Etching Rate Analysis Model Based on Quartz Bond Angle Characteristics
Abstract
:1. Introduction
2. Atomic Division of Crystal Plane and Analysis of Etching Rate Model
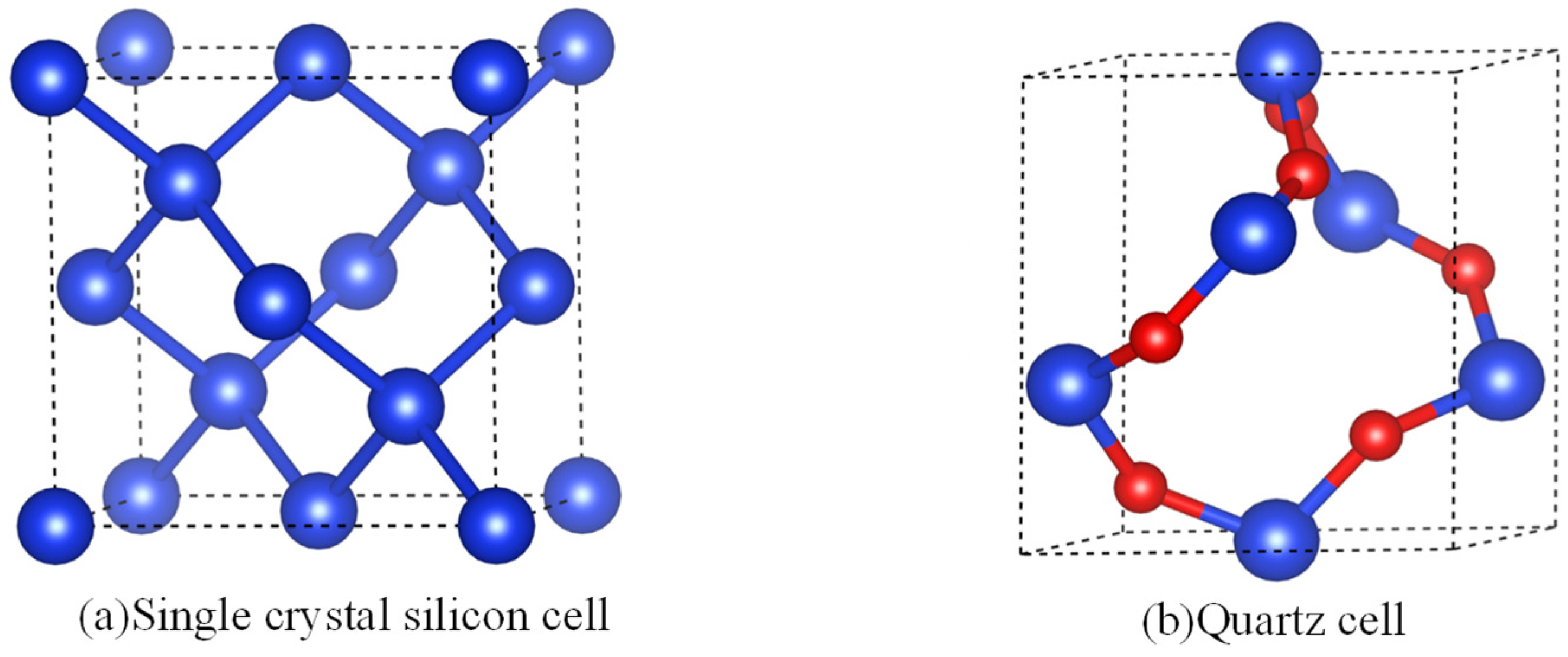
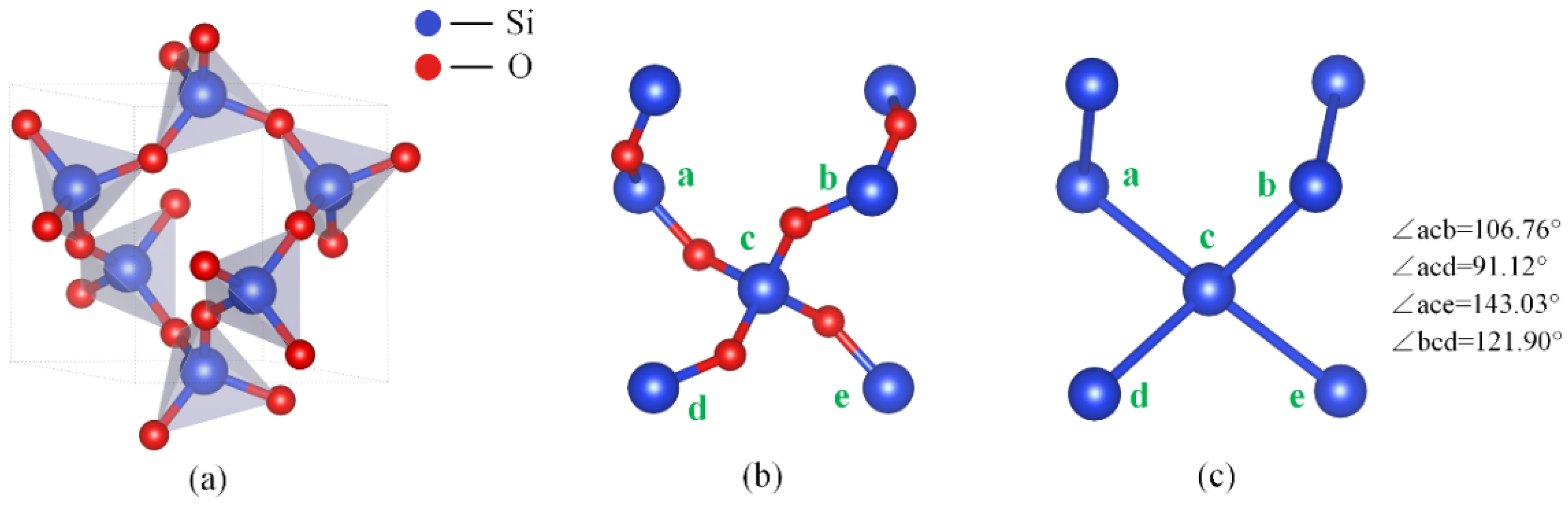
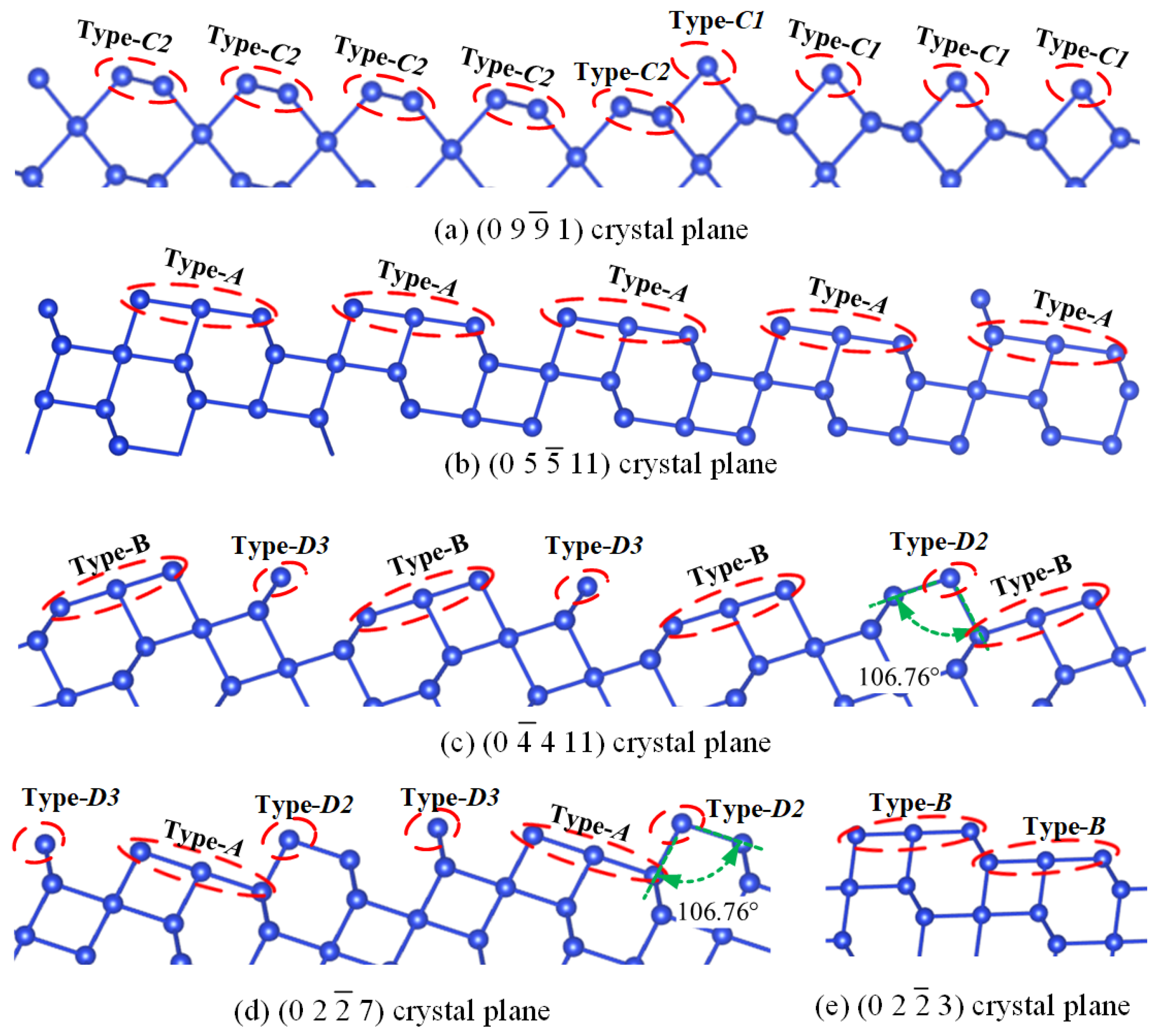
2.1. Method for Atomic Division of Crystal Surface
2.2. Analysis of Etching Mechanism
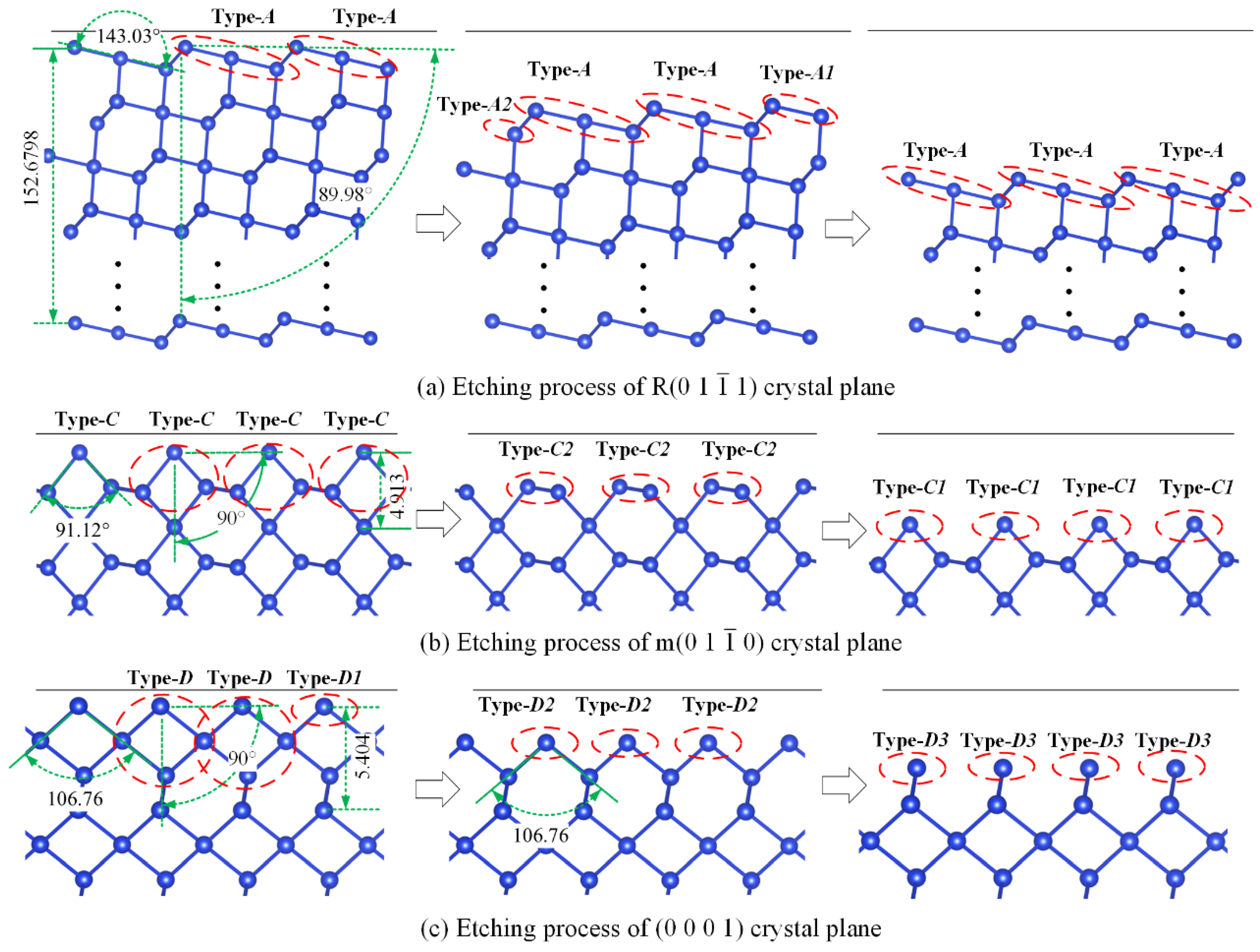
2.3. Partition of X_cut Crystal Plane and Selection of Basic Crystal Plane
3. Result and Discussion
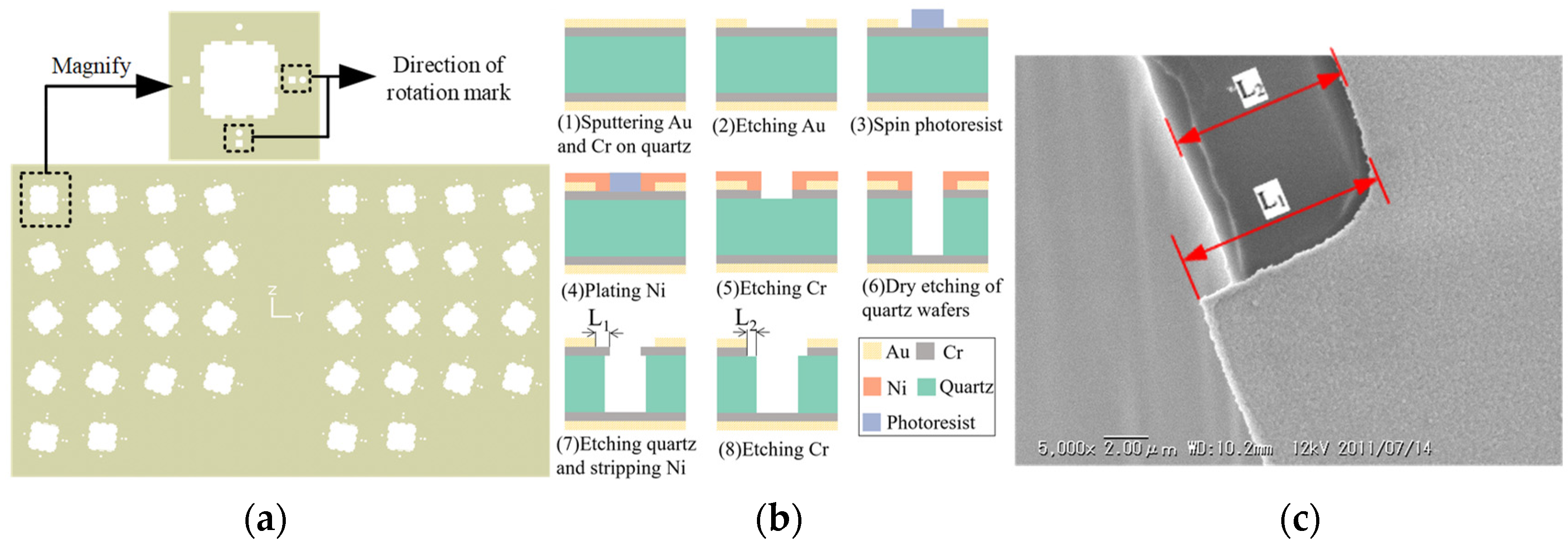
3.1. Experiment
3.2. Result Analysis and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, Y.; Wu, S.; Wang, W.; Wu, T.; Li, X. Piezoelectric Micromachined Ultrasonic Transducers with Micro-Hole Inter-Etch and Sealing Process on (111) Silicon Wafer. Micromachines 2024, 15, 482. [Google Scholar] [CrossRef]
- Zhang, Z.; Wei, S.; Tang, B.; Wei, X.; Zhuang, X. Surface Roughness of Z-Cut Quartz Etched by Ammonium Bifluoride and Ammonium Bifluoride Mixed with Isopropyl Alcohol Solutions. Micromachines 2017, 8, 122. [Google Scholar] [CrossRef]
