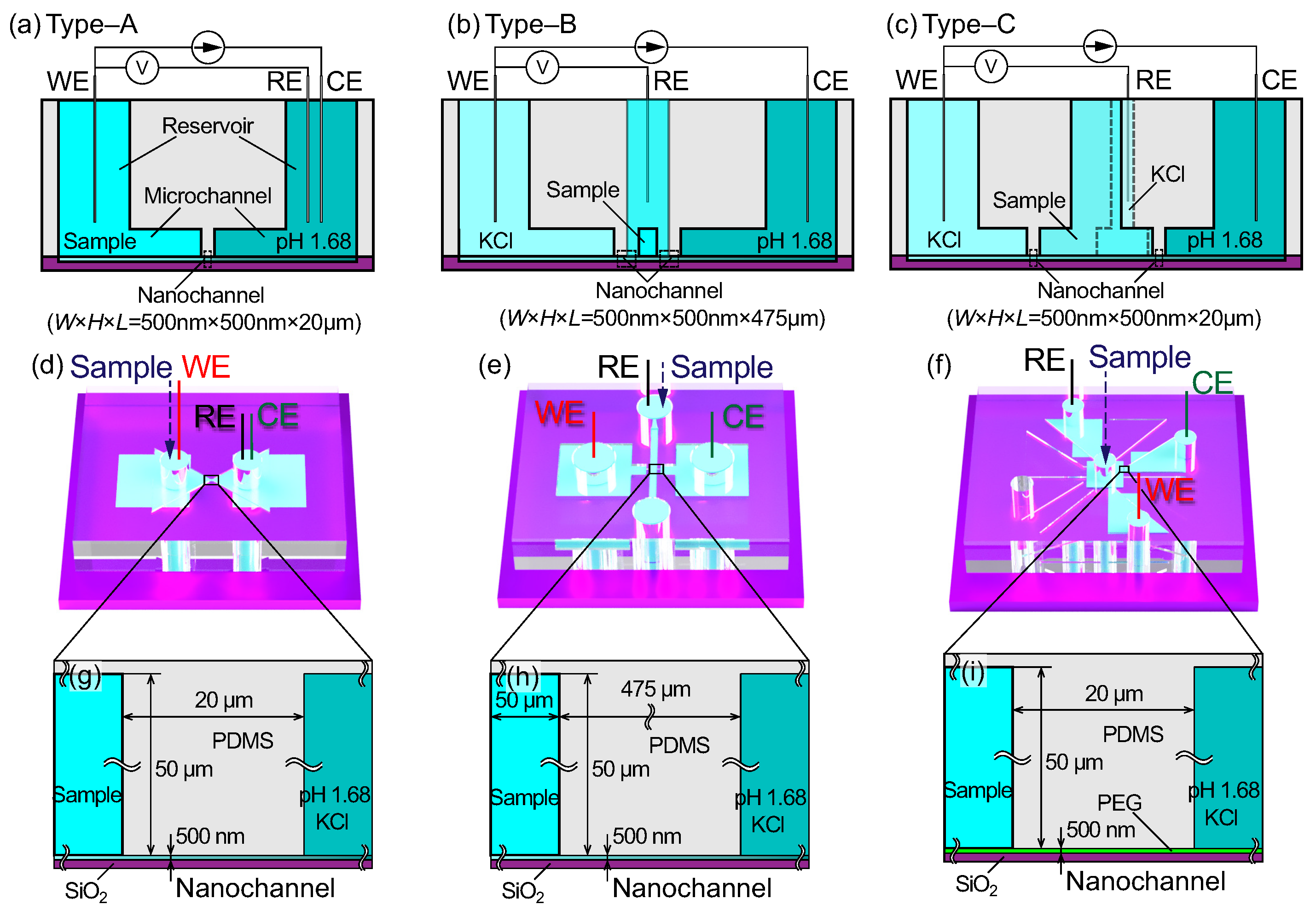
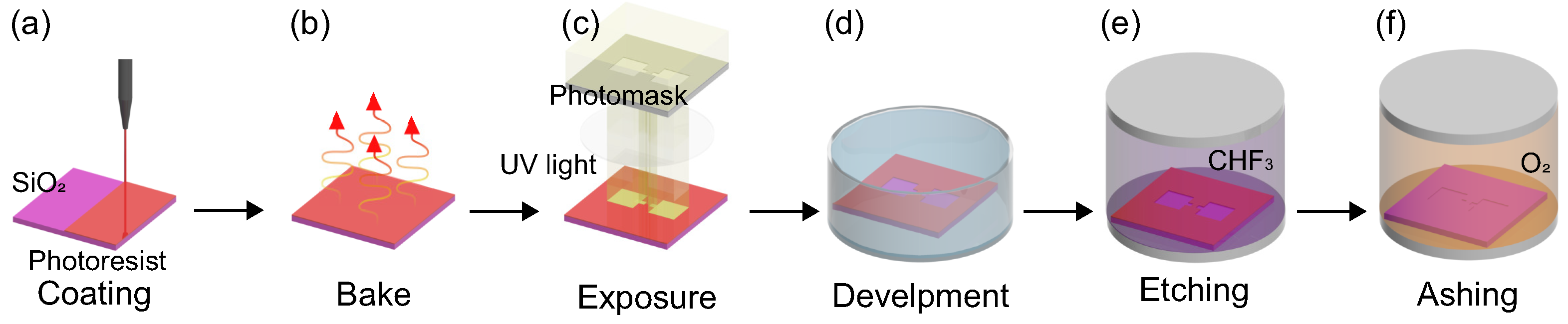
2.1. Nanochannels for the Test Section
2.2. Microchannels for Sample Transport
2.3. Preparation of Sample Solutions
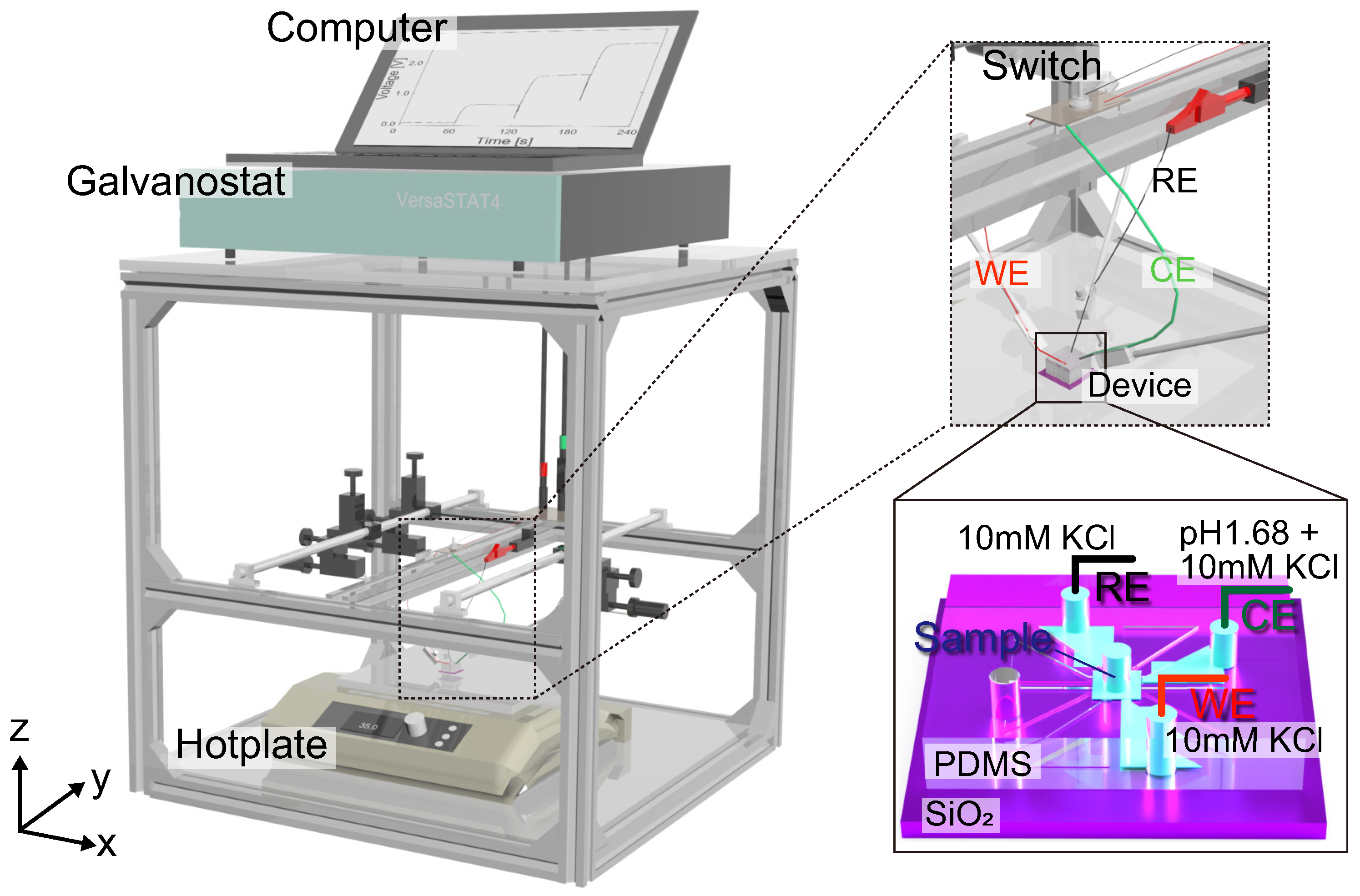
2.4. Experimental Methods
3. Results and Discussion
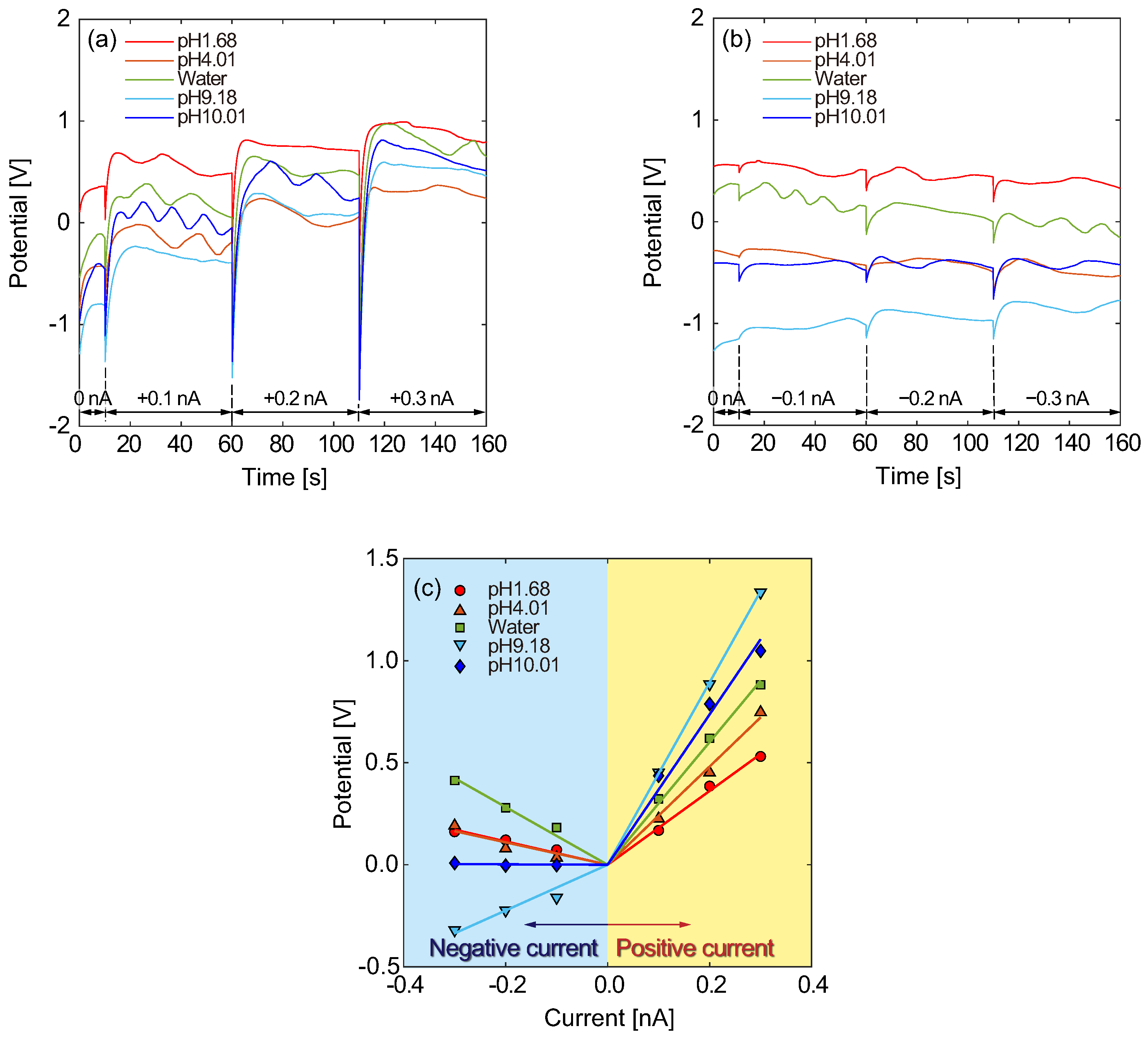
3.1. Electrical Responses of Type A Micro- and Nanofluidic Channels
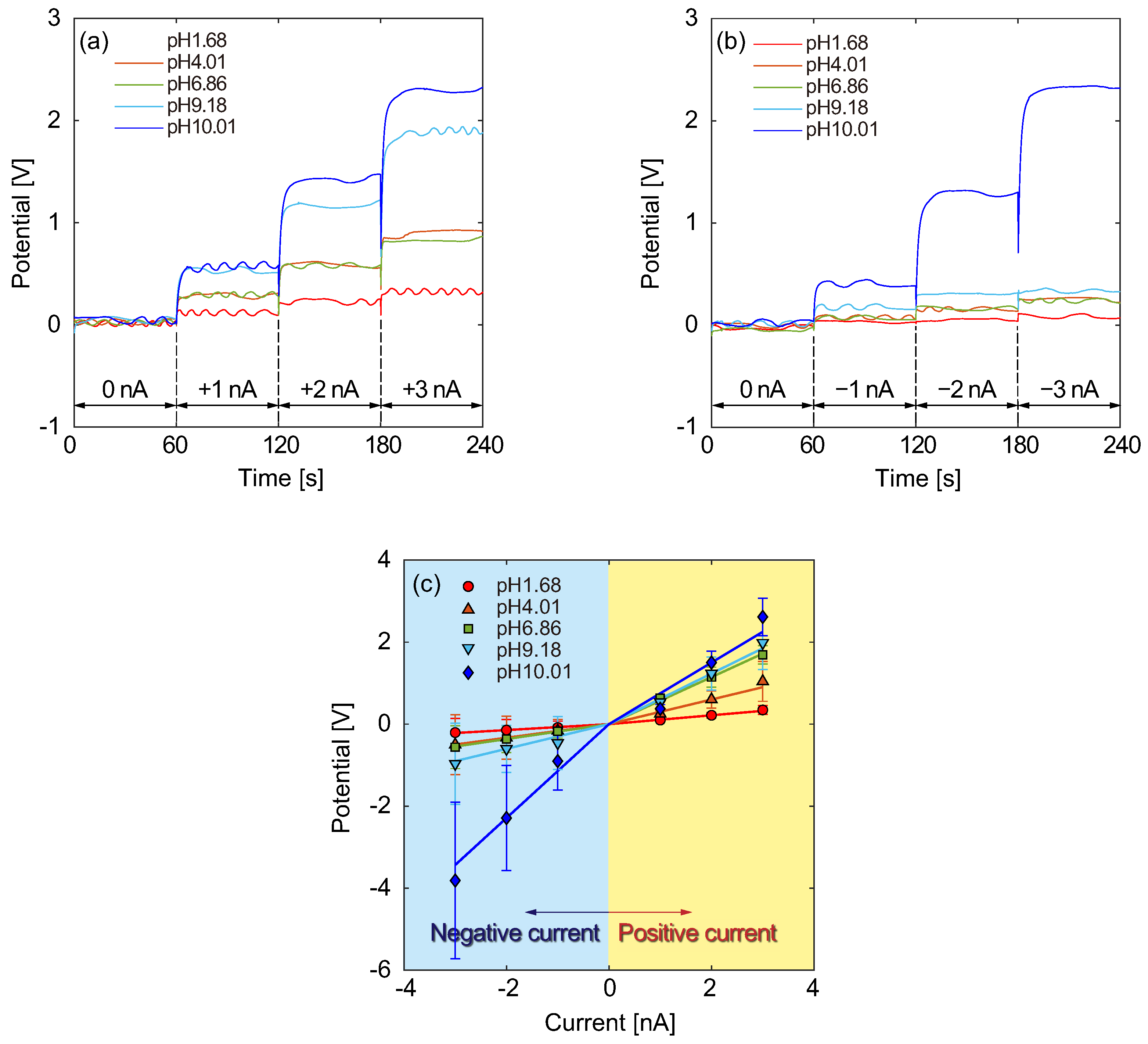
3.2. Electrical Responses of the Type B Structure
3.3. Electrical Responses of the Type C Structure
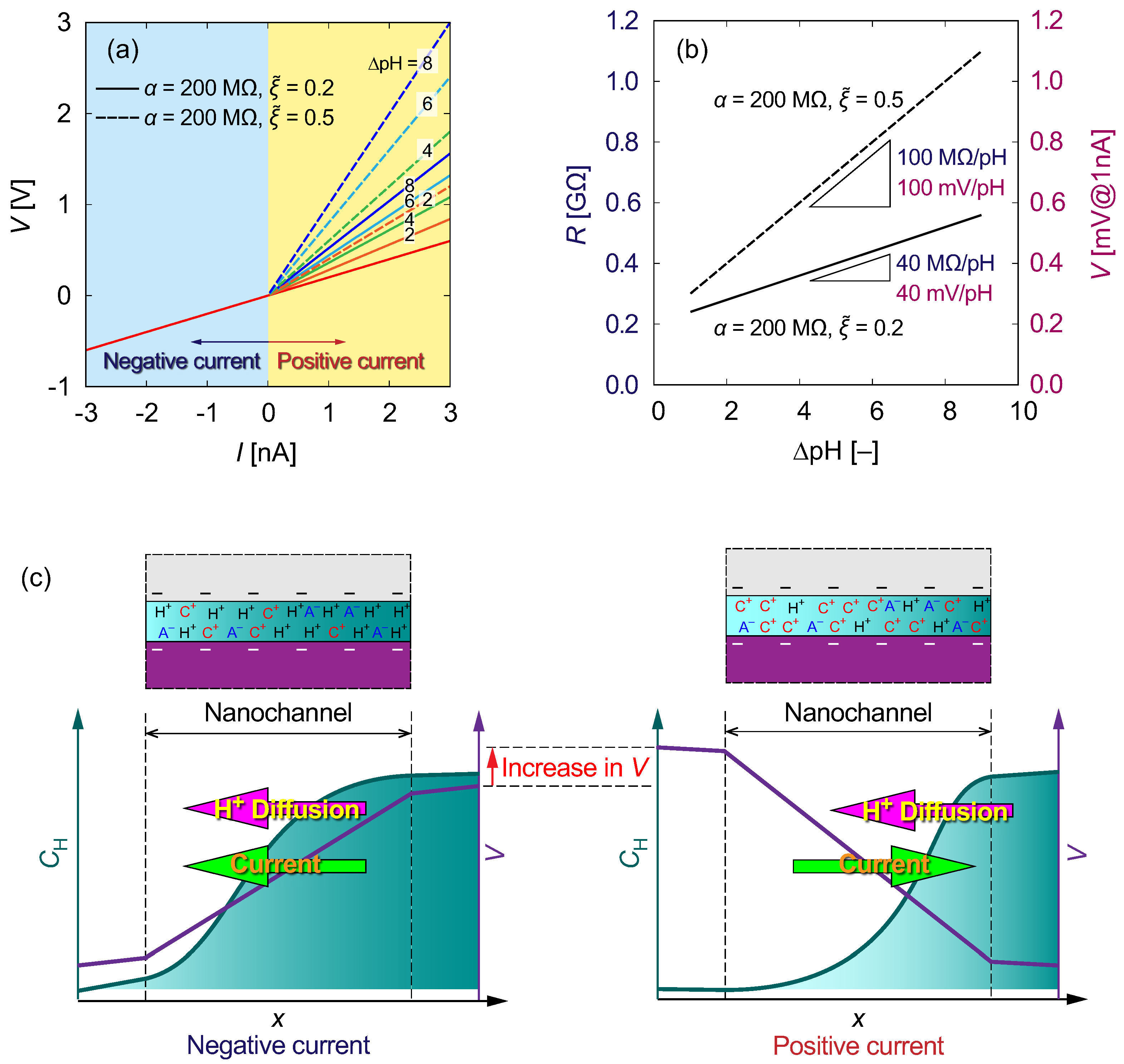
3.4. Theoretical Model of ICR
3.5. Comparison between Channel Types
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CE | Counter electrode |
| DNA | Deoxyribonucleic acid |
| EDL | Electric double layer |
| EHD | Electrohydrodynamics |
| EOF | Electroosmotic flow |
| ICR | Ionic current rectification |
| PDMS | Polydimethylsiloxane |
| PEG | Polyethylene glycol |
| RE | Reference electrode |
| RNA | Ribonucleic acid |
| WE | Working electrode |
Appendix A
References
- Wang, X.; Liu, Z.; Wang, B.; Cai, Y.; Song, Q. An overview on state-of-art of micromixer designs, characteristics and applications. Anal. Chim. Acta 2023, 1279, 341685. [Google Scholar] [CrossRef] [PubMed]
- Dedovets, D.; Li, Q.; Leclercq, L.; Nardello-Rataj, V.; Leng, J.; Zhao, S.; Pera-Titus, M. Multiphase microreactors based on liquid-liquid and gas-liquid dispersions stabilized by colloidal catalytic particles. Angew. Chem. Int. Ed. 2022, 61, e202107537. [Google Scholar] [CrossRef] [PubMed]
