Rapid Fabrication of Tungsten Oxide-Based Electrochromic Devices through Femtosecond Laser Processing
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
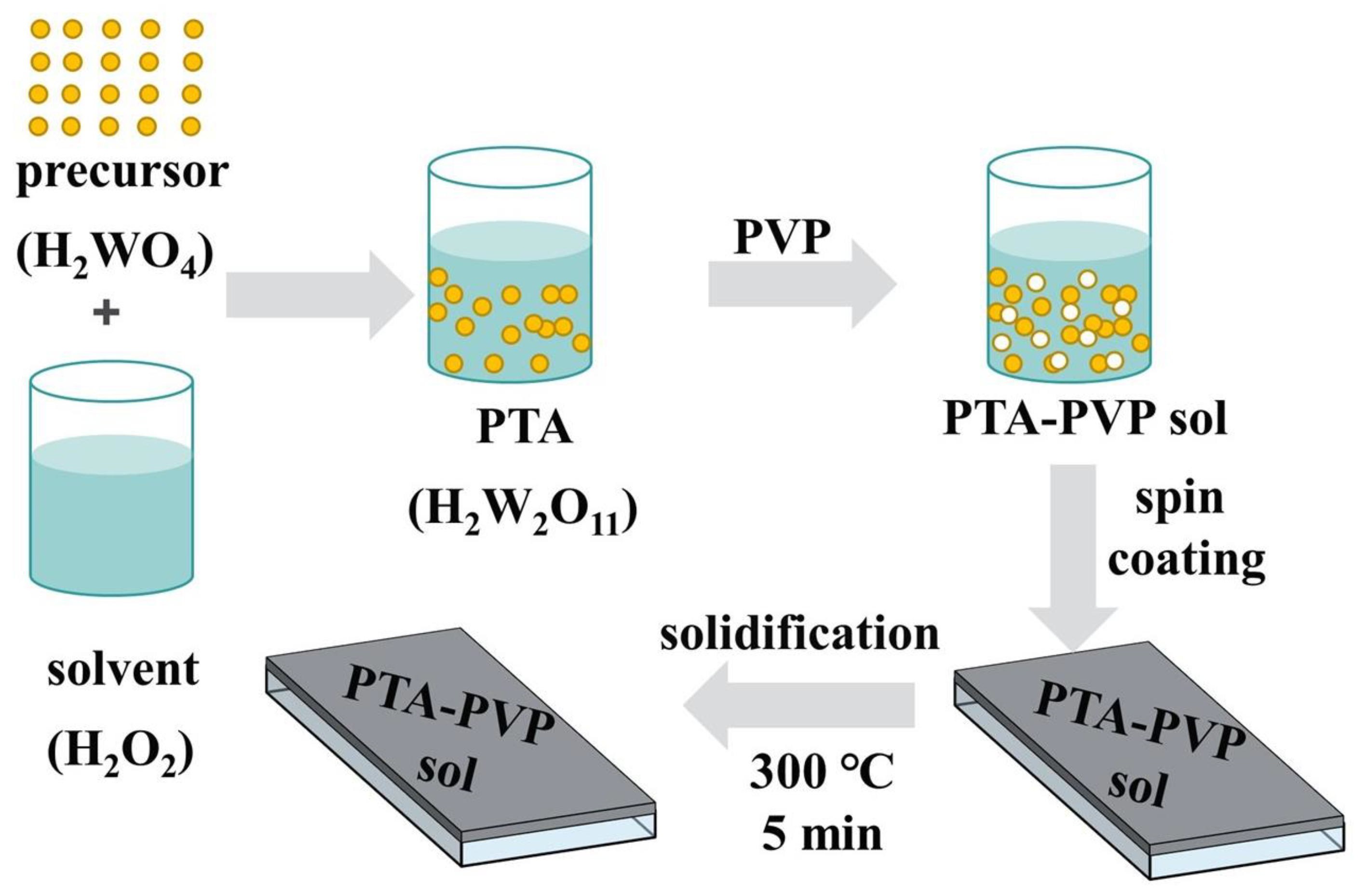
2.2. Preparation of WO3 Precursor Film
2.3. Femtosecond Laser Processing of WO3 Precursor Film
2.4. Characterization
2.5. Electrochemical Measurement
3. Results and Discussions
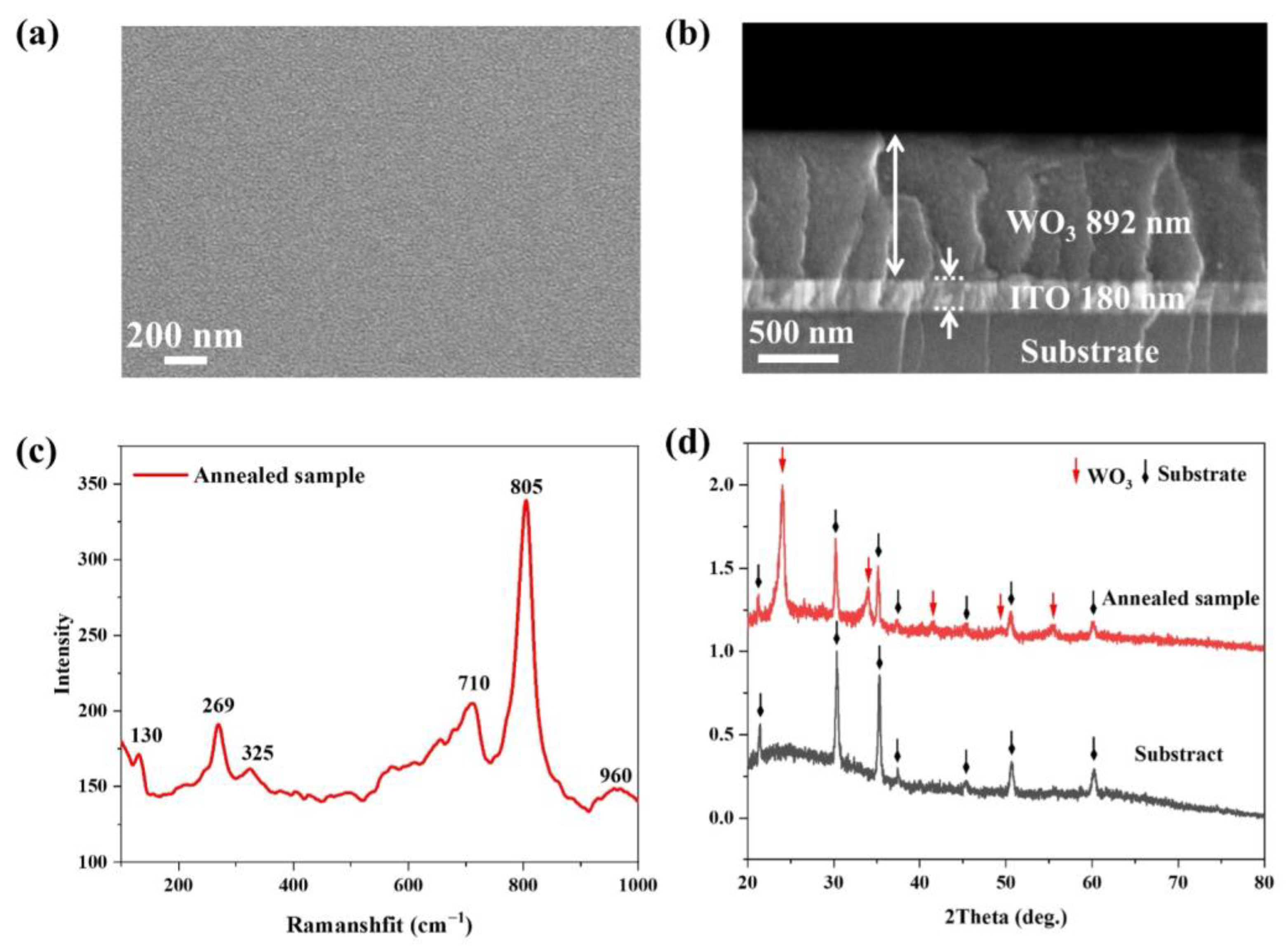
3.1. Characterization of WO3 Precursor Film
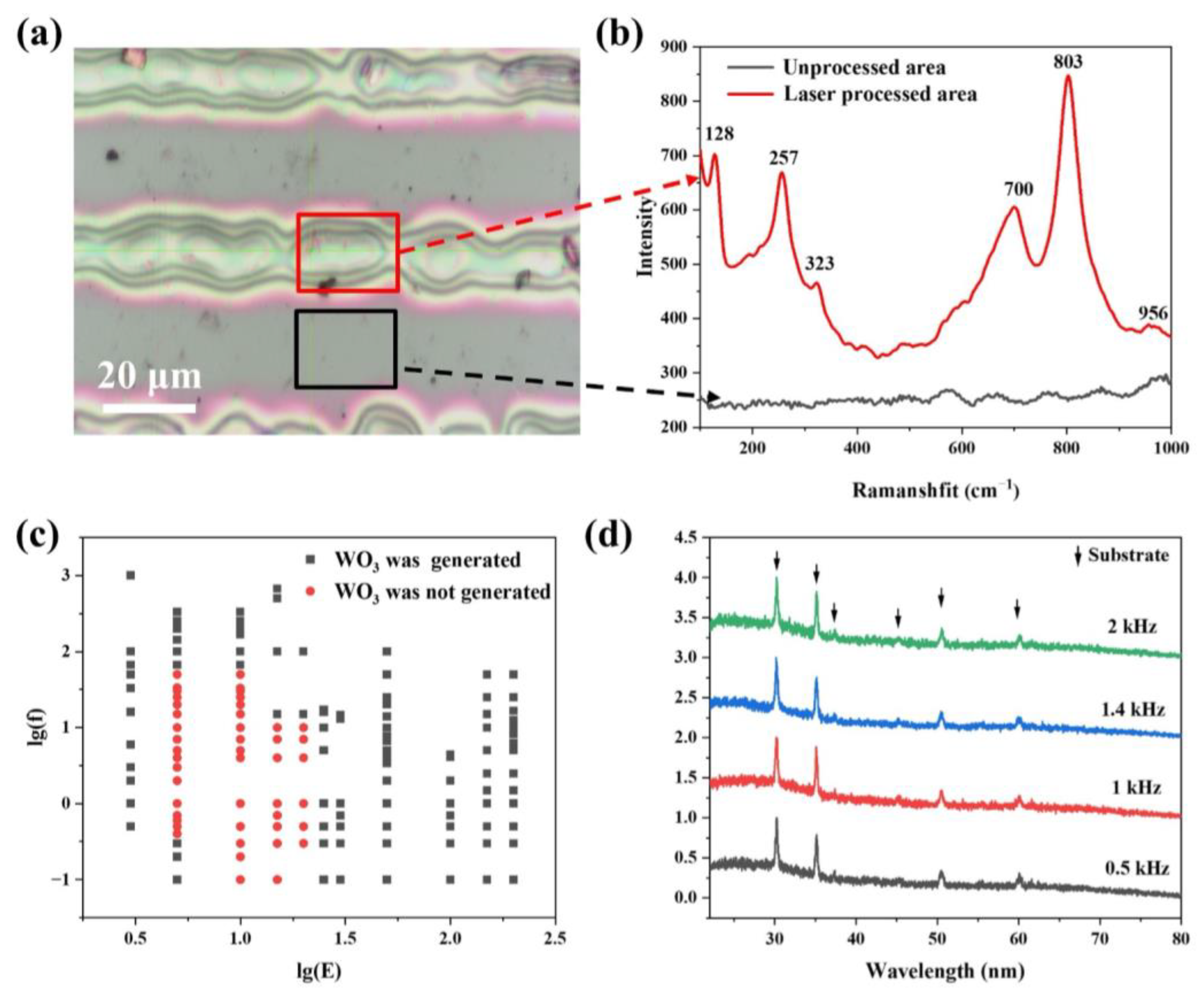
3.2. Femtosecond Laser Processing of PTA-PVP Sol Film
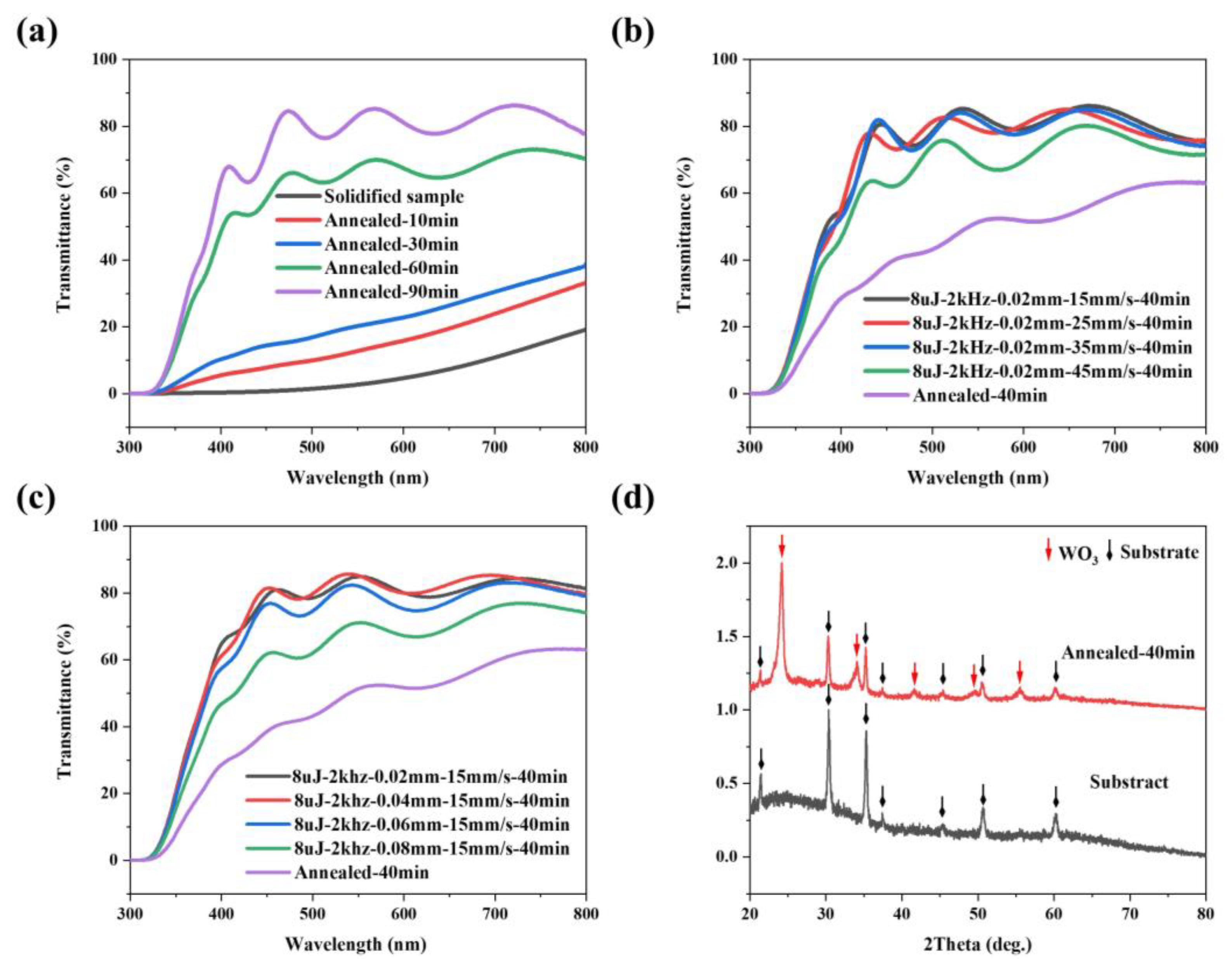
3.3. Characterization of Laser Processed WO3 Film
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rai, V.; Singh, R.S.; Blackwood, D.J.; Zhili, D. A review on recent advances in electrochromic devices: A material approach. Adv. Eng. Mater. 2020, 22, 2000082. [Google Scholar] [CrossRef]
- Hu, C.; Li, L.; Zhou, J.; Li, B.; Zhao, S.; Zou, C. Enhanced contrast of WO3-based smart windows by continuous Li-ion insertion and metal electroplating. ACS Appl. Mater. Interfaces 2022, 14, 32253–32260. [Google Scholar] [CrossRef] [PubMed]
- Arslan, M.; Firat, Y.; Tokgöz, S.R.; Peksoz, A. Fast electrochromic response and high coloration efficiency of Al-doped WO3 thin films for smart window applications. Ceram. Int. 2021, 47, 32570–32578. [Google Scholar] [CrossRef]
- Liu, Z.; Yang, J.; Leftheriotis, G.; Huang, H.; Xia, Y.; Gan, Y.; Zhang, W.; Zhang, J. A solar-powered multifunctional and multimode electrochromic smart window based on WO3/Prussian blue complementary structure. Sustain. Mater. Technol. 2022, 31, e00372. [Google Scholar] [CrossRef]
- Cai, G.; Wang, J.; Lee, P.S. Next-generation multifunctional electrochromic devices. Acc. Chem. Res. 2016, 49, 1469–1476. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Fang, H.; Ma, H.; Wu, L.; Wang, Q.; Wang, H. Self-powered rewritable electrochromic display based on WO3−x film with mechanochemically synthesized MoO3−y nanosheets. ACS Appl. Mater. Interfaces 2021, 13, 20326–20335. [Google Scholar] [CrossRef]
- Ran, X.; Ren, J.; Zhang, S.; Wu, Y.; Wu, S. Multicolor Electrochromic Display and Patterned Device Based on Hollow-SiO2-Supported WO3 Photonic Crystals. ACS Appl. Mater. Interfaces 2023, 15, 41763–41771. [Google Scholar] [CrossRef]