- Liang, J.X. Development of Quartz MEMS Microfabrication Technologies and Their Application to Capacitive Tilt Sensors; Waseda University: Tokyo, Japan, 2012; pp. 11–31. [Google Scholar]
- Burns, D.W. MEMS wet-etch processes and procedures. In MEMS Materials and Processes Handbook; Springer: Berlin/Heidelberg, Germany, 2011; pp. 457–665. [Google Scholar]
- Negi, S.; Bhandari, R. Silicon isotropic and anisotropic etching for MEMS applications. Microsyst. Technol. 2013, 19, 203–210. [Google Scholar] [CrossRef]
- Zhao, M.; Ji, J.; Ueda, T. Simulation-based Design of Quartz Resonator for Bionic Olfactory Sensor. Sens. Mater. 2021, 33, 1199–1207. [Google Scholar] [CrossRef]
- Linden, J.; Melech, N.; Sakaev, I. Femtosecond laser-assisted fabrication of piezoelectrically actuated crystalline quartz-based MEMS resonators. Microsyst. Nanoeng. 2023, 9, 38. [Google Scholar] [CrossRef]
- Kubena, R.L.; Stratton, F.P.; Nguyen, H.D. A Fully Integrated Quartz MEMS VHF TCXO. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2017, 65, 904–910. [Google Scholar] [CrossRef]