- Sheng, L.; Wang, K.; Deng, J.; Chen, G.; Luo, G. Gas–liquid microdispersion and microflow for carbon dioxide absorption and utilization: A review. Curr. Opin. Chem. Eng. 2023, 40, 100917. [Google Scholar] [CrossRef]
- Liao, Z.; Zhang, Y.; Li, Y.; Miao, Y.; Gao, S.; Lin, F.; Deng, Y.; Geng, L. Microfluidic chip coupled with optical biosensors for simultaneous detection of multiple analytes: A review. Biosens. Bioelectron. 2019, 126, 697–706. [Google Scholar] [CrossRef] [PubMed]
- Naresh, V.; Lee, N. A review on biosensors and recent development of nanostructured materials-enabled biosensors. Sensors 2021, 21, 1109. [Google Scholar] [CrossRef]
- Siwy, Z.; Gu, Y.; Spohr, H.A.; Baur, D.; Wolf-Reber, A.; Spohr, R.; Apel, P.; Korchev, Y.E. Rectification and voltage gating of ion currents in a nanofabricated pore. EPL 2002, 60, 349. [Google Scholar] [CrossRef]
- Siwy, Z.S.; Powell, M.R.; Kalman, E.; Astumian, R.D.; Eisenberg, R.S. Negative incremental resistance induced by calcium in asymmetric nanopores. Nano Lett. 2006, 6, 473–477. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.Y.; Wong, P.H.; Wang, P.H.; Siwy, Z.S.; Yeh, L.H. Electrodiffusioosmosis-induced gegative differential resistance in pH-regulated mesopores containing purely monovalent solutions. ACS Appl. Mater. Interfaces 2020, 12, 3198–3204. