Constrained Volume Micro- and Nanoparticle Collection Methods in Microfluidic Systems
Abstract
1. Introduction
2. Trapping Methods Introduction
3. Physical Methods
3.1. Barrier Trapping
3.2. Flow Trapping
4. Optical Methods
5. Electrical Methods
5.1. Dielectrophoresis
5.2. Electrokinetic Fluid Trapping
5.3. Other Electrical Methods
6. Magnetic Methods
7. Acoustic Methods
8. Other Methods
Comparison
- Passive: no external forces or fields are used for particle trapping.
- Structured: captured particles can be arbitrarily arranged into multiple configurations.
- Non-contact: particles do not come into direct contact with the solid portion of the device.
- Reversible: particles or cells may be freely released after capture.
- Size-selective: mechanism selectively traps particles in a specific size range.
- Complexity (fabrication): a general measure (low, medium, or high) of how complex the trapping platform is to initially fabricate, based on complicating factors such as small dimensions, a large number of process steps, and expensive fabrication facilities.
- Complexity (use): a general measure (low, medium, or high) of how complex the trapping platform is to implement, based on complicating factors like expensive equipment, active particle tracking, and the need for precise control mechanisms.
- Enrichment capability: a general measure (low, medium, or high) of the ability of a trapping mechanism to locally concentrate particles, including flow rate, enrichment factor, etc. Some numerical substantiation is given in Table 1.
9. Applications
9.1. Detection Enhancement
9.2. Particle Medium Exchange
9.3. Surface Modification and Labeling
9.4. Tissue Engineering
10. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Yin, H.; Marshall, D. Microfluidics for Single Cell Analysis. Curr. Opin. Biotechnol. 2012, 23, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Gholizadeh, S.; Shehata Draz, M.; Zarghooni, M.; Sanati-Nezhad, A.; Ghavami, S.; Shafiee, H.; Akbari, M. Microfluidic Approaches for Isolation, Detection, and Characterization of Extracellular Vesicles: Current Status and Future Directions. Biosens. Bioelectron. 2017, 91, 588–605. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhang, L.; Ge, X.; Xu, B.; Zhang, W.; Qu, L.; Choi, C.-H.; Xu, J.; Zhang, A.; Lee, H.; et al. Microfluidic Fabrication of Microparticles for Biomedical Applications. Chem. Soc. Rev. 2018, 47, 5646–5683. [Google Scholar] [CrossRef] [PubMed]
- Khizar, S.; Zine, N.; Errachid, A.; Jaffrezic-Renault, N.; Elaissari, A. Microfluidic-based Nanoparticle Synthesis and Their Potential Applications. Electrophoresis 2022, 43, 819–838. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, J.; Evander, M.; Hammarström, B.; Laurell, T. Review of Cell and Particle Trapping in Microfluidic Systems. Anal. Chim. Acta 2009, 649, 141–157. [Google Scholar] [CrossRef] [PubMed]
- Sackmann, E.K.; Fulton, A.L.; Beebe, D.J. The Present and Future Role of Microfluidics in Biomedical Research. Nature 2014, 507, 181–189. [Google Scholar] [CrossRef]
- Hoffmann, G.G. Raman Spectroscopy, Volume I: Principles and Applications in Chemistry, Physics, Materials Science, and Biology; Momentum Press: New York, NY, USA, 2017; ISBN 978-1-945612-01-5. [Google Scholar]
- Sampad, M.J.N.; Saiduzzaman, S.M.; Walker, Z.J.; Wells, T.N.; Wayment, J.X.; Ong, E.M.; Mdaki, S.D.; Tamhankar, M.A.; Yuzvinsky, T.D.; Patterson, J.L.; et al. Label-Free and Amplification-Free Viral RNA Quantification from Primate Biofluids Using a Trapping-Assisted Optofluidic Nanopore Platform. Proc. Natl. Acad. Sci. USA 2024, 121, e2400203121. [Google Scholar] [CrossRef]
- Park, J.; Destgeer, G.; Kim, H.; Cho, Y.; Jin Sung, H. In-Droplet Microparticle Washing and Enrichment Using Surface Acoustic Wave-Driven Acoustic Radiation Force. Lab. Chip 2018, 18, 2936–2945. [Google Scholar] [CrossRef]
- Evander, M.; Johansson, L.; Lilliehorn, T.; Piskur, J.; Lindvall, M.; Johansson, S.; Almqvist, M.; Laurell, T.; Nilsson, J. Noninvasive Acoustic Cell Trapping in a Microfluidic Perfusion System for Online Bioassays. Anal. Chem. 2007, 79, 2984–2991. [Google Scholar] [CrossRef]
- Mirsaidov, U.; Scrimgeour, J.; Timp, W.; Beck, K.; Mir, M.; Matsudaira, P.; Timp, G. Live Cell Lithography: Using Optical Tweezers to Create Synthetic Tissue. Lab. Chip 2008, 8, 2174–2181. [Google Scholar] [CrossRef]
- Wells, T.; Schmidt, H.; Hawkins, A. Nano/Microfluidic Device for High-Throughput Passive Trapping of Nanoparticles. Biomicrofluidics 2023, 17, 064101. [Google Scholar] [CrossRef]
- Mohtar, M.N.; Abdulhameed, A.; Halin, I.A.; Hamidon, M.N. Carbon Nanotube Collections by Electro-Osmosis in Microfluidic Systems. AIP Conf. Proc. 2020, 2203, 020031. [Google Scholar] [CrossRef]
- Fu, L.-M.; Hou, H.-H.; Chiu, P.-H.; Yang, R.-J. Sample Preconcentration from Dilute Solutions on Micro/Nanofluidic Platforms: A Review. Electrophoresis 2018, 39, 289–310. [Google Scholar] [CrossRef] [PubMed]
- Haywood, D.G.; Saha-Shah, A.; Baker, L.A.; Jacobson, S.C. Fundamental Studies of Nanofluidics: Nanopores, Nanochannels, and Nanopipets. Anal. Chem. 2015, 87, 172–187. [Google Scholar] [CrossRef] [PubMed]
- Sajeesh, P.; Sen, A.K. Particle Separation and Sorting in Microfluidic Devices: A Review. Microfluid. Nanofluidics 2014, 17, 1–52. [Google Scholar] [CrossRef]
- Cetin, B.; Özer, M.B.; Solmaz, M.E. Microfluidic Bio-Particle Manipulation for Biotechnology. Biochem. Eng. J. 2014, 92, 63–82. [Google Scholar] [CrossRef]
- Xie, Y.; Rufo, J.; Zhong, R.; Rich, J.; Li, P.; Leong, K.W.; Huang, T.J. Microfluidic Isolation and Enrichment of Nanoparticles. ACS Nano 2020, 14, 16220–16240. [Google Scholar] [CrossRef] [PubMed]
- Paiè, P.; Zandrini, T.; Vázquez, R.M.; Osellame, R.; Bragheri, F. Particle Manipulation by Optical Forces in Microfluidic Devices. Micromachines 2018, 9, 200. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Guo, Y.; Xu, B. Recent Development of Microfluidic Technology for Cell Trapping in Single Cell Analysis: A Review. Processes 2020, 8, 1253. [Google Scholar] [CrossRef]
- Fatoyinbo, H.O.; Li, X. 8—Microfluidic Devices for Cell Manipulation. In Microfluidic Devices for Biomedical Applications (Second Edition); Li, X., Zhou, Y., Eds.; Woodhead Publishing Series in Biomaterials; Woodhead Publishing: Sawston, UK, 2021; pp. 329–389. ISBN 978-0-12-819971-8. [Google Scholar]
- Gong, L.; Cretella, A.; Lin, Y. Microfluidic Systems for Particle Capture and Release: A Review. Biosens. Bioelectron. 2023, 236, 115426. [Google Scholar] [CrossRef]
- Lapizco-Encinas, B.H. On the Recent Developments of Insulator-based Dielectrophoresis: A Review. Electrophoresis 2019, 40, 358–375. [Google Scholar] [CrossRef] [PubMed]
- Yin, C.; Jiang, X.; Mann, S.; Tian, L.; Drinkwater, B.W. Acoustic Trapping: An Emerging Tool for Microfabrication Technology. Small 2023, 19, 2207917. [Google Scholar] [CrossRef] [PubMed]
- Squires, T.M.; Quake, S.R. Microfluidics: Fluid Physics at the Nanoliter Scale. Rev. Mod. Phys. 2005, 77, 977–1026. [Google Scholar] [CrossRef]
- Tabeling, P. Introduction to Microfluidics; Oxford University Press: Oxford, UK, 2023; ISBN 978-0-19-266003-9. [Google Scholar]
- Bocquet, L.; Charlaix, E. Nanofluidics, from Bulk to Interfaces. Chem. Soc. Rev. 2010, 39, 1073–1095. [Google Scholar] [CrossRef] [PubMed]
- Turner, A.P.F. Biosensors: Sense and Sensibility. Chem. Soc. Rev. 2013, 42, 3184–3196. [Google Scholar] [CrossRef] [PubMed]
- Mehrotra, P. Biosensors and Their Applications—A Review. J. Oral Biol. Craniofacial Res. 2016, 6, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Schmid, G. Nanoparticles: From Theory to Application; John Wiley & Sons: Hoboken, NJ, USA, 2011; ISBN 978-3-527-63236-7. [Google Scholar]
- Mohanraj, V.J.; Chen, Y. Nanoparticles—A Review. Trop. J. Pharm. Res. 2006, 5, 561–573. [Google Scholar] [CrossRef]
- Ahn, J.; Ko, J.; Lee, S.; Yu, J.; Kim, Y.; Jeon, N.L. Microfluidics in Nanoparticle Drug Delivery; From Synthesis to Pre-Clinical Screening. Adv. Drug Deliv. Rev. 2018, 128, 29–53. [Google Scholar] [CrossRef]
- Walker, Z.J.; Wells, T.; Belliston, E.; Romney, S.; Walker, S.B.; Sampad, M.J.N.; Saiduzzaman, S.M.; Losakul, R.; Schmidt, H.; Hawkins, A.R. Optofluidic Particle Manipulation Platform with Nanomembrane. Micromachines 2022, 13, 721. [Google Scholar] [CrossRef]
- Walker, Z.J.; Wells, T.; Belliston, E.; Walker, S.B.; Zeller, C.; Sampad, M.J.N.; Saiduzzaman, S.M.; Schmidt, H.; Hawkins, A.R. Optofluidic Particle Manipulation: Optical Trapping in a Thin-Membrane Microchannel. Biosensors 2022, 12, 690. [Google Scholar] [CrossRef]
- Melzer, J.E.; McLeod, E. Fundamental Limits of Optical Tweezer Nanoparticle Manipulation Speeds. ACS Nano 2018, 12, 2440–2447. [Google Scholar] [CrossRef] [PubMed]
- Hur, S.C.; Mach, A.J.; Di Carlo, D. High-Throughput Size-Based Rare Cell Enrichment Using Microscale Vortices. Biomicrofluidics 2011, 5, 022206. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Xuan, J.; Lee, M.L.; Tolley, H.D.; Hawkins, A.R.; Woolley, A.T. Thin-Film Microfabricated Nanofluidic Arrays for Size-Selective Protein Fractionation. Lab. Chip 2013, 13, 4591–4598. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kim, J.; Erath, J.; Rodriguez, A.; Yang, C. A High-Efficiency Microfluidic Device for Size-Selective Trapping and Sorting. Lab. Chip 2014, 14, 2480–2490. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Lin, H.; Liu, J.-Q.; Balic, M.; Datar, R.; Cote, R.J.; Tai, Y.-C. Membrane Microfilter Device for Selective Capture, Electrolysis and Genomic Analysis of Human Circulating Tumor Cells. J. Chromatogr. A 2007, 1162, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Wlodkowic, D.; Faley, S.; Zagnoni, M.; Wikswo, J.P.; Cooper, J.M. Microfluidic Single-Cell Array Cytometry for the Analysis of Tumor Apoptosis. Anal. Chem. 2009, 81, 5517–5523. [Google Scholar] [CrossRef] [PubMed]
- Tayebi, M.; Zhou, Y.; Tripathi, P.; Chandramohanadas, R.; Ai, Y. Exosome Purification and Analysis Using a Facile Microfluidic Hydrodynamic Trapping Device. Anal. Chem. 2020, 92, 10733–10742. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.-H.; Takeuchi, S. A Trap-and-Release Integrated Microfluidic System for Dynamic Microarray Applications. Proc. Natl. Acad. Sci. USA 2007, 104, 1146–1151. [Google Scholar] [CrossRef]
- Hamblin, M.N.; Xuan, J.; Maynes, D.; Tolley, H.D.; Belnap, D.M.; Woolley, A.T.; Lee, M.L.; Hawkins, A.R. Selective Trapping and Concentration of Nanoparticles and Viruses in Dual-Height Nanofluidic Channels. Lab. Chip 2009, 10, 173–178. [Google Scholar] [CrossRef]
