Harnessing Standing Sound Waves to Treat Intraocular Blood Cell Accumulation
Abstract
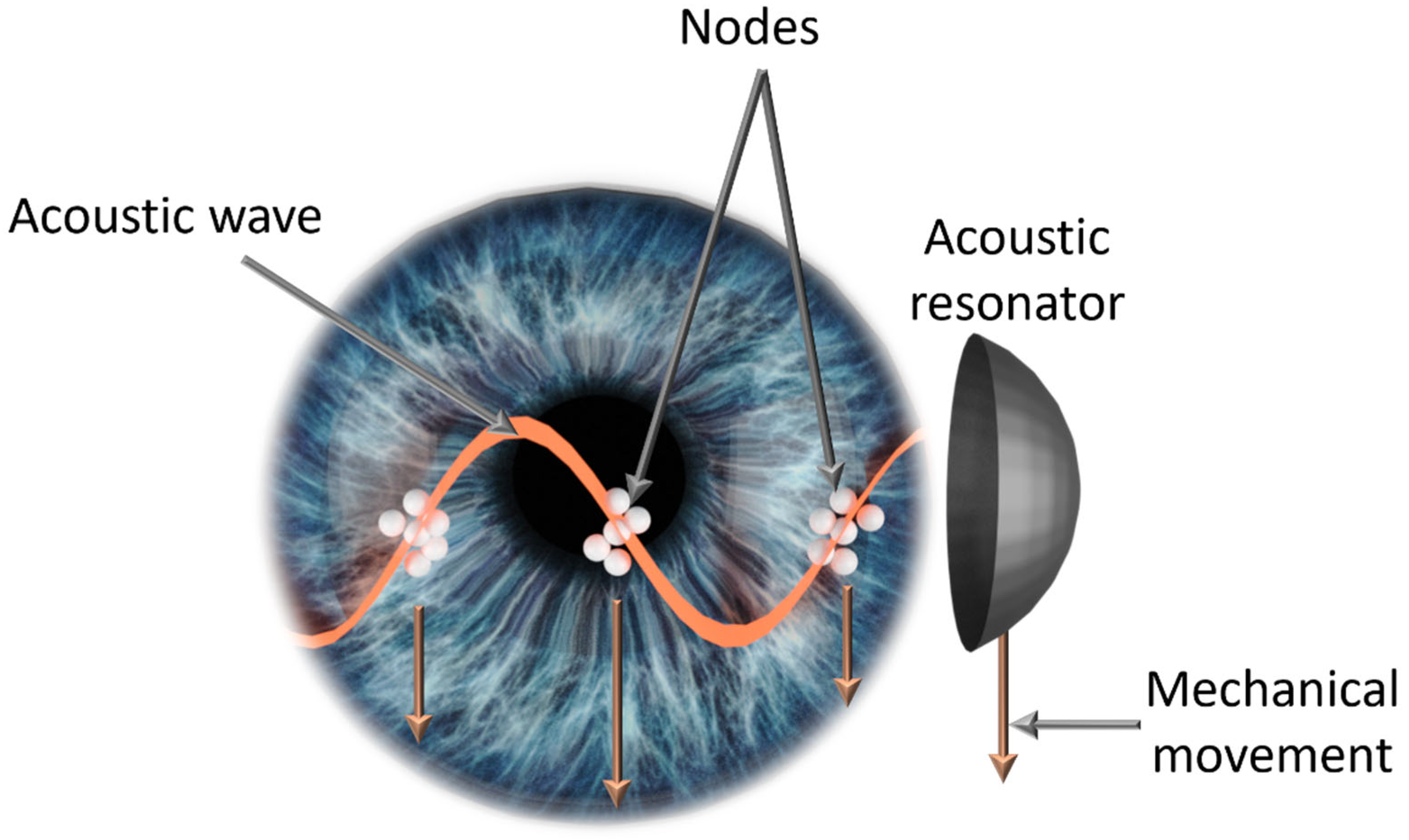
:1. Introduction
2. Materials and Methods
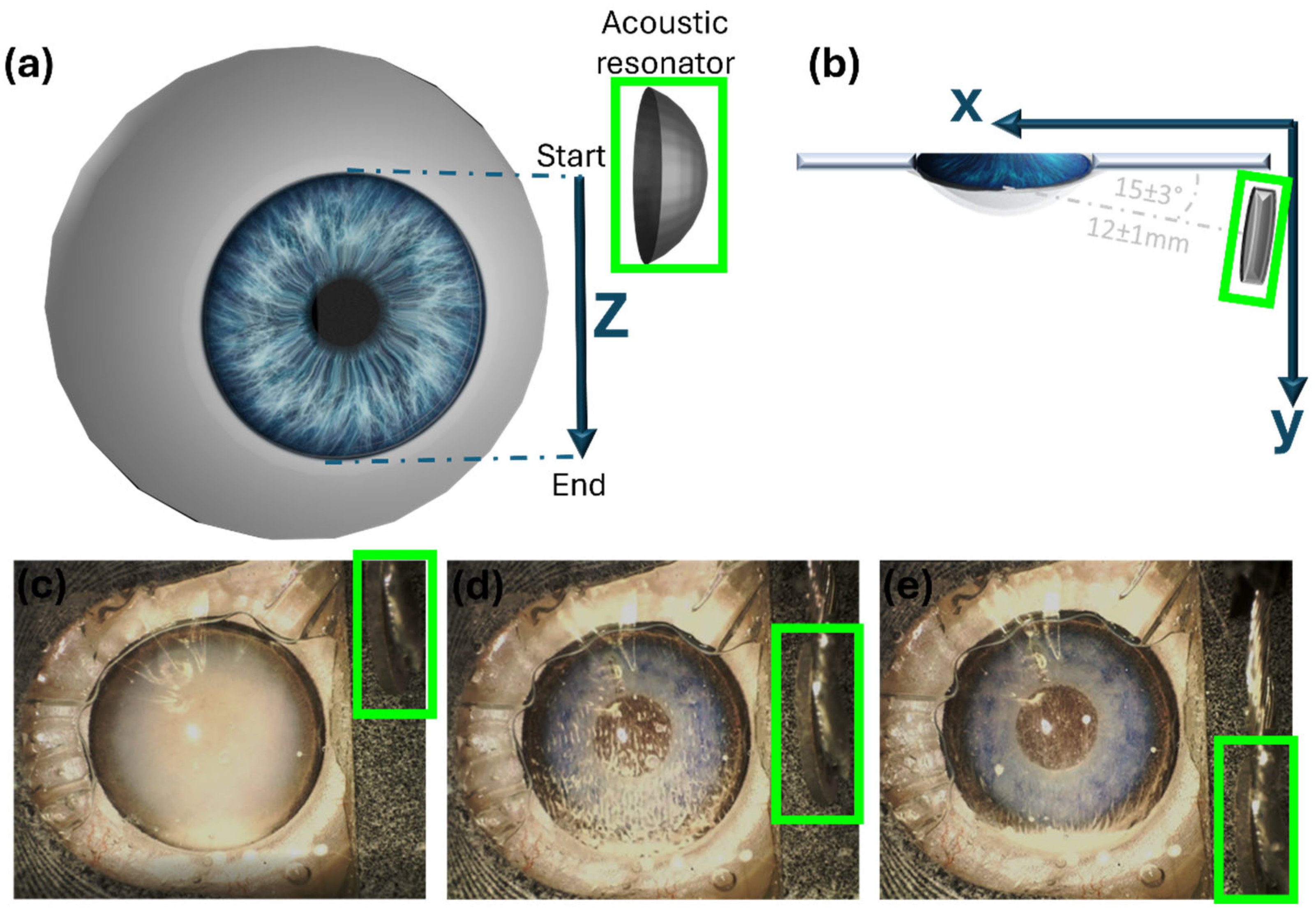
2.1. Intraocular Manipulation Device
2.2. In Vitro Model
2.3. Ex Vivo Model
3. Results
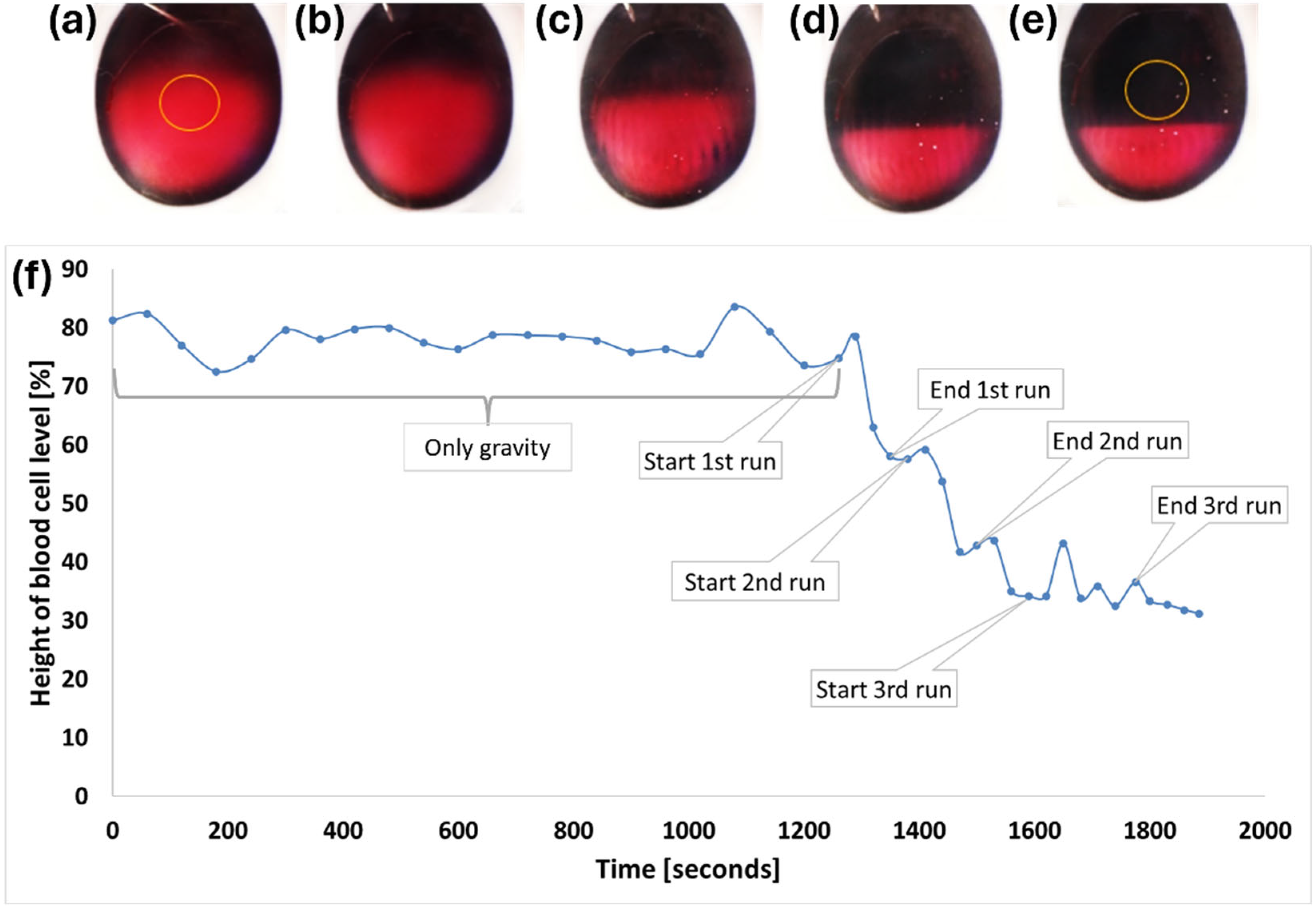
3.1. In Vitro Model
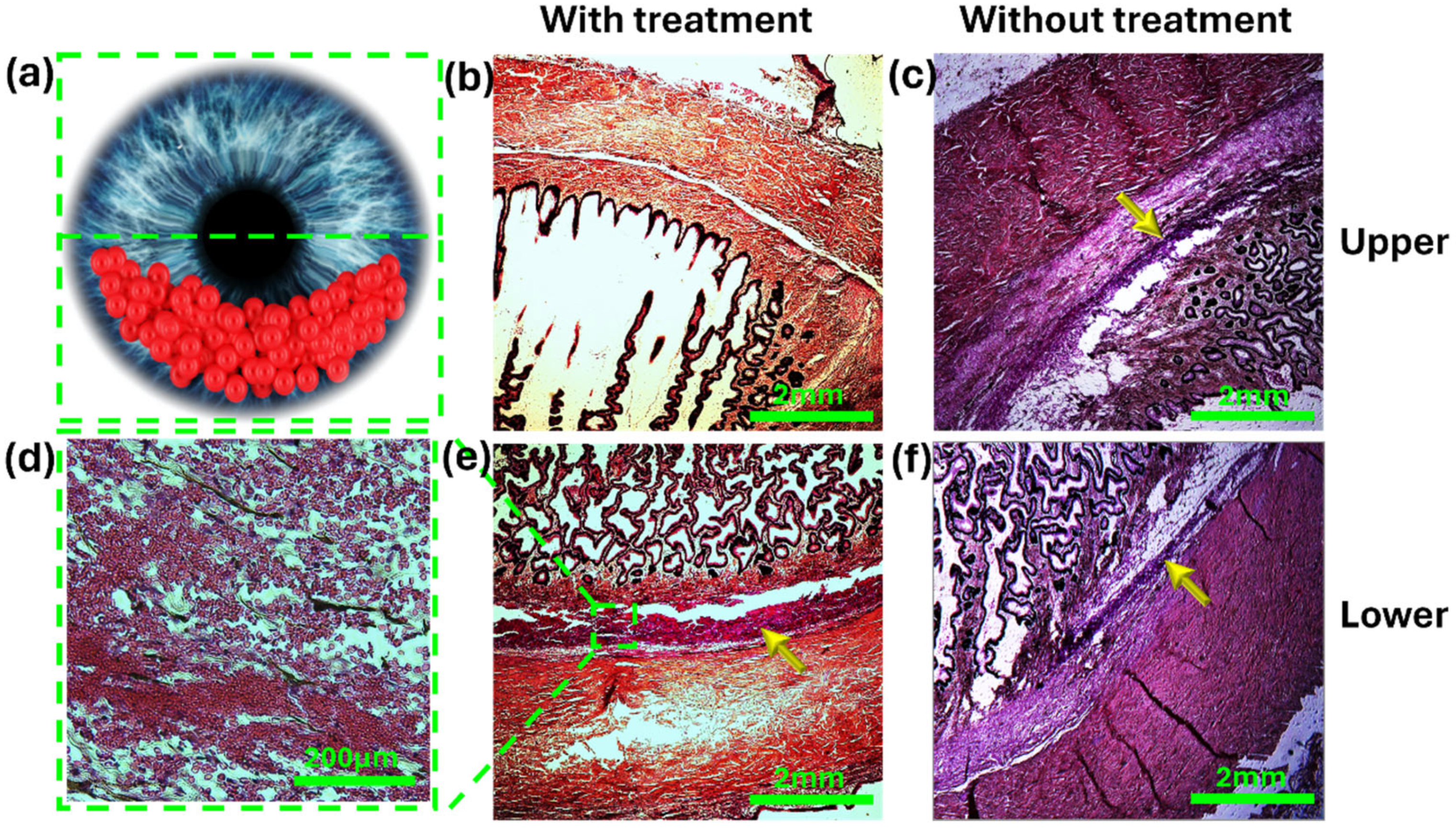
3.2. Ex Vivo Model
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| SOAG | secondary open-angle glaucoma |
| PS | polystyrene |
| IOP | intraocular pressure |
| AH | aqueous humor |
| TM | trabecular meshwork |
| HIFU | high-intensity focused ultrasound |
| Vpp | volt peak-to-peak |
References
- Okafor, K.; Vinod, K.; Gedde, S.J. Update on Pigment Dispersion Syndrome and Pigmentary Glaucoma. Curr. Opin. Ophthalmol. 2017, 28, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Ritch, R.; Schlötzer-Schrehardt, U. Exfoliation Syndrome. Surv. Ophthalmol. 2001, 45, 265–315. