Process Development of Aluminum Electroplating from an Ionic Liquid on 150 mm Wafer Level
Abstract
:1. Introduction
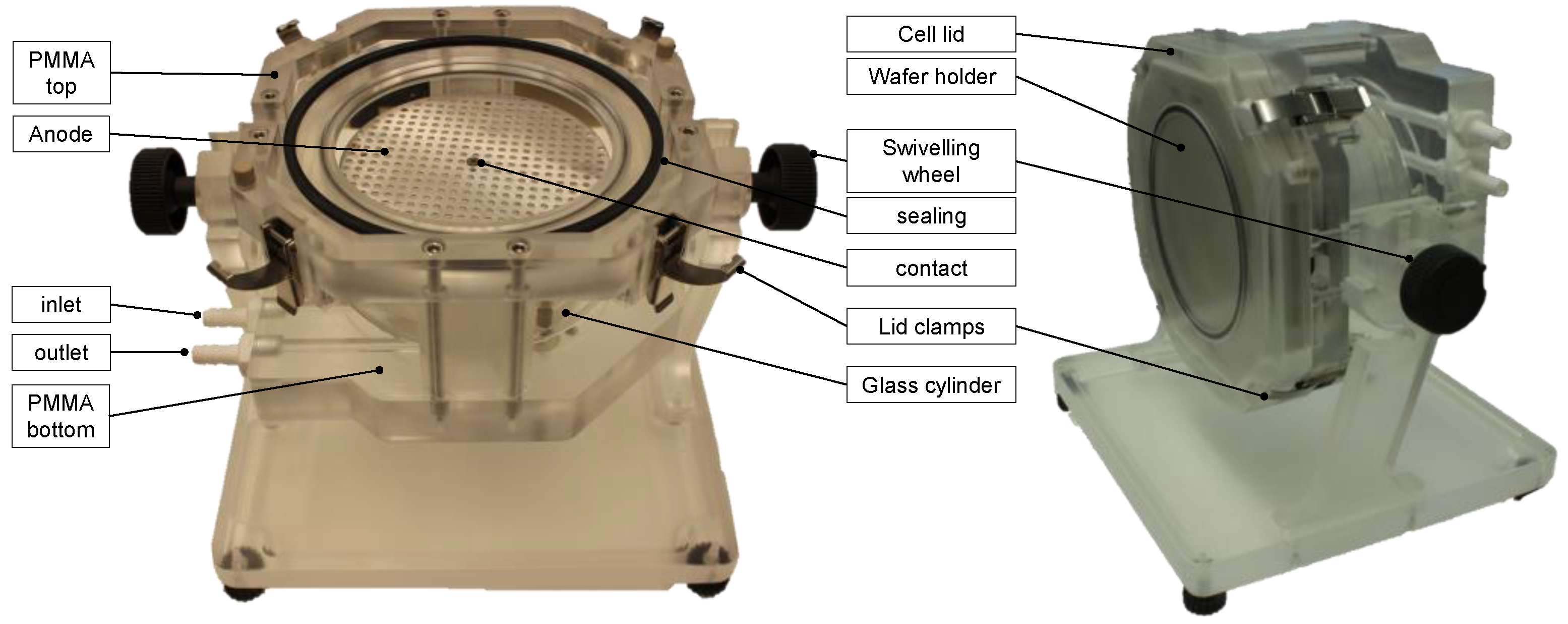
2. Materials and Methods
3. Results and Discussion
3.1. Electroplating on Gold Seed Layer
3.2. Investigation of the Aluminum Oxide Removal on Chip Level
3.2.1. Current-Controlled Oxide Removal
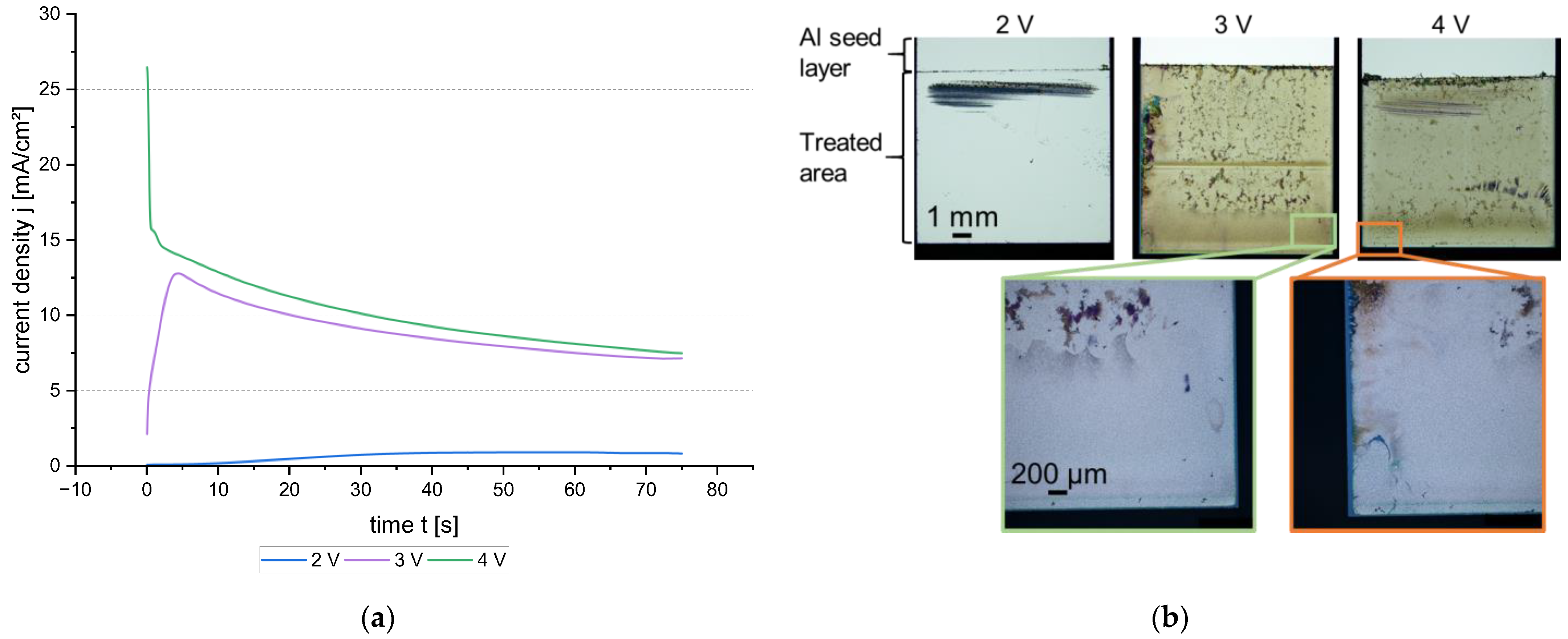
3.2.2. Potential-Controlled Anodic Pulse
3.3. Electroplating on Aluminum Seed Layer
3.3.1. Process Development
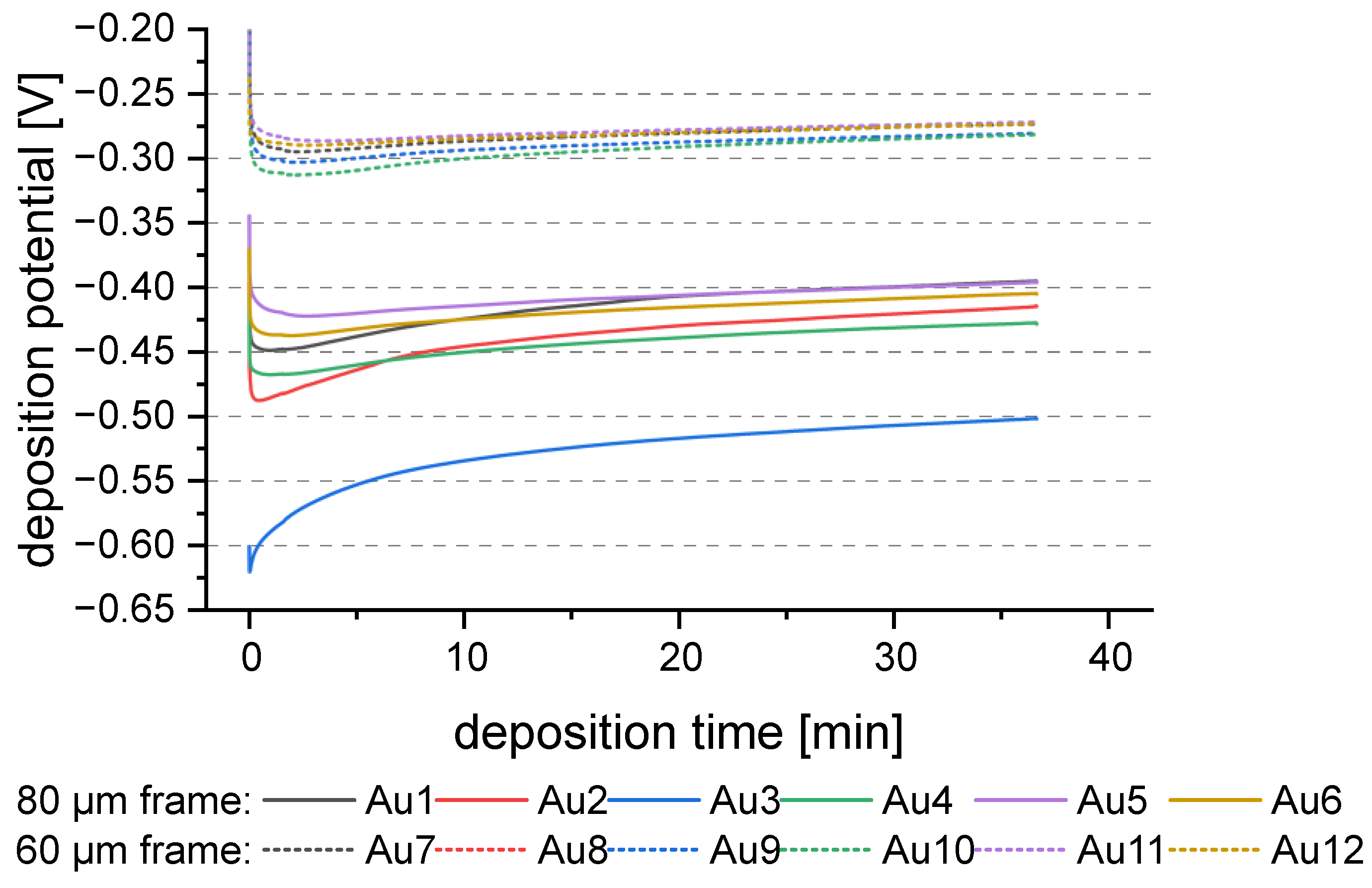
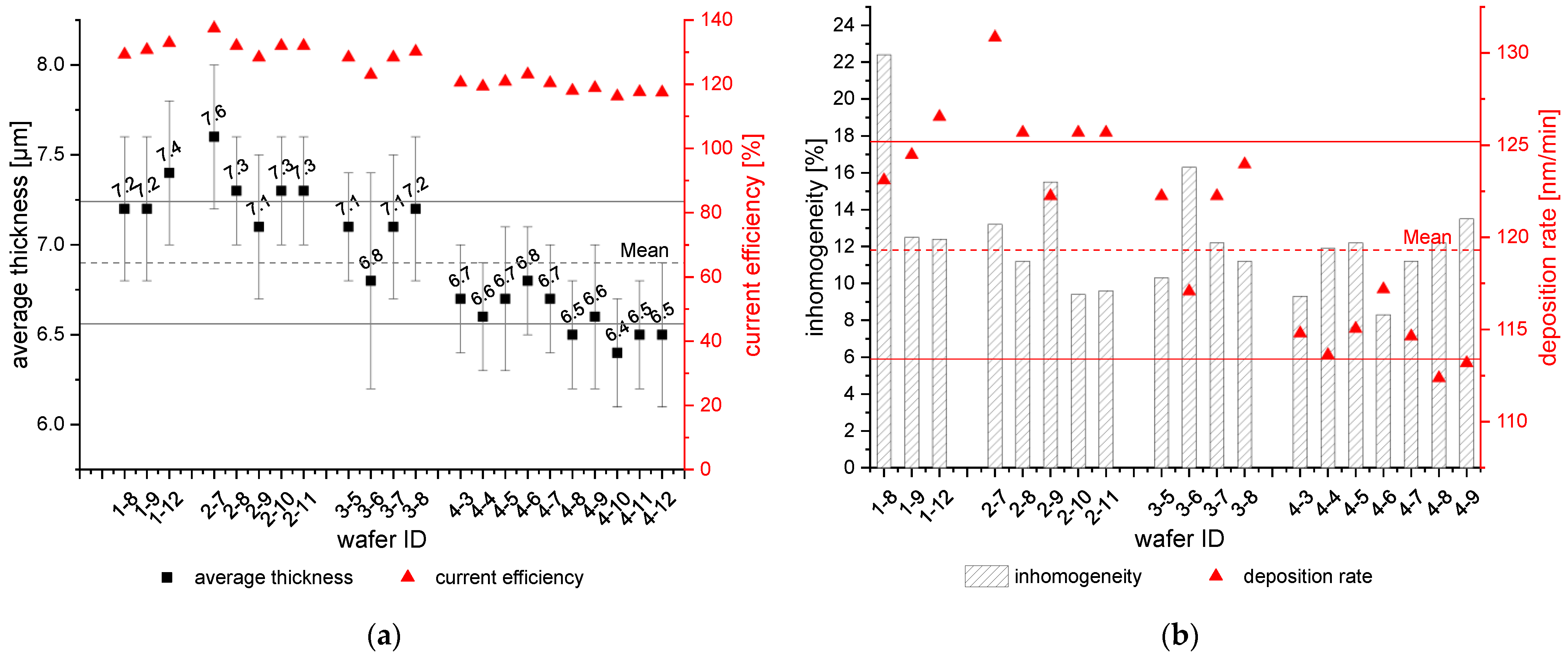
3.3.2. Plating Reproducibility
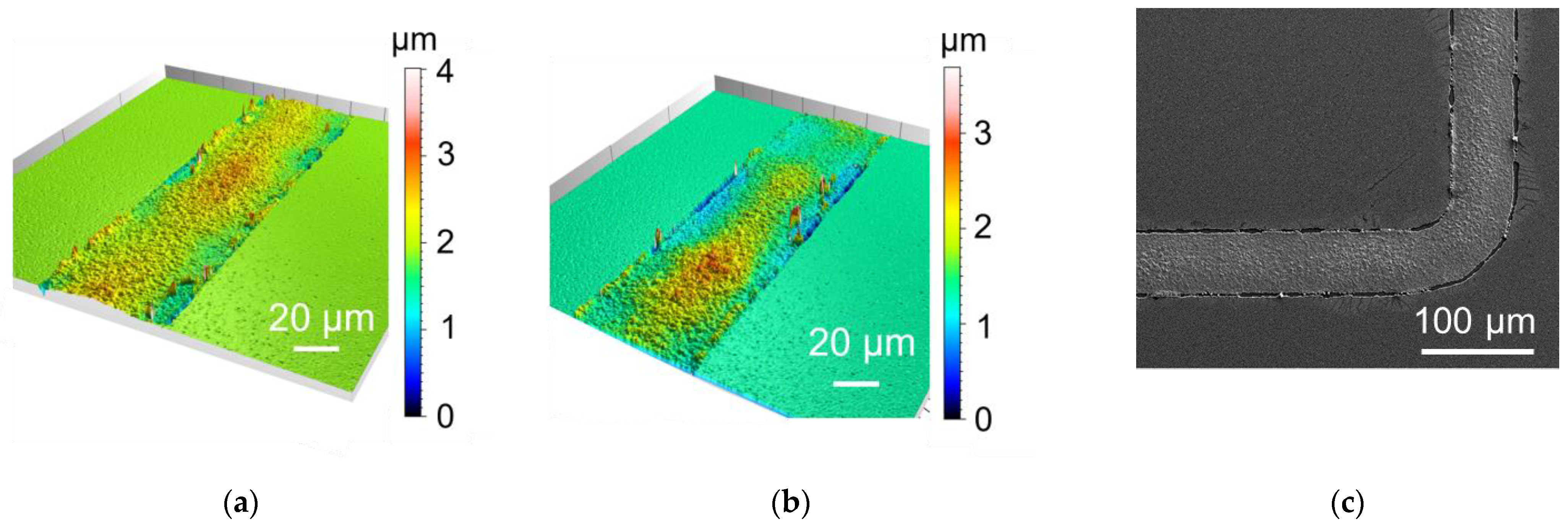
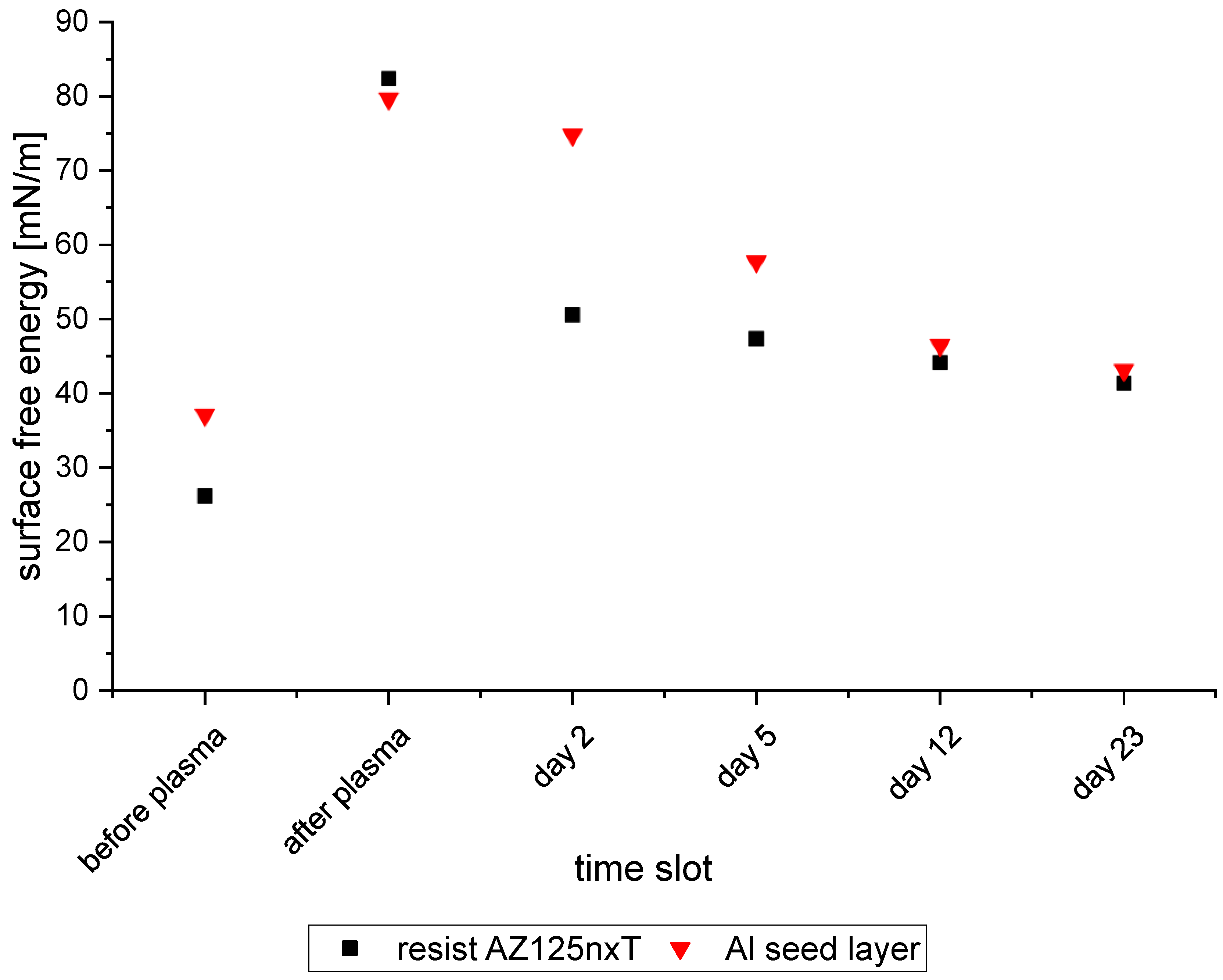
3.3.3. Influence of Plasma Treatment
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Endres, F.; Abbott, A.P.; MacFarlane, D.R. Electrodeposition from Ionic Liquids; Wiley-YCH: Hoboken, NJ, USA, 2008. [Google Scholar]
- Krauss, W.; Konys, J.; Holstein, N.; Zimmermann, H. Al-based anti-corrosion and T-permeation barrier development for future DEMO blankets. J. Nucl. Mater. 2011, 417, 1233–1236. [Google Scholar] [CrossRef]
- Tang, J.; Azumi, K. Improvement of Al coating adhesive strength on the AZ91D magnesium alloy electrodeposited from ionic liquid. Surf. Coat. Technol. 2012, 208, 1–6. [Google Scholar] [CrossRef]