- Chou, H.-H.; Nguyen, A.; Chortos, A.; To, J.W.; Lu, C.; Mei, J.; Kurosawa, T.; Bae, W.-G.; Tok, J.B.-H.; Bao, Z. A chameleon-inspired stretchable electronic skin with interactive colour changing controlled by tactile sensing. Nat. Commun. 2015, 6, 8011. [Google Scholar] [CrossRef] [PubMed]
- Koo, B.-R.; Jo, M.-H.; Kim, K.-H.; Ahn, H.-J. Amorphous-quantized WO3· H2O films as novel flexible electrode for advanced electrochromic energy storage devices. Chem. Eng. J. 2021, 424, 130383. [Google Scholar] [CrossRef]
- Li, W.; Zhang, J.; Zheng, Y.; Cui, Y. High performance electrochromic energy storage devices based on Mo-doped crystalline/amorphous WO3 core-shell structures. Sol. Energy Mater. Sol. Cells 2022, 235, 111488. [Google Scholar] [CrossRef]
- Rudra, S.; Janani, K.; Thamizharasan, G.; Pradhan, M.; Rani, B.; Sahu, N.K.; Nayak, A.K. Fabrication of Mn3O4-WO3 nanoparticles based nanocomposites symmetric supercapacitor device for enhanced energy storage performance under neutral electrolyte. Electrochim. Acta 2022, 406, 139870. [Google Scholar] [CrossRef]
- Yao, Y.; Sang, D.; Zou, L.; Wang, Q.; Liu, C. A review on the properties and applications of WO3 nanostructure-based optical and electronic devices. Nanomaterials 2021, 11, 2136. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.Y.; Sun, Q.; Cui, J.; Yu, X.; Li, S.; Zhang, L.; Jiang, S.; Ma, W.; Ma, R. Review on recent progress in WO3-based electrochromic films: Preparation methods and performance enhancement strategies. Nanoscale 2023, 15, 63–79. [Google Scholar] [CrossRef] [PubMed]
- Crandall, R.; Wojtowicz, P.; Faughnan, B. Theory and measurement of the change in chemical potential of hydrogen in amorphous HxWO3 as a function of the stoichiometric parameter x. Solid State Commun. 1976, 18, 1409–1411. [Google Scholar] [CrossRef]
- Yao, Y.; Zhao, Q.; Wei, W.; Chen, Z.; Zhu, Y.; Zhang, P.; Zhang, Z.; Gao, Y. WO3 quantum-dots electrochromism. Nano Energy 2020, 68, 104350. [Google Scholar] [CrossRef]
- Wang, S.; Fan, W.; Liu, Z.; Yu, A.; Jiang, X. Advances on tungsten oxide based photochromic materials: Strategies to improve their photochromic properties. J. Mater. Chem. C 2018, 6, 191–212. [Google Scholar] [CrossRef]
