Label-Free Continuous Cell Sorting Using Optofluidic Chip
Abstract
:1. Introduction
2. Models
3. Materials and Methods Results
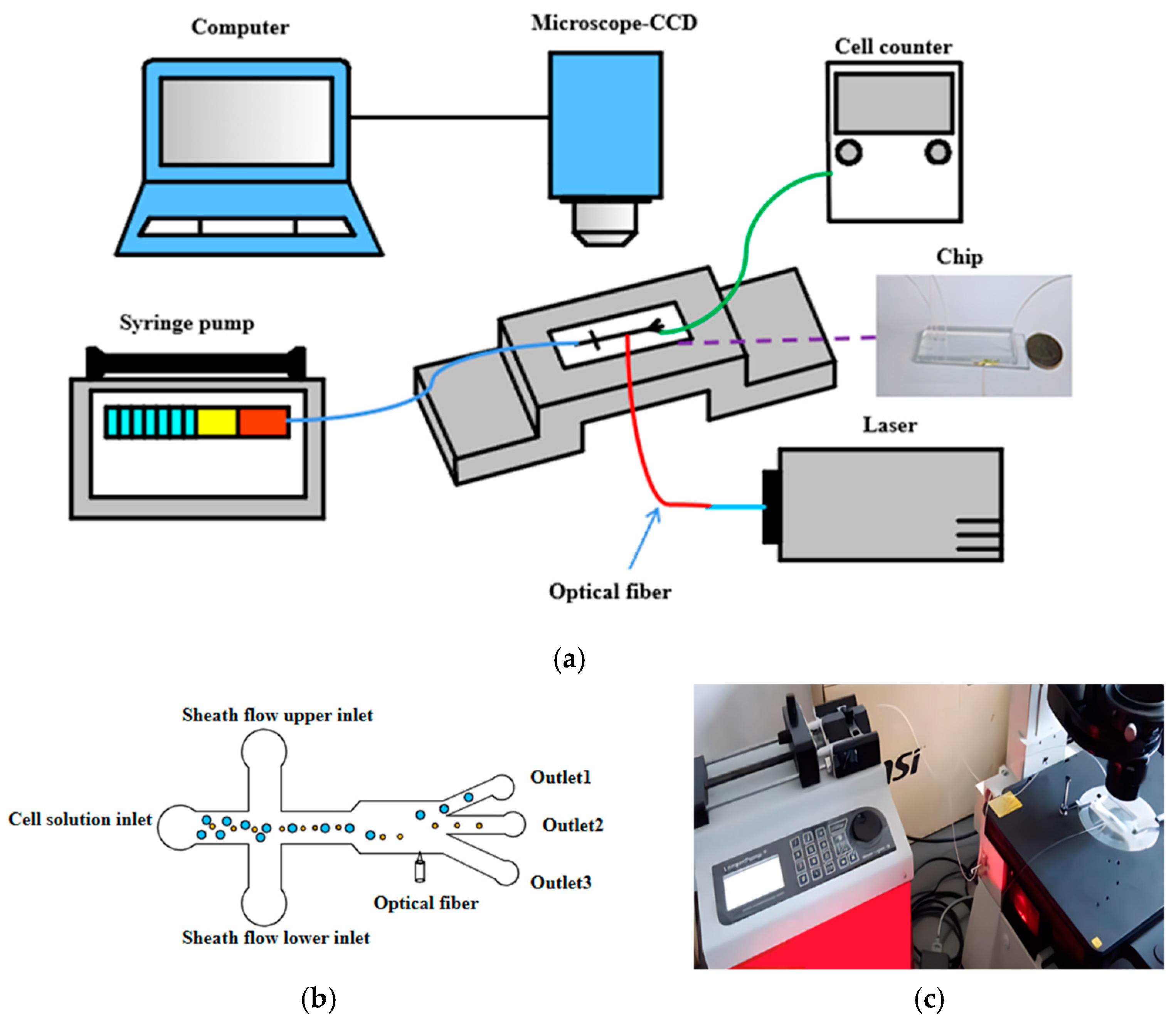
3.1. Experimental Setup
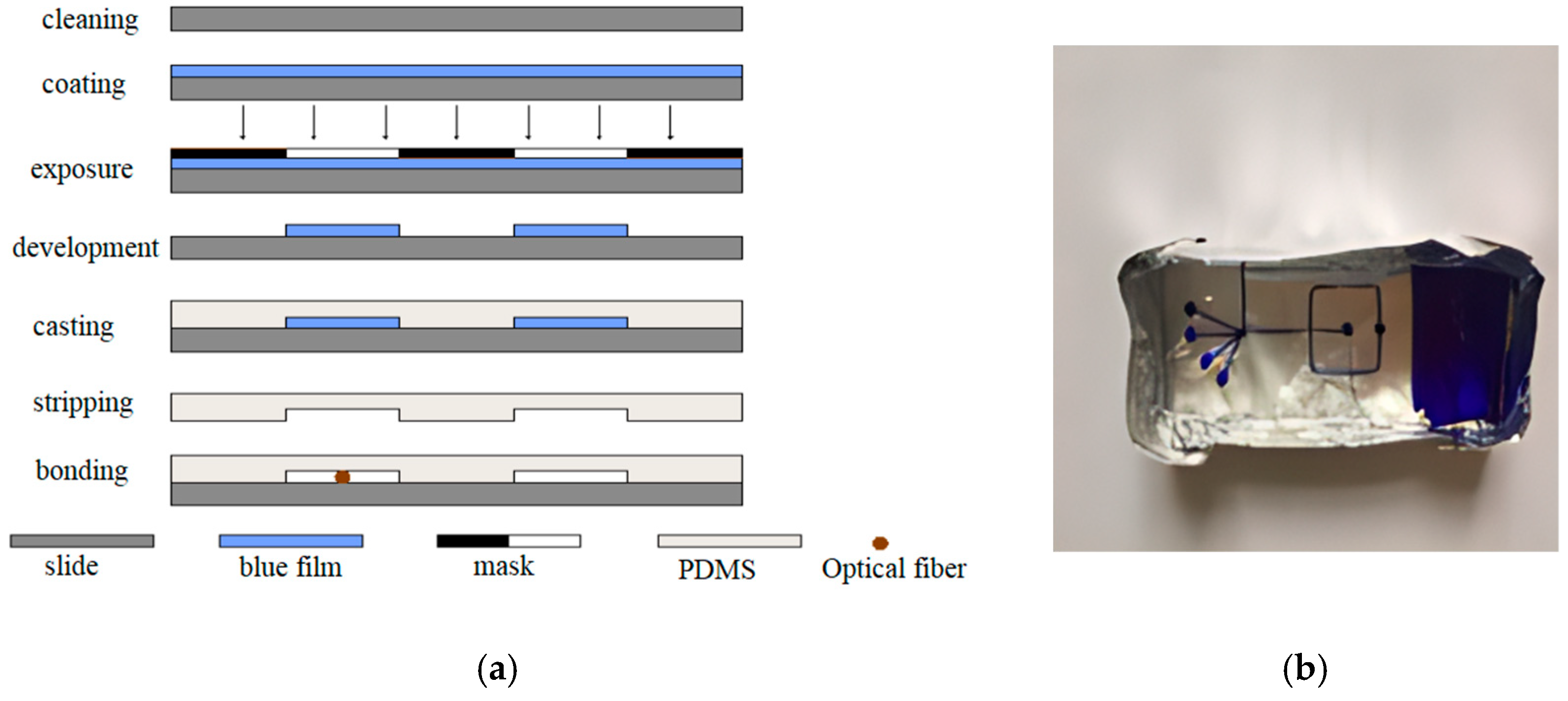
3.2. Fabrication of the Optofluidic Chip
3.2.1. Channel Structure Design
3.2.2. Bonding and Pretreatment
4. Analysis and Discussion
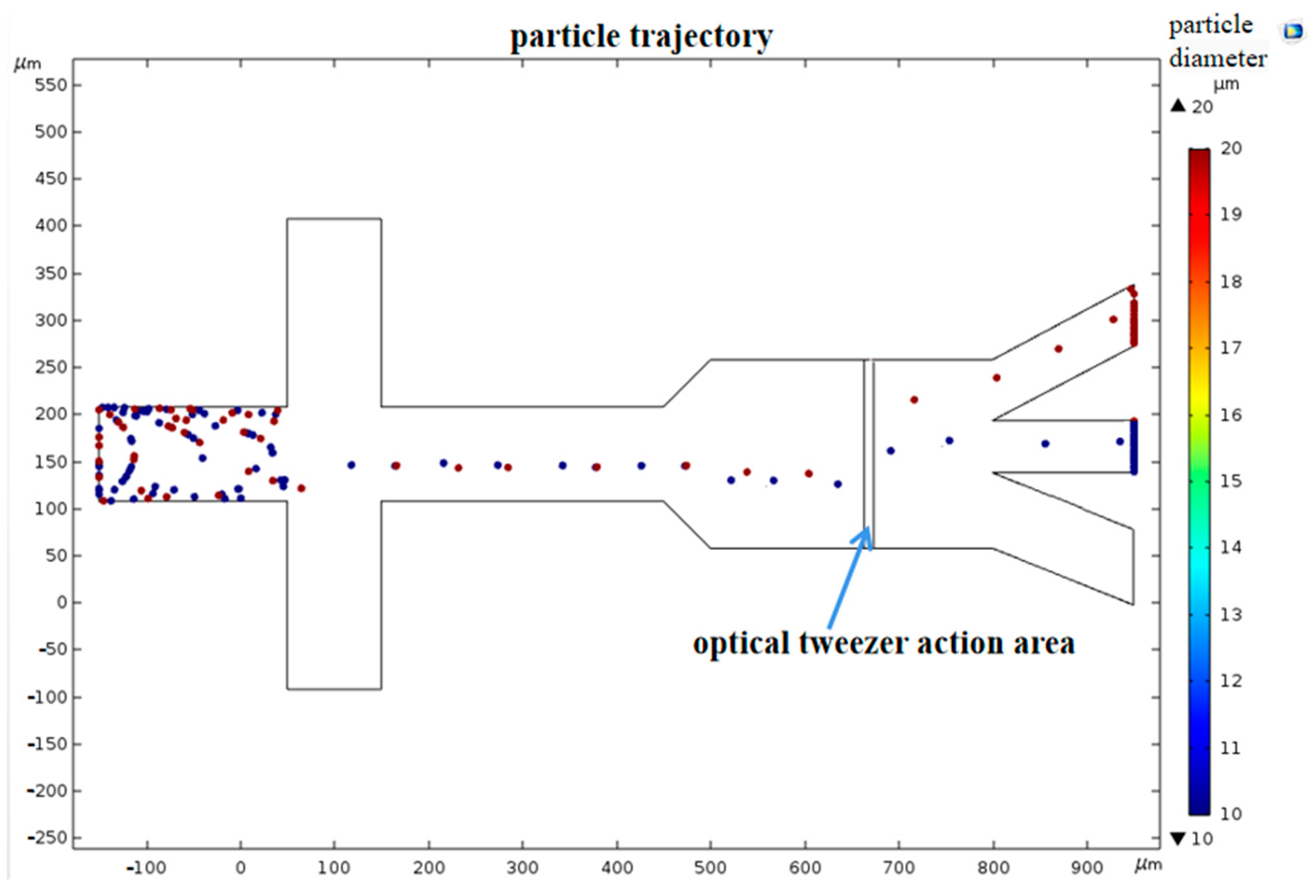