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.J.; Guo, L.J. Rectified ion transport through concentration gradient in homogeneous silica nanochannels. Nano Lett. 2007, 7, 3165–3171. [Google Scholar] [CrossRef]
- Smeets, R.M.; Keyser, U.F.; Krapf, D.; Wu, M.Y.; Dekker, N.H.; Dekker, C. Salt dependence of ion transport and DNA translocation through solid-state nanopores. Nano Lett. 2006, 6, 89–95. [Google Scholar] [CrossRef]
- Schoch, R.B.; Han, J.; Renaud, P. Transport phenomena in nanofluidics. Rev. Mod. Phys. 2008, 80, 839–883. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods, 2nd ed.; John Wiley & Sons: Danvers, MA, USA, 2001; pp. 362–363. [Google Scholar]
- Doi, K.; Nito, F.; Kawano, S. Cation-induced electrohydrodynamic flow in aqueous solutions. J. Chem. Phys. 2018, 148, 204512. [Google Scholar] [CrossRef] [PubMed]
- Slouka, Z.; Senapati, S.; Chang, H.C. Microfluidic systems with ion-selective membranes. Annu. Rev. Chem. 2014, 7, 317–335. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Yang, K.; Wang, J.; Sun, H.; Xia, X.H.; Wang, C. In Situ Growth of Imine-Bridged Anion-Selective COF/AAO Membrane for Ion Current Rectification and Nanofluidic Osmotic Energy Conversion. Adv. Funct. Mater. 2023, 33, 2302427. [Google Scholar] [CrossRef]
- Bakker, E.; Meyerhoff, M.E. Ionophore-based membrane electrodes: New analytical concepts and non-classical response mechanisms. Anal. Chim. Acta 2000, 416, 121–137. [Google Scholar] [CrossRef]