- Chang, C.-M.; Chiang, Y.-C.; Cheng, M.-H.; Lin, S.-H.; Jian, W.-B.; Chen, J.-T.; Cheng, Y.-J.; Ma, Y.-R.; Tsukagoshi, K. Fabrication of WO3 electrochromic devices using electro-exploding wire techniques and spray coating. Sol. Energy Mater. Sol. Cells 2021, 223, 110960. [Google Scholar] [CrossRef]
- Zhen, Y.; Jelle, B.P.; Gao, T. Electrochromic properties of WO3 thin films: The role of film thickness. Anal. Sci. Adv. 2020, 1, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Cossari, P.; Pugliese, M.; Simari, C.; Mezzi, A.; Maiorano, V.; Nicotera, I.; Gigli, G. Simplified all-solid-state WO3 based electrochromic devices on single substrate: Toward large area, low voltage, high contrast, and fast switching dynamics. Adv. Mater. Interfaces 2020, 7, 1901663. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, H.; Lin, Z.; Guan, X.; Zhang, J.; Tang, X.; Zhan, Y.; Luo, J. Pivotal Role of the Granularity Uniformity of the WO3 Film Electrode upon the Cyclic Stability during Cation Insertion/Extraction. Nanomaterials 2023, 13, 973. [Google Scholar] [CrossRef] [PubMed]
- Naveen Kumar, K.; Shaik, H.; Madhavi, V.; Imran Jafri, R.; Gupta, J.; Nithya, G.; Sattar, S.A.; Ashok Reddy, G. Glancing angle sputter deposited tungsten trioxide (WO3) thin films for electrochromic applications. Appl. Phys. A 2022, 128, 985. [Google Scholar] [CrossRef]
- Louloudakis, D.; Mouratis, K.; Gil-Rostra, J.; Koudoumas, E.; Alvarez, R.; Palmero, A.; Gonzalez-Elipe, A.R. Electrochromic response and porous structure of WO3 cathode layers. Electrochim. Acta 2021, 376, 138049. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, X.; Li, W.; Li, Z.; Zhang, H.; Chen, M.; Sun, W.; Xiao, Y.; Zhao, J.; Li, Y. High-performance electrochromic WO3 film driven by controllable crystalline structure and its all-solid-state device. Sol. Energy Mater. Sol. Cells 2022, 237, 111564. [Google Scholar] [CrossRef]
- Park, Y.J.; Kang, K.-M.; Kang, J.H.; Lee, D.; Na, S.H.; Han, S.H.; Nah, Y.-C.; Kim, D.H. Structure and electrochromic properties of WO3 thin films by direct current, pulsed direct current, and radio frequency magnetron sputtering from metallic and ceramic targets. Thin Solid Film. 2022, 763, 139596. [Google Scholar] [CrossRef]