- Bakkar, A.; Neubert, V. Electrodeposition and corrosion characterisation of micro- and nano-crystalline aluminium from AlCl3/1-ethyl-3-methylimidazolium chloride ionic liquid. Electrochim. Acta 2013, 103, 211–218. [Google Scholar] [CrossRef]
- Böttcher, R.; Valitova, A.; Ispas, A.; Bund, A. Electrodeposition of aluminium from ionic liquids on high strength steel. Trans. Inst. Met. Finish. 2019, 97, 82–88. [Google Scholar] [CrossRef]
- Sun, W.-C.; Han, X.; Tao, M. Electroplating of Aluminium on Silicon in an Ionic Liquid. ECS Electrochem. Lett. 2015, 4, D5–D7. [Google Scholar] [CrossRef]
- Al Farisi, M.S.; Tsukamoto, T.; Tanaka, S. Electrochemically Deposited Aluminum for MEMS Thermal Actuator. In Proceedings of the 2021 Smart Systems Integration, SSI 2021, Grenoble, France, 27–29 April 2021. [Google Scholar] [CrossRef]
- Xu, M.; Yue, G.; Bizzotto, D.; Asselin, E. Electrodeposition of Aluminum onto Copper-Coated Printed Circuit Boards. J. Electrochem. Soc. 2017, 164, D729–D736. [Google Scholar] [CrossRef]
- Al Farisi, M.S.; Hertel, S.; Wiemer, M.; Otto, T. Aluminum patterned electroplating from AlCl3-[EMIm]Cl ionic liquid towards microsystems application. Micromachines 2018, 9, 589. [Google Scholar] [CrossRef] [PubMed]