- Johnson, R.D.; Bachas, L.G. Ionophore-based ion-selective potentiometric and optical sensors. Anal. Bioanal. Chem. 2003, 376, 328–341. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.H.; Cao, S.H.; Cai, S.L.; Wu, Y.Y.; Ajmal, M.; Li, Y.Q. Reversing Current Rectification to Improve DNA-Sensing Sensitivity in Conical Nanopores. Electrophoresis 2019, 40, 2098–2103. [Google Scholar] [CrossRef]
- Ma, W.; Xie, W.; Tian, R.; Zeng, X.; Liang, L.; Hou, C.; Huo, D.; Wang, D. An Ultrasensitive Aptasensor of SARS-CoV-2 N Protein Based on Ion Current Rectification with Nanopipettes. Sens. Actuators B Chem. 2023, 377, 133075. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.T.; Ruan, Y.F.; Zhang, T.Y.; Shi, X.M.; Wang, H.Y.; Zhao, W.W.; Chen, H.Y.; Xu, J.J. Ion Current Rectification-Nanopipette Technique for Single-Cell Analysis. Trends Anal. Chem. 2023, 167, 117217. [Google Scholar] [CrossRef]
- Venkatesan, B.M.; Bashir, R. Nanopore Sensors for Nucleic Acid Analysis. Nat. Nanotechnol. 2011, 6, 615–624. [Google Scholar] [CrossRef]
- Dekker, C. Solid-State Nanopores. Nat. Nanotechnol. 2007, 2, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Qian, W.; Doi, K.; Kawano, S. Effects of polymer length and salt concentration on the transport of ssDNA in nanofluidic channels. Biophys. J. 2017, 112, 838–849. [Google Scholar] [CrossRef] [PubMed]