- Wang, C.-H.; Yen, H.-K.; Yang, S.-M.; Lu, K.-C. Catalyst-free synthesis of tungsten oxide nanowires via thermal evaporation for fast-response electrochromic devices. CrystEngComm 2022, 24, 8213–8218. [Google Scholar] [CrossRef]
- Kim, K.-W.; Yun, T.Y.; You, S.-H.; Tang, X.; Lee, J.; Seo, Y.; Kim, Y.-T.; Kim, S.H.; Moon, H.C.; Kim, J.K. Extremely fast electrochromic supercapacitors based on mesoporous WO3 prepared by an evaporation-induced self-assembly. NPG Asia Mater. 2020, 12, 84. [Google Scholar] [CrossRef]
- Shakoury, R.; Arman, A.; Rezaee, S.; Korpi, A.G.; Kulesza, S.; Luna, C.; Bramowicz, M.; Mardani, M. Optical properties and morphology analysis of hexagonal WO3 thin films obtained by electron beam evaporation. J. Mater. Sci. Mater. Electron. 2021, 32, 798–805. [Google Scholar] [CrossRef]
- Adilakshmi, G.; Reddy, A.S.; Reddy, P.S.; Reddy, C.S. Electron beam evaporated nanostructure WO3 films for gas sensor application. Mater. Sci. Eng. B 2021, 273, 115421. [Google Scholar] [CrossRef]
- Chang, J.-Y.; Chen, Y.-C.; Wang, C.-M.; Wang, W.-N.; Wen, C.-Y.; Lin, J.-M. Electrochromic properties of Lithium-doped tungsten oxide prepared by electron beam evaporation. Coatings 2019, 9, 191. [Google Scholar] [CrossRef]
- Huang, L.; Tang, H.; Bai, Y.; Pu, Y.; Li, L.; Cheng, J. Preparation of monodispersed Cs0.33WO3 nanocrystals by mist chemical vapor deposition for near-infrared shielding application. Nanomaterials 2020, 10, 2295. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Kim, E.-B.; Kwak, D.-H.; Akhtar, M.S.; Ameen, S. Controlled growth of WO3 pyramidal thin film via hot-filament chemical vapor deposition: Electrochemical detection of ethylenediamine. Chemosensors 2021, 9, 257. [Google Scholar] [CrossRef]
- Abbaspoor, M.; Aliannezhadi, M.; Tehrani, F.S. Effect of solution pH on as-synthesized and calcined WO3 nanoparticles synthesized using sol-gel method. Opt. Mater. 2021, 121, 111552. [Google Scholar] [CrossRef]
- Aliannezhadi, M.; Abbaspoor, M.; Shariatmadar Tehrani, F.; Jamali, M. High photocatalytic WO3 nanoparticles synthesized using Sol-gel method at different stirring times. Opt. Quantum Electron. 2023, 55, 250. [Google Scholar] [CrossRef]