- Xuan, J.; Hamblin, M.N.; Stout, J.M.; Tolley, H.D.; Maynes, R.D.; Woolley, A.T.; Hawkins, A.R.; Lee, M.L. Surfactant Addition and Alternating Current Electrophoretic Oscillation during Size Fractionation of Nanoparticles in Channels with Two or Three Different Height Segments. J. Chromatogr. A 2011, 1218, 9102–9110. [Google Scholar] [CrossRef]
- Stout, J.M.; Johnson, J.E.; Kumar, S.; Woolley, A.T.; Hawkins, A.R. Particle Trapping in Electrostatically Actuated Nanofluidic Barriers. In Proceedings of the 2015 IEEE 58th International Midwest Symposium on Circuits and Systems (MWSCAS), Fort Collins, CO, USA, 2–5 August 2015; pp. 1–4. [Google Scholar]
- Tonomura, W.; Tsutsui, M.; Arima, A.; Yokota, K.; Taniguchi, M.; Washio, T.; Kawai, T. High-Throughput Single-Particle Detections Using a Dual-Height-Channel-Integrated Pore. Lab. Chip 2019, 19, 1352–1358. [Google Scholar] [CrossRef] [PubMed]
- Han, J.Y.; Yeh, M.; DeVoe, D.L. Nanogap Traps for Passive Bacteria Concentration and Single-Point Confocal Raman Spectroscopy. Biomicrofluidics 2023, 17, 024101. [Google Scholar] [CrossRef] [PubMed]
- Bruus, H. Theoretical Microfluidics; Oxford University Press: Oxford, UK, 2007; Volume 18, ISBN 978-0-19-152858-3. [Google Scholar]
- Sollier, E.; Go, D.E.; Che, J.; Gossett, D.R.; O’Byrne, S.; Weaver, W.M.; Kummer, N.; Rettig, M.; Goldman, J.; Nickols, N.; et al. Size-Selective Collection of Circulating Tumor Cells Using Vortex Technology. Lab. Chip 2014, 14, 63–77. [Google Scholar] [CrossRef] [PubMed]
- Raihan, M.K.; Li, D.; Kummetz, A.J.; Song, L.; Yu, L.; Xuan, X. Vortex Trapping and Separation of Particles in Shear Thinning Fluids. Appl. Phys. Lett. 2020, 116, 183701. [Google Scholar] [CrossRef]
- Shen, F.; Li, Z.; Xue, S.; Li, M.; Liu, Z. Particle Recirculating Orbits within Microvortices Using Microfluidics. J. Phys. Appl. Phys. 2020, 54, 025401. [Google Scholar] [CrossRef]
- Shen, F.; Gao, J.; Ai, M.; Li, Z.; Liu, Z. Mechanism of Particle Dual-Orbital Motion in a Laminar Microvortex. Phys. Fluids 2023, 35, 073325. [Google Scholar] [CrossRef]
- Kwon, T.; Jeon, H.; Hamel, J.-F.P.; Han, J. Removal of Cell Clusters from CHO Suspension Cultures Based on Large-Particle Trapping Effect in Spiral Inertial Microfluidics. Sep. Purif. Technol. 2024, 329, 125162. [Google Scholar] [CrossRef]
- Ashkin, A.; Dziedzic, J.M. Optical Levitation by Radiation Pressure. Appl. Phys. Lett. 1971, 19, 283–285. [Google Scholar] [CrossRef]
- Ashkin, A.; Dziedzic, J.M.; Bjorkholm, J.E.; Chu, S. Observation of a Single-Beam Gradient Force Optical Trap for Dielectric Particles. Opt. Lett. 1986, 11, 288–290. [Google Scholar] [CrossRef]
- Cai, H.; Leake, K.D.; Schmidt, H. Planar Optofluidics for On-Chip Particle Manipulation. In Biomedical Optical Sensors: Differentiators for Winning Technologies; De La Rue, R., Herzig, H.P., Gerken, M., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 181–210. ISBN 978-3-030-48387-6. [Google Scholar]
- Werner, M.; Merenda, F.; Piguet, J.; Salathé, R.-P.; Vogel, H. Microfluidic Array Cytometer Based on Refractive Optical Tweezers for Parallel Trapping, Imaging and Sorting of Individual Cells. Lab. Chip 2011, 11, 2432–2439. [Google Scholar] [CrossRef]
- Mandal, S.; Serey, X.; Erickson, D. Nanomanipulation Using Silicon Photonic Crystal Resonators. Nano Lett. 2010, 10, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Renaut, C.; Dellinger, J.; Cluzel, B.; Honegger, T.; Peyrade, D.; Picard, E.; de Fornel, F.; Hadji, E. Assembly of Microparticles by Optical Trapping with a Photonic Crystal Nanocavity. Appl. Phys. Lett. 2012, 100, 101103. [Google Scholar] [CrossRef]
- Kang, J.-H.; Kim, K.; Ee, H.-S.; Lee, Y.-H.; Yoon, T.-Y.; Seo, M.-K.; Park, H.-G. Low-Power Nano-Optical Vortex Trapping via Plasmonic Diabolo Nanoantennas. Nat. Commun. 2011, 2, 582. [Google Scholar] [CrossRef]
- Kawata, S.; Tani, T. Optically Driven Mie Particles in an Evanescent Field along a Channeled Waveguide. Opt. Lett. 1996, 21, 1768–1770. [Google Scholar] [CrossRef] [PubMed]
- Hellesø, O.G.; Løvhaugen, P.; Subramanian, A.Z.; Wilkinson, J.S.; Ahluwalia, B.S. Surface Transport and Stable Trapping of Particles and Cells by an Optical Waveguide Loop. Lab. Chip 2012, 12, 3436–3440. [Google Scholar] [CrossRef] [PubMed]
- Sergides, M.; Truong, V.G.; Chormaic, S.N. Highly Tunable Plasmonic Nanoring Arrays for Nanoparticle Manipulation and Detection. Nanotechnology 2016, 27, 365301. [Google Scholar] [CrossRef] [PubMed]
- Kühn, S.; Lunt, E.J.; Phillips, B.S.; Hawkins, A.R.; Schmidt, H. Optofluidic Particle Concentration by a Long-Range Dual-Beam Trap. Opt. Lett. 2009, 34, 2306–2308. [Google Scholar] [CrossRef]
- Kühn, S.; Phillips, B.S.; Lunt, E.J.; Hawkins, A.R.; Schmidt, H. Ultralow Power Trapping and Fluorescence Detection of Single Particles on an Optofluidic Chip. Lab. Chip 2010, 10, 189–194. [Google Scholar] [CrossRef]
- Rahman, M.; Stott, M.A.; Harrington, M.; Li, Y.; Sampad, M.J.N.; Lancaster, L.; Yuzvinsky, T.D.; Noller, H.F.; Hawkins, A.R.; Schmidt, H. On Demand Delivery and Analysis of Single Molecules on a Programmable Nanopore-Optofluidic Device. Nat. Commun. 2019, 10, 3712. [Google Scholar] [CrossRef]
- Rahman, M.; Harrington, M.; Stott, M.A.; Li, Y.; Sampad, M.J.N.; Yuzvinsky, T.D.; Hawkins, A.R.; Schmidt, H. Optical Trapping Assisted Detection Rate Enhancement of Single Molecules on a Nanopore Optofluidic Chip. Optica 2019, 6, 1130–1131. [Google Scholar] [CrossRef]
- Sampad, M.J.N.; Zhang, H.; Yuzvinsky, T.D.; Stott, M.A.; Hawkins, A.R.; Schmidt, H. Optical Trapping Assisted Label-Free and Amplification-Free Detection of SARS-CoV-2 RNAs with an Optofluidic Nanopore Sensor. Biosens. Bioelectron. 2021, 194, 113588. [Google Scholar] [CrossRef] [PubMed]
- Morgan, H.; Hughes, M.P.; Green, N.G. Separation of Submicron Bioparticles by Dielectrophoresis. Biophys. J. 1999, 77, 516–525. [Google Scholar] [CrossRef] [PubMed]
- Pohl, H.A. The Motion and Precipitation of Suspensoids in Divergent Electric Fields. J. Appl. Phys. 1951, 22, 869–871. [Google Scholar] [CrossRef]
- Huang, Y.; Pethig, R. Electrode Design for Negative Dielectrophoresis. Meas. Sci. Technol. 1991, 2, 1142–1146. [Google Scholar] [CrossRef]
- Schnelle, T.; Hagedorn, R.; Fuhr, G.; Fiedler, S.; Müller, T. Three-Dimensional Electric Field Traps for Manipulation of Cells—Calculation and Experimental Verification. Biochim. Biophys. Acta BBA Gen. Subj. 1993, 1157, 127–140. [Google Scholar] [CrossRef] [PubMed]
- Müller, T.; Gerardino, A.; Schnelle, T.; Shirley, S.G.; Bordoni, F.; De Gasperis, G.; Leoni, R.; Fuhr, G. Trapping of Micrometre and Sub-Micrometre Particles by High-Frequency Electric Fields and Hydrodynamic Forces. J. Phys. Appl. Phys. 1996, 29, 340. [Google Scholar] [CrossRef]
- Müller, T.; Fiedler, S.; Schnelle, T.; Ludwig, K.; Jung, H.; Fuhr, G. High Frequency Electric Fields for Trapping of Viruses. Biotechnol. Technol. 1996, 10, 221–226. [Google Scholar] [CrossRef]
- Schnelle, T.; Muller, T.; Kentsch, J.; Grom, F.; Stelzle, M. Method and Device for Collecting Suspended Particles. U.S. Patent 7,879,214, 1 February 2011. [Google Scholar]
- Hughes, M.P.; Hoettges, F.; Wattingham, R. Device for Dielectrophoretic Manipulation of Particles. U.S. Patent 8,864,973, 21 October 2014. [Google Scholar]
- Hughes, M.P. Apparatus for Collecting Particles 2009. U.S. Patent 7,488,406, 10 February 2009. [Google Scholar]
- Masuda, S.; Washizu, M.; Nanba, T. Novel Method of Cell Fusion in Field Constriction Area in Fluid Integration Circuit. IEEE Trans. Ind. Appl. 1989, 25, 732–737. [Google Scholar] [CrossRef]
- Ogle, B.M.; Cascalho, M.; Platt, J.L. Biological Implications of Cell Fusion. Nat. Rev. Mol. Cell Biol. 2005, 6, 567–575. [Google Scholar] [CrossRef]
- Nakidde, D.; Zellner, P.; Alemi, M.M.; Shake, T.; Hosseini, Y.; Riquelme, M.V.; Pruden, A.; Agah, M. Three Dimensional Passivated-Electrode Insulator-Based Dielectrophoresis. Biomicrofluidics 2015, 9, 014125. [Google Scholar] [CrossRef]
- Chiou, C.-H.; Chien, L.-J.; Kuo, J.-N. Nanoconstriction-Based Electrodeless Dielectrophoresis Chip for Nanoparticle and Protein Preconcentration. Appl. Phys. Express 2015, 8, 085201. [Google Scholar] [CrossRef]
- Cummings, E.B.; Singh, A.K. Dielectrophoresis in Microchips Containing Arrays of Insulating Posts: Theoretical and Experimental Results. Anal. Chem. 2003, 75, 4724–4731. [Google Scholar] [CrossRef] [PubMed]
- Lapizco-Encinas, B.H.; Simmons, B.A.; Cummings, E.B.; Fintschenko, Y. Insulator-based Dielectrophoresis for the Selective Concentration and Separation of Live Bacteria in Water. Electrophoresis 2004, 25, 1695–1704. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Du, H. A Microfluidic Device for Rapid Concentration of Particles in Continuous Flow by DC Dielectrophoresis. Microfluid. Nanofluidics 2010, 9, 281–291. [Google Scholar] [CrossRef]
- Hoettges, K.F.; Hughes, M.P.; Cotton, A.; Hopkins, N.A.E.; McDonnell, M.B. Optimizing Particle Collection for Enhanced Surface-Based Biosensors. IEEE Eng. Med. Biol. Mag. 2003, 22, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Mohtar, M.N.; Hoettges, K.F.; Hughes, M.P. Factors Affecting Particle Collection by Electro-Osmosis in Microfluidic Systems. Electrophoresis 2014, 35, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Hübner, Y.; Hoettges, K.F.; McDonnell, M.B.; Carter, M.J.; Hughes, M.P. Applications of Dielectrophoretic/Electro-Hydrodynamic “Zipper” Electrodes for Detection of Biological Nanoparticles. Int. J. Nanomed. 2007, 2, 427–431. [Google Scholar]
- Wong, P.K.; Chen, C.-Y.; Wang, T.-H.; Ho, C.-M. An AC Electroosmotic Processor for Biomolecules. In Proceedings of the TRANSDUCERS ’03. 12th International Conference on Solid-State Sensors, Actuators and Microsystems. Digest of Technical Papers (Cat. No.03TH8664), Boston, MA, USA, 8–12 June 2003; Volume 1, pp. 20–23. [Google Scholar]
- Wong, P.K.; Chen, C.-Y.; Wang, T.-H.; Ho, C.-M. Electrokinetic Bioprocessor for Concentrating Cells and Molecules. Anal. Chem. 2004, 76, 6908–6914. [Google Scholar] [CrossRef] [PubMed]
- Gagnon, Z.; Chang, H.-C. Aligning Fast Alternating Current Electroosmotic Flow Fields and Characteristic Frequencies with Dielectrophoretic Traps to Achieve Rapid Bacteria Detection. Electrophoresis 2005, 26, 3725–3737. [Google Scholar] [CrossRef]
- Cheng, I.-F.; Chang, H.-C.; Chen, T.-Y.; Hu, C.; Yang, F.-L. Rapid (<5 Min) Identification of Pathogen in Human Blood by Electrokinetic Concentration and Surface-Enhanced Raman Spectroscopy. Sci. Rep. 2013, 3, 2365. [Google Scholar] [CrossRef]
- Park, S.; Koklu, M.; Beskok, A. Particle Trapping in High-Conductivity Media with Electrothermally Enhanced Negative Dielectrophoresis. Anal. Chem. 2009, 81, 2303–2310. [Google Scholar] [CrossRef] [PubMed]
- Electrokinetic. Merriam-Webstercom Online Dict. Available online: https://www.merriam-webster.com/dictionary/electrokinetic (accessed on 8 March 2024).