- Dong, Y.; Zhou, Y.; Huang, H.; Zhang, B.; Li, X.; Chen, K.; Sun, L.; Dou, G. Etching of quartz crystals in liquid phase environment: A review. Nanotechnol. Precis. Eng. 2024, 7, 025001. [Google Scholar] [CrossRef]
- Xing, Y.; Qian, J.; Gosálvez, M.A.; Zhang, J.; Zhang, Y. Simulation-based optimization of out-of-plane, variable-height, convoluted quartz micro needle arrays via single-step anisotropic wet etching. Microelectron. Eng. 2020, 231, 111375. [Google Scholar] [CrossRef]
- Xue, H.; Zhang, Z.; Ai, J.; Li, C.; Li, B.; Zhao, Y.; Wang, A. Dynamic process of wet etching using BOE solutions to control the etch rate, roughness, and surface morphology of a Z-cut α-quartz wafer. Ceram. Int. 2024, 4, 340. [Google Scholar] [CrossRef]
- Liang, J.; Zhang, L.; Wang, L.; Dong, Y.; Ueda, T. Flip Chip Bonding of a Quartz MEMS-Based Vibrating Beam Accelerometer. Sensors 2015, 15, 22049–22059. [Google Scholar] [CrossRef]
- Xing, Y.; Gosálvez, M.A.; Zhang, H.; Li, Y.; Qiu, X. Transient and stable profiles during anisotropic wet etching of quartz. J. Microelectromech. Syst. 2017, 26, 1063–1072. [Google Scholar] [CrossRef]