- Zheng, J.Y.; Sun, Q.; Yang, H.; Yu, S.; Li, M.; Yu, X.; Wang, C.; Liu, T.; Li, S. Amorphous bismuth and GO co-doped WO3 electrochromic film with fast-switching time and long-term stability. Dalton Trans. 2024, 53, 2460–2464. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Li, S.; Yu, X.; Zhang, Y.; Liu, T.; Zheng, J.Y. Amorphous bismuth-doped WO3 film: Fast-switching time and high-performance proton-based aqueous electrochromic device. Appl. Surf. Sci. 2023, 641, 158510. [Google Scholar] [CrossRef]
- Vankova, S.; Zanarini, S.; Amici, J.; Camara, F.; Arletti, R.; Bodoardo, S.; Penazzi, N. WO3 nanorolls self-assembled as thin films by hydrothermal synthesis. Nanoscale 2015, 7, 7174–7177. [Google Scholar] [CrossRef] [PubMed]
- Shembade, U.V.; Dhas, S.D.; Gurav, S.R.; Sonkawade, R.G.; Wategaonkar, S.B.; Ghatage, S.R.; Gaikwad, M.A.; Kim, J.H.; Parale, V.G.; Park, H.-H. Acid substitutions for WO3 nanostructures synthesis by the hydrothermal route and its effect on physio-chemical and electrochemical properties for supercapacitors. J. Energy Storage 2023, 72, 108432. [Google Scholar] [CrossRef]
- Zakharova, G.S.; Natal’ya, V.; Tat’yana, I.G.; Pervova, M.G.; Murzakaev, A.M.; Enyashin, A.N. Morphology-controlling hydrothermal synthesis of h-WO3 for photocatalytic degradation of 1,2,4-trichlorobenzene. J. Alloys Compd. 2023, 938, 168620. [Google Scholar] [CrossRef]
- Kyaw, A.M.M.; Panomsuwan, G.; Munprom, R. Effect of hydrothermal reaction time on the photoelectrochemical performance of nanostructured WO3 photoanode. Mater. Today Proc. 2022, 65, 2456–2460. [Google Scholar] [CrossRef]
- Palharim, P.H.; Caira, M.C.D.A.; de Araújo Gusmão, C.; Ramos, B.; dos Santos, G.T.; Rodrigues, O., Jr.; Teixeira, A.C.S.C. Effect of temperature and time on the hydrothermal synthesis of WO3-AgCl photocatalysts regarding photocatalytic activity. Chem. Eng. Res. Des. 2022, 188, 935–953. [Google Scholar] [CrossRef]
- Kolhe, P.S.; Mutadak, P.; Maiti, N.; Sonawane, K.M. Synthesis of WO3 nanoflakes by hydrothermal route and its gas sensing application. Sens. Actuators A Phys. 2020, 304, 111877. [Google Scholar] [CrossRef]
- Tehrani, F.S.; Ahmadian, H.; Aliannezhadi, M. Hydrothermal synthesis and characterization of WO3 nanostructures: Effect of reaction time. Mater. Res. Express 2020, 7, 015911. [Google Scholar] [CrossRef]
- Wei, Y.; Zhou, J.; Zheng, J.; Xu, C. Improved stability of electrochromic devices using Ti-doped V2O5 film. Electrochim. Acta 2015, 166, 277–284. [Google Scholar] [CrossRef]