4.1. FE Analysis
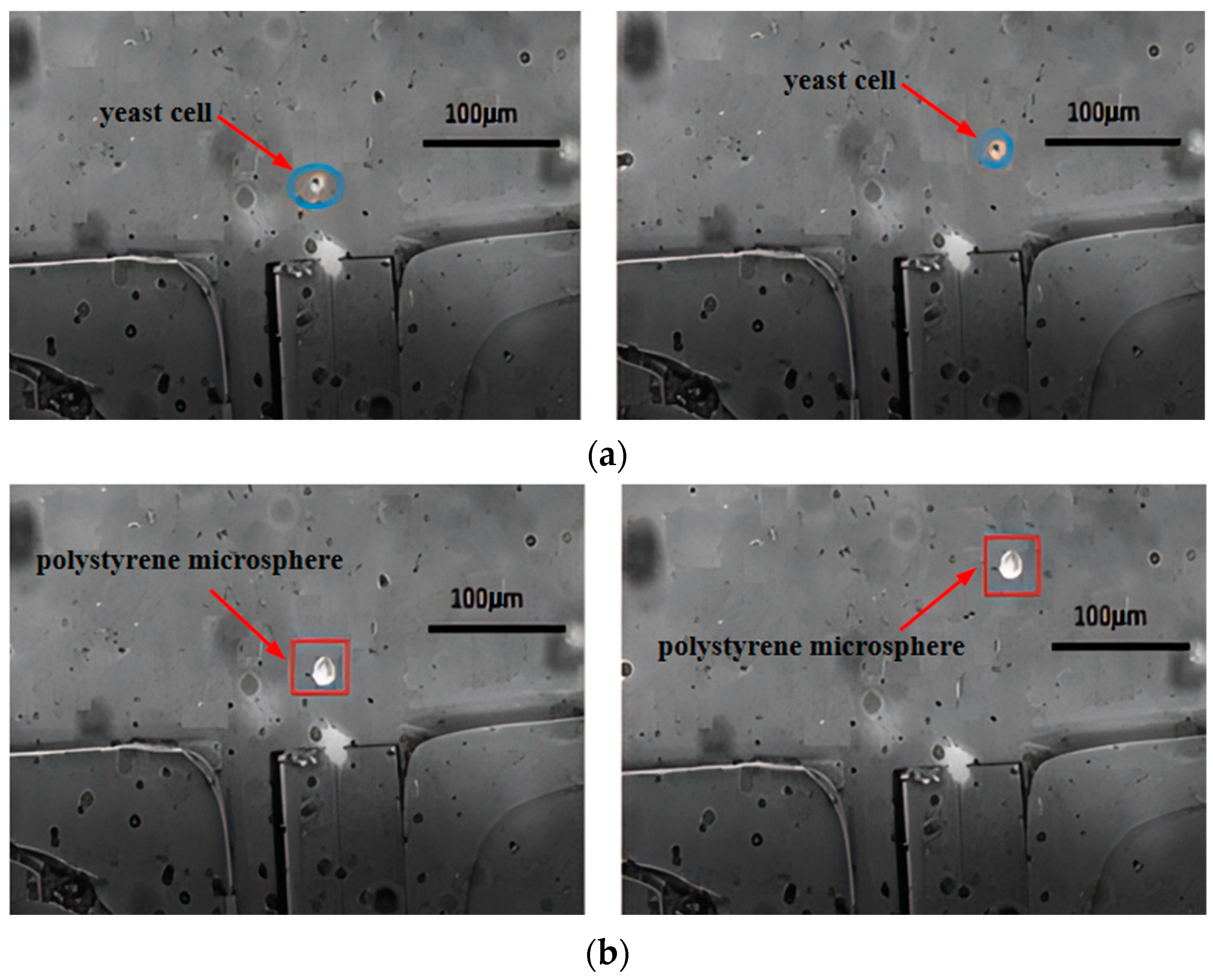
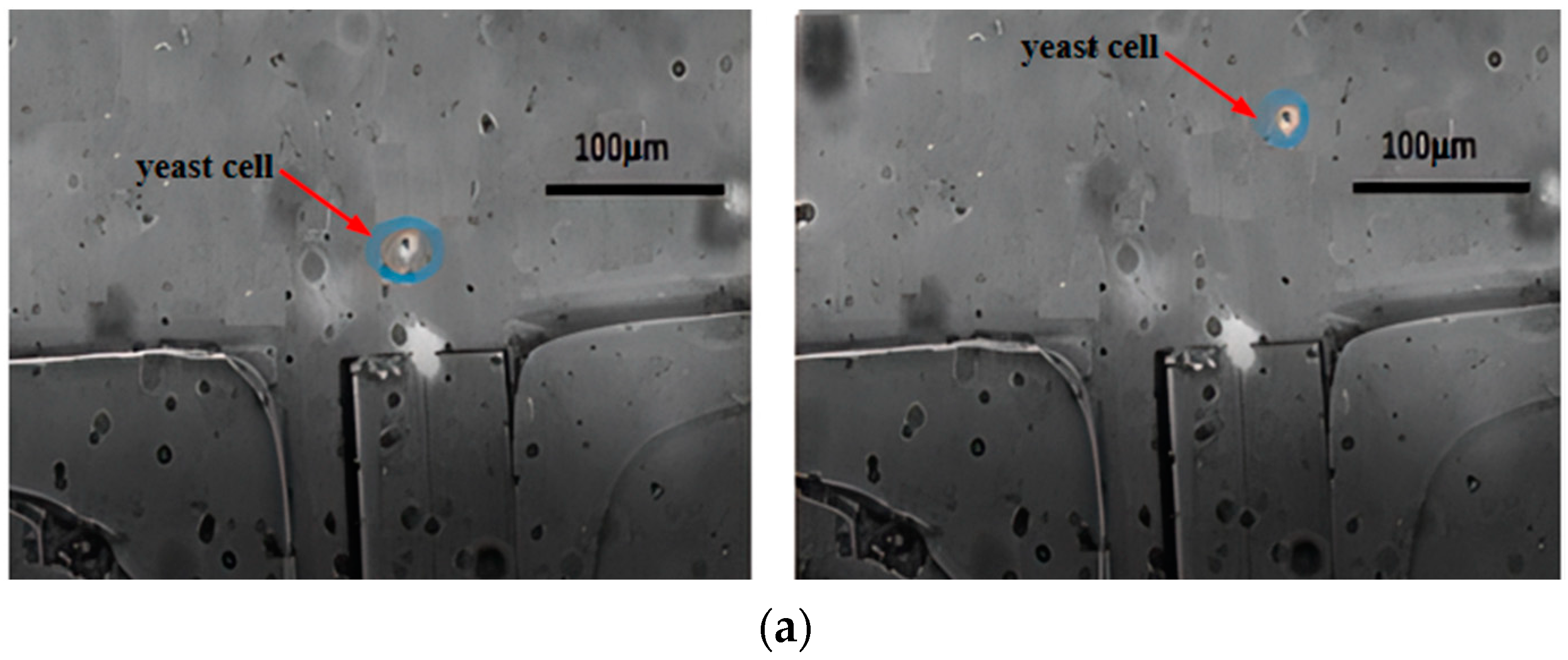
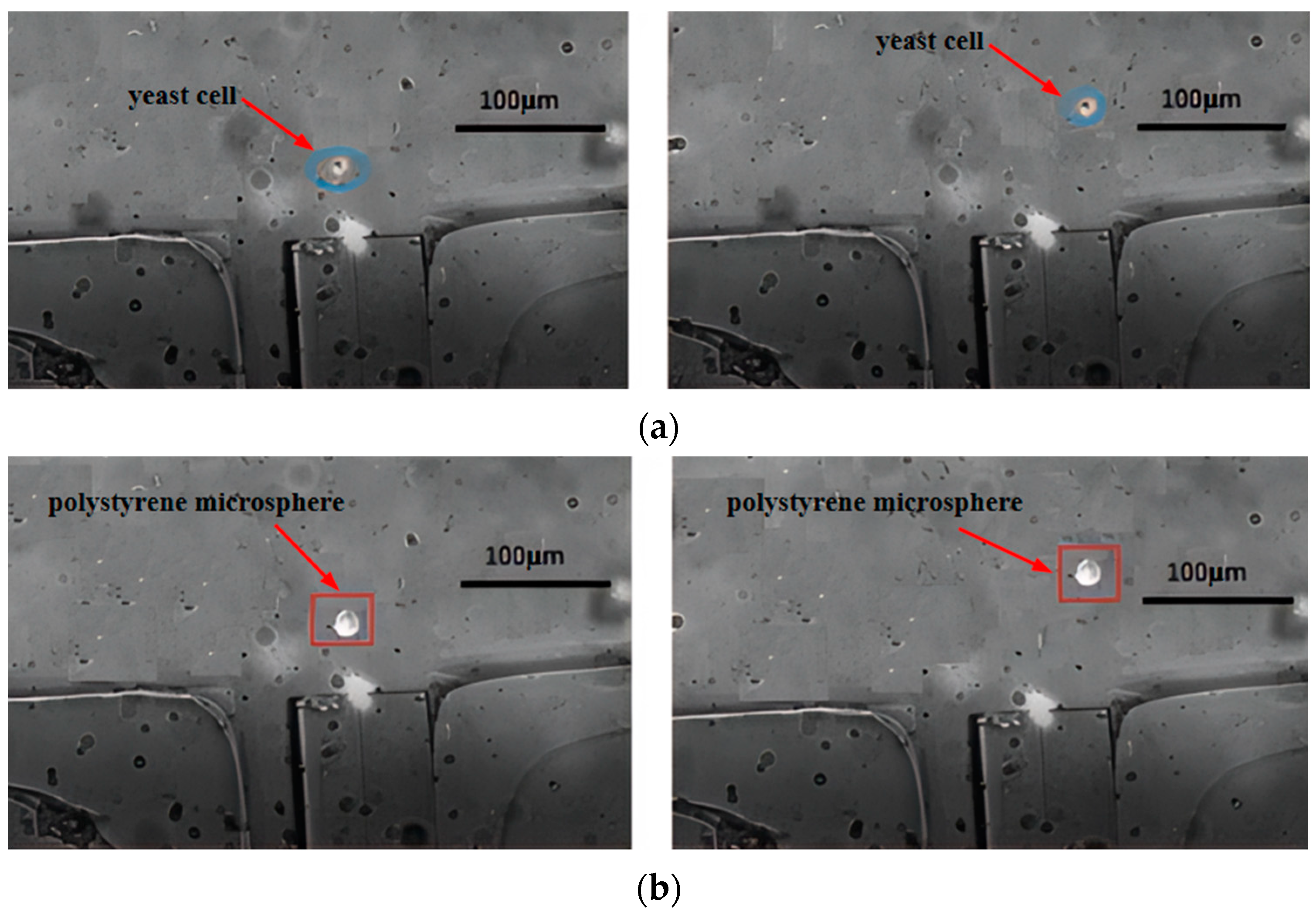
4.2. Separation Efficiency
4.3. Parameter Analysis
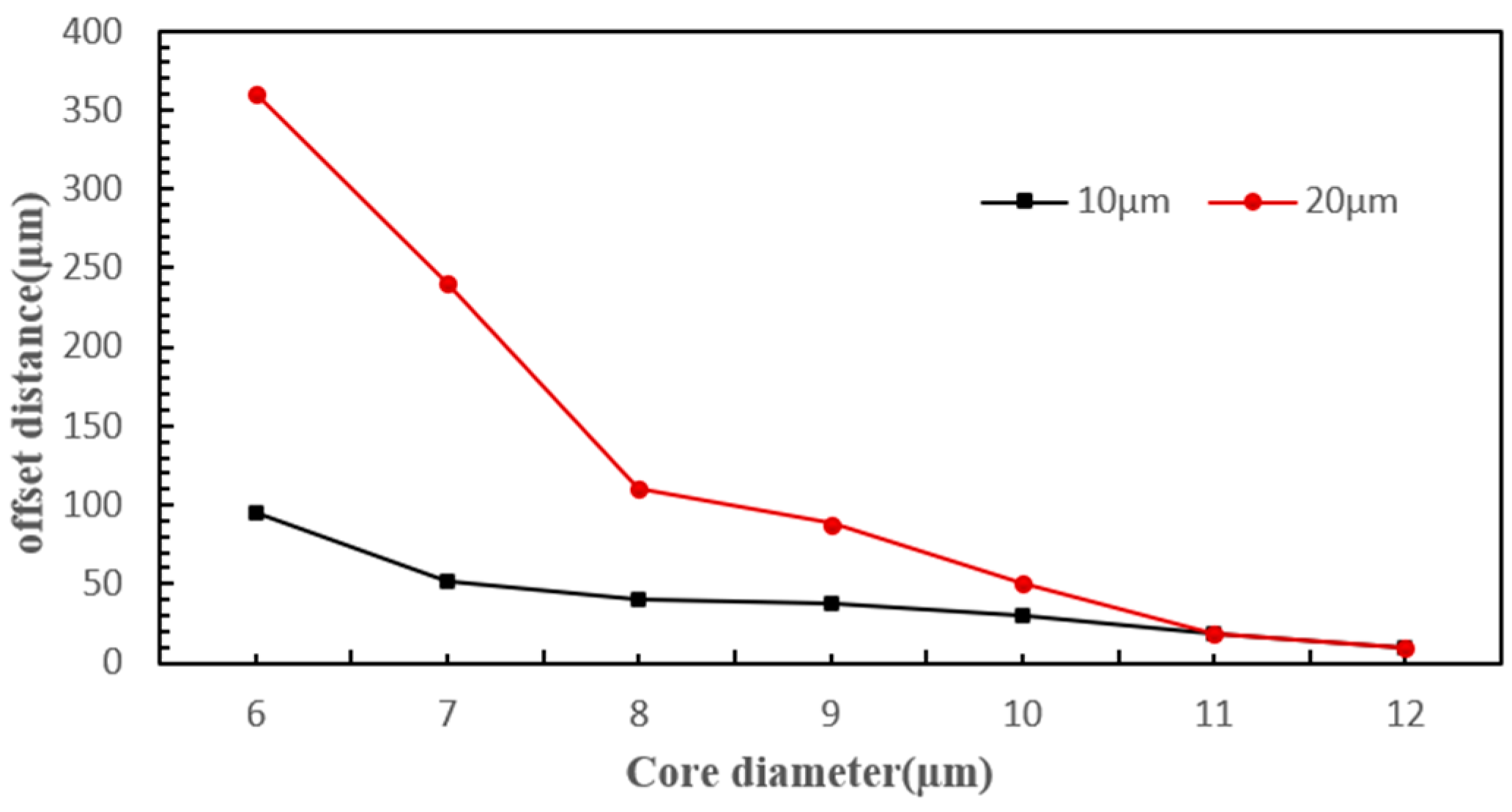
4.3.1. Cell Diameter
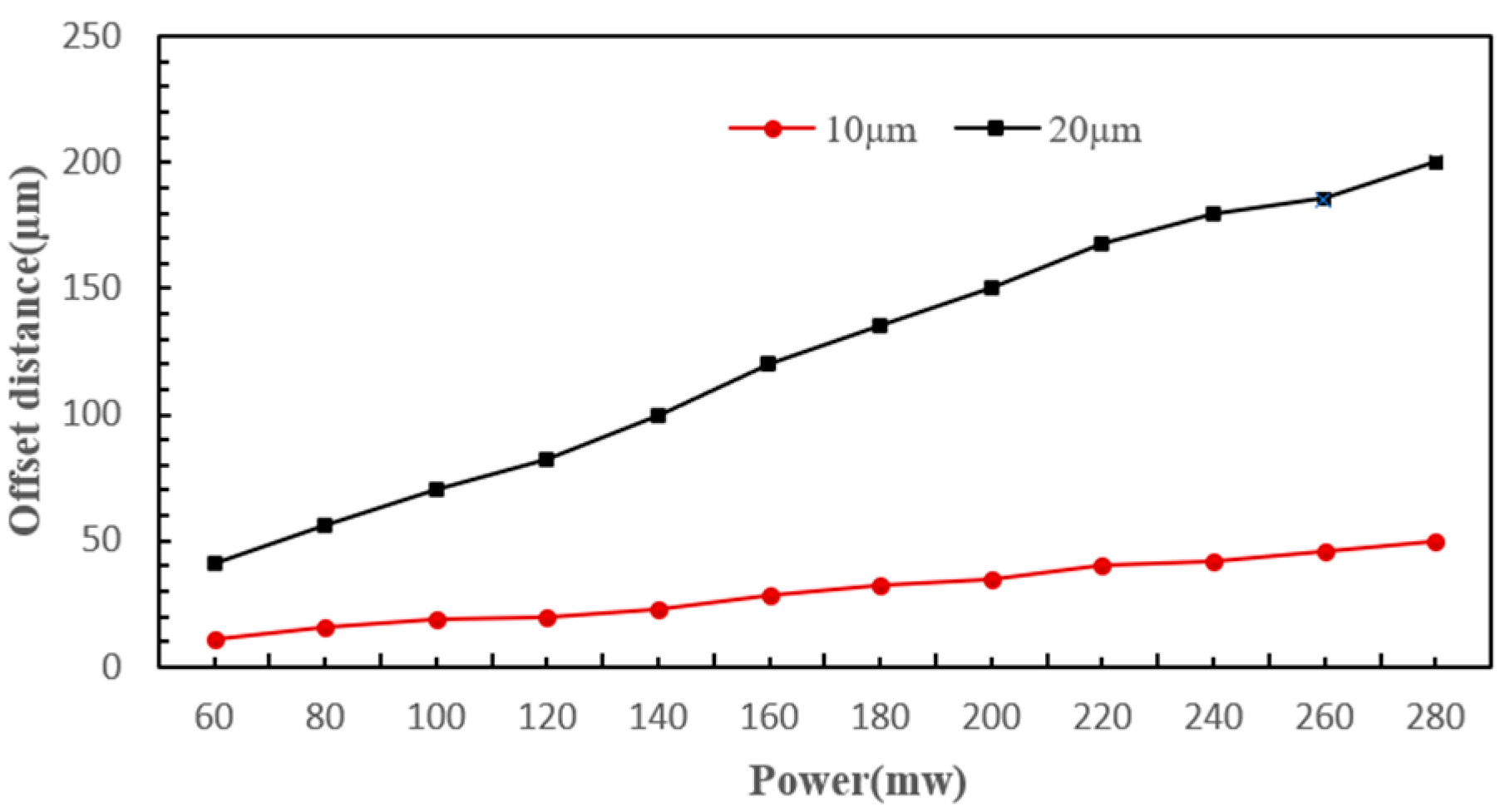
4.3.2. Laser Power
4.3.3. Fiber Core Diameter
5. Conclusions
- (1)