- Liang, J.; Kohsaka, F.; Matsuo, T. Deep wet etching of Z cut quartz wafer for MEMS applications. In Proceedings of the 23rd Sensor Symposium on Sensors, Micromachines, and Applied Systems, Takamatsu, Japan, 5–6 October 2006. [Google Scholar]
- Ueda, T.; Kohsaka, F.; Iino, T. Theory to Predict Etching Shapes in Quartz Crystal and Its Application to Design Devices. Trans. Soc. Instrum. Contr. Eng. 1987, 23, 1233–1238. [Google Scholar]
- Biswas, K.; Kal, S. Etch characteristics of KOH, TMAH and dual doped TMAH for bulk micromachining of silicon. Microelectron. J. 2006, 6, 519–525. [Google Scholar] [CrossRef]
- Kramkowska, M.; Zubel, I. Silicon anisotropic etching in KOH and TMAH with modified surface tension. Procedia Chem. 2009, 9, 774–777. [Google Scholar] [CrossRef]
- Zubel, I. The model of etching of (hkl) planes in monocrystalline silicon. J. Electrochem. Soc. 2003, 150, C391. [Google Scholar] [CrossRef]
- Zhou, Z.; Huang, Q.; Li, W.; Deng, W. A cellular automaton-based simulator for silicon anisotropic etching processes considering high index planes. J. Micromech. Microeng. 2007, 17, S38. [Google Scholar] [CrossRef]