- Tong, M.; Dai, G.; Gao, D. WO3 thin film sensor prepared by sol–gel technique and its low-temperature sensing properties to trimethylamine. Mater. Chem. Phys. 2001, 69, 176–179. [Google Scholar] [CrossRef]
- Deepa, M.; Sharma, R.; Basu, A.; Agnihotry, S. Effect of oxalic acid dihydrate on optical and electrochemical properties of sol–gel derived amorphous electrochromic WO3 films. Electrochim. Acta 2005, 50, 3545–3555. [Google Scholar] [CrossRef]
- Deepa, M.; Kar, M.; Agnihotry, S. Electrodeposited tungsten oxide films: Annealing effects on structure and electrochromic performance. Thin Solid Film. 2004, 468, 32–42. [Google Scholar] [CrossRef]
- Krašovec, U.O.; Vuk, A.Š.; Orel, B. IR Spectroscopic studies of charged–discharged crystalline WO3 films. Electrochim. Acta 2001, 46, 1921–1929. [Google Scholar] [CrossRef]
- Choi, Y.-G.; Sakai, G.; Shimanoe, K.; Miura, N.; Yamazoe, N. Wet process-prepared thick films of WO3 for NO2 sensing. Sens. Actuators B Chem. 2003, 95, 258–265. [Google Scholar] [CrossRef]
- Ning, H.; Zhang, X.; Zhang, G.; Shi, M.; Huang, Z.; Ni, H.; Tao, R.; Yao, R.; Peng, J.; Fang, Z. The characters of WO3 electrochromic film prepared by sol-gel method. In Proceedings of the 2018 19th International Conference on Electronic Packaging Technology (ICEPT), Shanghai, China, 8–11 August 2018; pp. 607–609. [Google Scholar]
- Dutta Majumdar, J.; Manna, I. Laser processing of materials. Sadhana 2003, 28, 495–562. [Google Scholar] [CrossRef]
- Cho, H.; Min, J.; Won, D.; Kwon, J.; Ko, S.H. Selective photo-thermal conversion of tungsten oxide sol precursor for electrochromic smart window applications. Acta Mater. 2020, 201, 528–534. [Google Scholar] [CrossRef]
- Zheng, J.Y.; Pawar, A.U.; Kim, C.W.; Kim, Y.J.; Kang, Y.S. Highly enhancing photoelectrochemical performance of facilely-fabricated Bi-induced (002)-oriented WO3 film with intermittent short-time negative polarization. Appl. Catal. B Environ. 2018, 233, 88–98. [Google Scholar] [CrossRef]
- Zheng, J.Y.; Kim, C.W.; Pawar, A.U.; Kang, Y.S. Fabrication of p-Cu2O/n-Bi-WO3 heterojunction thin films: Optical and photoelectrochemical properties. New J. Chem. 2017, 41, 755–762. [Google Scholar] [CrossRef]