- Al Farisi, M.S.; Tsukamoto, T.; Tanaka, S. Fully CMOS-Compatible Wafer Bonding Based on Press Marking Using Thick Electroplated Aluminum; Fully CMOS-Compatible Wafer Bonding Based on Press Marking Using Thick Electroplated Aluminum. In Proceedings of the 2021 21st International Conference on Solid-State Sensors, Actuators and Microsystems (Transducers), Orlando, FL, USA, 20–24 June 2021. [Google Scholar] [CrossRef]
- Hertel, S.; Wünsch, D.; Wiemer, M.; Otto, T. Galvanische Abscheidung von Aluminium für die Mikrosystem- und Leiterplattentechnik. In MikroSystemTechnik Kongress; VDE Verlag GMbH: Berlin, Germany, 2017; pp. 666–669. [Google Scholar]
- El Abedin, S.Z.; Moustafa, E.M.; Hempelmann, R.; Natter, H.; Endres, F. Electrodeposition of nano- and macrocrystalline aluminium in three different air and water stable ionic liquids. ChemPhysChem 2006, 7, 1535–1543. [Google Scholar] [CrossRef] [PubMed]
- El Abedin, S.Z.; Moustafa, E.M.; Hempelmann, R.; Natter, H.; Endres, F. Additive free electrodeposition of nanocrystalline aluminium in a water and air stable ionic liquid. Electrochem. Commun. 2005, 7, 1111–1116. [Google Scholar] [CrossRef]
- Jiang, T.; Brym, M.J.C.; Dubé, G.; Lasia, A.; Brisard, G.M. Electrodeposition of aluminium from ionic liquids: Part II—Studies on the electrodeposition of aluminum from aluminum chloride (AICl3)—Trimethylphenylammonium chloride (TMPAC) ionic liquids. Surf. Coat. Technol. 2006, 201, 10–18. [Google Scholar] [CrossRef]