- Hasunuma, R.; Yamabe, K. Evaluation of Kink Generation Rate and Step Flow Velocity on Si (111) during Wet Etching. Jpn. J. Appl. Phys. 2013, 52, 110127. [Google Scholar] [CrossRef]
- Gosálvez, M.A.; Xing, Y.; Sato, K. Analytical solution of the continuous cellular automaton for anisotropic etching. J. Microelectromech. Syst. 2008, 17, 410–431. [Google Scholar] [CrossRef]
- Wu, G.; Xing, Y. The model of etch rates of crystal planes of sapphire based on step flow mechanism. J. Microelectromechanical Syst. 2020, 29, 1234–1244. [Google Scholar] [CrossRef]
- Guo, X.; Gosalvez, M.A.; Xing, Y.; Chen, Y. Etch and growth rates of GaN for surface orientations in the <0001> crystallographic zone: Step flow and terrace erosion/filling via the Continuous Cellular Automaton. Mat. Sci. Semicon. Proc. 2023, 153, 107173. [Google Scholar]
- Bean, K.E. Anisotropic etching of silicon. IEEE. Trans. Electron. Dev. 1978, 25, 1185–1193. [Google Scholar] [CrossRef]
- Zhang, H.; Xing, Y.; Li, Y.; Gosálvez, M.A.; Qiu, X. Kinetic Monte Carlo method for the simulation of anisotropic wet etching of quartz. Sens. Actuators A Phys. 2017, 256, 24–34. [Google Scholar] [CrossRef]