- Song, W.; Zhang, R.; Bai, X.; Jia, Q.; Ji, H. Exposed crystal facets of WO3 nanosheets by phase control on NO2-sensing performance. J. Mater. Sci. Mater. Electron. 2020, 31, 610–620. [Google Scholar] [CrossRef]
- Rougier, A.; Portemer, F.; Quédé, A.; El Marssi, M. Characterization of pulsed laser deposited WO3 thin films for electrochromic devices. Appl. Surf. Sci. 1999, 153, 1–9. [Google Scholar] [CrossRef]
- Zheng, J.Y.; Song, G.; Kim, C.W.; Kang, Y.S. Fabrication of (001)-oriented monoclinic WO3 films on FTO substrates. Nanoscale 2013, 5, 5279–5282. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Lu, K.; Zhang, X.; Yuan, W.; Shi, M.; Ning, H.; Tao, R.; Liu, X.; Yao, R.; Peng, J. Effects of annealing temperature on optical band gap of sol-gel tungsten trioxide films. Micromachines 2018, 9, 377. [Google Scholar] [CrossRef] [PubMed]
- Al Mohammad, A.; Gillet, M. Phase transformations in WO3 thin films during annealing. Thin Solid Film. 2002, 408, 302–309. [Google Scholar] [CrossRef]
- Pecquenard, B.; Castro-Garcia, S.; Livage, J.; Zavalij, P.Y.; Whittingham, M.S.; Thouvenot, R. Structure of Hydrated Tungsten Peroxides [WO2(O2)H2O]·nH2O. Chem. Mater. 1998, 10, 1882–1888. [Google Scholar] [CrossRef]
- Yang, H.; Shang, F.; Gao, L.; Han, H. Structure, electrochromic and optical properties of WO3 film prepared by dip coating-pyrolysis. Appl. Surf. Sci. 2007, 253, 5553–5557. [Google Scholar] [CrossRef]
- Siegel, J.; Gawelda, W.; Puerto, D.; Dorronsoro, C.; Solis, J.; Afonso, C.N.; De Sande, J.; Bez, R.; Pirovano, A.; Wiemer, C. Amorphization dynamics of Ge2Sb2Te5 films upon nano-and femtosecond laser pulse irradiation. J. Appl. Phys. 2008, 103, 023516. [Google Scholar] [CrossRef]
- Bonse, J.; Bachelier, G.; Siegel, J.; Solis, J. Time-and space-resolved dynamics of melting, ablation, and solidification phenomena induced by femtosecond laser pulses in germanium. Phys. Rev. B 2006, 74, 134106. [Google Scholar] [CrossRef]
- Ou, J.Z.; Balendhran, S.; Field, M.R.; McCulloch, D.G.; Zoolfakar, A.S.; Rani, R.A.; Zhuiykov, S.; O’Mullane, A.P.; Kalantar-Zadeh, K. The anodized crystalline WO3 nanoporous network with enhanced electrochromic properties. Nanoscale 2012, 4, 5980–5988. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Liu, Z.; Zhao, L.; Chen, Y.; Li, J.; Yan, W. Efficient electrochromic efficiency and stability of amorphous/crystalline tungsten oxide film. J. Alloys Compd. 2023, 930, 167405. [Google Scholar] [CrossRef]