- Lai, P.K.; Skyllas-Kazacos, M. Aluminium deposition and dissolution in aluminium chloride-n-butylpyridinium chloride melts. Electrochim. Acta 1987, 32, 1443–1449. [Google Scholar] [CrossRef]
- Zhao, Y.; VanderNoot, T.J. Review: Electrodeposition of aluminium from nonaqueous organic electrolytic systems and room temperature molten salts. Electrochim. Acta 1997, 42, 3–13. [Google Scholar] [CrossRef]
- Maniam, K.K.; Paul, S. A Review on the Electrodeposition of Aluminum and Aluminum Alloys in Ionic Liquids. Coatings 2021, 11, 80. [Google Scholar] [CrossRef]
- Jiang, T.; Brym, M.J.C.; Dubé, G.; Lasia, A.; Brisard, G.M. Electrodeposition of aluminium from ionic liquids: Part I—Electrodeposition and surface morphology of aluminium from aluminium chloride (AlCl3)-1-ethyl-3-methylimidazolium chloride ([EMIm]Cl) ionic liquids. Surf. Coat. Technol. 2006, 201, 1–9. [Google Scholar] [CrossRef]
- Pradhan, D.; Reddy, R.G. Mechanistic study of Al electrodeposition from EMIC-AlCl3 and BMIC-AlCl3 electrolytes at low temperature. Mater. Chem. Phys. 2014, 143, 564–569. [Google Scholar] [CrossRef]
- Pradhan, D.; Reddy, R.G. Dendrite-free aluminum electrodeposition from AlCl 3-1-ethyl-3- methyl-imidazolium chloride ionic liquid electrolytes. Metall. Mater. Trans. B Process Metall. Mater. Process. Sci. 2012, 43, 519–531. [Google Scholar] [CrossRef]