- Xuan, X. Recent Advances in Direct Current Electrokinetic Manipulation of Particles for Microfluidic Applications. Electrophoresis 2019, 40, 2484–2513. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Chen, P.; Chung, M.T.; Nidetz, R.; Park, Y.; Liu, Z.; McHugh, W.; Cornell, T.T.; Fu, J.; Kurabayashi, K. AC Electroosmosis-Enhanced Nanoplasmofluidic Detection of Ultralow-Concentration Cytokine. Nano Lett. 2017, 17, 2374–2380. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Ben, Y.; Battigelli, D.; Chang, H.-C. Long-Range AC Electroosmotic Trapping and Detection of Bioparticles. Ind. Eng. Chem. Res. 2005, 44, 2815–2822. [Google Scholar] [CrossRef]
- Wu, J.; Ben, Y.; Chang, H.-C. Particle Detection by Electrical Impedance Spectroscopy with Asymmetric-Polarization AC Electroosmotic Trapping. Microfluid. Nanofluidics 2005, 1, 161–167. [Google Scholar] [CrossRef]
- Bhatt, K.H.; Grego, S.; Velev, O.D. An AC Electrokinetic Technique for Collection and Concentration of Particles and Cells on Patterned Electrodes. Langmuir 2005, 21, 6603–6612. [Google Scholar] [CrossRef]
- Hou, D.; Maheshwari, S.; Chang, H.-C. Rapid Bioparticle Concentration and Detection by Combining a Discharge Driven Vortex with Surface Enhanced Raman Scattering. Biomicrofluidics 2007, 1, 014106. [Google Scholar] [CrossRef] [PubMed]
- Dey, R.; Shaik, V.A.; Chakraborty, D.; Ghosal, S.; Chakraborty, S. AC Electric Field-Induced Trapping of Microparticles in Pinched Microconfinements. Langmuir 2015, 31, 5952–5961. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Wu, J. In Situ Electrokinetic Preconcentrator for Conductive Biofluids. In Proceedings of the American Society of Mechanical Engineers Digital Collection, Shanghai, China, 18–21 December 2009; pp. 651–657. [Google Scholar]
- Yang, K.; Wu, J. Numerical Study of in Situ Preconcentration for Rapid and Sensitive Nanoparticle Detection. Biomicrofluidics 2010, 4, 034106. [Google Scholar] [CrossRef]
- Sun, H.; Ren, Y.; Tao, Y.; Jiang, T.; Jiang, H. Flexible Online In-Droplet Cell/Synthetic Particle Concentration Utilizing Alternating Current Electrothermal-Flow Field-Effect Transistor. Lab. Chip 2021, 21, 1987–1997. [Google Scholar] [CrossRef]
- Abdelghany, A.; Ichikawa, Y.; Motosuke, M. Tuning AC Electrokinetic Flow to Enhance Nanoparticle Accumulation in Low-Conductivity Solutions. Adv. Mater. Interfaces 2023, 10, 2300478. [Google Scholar] [CrossRef]
- Richetti, P.; Prost, J.; Barois, P. Two-Dimensional Aggregation and Crystallization of a Colloidal Suspension of Latex Spheres. J. Phys. Lett. 1984, 45, 1137–1143. [Google Scholar] [CrossRef]
- Trau, M.; Saville, D.A.; Aksay, I.A. Field-Induced Layering of Colloidal Crystals. Science 1996, 272, 706–709. [Google Scholar] [CrossRef]
- Trau, M.; Saville, D.A.; Aksay, I.A. Assembly of Colloidal Crystals at Electrode Interfaces. Langmuir 1997, 13, 6375–6381. [Google Scholar] [CrossRef]
- Williams, S.J.; Kumar, A.; Wereley, S.T. Electrokinetic Patterning of Colloidal Particles with Optical Landscapes. Lab. Chip 2008, 8, 1879–1882. [Google Scholar] [CrossRef] [PubMed]
- Velasco, V.; Williams, S.J. Electrokinetic Concentration, Patterning, and Sorting of Colloids with Thin Film Heaters. J. Colloid Interface Sci. 2013, 394, 598–603. [Google Scholar] [CrossRef] [PubMed]
- Guan, W.; Park, J.H.; Krstić, P.S.; Reed, M.A. Non-Vanishing Ponderomotive AC Electrophoretic Effect for Particle Trapping. Nanotechnology 2011, 22, 245103. [Google Scholar] [CrossRef]