- Constantin, D.; Siwy, Z.S. Poisson-Nernst-Planck model of ion current rectification through a nanofluidic diode. Phys. Rev. E 2007, 76, 041202. [Google Scholar] [CrossRef] [PubMed]
- Daiguji, H. Ion transport in nanofluidic channels. Chem. Soc. Rev. 2009, 39, 901–911. [Google Scholar] [CrossRef] [PubMed]
- Guan, W.; Fan, R.; Reed, M.A. Field-effect reconfigurable nanofluidic ionic diodes. Nat. Commun. 2011, 2, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.Y.; Yeh, L.H.; Hsu, J.P.; Tseng, S. Regulating Current Rectifi cation and Nanoparticle Transport Through a Salt Gradient in Bipolar Nanopores. Small 2015, 11, 4594–4602. [Google Scholar] [CrossRef] [PubMed]
- Yen, W.K.; Huang, W.C.; Hsu, J.P. Ion Current Rectification Behavior of a Nanochannel Having Nonuniform Cross-Section. Electrophoresis 2020, 41, 802–810. [Google Scholar] [CrossRef] [PubMed]
- Qiao, N.; Li, Z.; Zhang, Z.; Guo, H.; Liao, J.; Lu, W.; Li, C. Effect of Membrane Thermal Conductivity on Ion Current Rectification in Conical Nanochannels under Asymmetric Temperature. Anal. Chim. Acta 2023, 1278, 341724. [Google Scholar] [CrossRef] [PubMed]
- Han, W.; Chen, X. A Review: Applications of Ion Transport in Micro-Nanofluidic Systems Based on Ion Concentration Polarization. J. Chem. Technol. Biotechnol. 2020, 95, 1622–1631. [Google Scholar] [CrossRef]
- Doi, K.; Yano, A.; Kawano, S. Electrohydrodynamic flow through a 1 mm2 cross-section pore placed in an ion-exchange membrane. J. Phys. Chem. B 2015, 119, 228–237. [Google Scholar] [CrossRef]