- Xu, B.; Zhang, M.; Ling, G. Electrolytic etching of AZ91D Mg alloy in AlCl3-EMIC ionic liquid for the electrodeposition of adhesive Al coating. Surf. Coat. Technol. 2014, 239, 1–6. [Google Scholar] [CrossRef]
- Ueda, M.; Hariyama, S.; Ohtsuka, T. Al electroplating on the AZ121 Mg alloy in an EMIC-AlCl 3 ionic liquid containing ethylene glycol. J. Solid State Electrochem. 2012, 16, 3423–3427. [Google Scholar] [CrossRef]
- Karthika, B.; Mohan, S.; Bera, A.; Rajasekaran, N. Structure, microstructure and electrodeposition behaviours of aluminium from AlCl3-EMIC and AlCl3-BMIC ionic liquid electrolytes—A comparative study. Surf. Eng. 2020, 36, 1012–1021. [Google Scholar] [CrossRef]
- Boettcher, R.; Mai, S.; Ispas, A.; Bund, A. Aluminum Deposition and Dissolution in [EMIm]Cl-Based Ionic Liquids-Kinetics of Charge-Transfer and the Rate-Determining Step. J. Electrochem. Soc. 2020, 167. [Google Scholar] [CrossRef]
- Hertel, S.; Wiemer, M.; Otto, T. Electroplating of Aluminium for Wafer Level Thermocompression Bonding. In Proceedings of the Waferbond Workshop, Halle/Saale, Germany, 21–25 May 2019. [Google Scholar]
- Braun, S.; Cirulis, I.; Liedtke, J.E.; Hiller, K.; Wiemer, M.; Kuhn, H. Electroplated Aluminum Pillars for Ultrasonic Flip Chip Bonding. In Proceedings of the 55th International Symposium on Microelectronics and Packaging (IMAPS), Boston, MA, USA, 3–6 October 2022; pp. 294–299. [Google Scholar]