- Aïzel, K.; Fouillet, Y.; Pudda, C. Electropreconcentration of Nanoparticles Using a Radial Micro-Nanofluidic Device. J. Nanoparticle Res. 2014, 16, 2731. [Google Scholar] [CrossRef]
- Tarn, M.D.; Peyman, S.A.; Pamme, N. Simultaneous Trapping of Magnetic and Diamagnetic Particle Plugs for Separations and Bioassays. RSC Adv. 2013, 3, 7209–7214. [Google Scholar] [CrossRef]
- Watarai, H.; Namba, M. Capillary Magnetophoresis of Human Blood Cells and Their Magnetophoretic Trapping in a Flow System. J. Chromatogr. A 2002, 961, 3–8. [Google Scholar] [CrossRef]
- Hejazian, M.; Nguyen, N.-T. Magnetofluidic Concentration and Separation of Non-Magnetic Particles Using Two Magnet Arrays. Biomicrofluidics 2016, 10, 044103. [Google Scholar] [CrossRef] [PubMed]
- Kimura, T.; Sato, Y.; Kimura, F.; Iwasaka, M.; Ueno, S. Micropatterning of Cells Using Modulated Magnetic Fields. Langmuir 2005, 21, 830–832. [Google Scholar] [CrossRef]
- Kimura, T.; Yamato, M.; Nara, A. Particle Trapping and Undulation of a Liquid Surface Using a Microscopically Modulated Magnetic Field. Langmuir 2004, 20, 572–574. [Google Scholar] [CrossRef] [PubMed]
- Ramadan, Q.; Samper, V.; Poenar, D.P.; Yu, C. An Integrated Microfluidic Platform for Magnetic Microbeads Separation and Confinement. Biosens. Bioelectron. 2006, 21, 1693–1702. [Google Scholar] [CrossRef] [PubMed]
- Gooneratne, C.P.; Liang, C.; Giouroudi, I.; Kosel, J. A Magnetic Particle Micro-Trap for Large Trapping Surfaces. Procedia Eng. 2011, 25, 1201–1204. [Google Scholar] [CrossRef][Green Version]
- Gooneratne, C.P.; Giouroudi, I.; Liang, C.; Kosel, J. A Giant Magnetoresistance Ring-Sensor Based Microsystem for Magnetic Bead Manipulation and Detection. J. Appl. Phys. 2011, 109, 07E517. [Google Scholar] [CrossRef]
- Li, F.; Kodzius, R.; Gooneratne, C.P.; Foulds, I.G.; Kosel, J. Magneto-Mechanical Trapping Systems for Biological Target Detection. Microchim. Acta 2014, 181, 1743–1748. [Google Scholar] [CrossRef]
- Gooneratne, C.P.; Kosel, J. A Micro-Pillar Array to Trap Magnetic Beads in Microfluidic Systems. In Proceedings of the 2012 Sixth International Conference on Sensing Technology (ICST), Kolkata, India, 18–21 December 2012; pp. 97–101. [Google Scholar]
- Yu, X.; He, R.; Li, S.; Cai, B.; Zhao, L.; Liao, L.; Liu, W.; Zeng, Q.; Wang, H.; Guo, S.-S.; et al. Magneto-Controllable Capture and Release of Cancer Cells by Using a Micropillar Device Decorated with Graphite Oxide-Coated Magnetic Nanoparticles. Small 2013, 9, 3895–3901. [Google Scholar] [CrossRef] [PubMed]
- Faivre, M.; Gelszinnis, R.; Degouttes, J.; Terrier, N.; Rivière, C.; Ferrigno, R.; Deman, A.-L. Magnetophoretic Manipulation in Microsystem Using Carbonyl Iron-Polydimethylsiloxane Microstructures. Biomicrofluidics 2014, 8, 054103. [Google Scholar] [CrossRef]
- Smistrup, K.; Bruus, H.; Hansen, M.F. Towards a Programmable Magnetic Bead Microarray in a Microfluidic Channel. J. Magn. Magn. Mater. 2007, 311, 409–415. [Google Scholar] [CrossRef]
- Lefebvre, O.; Cao, H.H.; Cortés Francisco, M.; Woytasik, M.; Dufour-Gergam, E.; Ammar, M.; Martincic, E. Reusable Embedded Microcoils for Magnetic Nano-Beads Trapping in Microfluidics: Magnetic Simulation and Experiments. Micromachines 2020, 11, 257. [Google Scholar] [CrossRef]
- Song, S.-H.; Kwak, B.-S.; Park, J.-S.; Kim, W.; Jung, H.-I. Novel Application of Joule Heating to Maintain Biocompatible Temperatures in a Fully Integrated Electromagnetic Cell Sorting System. Sens. Actuators Phys. 2009, 151, 64–70. [Google Scholar] [CrossRef]