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Acharya, S.; Beuerman, R. Deposition of Particles on Ocular Tissues and Formation of Krukenberg Spindle, Hyphema, and Hypopyon. J. Biomech. Eng. 2007, 129, 174–186. [Google Scholar] [CrossRef] [PubMed]
- Zemba, M.; Camburu, G. Uveitis–Glaucoma–Hyphaema Syndrome. General Review. Rom. J. Ophthalmol. 2017, 61, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Sunderland, D.K.; Sapra, A. Physiology, Aqueous Humor Circulation; StatPearls: Treasure Island, FL, USA, 2020. [Google Scholar]
- Saccà, S.C.; Gandolfi, S.; Bagnis, A.; Manni, G.; Damonte, G.; Traverso, C.E.; Izzotti, A. From DNA Damage to Functional Changes of the Trabecular Meshwork in Aging and Glaucoma. Ageing Res. Rev. 2016, 29, 26–41. [Google Scholar] [CrossRef] [PubMed]
- McNally, T.W.; Liu, X.; Beese, S.; Keane, P.A.; Moore, D.J.; Denniston, A.K. Instrument-Based Tests for Quantifying Aqueous Humour Protein Levels in Uveitis: A Systematic Review Protocol. Syst. Rev. 2019, 8, 287. [Google Scholar] [CrossRef] [PubMed]