- Hedlund, C.; Lindberg, U.; Bucht, U.; Soderkvist, J. Anisotropic etching of Z-cut quartz. J. Micromech. Microeng. 1993, 3, 65. [Google Scholar] [CrossRef]
- Gosálvez, M.A.; Sato, K.; Foster, A.S.; Nieminen, R.M.; Tanaka, H. An atomistic introduction to anisotropic etching. J. Micromech. Microeng. 2007, 17, S1. [Google Scholar] [CrossRef]
- Pal, P.; Sato, K. A comprehensive review on convex and concave corners in silicon bulk micromachining based on anisotropic wet chemical etching. Micro Nano Syst. Lett. 2015, 3, 6. [Google Scholar] [CrossRef]
- Xing, Y.; Guo, Z.; Gosalvez, M.A.; Wu, G.; Qiu, X. Characterization of anisotropic wet etching of single-crystal sapphire. Sens. Actuators A Phys. 2020, 303, 111667. [Google Scholar] [CrossRef]
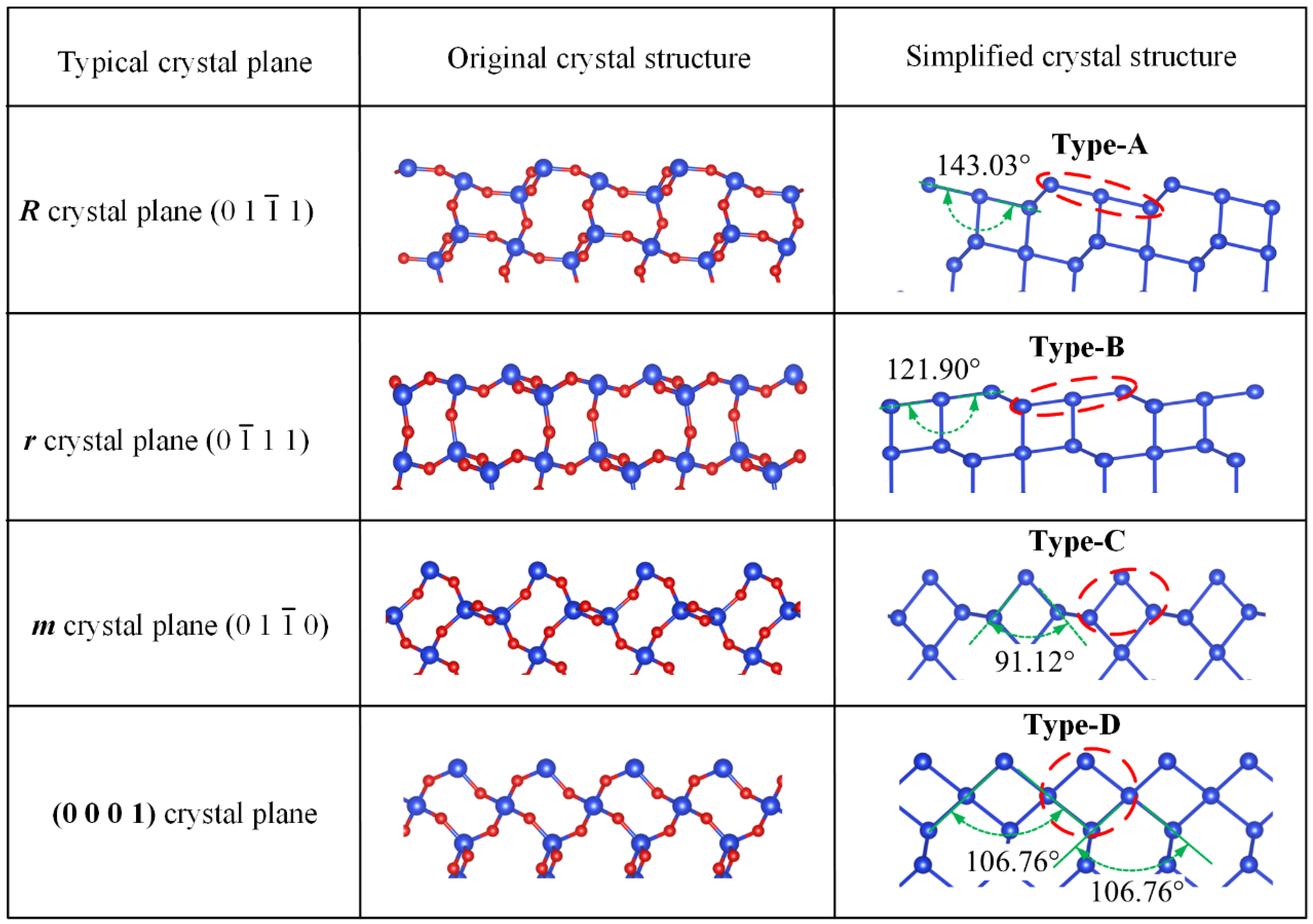


| Basic Crystal Plane | ||||
|---|---|---|---|---|
| Group type | Type-A | Type-B | Type-C | Type-D |
| Depth information (Å) | 152.679 | 152.679 | 54.3125 | 140.847 |
| Layer information | 42 | 42 | 54.3125/4.913 | 140.847/5.404 |
| Corresponding position angle (°) | 89.984 | 90.015 | 89.942 | 89.932 |