- Yano, A.; Shirai, H.; Imoto, M.; Doi, K.; Kawano, S. Concentration dependence of cation-induced electrohydrodynamic flow passing through an anion exchange membrane. Jpn. J. Appl. Phys. 2017, 56, 097201. [Google Scholar] [CrossRef]
- Doi, K.; Asano, N.; Kawano, S. Development of glass micro-electrodes for local electric field, electrical conductivity, and pH measurements. Sci. Rep. 2020, 10, 4110. [Google Scholar] [CrossRef] [PubMed]
- Kishimoto, T.; Doi, K. pH Sensing Based on Ionic Current Rectification Using Triple-Barrelled Glass Microelectrodes. J. Phys. Chem. C 2023, 128, 346–354. [Google Scholar] [CrossRef]
- Anderson, J.; Parks, G. The electrical conductivity of silica Gel in the presence of adsorbed water. J. Phys. Chem. 1968, 72, 3662–3668. [Google Scholar] [CrossRef]
- Bernal, J.; Fowler, R. A theory of water and ionic solution, with particular reference to hydrogen and hydroxyl ions. J. Chem. Phys. 1933, 1, 515–548. [Google Scholar] [CrossRef]
- Agmon, N. The Grotthuss mechanism. Chem. Phys. Lett. 1995, 244, 456–462. [Google Scholar] [CrossRef]
- Xiao, W.; Dong, Q. Iridium oxide and cobalt hydroxide microfluidic-based potentiometric pH sensor. Michrochim. Acta 2023, 190, 457. [Google Scholar] [CrossRef]
- Doi, K.; Yamamoto, K.; Yamazaki, H.; Kawano, S. Micro-to-nano bimodal single-particle sensing using optical tweezers. J. Phys. Chem. C 2022, 126, 10713–10721. [Google Scholar] [CrossRef]