- Zheng, Y.; Sawan, M. Planar Microcoil Array Based Temperature-Controllable Lab-on-Chip Platform. IEEE Trans. Magn. 2013, 49, 5236–5242. [Google Scholar] [CrossRef]
- Bücks, K.; Müller, H. Über einige Beobachtungen an schwingenden Piezoquarzen und ihrem Schallfeld. Z. Für Phys. 1933, 84, 75–86. [Google Scholar] [CrossRef]
- Sarvazyan, A.P.; Rudenko, O.V.; Fatemi, M. Acoustic Radiation Force: A Review of Four Mechanisms for Biomedical Applications. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2021, 68, 3261–3269. [Google Scholar] [CrossRef]
- Lilliehorn, T.; Nilsson, M.; Simu, U.; Johansson, S.; Almqvist, M.; Nilsson, J.; Laurell, T. Dynamic Arraying of Microbeads for Bioassays in Microfluidic Channels. Sens. Actuators B Chem. 2005, 106, 851–858. [Google Scholar] [CrossRef]
- Shilton, R.; Tan, M.K.; Yeo, L.Y.; Friend, J.R. Particle Concentration and Mixing in Microdrops Driven by Focused Surface Acoustic Waves. J. Appl. Phys. 2008, 104, 014910. [Google Scholar] [CrossRef]
- Raghavan, R.V.; Friend, J.R.; Yeo, L.Y. Particle Concentration via Acoustically Driven Microcentrifugation: microPIV Flow Visualization and Numerical Modelling Studies. Microfluid. Nanofluidics 2010, 8, 73–84. [Google Scholar] [CrossRef]
- Rogers, P.R.; Friend, J.R.; Yeo, L.Y. Exploitation of Surface Acoustic Waves to Drive Size-Dependent Microparticle Concentration within a Droplet. Lab. Chip 2010, 10, 2979–2985. [Google Scholar] [CrossRef]
- Destgeer, G.; Cho, H.; Hang Ha, B.; Ho Jung, J.; Park, J.; Jin Sung, H. Acoustofluidic Particle Manipulation inside a Sessile Droplet: Four Distinct Regimes of Particle Concentration. Lab. Chip 2016, 16, 660–667. [Google Scholar] [CrossRef]
- Whitehill, J.; Neild, A.; Ng, T.W.; Stokes, M. Collection of Suspended Particles in a Drop Using Low Frequency Vibration. Appl. Phys. Lett. 2010, 96, 053501. [Google Scholar] [CrossRef]
- Hammarström, B.; Laurell, T.; Nilsson, J. Seed Particle-Enabled Acoustic Trapping of Bacteria and Nanoparticles in Continuous Flow Systems. Lab. Chip 2012, 12, 4296–4304. [Google Scholar] [CrossRef] [PubMed]
- Evander, M.; Gidlöf, O.; Olde, B.; Erlinge, D.; Laurell, T. Non-Contact Acoustic Capture of Microparticles from Small Plasma Volumes. Lab. Chip 2015, 15, 2588–2596. [Google Scholar] [CrossRef] [PubMed]
- Cui, W.; Mu, L.; Duan, X.; Pang, W.; Reed, M.A. Trapping of Sub-100 Nm Nanoparticles Using Gigahertz Acoustofluidic Tweezers for Biosensing Applications. Nanoscale 2019, 11, 14625–14634. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Ma, Z.; Ai, Y. Submicron Particle Concentration and Patterning with Ultralow Frequency Acoustic Vibration. Anal. Chem. 2020, 92, 12795–12800. [Google Scholar] [CrossRef] [PubMed]
- Fakhfouri, A.; Devendran, C.; Collins, D.J.; Ai, Y.; Neild, A. Virtual Membrane for Filtration of Particles Using Surface Acoustic Waves (SAW). Lab. Chip 2016, 16, 3515–3523. [Google Scholar] [CrossRef] [PubMed]
- Collins, D.J.; Luan Khoo, B.; Ma, Z.; Winkler, A.; Weser, R.; Schmidt, H.; Han, J.; Ai, Y. Selective Particle and Cell Capture in a Continuous Flow Using Micro-Vortex Acoustic Streaming. Lab. Chip 2017, 17, 1769–1777. [Google Scholar] [CrossRef] [PubMed]
- Kane, R.S.; Takayama, S.; Ostuni, E.; Ingber, D.E.; Whitesides, G.M. Patterning Proteins and Cells Using Soft Lithography. Biomaterials 1999, 20, 2363–2376. [Google Scholar] [CrossRef]