- Gadia, R.; Sihota, R.; Dada, T.; Gupta, V. Current Profile of Secondary Glaucomas. Indian J. Ophthalmol. 2008, 56, 285. [Google Scholar] [CrossRef] [PubMed]
- Woreta, F.A.; Lindsley, K.B.; Gharaibeh, A.; Ng, S.M.; Scherer, R.W.; Goldberg, M.F. Medical Interventions for Traumatic Hyphema. Cochrane Database Syst. Rev. 2023, 2023. [Google Scholar] [CrossRef] [PubMed]
- Beach, K.; Dunmire, B. Medical Acoustics. In Springer Handbook of Acoustics; Rossing, T.D., Ed.; Springer: New York, NY, USA, 2007; pp. 839–898. ISBN 978-0-387-30446-5. [Google Scholar]
- Leshno, A.; Rubinstein, Y.; Singer, R.; Sher, I.; Rotenstreich, Y.; Melamed, S.; Skaat, A. High-Intensity Focused Ultrasound Treatment in Moderate Glaucoma Patients: Results of a 2-Year Prospective Clinical Trial. J. Glaucoma 2020, 29, 556–560. [Google Scholar] [CrossRef] [PubMed]
- Hazan, Z.; Zumeris, J.; Jacob, H.; Raskin, H.; Kratysh, G.; Vishnia, M.; Dror, N.; Barliya, T.; Mandel, M.; Lavie, G. Effective Prevention of Microbial Biofilm Formation on Medical Devices by Low-Energy Surface Acoustic Waves. Antimicrob. Agents Chemother. 2006, 50, 4144–4152. [Google Scholar] [CrossRef] [PubMed]
- Hanaki, T.; Tsuda, A.; Sunaguchi, T.; Goto, K.; Morimoto, M.; Murakami, Y.; Kihara, K.; Matsunaga, T.; Yamamoto, M.; Tokuyasu, N.; et al. Influence of the Water Jet System vs. Cavitron Ultrasonic Surgical Aspirator for Liver Resection on the Remnant Liver. World J. Clin. Cases 2022, 10, 6855–6864. [Google Scholar] [CrossRef] [PubMed]
- Leshno, A.; Kenigsberg, A.; Peleg-Levy, H.; Piperno, S.; Skaat, A.; Shpaisman, H. Acoustic Manipulation of Intraocular Particles. Micromachines 2022, 13, 1362. [Google Scholar] [CrossRef] [PubMed]
- Piperno, S.; Sazan, H.; Shpaisman, H. Simultaneous Polymerization and Patterning: A One Step Acoustic Directed Assembly Method. Polymer 2019, 173, 20–26. [Google Scholar] [CrossRef]