- Wei, C.; Bard, A.J.; Feldberg, S.W. Current rectification at quartz nanopipet electrodes. Anal. Chem. 1997, 69, 4627–4633. [Google Scholar] [CrossRef]
- White, H.S.; Bund, A. Ion current rectification at nanopores in glass membranes. Langmuir 2008, 24, 2212–2218. [Google Scholar] [CrossRef]
- Yusko, E.C.; An, R.; Mayer, M. Electroosmotic flow can generate ion current rectification in nano- and micropores. ACS Nano 2010, 4, 477–487. [Google Scholar] [CrossRef] [PubMed]
- Chang, F.; Chen, C.; Xie, X.; Chen, L.; Li, M.; Zhu, Z. A bidirection-adjustable ionic current rectification system based on biconical micro-channel. Chem. Commun. 2015, 51, 15316–15319. [Google Scholar] [CrossRef] [PubMed]
- Ndobo-Epoy, J.P.; Lesniewska, E.; Guicquero, J.P. Nano-pH sensor for the study of reactive materials. Anal. Chem. 2007, 79, 7560–7564. [Google Scholar] [CrossRef] [PubMed]
- Knopfmacher, O.; Tarasov, A.; Fu, W.; Wipf, M.; Niesen, B.; Calame, M.; Schönenberger, C. Nernst limit in dual-gated Si-nanowire FET sensors. Nano Lett. 2010, 10, 2268–2274. [Google Scholar] [CrossRef] [PubMed]
- Manjakkal, L.; Dervin, S.; Dahiya, R. Flexible potentiometric pH sensors for wearable systems. RSC Adv. 2020, 10, 8594–8617. [Google Scholar] [CrossRef] [PubMed]
- Antohe, I.; Jinga, L.I.; Antohe, V.A.; Socol, G. Sensitive pH monitoring using a polyaniline-functionalized fiber optic-surface plasmon resonance detector. Sensors 2021, 21, 4218. [Google Scholar] [CrossRef]