- Dharmasiri, U.; Njoroge, S.K.; Witek, M.A.; Adebiyi, M.G.; Kamande, J.W.; Hupert, M.L.; Barany, F.; Soper, S.A. High-Throughput Selection, Enumeration, Electrokinetic Manipulation, and Molecular Profiling of Low-Abundance Circulating Tumor Cells Using a Microfluidic System. Anal. Chem. 2011, 83, 2301–2309. [Google Scholar] [CrossRef]
- Xu, Y.; Phillips, J.A.; Yan, J.; Li, Q.; Fan, Z.H.; Tan, W. Aptamer-Based Microfluidic Device for Enrichment, Sorting, and Detection of Multiple Cancer Cells. Anal. Chem. 2009, 81, 7436–7442. [Google Scholar] [CrossRef]
- Mu, X.; Zheng, W.; Sun, J.; Zhang, W.; Jiang, X. Microfluidics for Manipulating Cells. Small 2013, 9, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Sigurdson, M.; Meinhart, C.; Wang, D.; Liu, X.; Feng, J.J.; Krishnamoorthy, S.; Sundaram, S. AC Electrokinetics for Microfluidic Immunosensors. In Proceedings of the American Society of Mechanical Engineers Digital Collection, Washington, DC, USA, 15–21 November 2003; pp. 479–483. [Google Scholar]
- Syed, A.; Mangano, L.; Mao, P.; Han, J.; Song, Y.-A. Creating Sub-50 Nm Nanofluidic Junctions in a PDMS Microchip via Self-Assembly Process of Colloidal Silica Beads for Electrokinetic Concentration of Biomolecules. Lab. Chip 2014, 14, 4455–4460. [Google Scholar] [CrossRef] [PubMed]
- Gerspach, M.A.; Mojarad, N.; Sharma, D.; Ekinci, Y.; Pfohl, T. Pneumatically Controlled Nanofluidic Devices for Contact-Free Trapping and Manipulation of Nanoparticles. Part. Part. Syst. Charact. 2018, 35, 1800161. [Google Scholar] [CrossRef]
- Krafft, B.; Tycova, A.; Urban, R.D.; Dusny, C.; Belder, D. Microfluidic Device for Concentration and SERS-based Detection of Bacteria in Drinking Water. Electrophoresis 2021, 42, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Habibi, R.; Neild, A. Sound Wave Activated Nano-Sieve (SWANS) for Enrichment of Nanoparticles. Lab. Chip 2019, 19, 3032–3044. [Google Scholar] [CrossRef] [PubMed]
- Allahrabbi, N.; Chia, Y.S.M.; Saifullah, M.S.M.; Lim, K.-M.; Yung, L.Y.L. A Hybrid Dielectrophoretic System for Trapping of Microorganisms from Water. Biomicrofluidics 2015, 9, 034110. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Williams, S.J.; Chuang, H.S.; Green, N.G.; Wereley, S.T. Hybrid Opto-Electric Manipulation in Microfluidics—Opportunities and Challenges. Lab. Chip 2011, 11, 2135–2148. [Google Scholar] [CrossRef] [PubMed]
- Al-Ali, A.; Waheed, W.; Abu-Nada, E.; Alazzam, A. A Review of Active and Passive Hybrid Systems Based on Dielectrophoresis for the Manipulation of Microparticles. J. Chromatogr. A 2022, 1676, 463268. [Google Scholar] [CrossRef] [PubMed]
- Niculescu, A.-G.; Chircov, C.; Bîrcă, A.C.; Grumezescu, A.M. Fabrication and Applications of Microfluidic Devices: A Review. Int. J. Mol. Sci. 2021, 22, 2011. [Google Scholar] [CrossRef]
- Baron, V.O.; Chen, M.; Hammarstrom, B.; Hammond, R.J.H.; Glynne-Jones, P.; Gillespie, S.H.; Dholakia, K. Real-Time Monitoring of Live Mycobacteria with a Microfluidic Acoustic-Raman Platform. Commun. Biol. 2020, 3, 236. [Google Scholar] [CrossRef]
- Branton, D.; Deamer, D.W.; Marziali, A.; Bayley, H.; Benner, S.A.; Butler, T.; Di Ventra, M.; Garaj, S.; Hibbs, A.; Huang, X.; et al. The Potential and Challenges of Nanopore Sequencing. Nat. Biotechnol. 2008, 26, 1146–1153. [Google Scholar] [CrossRef] [PubMed]