- Granqvist, C.G. Electrochromics for smart windows: Oxide-based thin films and devices. Thin Solid Film. 2014, 564, 1–38. [Google Scholar] [CrossRef]
- Haro-Poniatowski, E.; Jouanne, M.; Morhange, J.; Julien, C.; Diamant, R.; Fernández-Guasti, M.; Fuentes, G.; Alonso, J.C. Micro-Raman characterization of WO3 and MoO3 thin films obtained by pulsed laser irradiation. Appl. Surf. Sci. 1998, 127, 674–678. [Google Scholar] [CrossRef]
- Tong, Z.; Tian, Y.; Zhang, H.; Li, X.; Ji, J.; Qu, H.; Li, N.; Zhao, J.; Li, Y. Recent advances in multifunctional electrochromic energy storage devices and photoelectrochromic devices. Sci. China Chem. 2017, 60, 13–37. [Google Scholar] [CrossRef]
- Zhou, D.; Xie, D.; Xia, X.; Wang, X.; Gu, C.; Tu, J. All-solid-state electrochromic devices based on WO3|| NiO films: Material developments and future applications. Sci. China Chem. 2017, 60, 3–12. [Google Scholar] [CrossRef]
- Ma, D.; Wang, J. Inorganic electrochromic materials based on tungsten oxide and nickel oxide nanostructures. Sci. China Chem. 2017, 60, 54–62. [Google Scholar] [CrossRef]
- Kim, M.H.; Choi, H.W.; Kim, K.H. Thickness dependence of WO3−x thin films for electrochromic device application. Mol. Cryst. Liq. Cryst. 2014, 598, 54–61. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Zhai, Z.; Li, L. Rapid Fabrication of Tungsten Oxide-Based Electrochromic Devices through Femtosecond Laser Processing. Micromachines 2024, 15, 785. https://doi.org/10.3390/mi15060785
Wang L, Zhai Z, Li L. Rapid Fabrication of Tungsten Oxide-Based Electrochromic Devices through Femtosecond Laser Processing. Micromachines. 2024; 15(6):785. https://doi.org/10.3390/mi15060785
Chicago/Turabian StyleWang, Liqun, Zihao Zhai, and Longnan Li. 2024. "Rapid Fabrication of Tungsten Oxide-Based Electrochromic Devices through Femtosecond Laser Processing" Micromachines 15, no. 6: 785. https://doi.org/10.3390/mi15060785
APA StyleWang, L., Zhai, Z., & Li, L. (2024). Rapid Fabrication of Tungsten Oxide-Based Electrochromic Devices through Femtosecond Laser Processing. Micromachines, 15(6), 785. https://doi.org/10.3390/mi15060785