- Kenigsberg, A.; Peleg-Levy, H.; Sazan, H.; Piperno, S.; Kenigsberg, L.; Shpaisman, H. One-Pot Approach for Acoustic Directed Assembly of Metallic and Composite Microstructures by Metal Ion Reduction. arXiv 2023, arXiv:2312.06839. [Google Scholar]
- Derakhshan, A.; Firoozi, J.; Esmaeili, S.; Bakhtiari, E.; Abbaspour, M.; Abrishami, M.; Zia, M.J. Systemic Prednisolone versus Topical Tranexamic Acid for Prevention of Rebleeding in Patients with Traumatic Hyphema: A Randomized Clinical Trial. J. Fr. d’Ophtalmol. 2022, 45, 9–12. [Google Scholar] [CrossRef] [PubMed]
- Farber, M.D.; Fiscella, R.; Goldberg, M.F. Aminocaproic Acid versus Prednisone for the Treatment of Traumatic Hyphema: A Randomized Clinical Trial. Ophthalmology 1991, 98, 279–286. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kenigsberg, A.; Shperling, S.; Nagler-Avramovitz, O.; Peleg-Levy, H.; Piperno, S.; Skaat, A.; Leshno, A.; Shpaisman, H.; Kapelushnik, N. Harnessing Standing Sound Waves to Treat Intraocular Blood Cell Accumulation. Micromachines 2024, 15, 786. https://doi.org/10.3390/mi15060786
Kenigsberg A, Shperling S, Nagler-Avramovitz O, Peleg-Levy H, Piperno S, Skaat A, Leshno A, Shpaisman H, Kapelushnik N. Harnessing Standing Sound Waves to Treat Intraocular Blood Cell Accumulation. Micromachines. 2024; 15(6):786. https://doi.org/10.3390/mi15060786
Chicago/Turabian StyleKenigsberg, Avraham, Shany Shperling, Ornit Nagler-Avramovitz, Heli Peleg-Levy, Silvia Piperno, Alon Skaat, Ari Leshno, Hagay Shpaisman, and Noa Kapelushnik. 2024. "Harnessing Standing Sound Waves to Treat Intraocular Blood Cell Accumulation" Micromachines 15, no. 6: 786. https://doi.org/10.3390/mi15060786
APA StyleKenigsberg, A., Shperling, S., Nagler-Avramovitz, O., Peleg-Levy, H., Piperno, S., Skaat, A., Leshno, A., Shpaisman, H., & Kapelushnik, N. (2024). Harnessing Standing Sound Waves to Treat Intraocular Blood Cell Accumulation. Micromachines, 15(6), 786. https://doi.org/10.3390/mi15060786