- Zhang, B.; Mumtaz, F.; Roman, M.; Alla, D.; Gerald, R.E., II; Huang, J. Miniaturized fluorescence pH sensor with assembly free ball lens on a tapered multimode optical fiber. Opt. Express 2024, 32, 4228–4241. [Google Scholar] [CrossRef]



| pH | Name | Composition |
|---|---|---|
| 1.68 | Oxalate | 49.61 mmol/L KH3(C2O4)2·2H2O |
| 4.01 | Phthalate | 49.55 mmol/L C6H4(COOK)(COOH) |
| 6.86 | Phosphate | 25 mmol/L KH2PO4 and 25 mmol/L Na2HPO4 |
| 9.01 | Tetraborate | 9.964 mmol/L Na2B4O7·10H2O |
| 10.01 | Carbonate | 24.90 mmol/L NaHCO3 and 24.91 mmol/L Na2CO3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takagi, T.; Kishimoto, T.; Doi, K. Micro- and Nanofluidic pH Sensors Based on Electrodiffusioosmosis. Micromachines 2024, 15, 698. https://doi.org/10.3390/mi15060698
Takagi T, Kishimoto T, Doi K. Micro- and Nanofluidic pH Sensors Based on Electrodiffusioosmosis. Micromachines. 2024; 15(6):698. https://doi.org/10.3390/mi15060698
Chicago/Turabian StyleTakagi, Tadashi, Tatsunori Kishimoto, and Kentaro Doi. 2024. "Micro- and Nanofluidic pH Sensors Based on Electrodiffusioosmosis" Micromachines 15, no. 6: 698. https://doi.org/10.3390/mi15060698
APA StyleTakagi, T., Kishimoto, T., & Doi, K. (2024). Micro- and Nanofluidic pH Sensors Based on Electrodiffusioosmosis. Micromachines, 15(6), 698. https://doi.org/10.3390/mi15060698