- Cirulis, I.; Zschenderlein, U.; Braun, S.; Radestock, M.; Pantou, R.; Vogel, K.; Selbmann, F.; Kurth, S.; Wunderle, B.; Kuhn, H. Investigation of Gold and Aluminum Flip-Chip bonding for Quantum device integration. In Proceedings of the 24th IEEE European Microelectronics Packaging Conference (EMPC), Cambridge, UK, 11–14 September 2023. [Google Scholar]
- Keller, F.; Zelley, W.G. Conditioning Aluminum Alloys for Electroplating. J. Electrochem. Soc. 1950, 97, 143–151. [Google Scholar] [CrossRef]
- Kamp, M.; Bartsch, J.; Cimiotti, G.; Keding, R.; Zogaj, A.; Reichel, C.; Kalio, A.; Glatthaar, M.; Glunz, S. Zincate processes for silicon solar cell metallization. Sol. Energy Mater. Sol. Cells 2014, 120, 332–338. [Google Scholar] [CrossRef]
- Li, B.; Fan, C.; Chen, Y.; Lou, J.; Yan, L. Pulse current electrodeposition of Al from an AlCl3-EMIC ionic liquid. Electrochim. Acta 2011, 56, 5478–5482. [Google Scholar] [CrossRef]
- Tang, J.; Azumi, K. Optimization of pulsed electrodeposition of aluminum from AlCl 3-1-ethyl-3-methylimidazolium chloride ionic liquid. Electrochim. Acta 2011, 56, 1130–1137. [Google Scholar] [CrossRef]