- Howorka, S.; Siwy, Z. Nanopore Analytics: Sensing of Single Molecules. Chem. Soc. Rev. 2009, 38, 2360–2384. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Wu, H.; Wu, L.; Xie, X.; Kong, J.; Ye, X.; Liu, L. Voltage-Driven Translocation of DNA through a High Throughput Conical Solid-State Nanopore. PLoS ONE 2012, 7, e46014. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Liu, Y.; Dai, M.; Yi, X.; Wang, C. Controlling DNA Translocation Through Solid-State Nanopores. Nanoscale Res. Lett. 2020, 15, 80. [Google Scholar] [CrossRef]
- Schmidt, H.; Sampad, M.; Saiduzzaman, M.S.; Hawkins, A.R.; Walker, Z.; Wells, T. Recent Advances in Waveguide-Based Optical Trapping for Molecular Biomarker Analysis. In Proceedings of the Optical Trapping and Optical Micromanipulation XIX; Dholakia, K., Spalding, G.C., Eds.; SPIE: San Diego, CA, USA, 2022; p. 17. [Google Scholar]
- Zhang, L.; Tian, Z.; Bachman, H.; Zhang, P.; Huang, T.J. A Cell-Phone-Based Acoustofluidic Platform for Quantitative Point-of-Care Testing. ACS Nano 2020, 14, 3159–3169. [Google Scholar] [CrossRef] [PubMed]
- Grünberger, A.; Paczia, N.; Probst, C.; Schendzielorz, G.; Eggeling, L.; Noack, S.; Wiechert, W.; Kohlheyer, D. A Disposable Picolitre Bioreactor for Cultivation and Investigation of Industrially Relevant Bacteria on the Single Cell Level. Lab. Chip 2012, 12, 2060–2068. [Google Scholar] [CrossRef] [PubMed]
- Hage, D.S. Immunoassays. Anal. Chem. 1999, 71, 294–304. [Google Scholar] [CrossRef] [PubMed]
- Wisdom, G.B. Enzyme-Immunoassay. Clin. Chem. 1976, 22, 1243–1255. [Google Scholar] [CrossRef] [PubMed]
- Nienhaus, G.U.; Nienhaus, K. Fluorescence Labeling. In Fluorescence Microscopy; Kubitscheck, U., Ed.; Wiley: Hoboken, NJ, USA, 2017; pp. 133–164. ISBN 978-3-527-33837-5. [Google Scholar]
- Liu, Y.; Guo, S.; Zhang, Z.; Huang, W.; Baigl, D.; Xie, M.; Chen, Y.; Pang, D. A Micropillar-integrated Smart Microfluidic Device for Specific Capture and Sorting of Cells. Electrophoresis 2007, 28, 4713–4722. [Google Scholar] [CrossRef]
- Garg, N.; Westerhof, T.M.; Liu, V.; Liu, R.; Nelson, E.L.; Lee, A.P. Whole-Blood Sorting, Enrichment and in Situ Immunolabeling of Cellular Subsets Using Acoustic Microstreaming. Microsyst. Nanoeng. 2018, 4, 17085. [Google Scholar] [CrossRef]
- Jung, D.R.; Kapur, R.; Adams, T.; Giuliano, K.A.; Mrksich, M.; Craighead, H.G.; Taylor, D.L. Topographical and Physicochemical Modification of Material Surface to Enable Patterning of Living Cells. Crit. Rev. Biotechnol. 2001, 21, 111–154. [Google Scholar] [CrossRef] [PubMed]
- Garvin, K.A.; Hocking, D.C.; Dalecki, D. Controlling the Spatial Organization of Cells and Extracellular Matrix Proteins in Engineered Tissues Using Ultrasound Standing Wave Fields. Ultrasound Med. Biol. 2010, 36, 1919–1932. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B.; Sharma, A. Microfluidics: Recent Advances Toward Lab-on-Chip Applications in Bioanalysis. Adv. Eng. Mater. 2022, 24, 2100738. [Google Scholar] [CrossRef]
- Mohammed, M.I.; Haswell, S.; Gibson, I. Lab-on-a-Chip or Chip-in-a-Lab: Challenges of Commercialization Lost in Translation. Procedia Technol. 2015, 20, 54–59. [Google Scholar] [CrossRef]
- Whitesides, G.M. The Origins and the Future of Microfluidics. Nature 2006, 442, 368–373. [Google Scholar] [CrossRef]
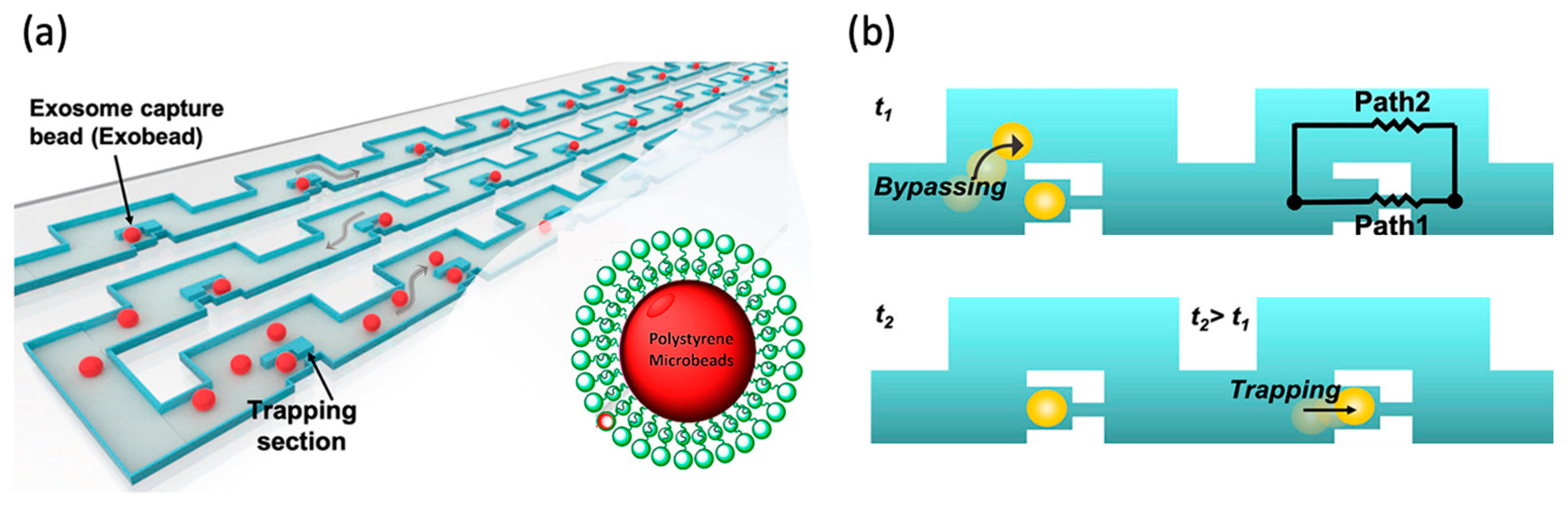

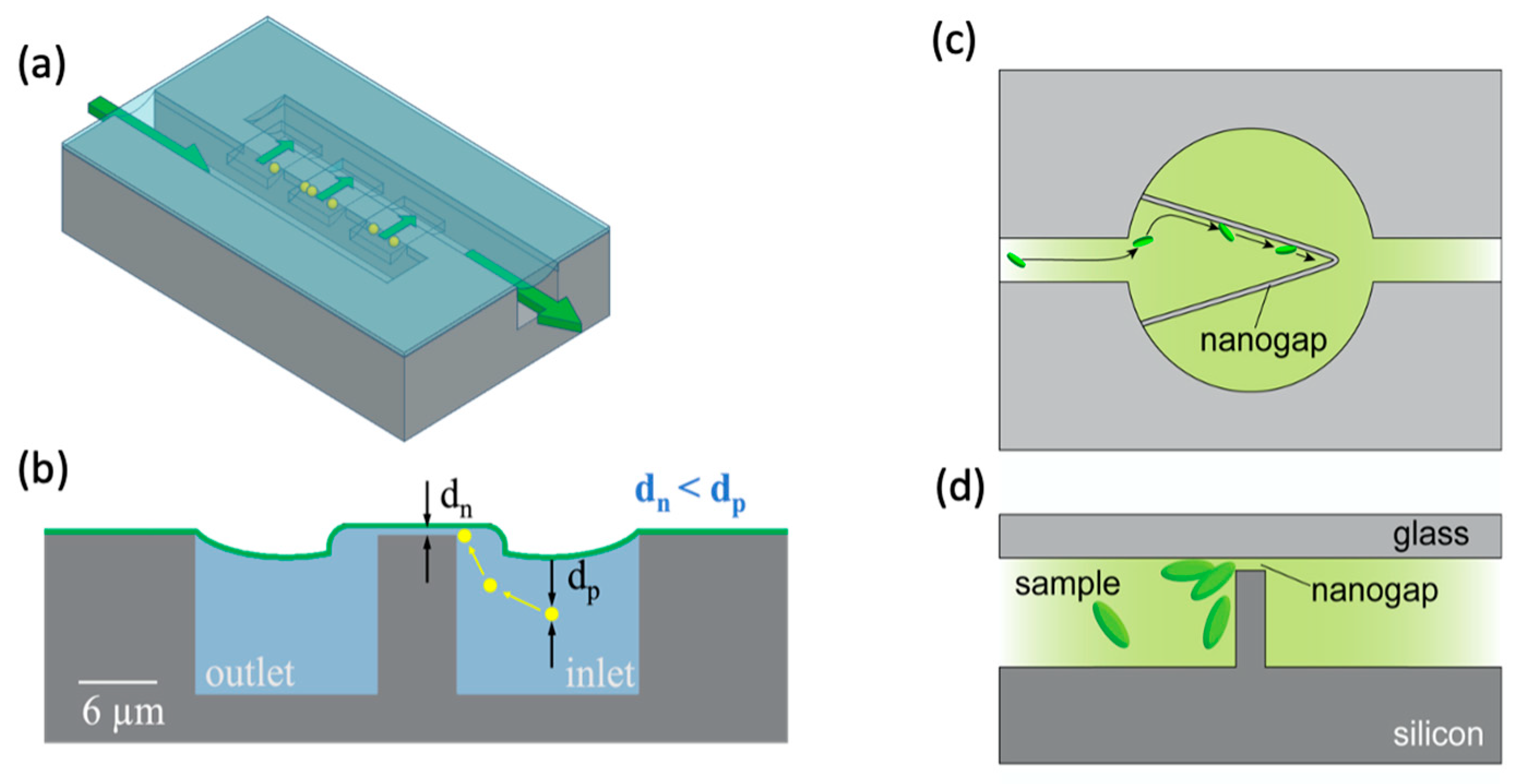

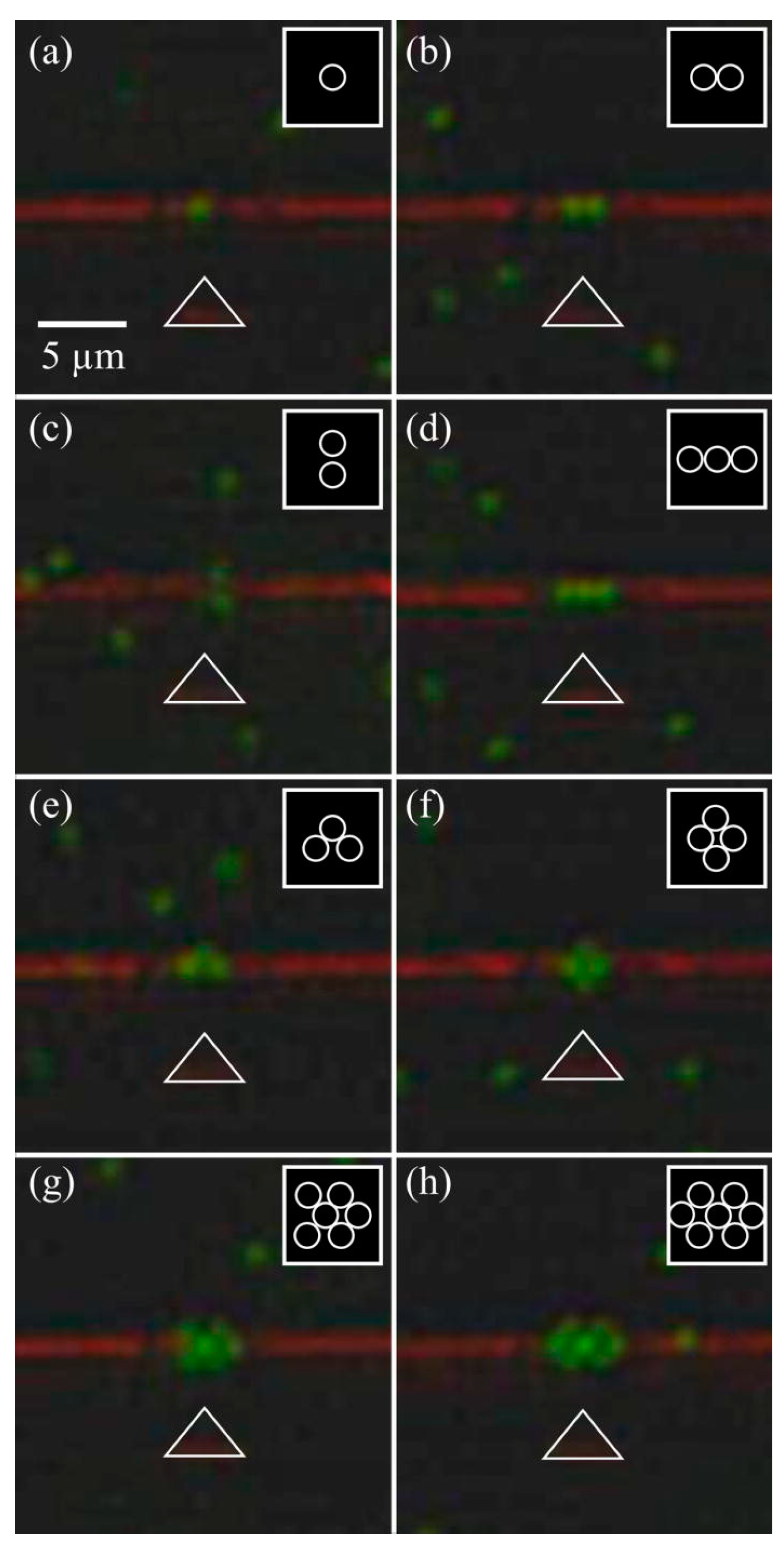
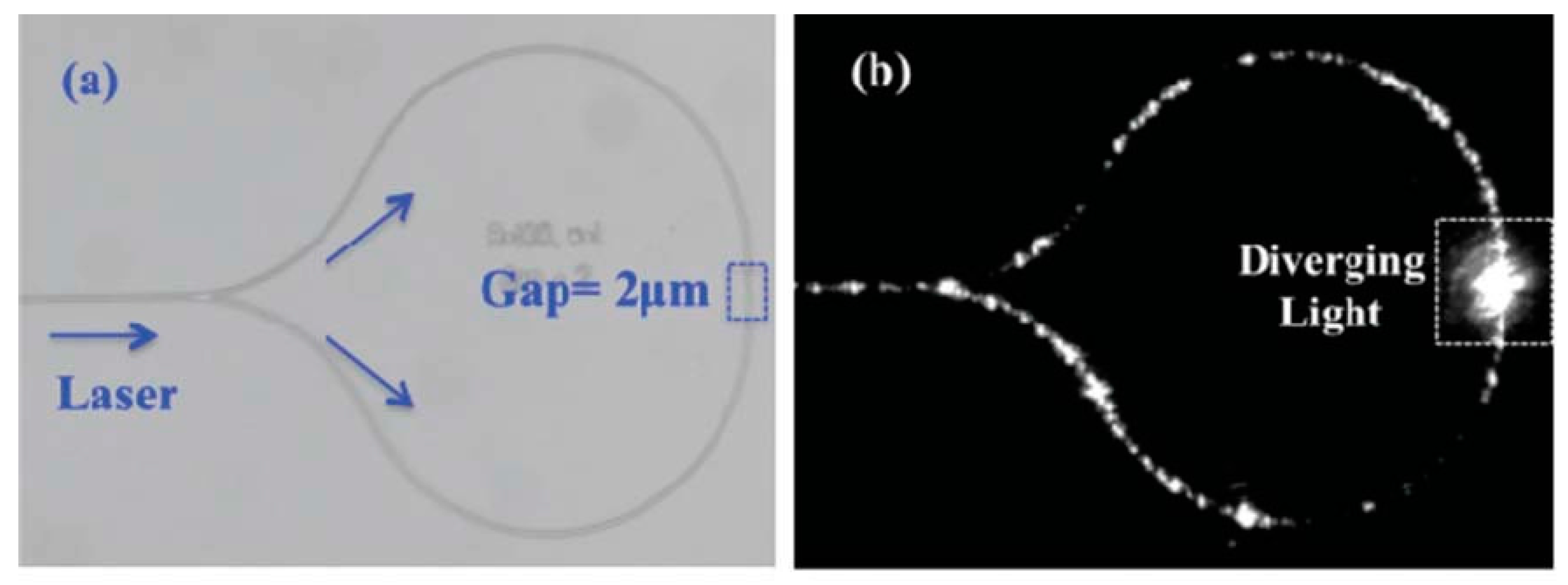

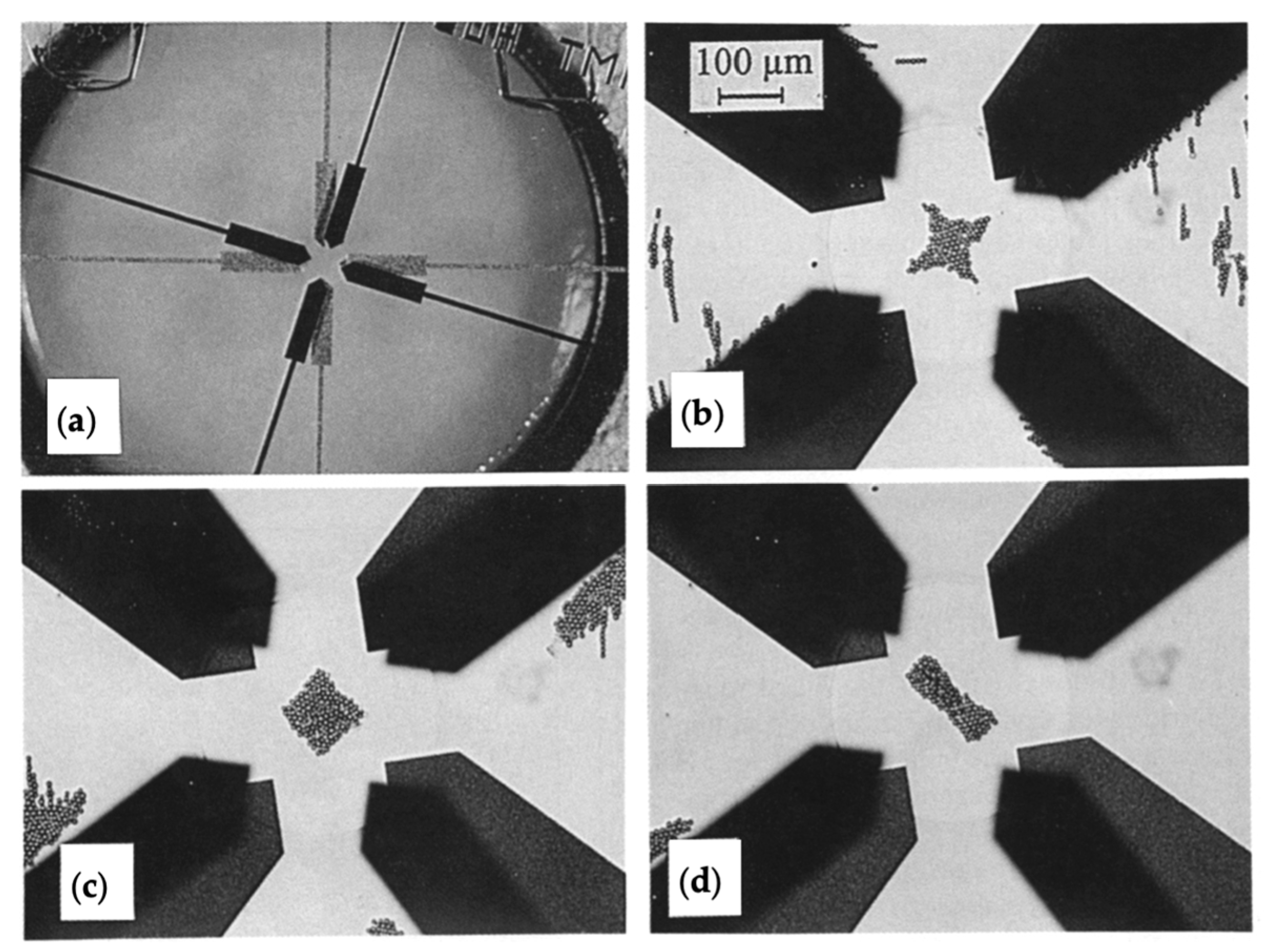
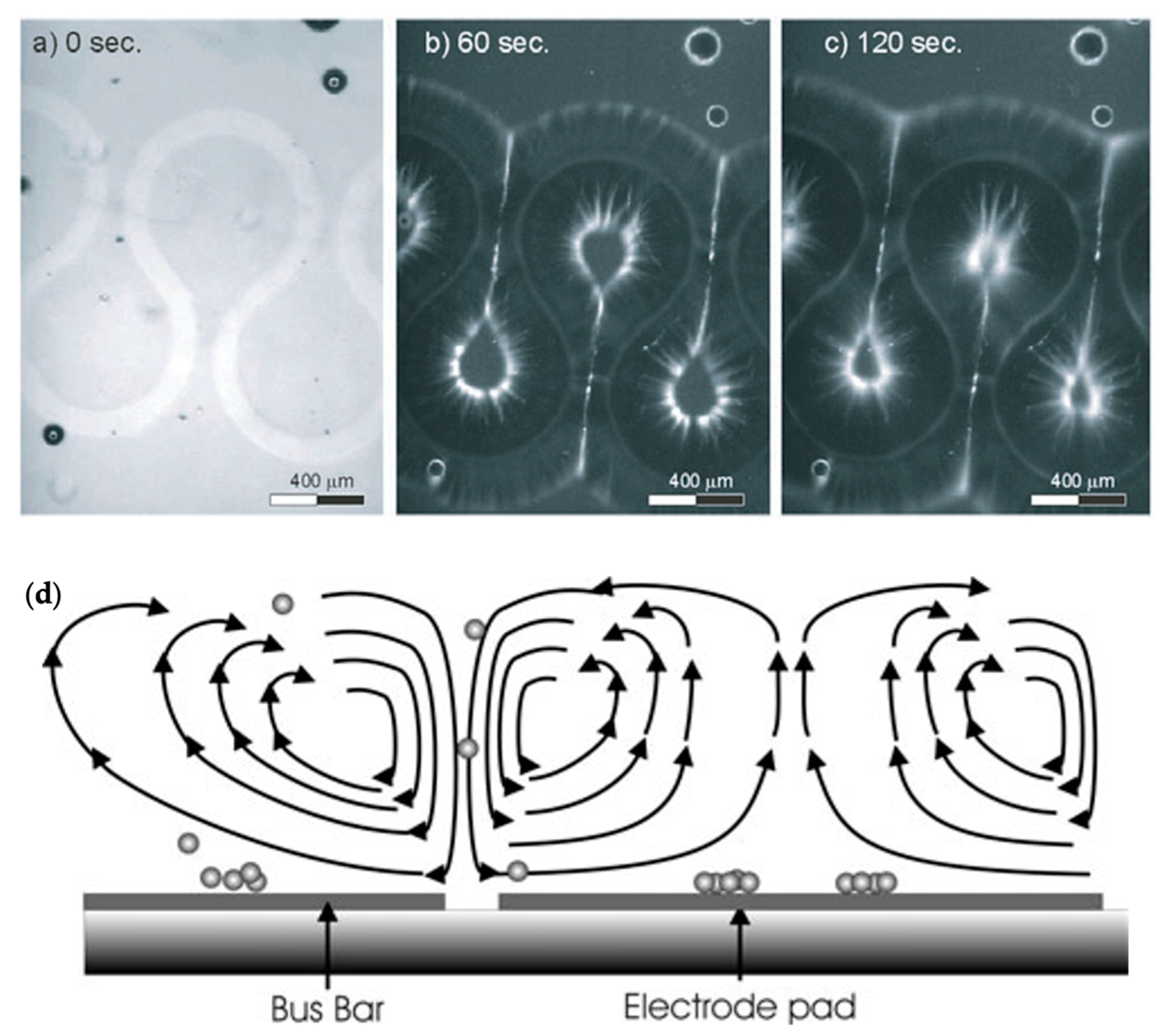
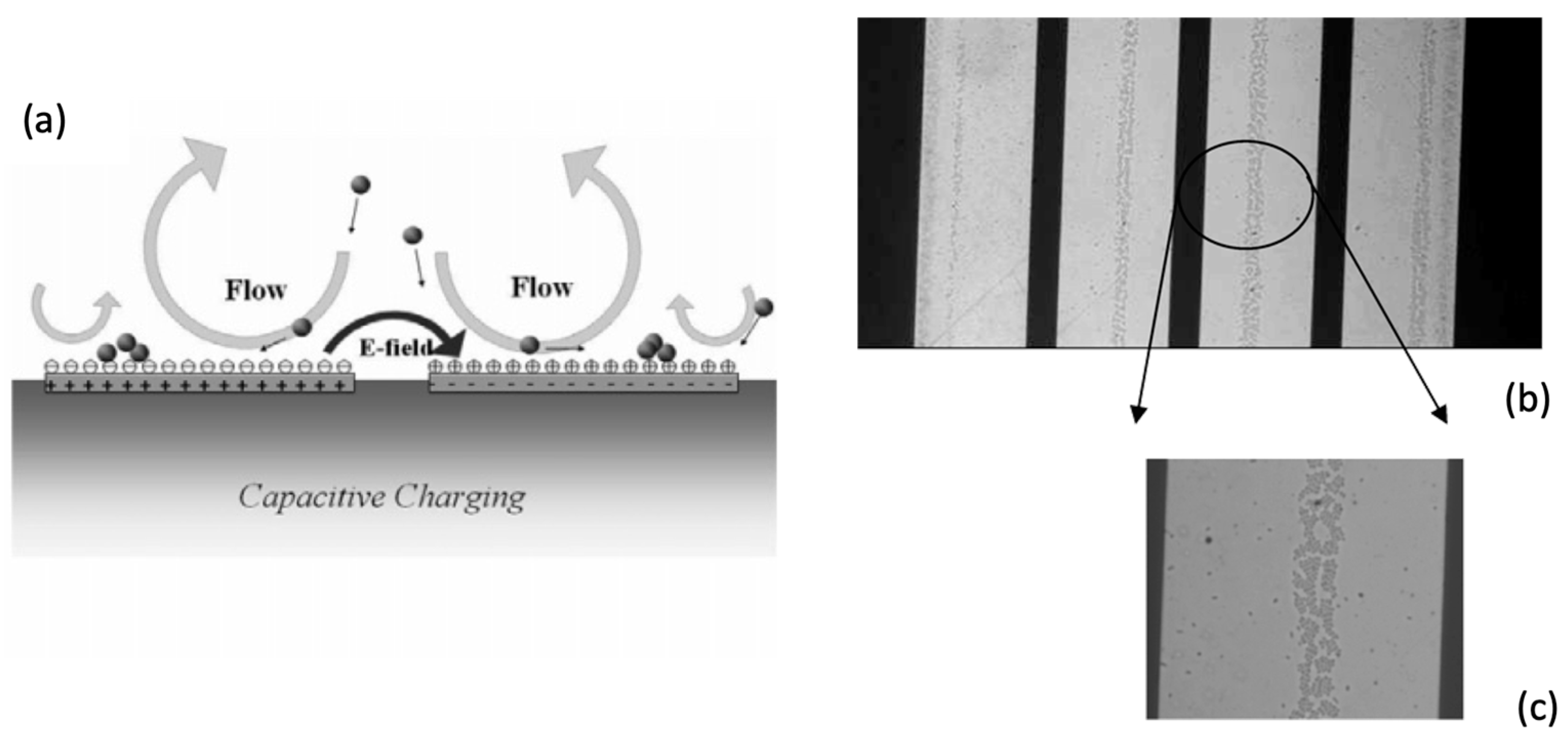