| Experimental data (μm/h) | 5.4 | 34.6 | 1.7 | 76 |
| Detachment rate | 0.00084 | 0.0053 | 0.003 | 0.0207 |
| Crystal Plane | Experimental Data (μm/h) | Model Calculation Result (μm/h) | Absolute Error |
|---|---|---|---|
| (0 17 −17 6) | 8 | 9.36 | 1.36 |
| (0 10 −10 9) | 7.2 | 4.91 | 2.29 |
| (0 4 −4 11) | 35.3 | 29.33 | 5.97 |
| (0 −2 2 15) | 73.2 | 71.39 | 1.81 |
| (0 −5 5 11) | 50.9 | 40.17 | 10.76 |
| (0 −10 10 9) | 37.1 | 31.45 | 5.56 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, X.; Lv, C.; Song, S.; Zhao, M.; Ji, J. Etching Rate Analysis Model Based on Quartz Bond Angle Characteristics. Micromachines 2024, 15, 768. https://doi.org/10.3390/mi15060768
Zhao X, Lv C, Song S, Zhao M, Ji J. Etching Rate Analysis Model Based on Quartz Bond Angle Characteristics. Micromachines. 2024; 15(6):768. https://doi.org/10.3390/mi15060768
Chicago/Turabian StyleZhao, Xinjia, Chengbao Lv, Shuanqiang Song, Meng Zhao, and Jing Ji. 2024. "Etching Rate Analysis Model Based on Quartz Bond Angle Characteristics" Micromachines 15, no. 6: 768. https://doi.org/10.3390/mi15060768