- Al Farisi, M.S.; Tsukamoto, T.; Tanaka, S. Tailoring material properties of electrochemically deposited Al film from chloroaluminate ionic liquid for microsystem technology using pulsed deposition. Sens. Actuators A Phys. 2020, 316. [Google Scholar] [CrossRef]
- Sato, N. A Theory for breakdown of anodic oxide films on metals. Electrochim. Acta 1971, 16, 1683–1692. [Google Scholar] [CrossRef]
- Welch, C.; Mohammad, A.K.; Hosmane, N.S.; Zhang, L.; Cho, K.T. Effect of aluminum oxide on the performance of ionic liquid-based aluminum-air battery. Energies 2020, 13, 2014. [Google Scholar] [CrossRef]
- Cibert, C.; Hidalgo, H.; Champeaux, C.; Tristant, P.; Tixier, C.; Desmaison, J.; Catherinot, A. Properties of aluminum oxide thin films deposited by pulsed laser deposition and plasma enhanced chemical vapor deposition. Thin Solid Films 2008, 516, 1290–1296. [Google Scholar] [CrossRef]









| Wafer ID | Jpeak [mA/cm2] | f [Hz] | Duty Cycle [%] | t [min] | davg [µm] | CE [%] |
|---|---|---|---|---|---|---|
| Al1 | 20 | 25 | 90 | 74 | 0.31 | 9.3 |
| Al2 | 10 | 25 | 90 | 42 | 6.50 | 71.4 |
| Al3 | 5 | 0,02 | 90 | 73 | 6.04 | 89.9 |
| Al4 | 20 | 0,016 | 75 | 201 | 3.00 | 11.0 |
| Wafer ID | Layout | Jpeak | Anodic Potential [V] | Removal Time [s] | Qdeposition [C] | daverage [µm] | Std. dev. [µm] | X [%] | CE [%] |
|---|---|---|---|---|---|---|---|---|---|