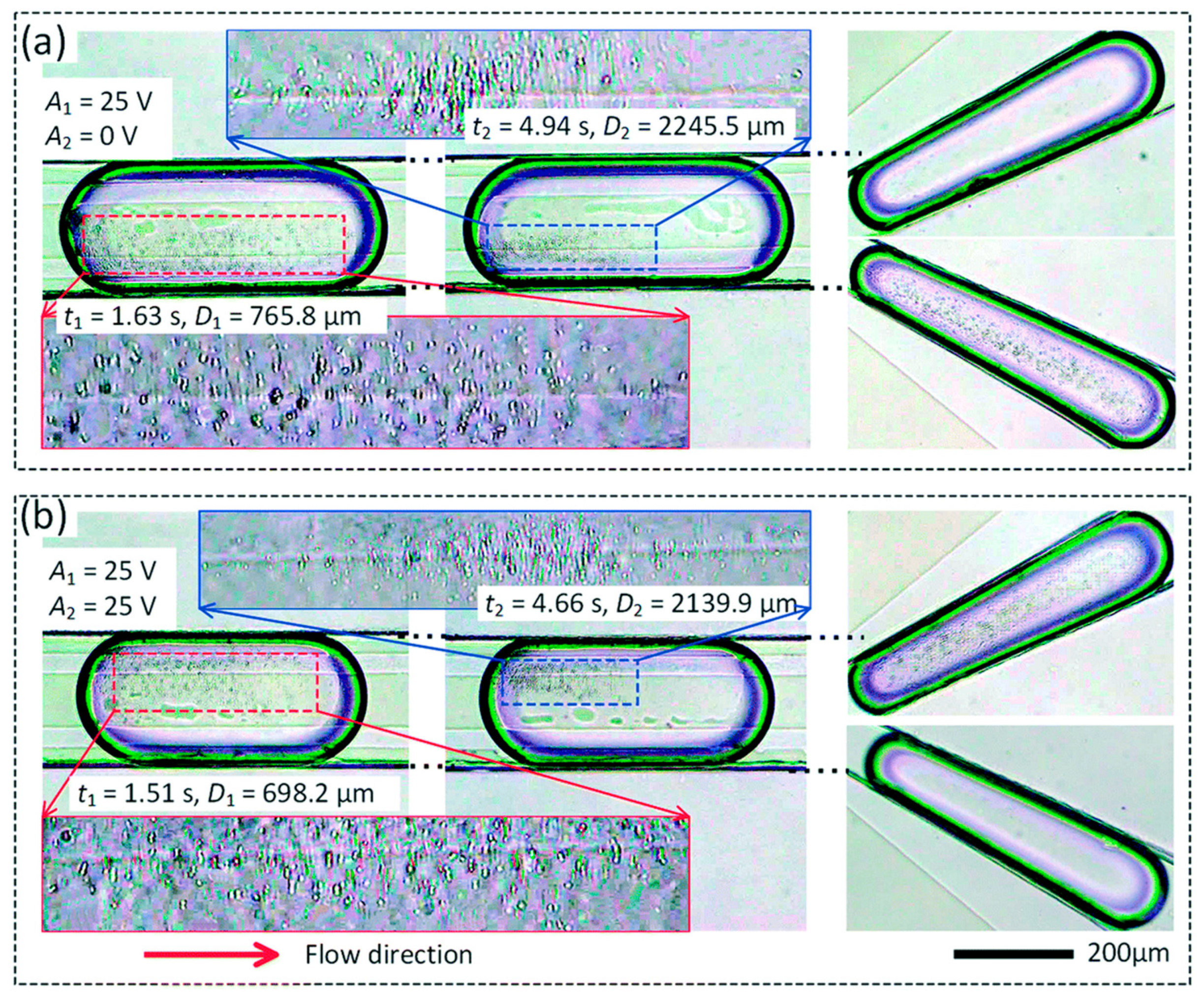
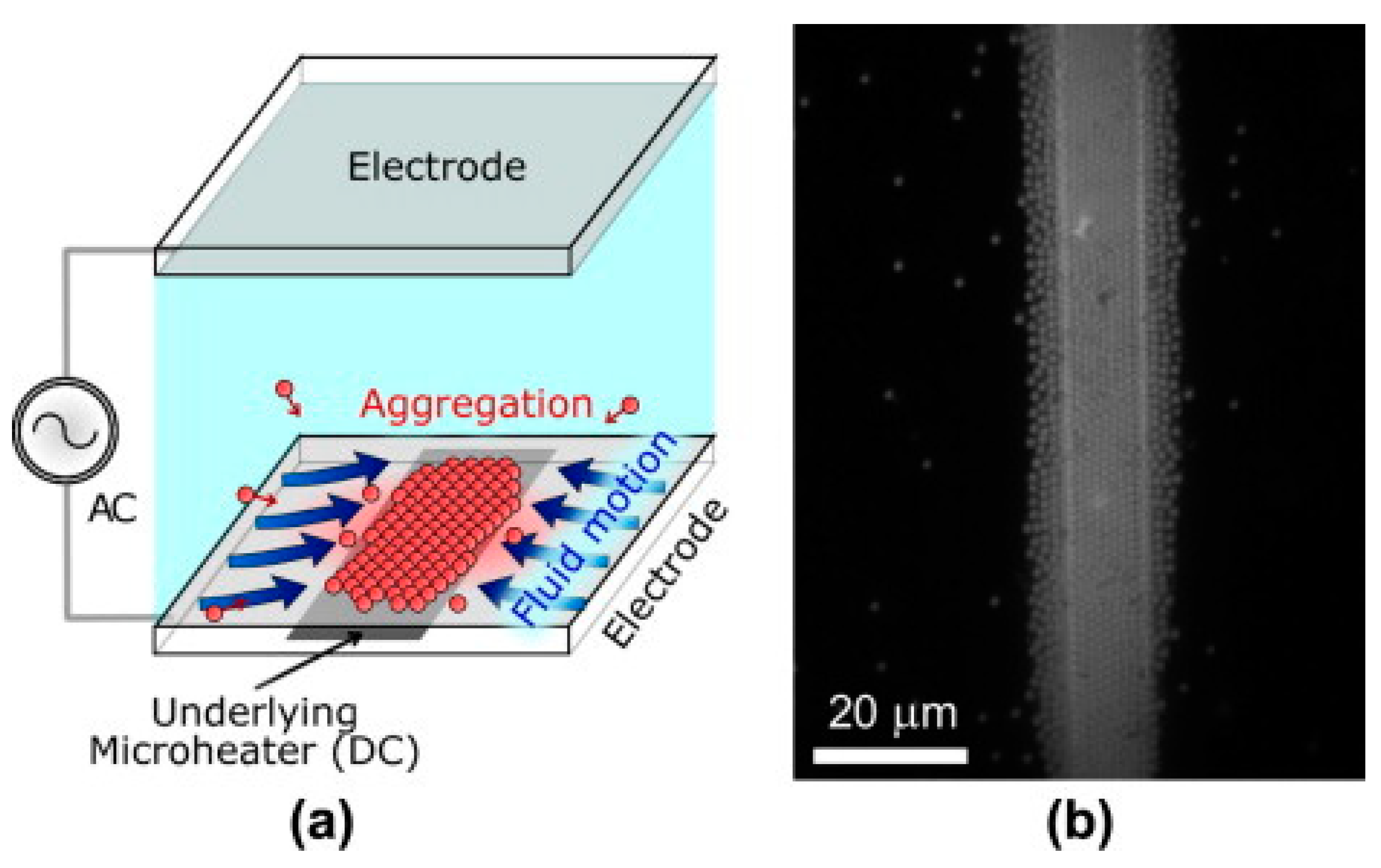
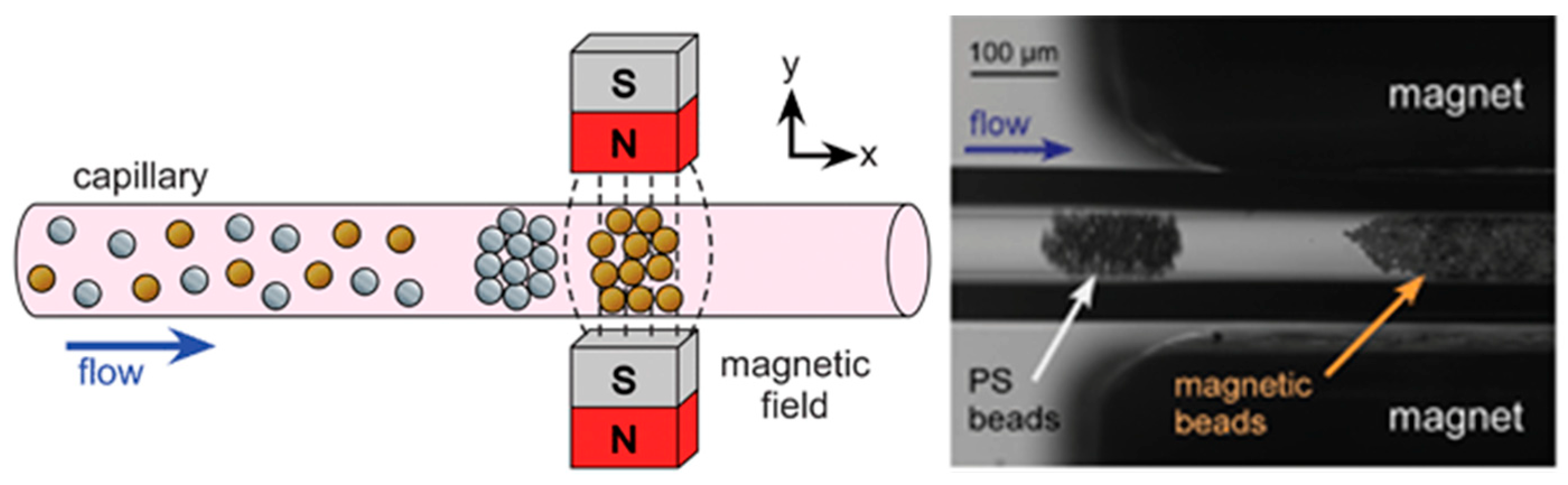


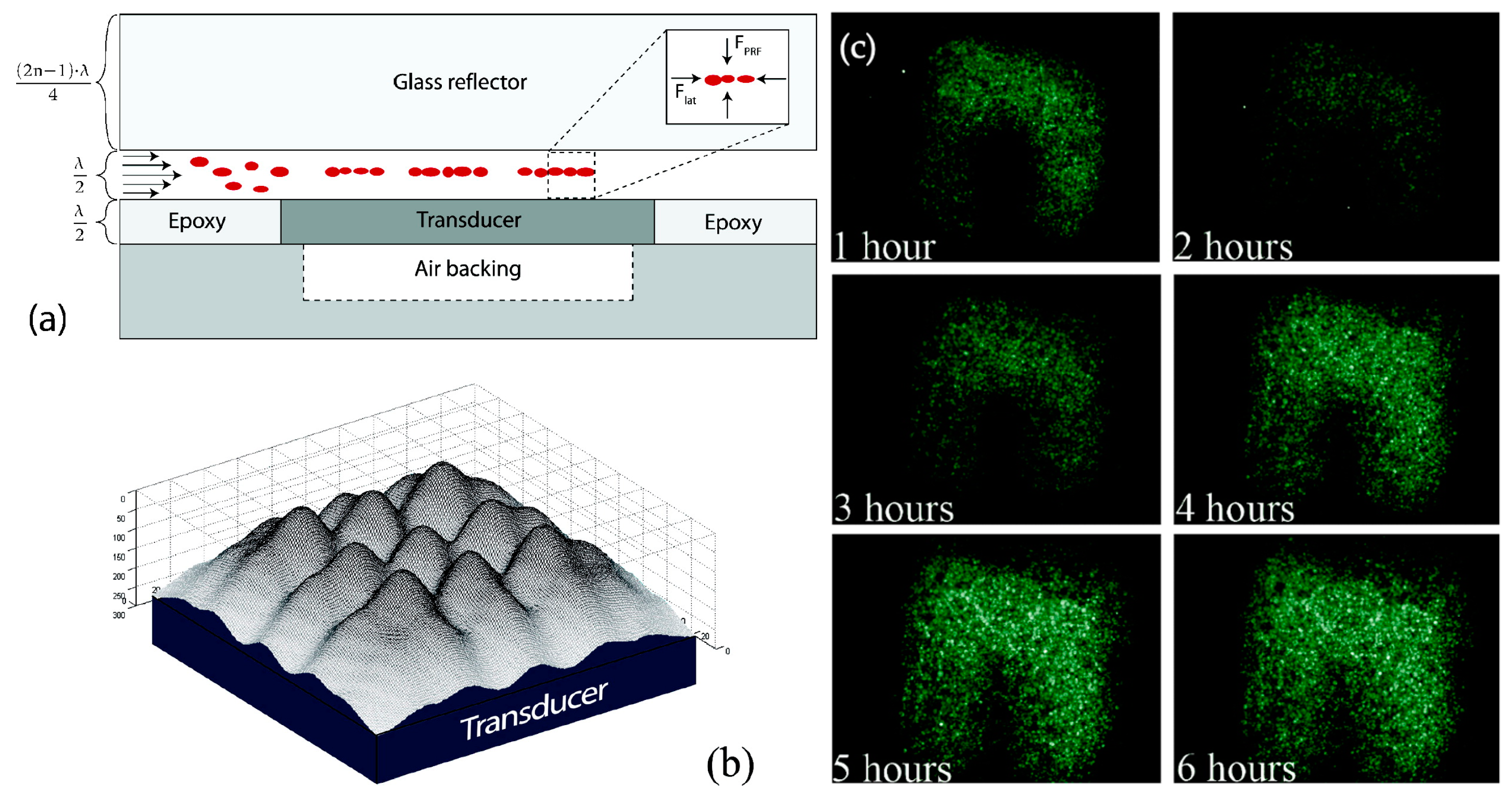
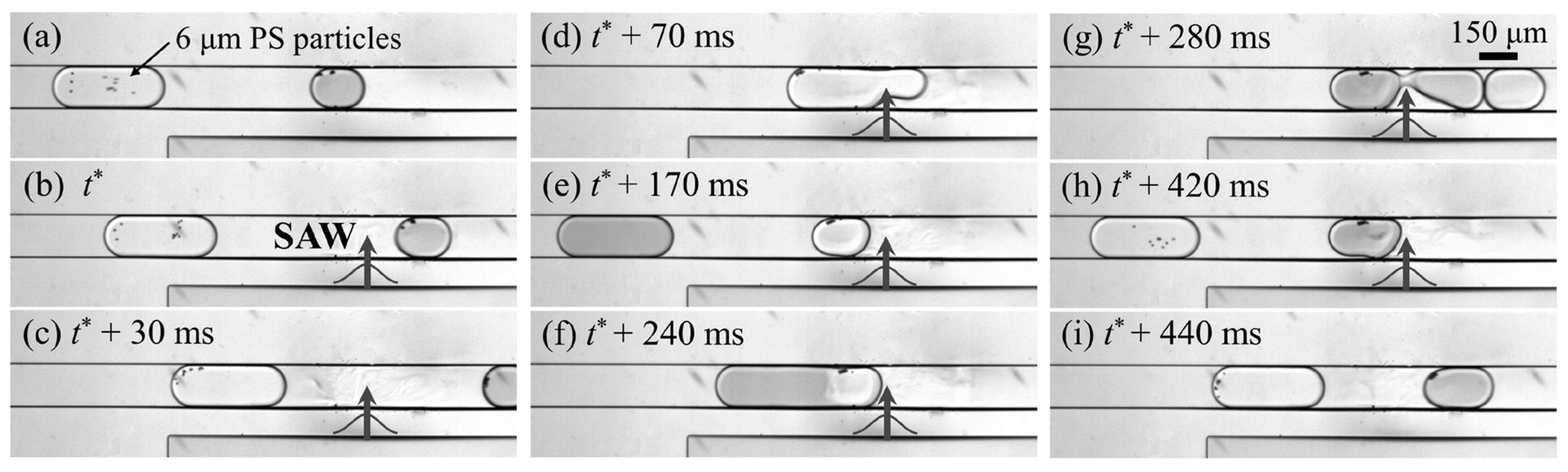

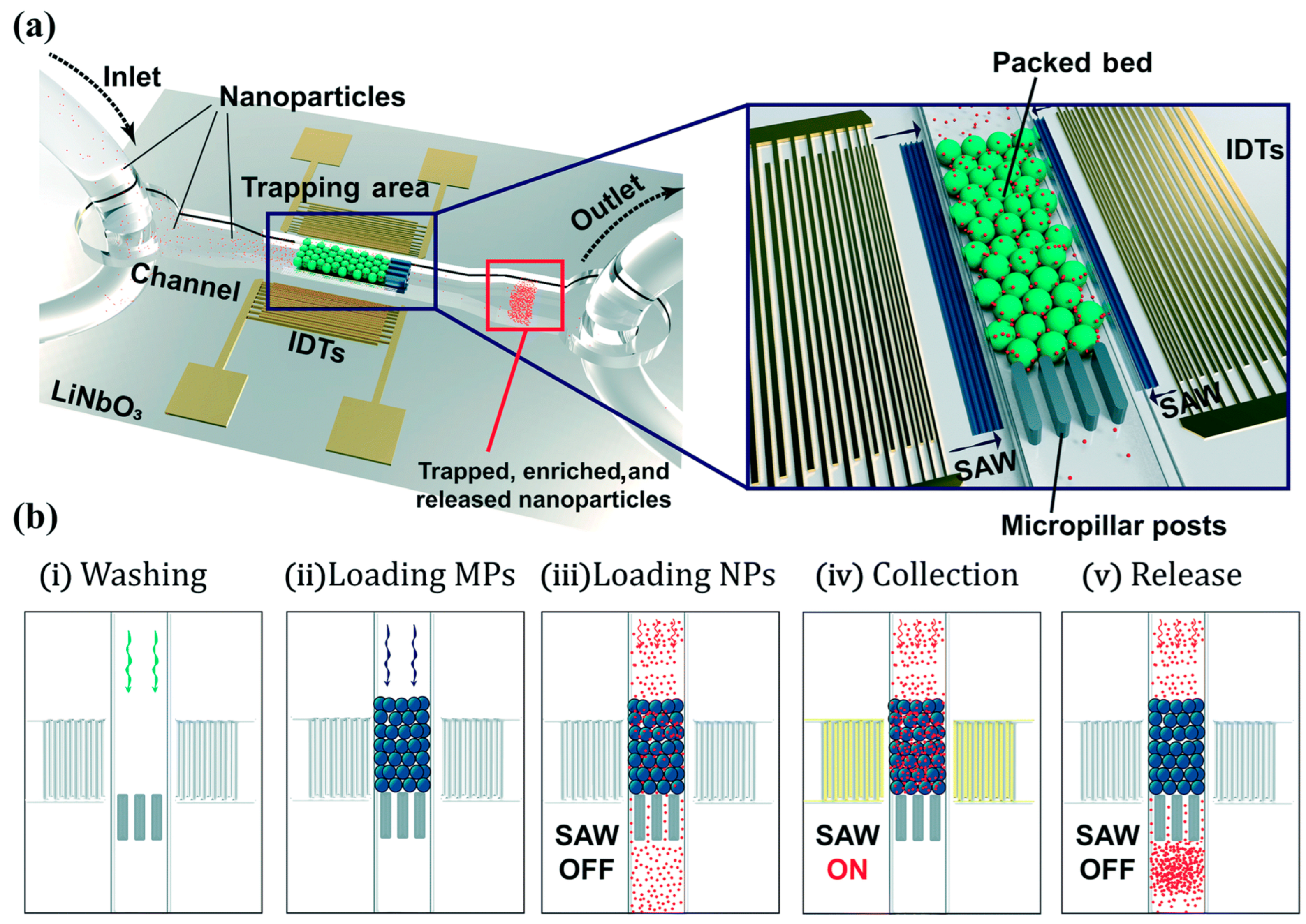
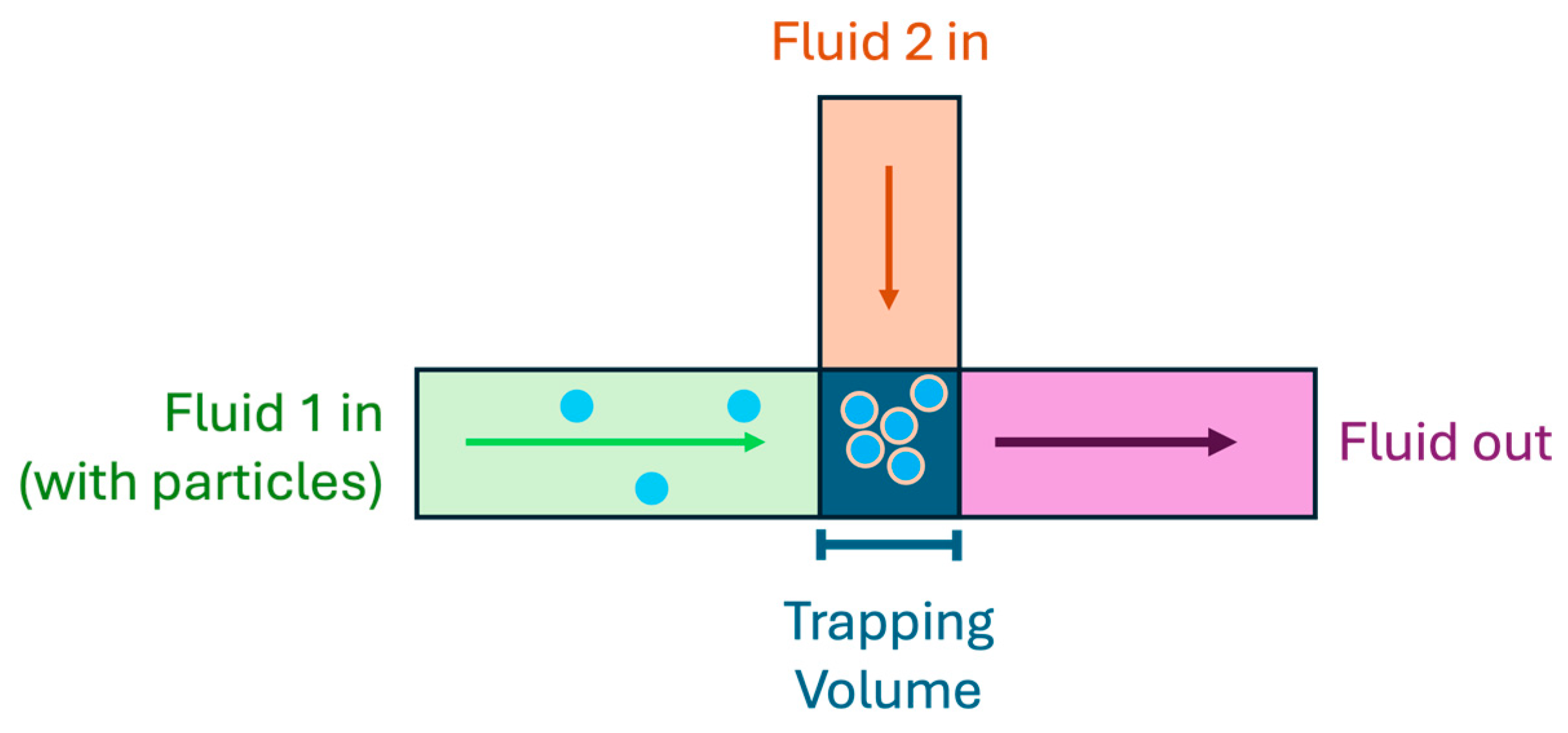
| Reference | Trap Mechanism | Particle Type, Diameter | Flow Rate (µL/min) | Trapping Efficiency | Trap Volume | Initial Concentration (particles/mL) | # of Trapped Particles | Enrichment Factor | Time | Notes |
|---|---|---|---|---|---|---|---|---|---|---|
| [38] | Physical | Bacteria ~0.5–2 µm | 10 | ~5 pL * | 1000 CFU/mL | ~100 CFU | ~106 * | 10 min | ||
| [25] | Optical | Polystyrene 1 µm | 6 × 10−4 | 98% | 1 pL * | 4 × 107 | ~140 | ~3600 * | 12 min | These values were taken from the results for the design with higher efficiency |
| [63] | Electrical | Bacteria ~1 µm | 12 | 90% | ~10 nL * | 109 | ~1000 * | 1 min | The values were from a high enrichment case | |
| [94] | Magnetic | Diamagnetic; paramagnetic 10 µm; 8 µm | 0.2 | 100% | ~1 nL * | 106 | ~1000 * | ~1000 | ~10 min | Paramagnetic particles could be trapped at higher flow rates, but not diamagnetic ones |
| [124] | Acoustic | Cancer cells 15–20 µm | 4 | ~100% | ~4 nL * | ~1000 | 1 min | A high rate, high enrichment case was used |
| Passive | Structured | Non- Contact | Reversible | Complexity (Fabrication) | Complexity (Use) | Enrichment Capability | References | ||
|---|---|---|---|---|---|---|---|---|---|
| Physical | Individual sites |  |  |  |  | Medium | Low | Low | [38,39,40,41,42] |
| Dual-height channel |  |  |  |  | High | Low | High | [12,43,44,45,46,47] | |
| Inertial microfluidics |  |  |  |  | Medium | Low | Medium | [36,49,50,51,52] | |
| Optical | Multiple optical tweezers |  |  |  |  | Low | High | Low | [11,57] |
| Integrated waveguide |  |  |  |  | High | Medium | High | [8,33,34,66,67] | |
| Electrical | DEP |  |  |  |  | High | Medium | High | [71,72,73,74,85,86,87,88,89,90,91,92] |
| iDEP |  |  |  |  | Medium | Low | High | [78,80,81,82,83,84] | |
| Electrokinetic flow |  |  |  |  | Medium | Medium | Medium | [96,97,98,99,100,101,102,103,104] | |
| Magnetic | External magnet |  |  |  |  | Low | Low | Medium | [112,113,114,115,116] |
| External magnet, micropillars |  |  |  |  | Medium | Low | High | [121,122,123] | |
| Integrated electromagnet |  |  |  |  | Medium | Medium | High | [117,118,119,120,124,125,126,127] | |
| Acoustic | ARF |  |  |  |  | High | High | High | [9,10,130,134,136,137] |
| Acoustic streaming |  |  |  |  | High | Medium | Medium | [131,132,133,134,135,141] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wells, T.N.; Schmidt, H.; Hawkins, A.R. Constrained Volume Micro- and Nanoparticle Collection Methods in Microfluidic Systems. Micromachines 2024, 15, 699. https://doi.org/10.3390/mi15060699
Wells TN, Schmidt H, Hawkins AR. Constrained Volume Micro- and Nanoparticle Collection Methods in Microfluidic Systems. Micromachines. 2024; 15(6):699. https://doi.org/10.3390/mi15060699
Chicago/Turabian StyleWells, Tanner N., Holger Schmidt, and Aaron R. Hawkins. 2024. "Constrained Volume Micro- and Nanoparticle Collection Methods in Microfluidic Systems" Micromachines 15, no. 6: 699. https://doi.org/10.3390/mi15060699
APA StyleWells, T. N., Schmidt, H., & Hawkins, A. R. (2024). Constrained Volume Micro- and Nanoparticle Collection Methods in Microfluidic Systems. Micromachines, 15(6), 699. https://doi.org/10.3390/mi15060699