| Al5 | 80 µm frames | 5 | 4 | 120 | 327 | 8.8 | 1.0 | 37.8 | 123.8 |
| Al6 | 5 | 3 | 75 | 327 | 9.5 | 1.1 | 38.4 | 133.6 | |
| Al7 | 7.5 | 3 | 75 | 327 | 10.0 | 1.6 | 35.2 | 140.7 | |
| Al8 | 7.5 | 3 | 75 | 981 | 23.1 | 1.6 | 29.0 | 108.3 | |
| Al9 | 60 µm frames | 5 | 3 | 75 | 206 | 8.0 | 0.4 | 8.4 | 105.5 |
| Al10 | 7.5 | 3 | 75 | 213 | 8.2 | 0.3 | 9.0 | 104.6 | |
| Al11 | 5.0 | 3 | 75 | 412 | 16.1 | 0.6 | 7.7 | 106.2 | |
| Al12 | 7.5 | 3 | 75 | 425 | 16.0 | 1.1 | 15.7 | 102.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Braun, S.; Wiemer, M.; Schulz, S.E. Process Development of Aluminum Electroplating from an Ionic Liquid on 150 mm Wafer Level. Micromachines 2024, 15, 746. https://doi.org/10.3390/mi15060746
Braun S, Wiemer M, Schulz SE. Process Development of Aluminum Electroplating from an Ionic Liquid on 150 mm Wafer Level. Micromachines. 2024; 15(6):746. https://doi.org/10.3390/mi15060746
Chicago/Turabian StyleBraun, Silvia, Maik Wiemer, and Stefan E. Schulz. 2024. "Process Development of Aluminum Electroplating from an Ionic Liquid on 150 mm Wafer Level" Micromachines 15, no. 6: 746. https://doi.org/10.3390/mi15060746
APA StyleBraun, S., Wiemer, M., & Schulz, S. E. (2024). Process Development of Aluminum Electroplating from an Ionic Liquid on 150 mm Wafer Level. Micromachines, 15(6), 746. https://doi.org/10.3390/mi15060746