- The scattering force experienced by cells primarily depends on laser power, cell radius, radiation pressure coefficient, cell position, beam waist radius, and wavelength. Hence, cells of different diameters experience varying scattering forces from the same laser, causing them to shift different distances along the laser’s axis and thus enter distinct sorting channels.
- (2)
- After repeated experiments, we successfully achieved the effective integration of single-mode fibers with microfluidic chips, fabricated an optofluidic chip, and established a cell sorting experimental platform. We optimized the traditional cross-channel chip structure, creating an expanded cross-channel that enabled continuous sorting of yeast cells and polystyrene microspheres. Compared to other sorting systems, this system does not require labeling and can achieve high-viability cell sorting.
- (3)
- Through simulations and experiments, we analyzed the effects of various parameters such as cell diameter, optical tweezers laser power, and fiber core diameter on the cell displacement distance, validating the rationality of the formula.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dholakia, K.; Čižmár, T. Shaping the Future of Manipulation. Nat. Photonics 2011, 5, 335–342. [Google Scholar] [CrossRef]
- Farasat, M.; Aalaei, E.; Kheirati Ronizi, S.; Bakhshi, A.; Mirhosseini, S.; Zhang, J.; Nguyen, N.-T.; Kashaninejad, N. Signal-Based Methods in Dielectrophoresis for Cell and Particle Separation. Biosensors 2022, 12, 510. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Wei, S.; Li, J.; Zhang, Z.; Gong, L.; He, L. High-Throughput Sorting of Nanoparticles with Light-Patterned Dielectrophoresis Force. Opt. Express 2023, 31, 41026. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.; Wang, X.; Wang, Q.; Kong, B.; Yuan, M.; Zhang, S.; Huang, J.; Wang, R.; Wu, Z.; Wang, Z.; et al. Fabrication and Separation of Egain Microparticles from Human Blood Based on Dielectrophoresis Force and a W-Type Electrode. Langmuir 2023, 39, 3151–3161. [Google Scholar] [CrossRef]
- Aghaamoo, M.; Cardenas-Benitez, B.; Lee, A.P. A High-Throughput Microfluidic Cell Sorter Using a Three-Dimensional Coupled Hydrodynamic-Dielectrophoretic Pre-Focusing Module. Micromachines 2023, 14, 1813. [Google Scholar] [CrossRef]
- Chu, P.-Y.; Nguyen, T.N.; Wu, A.-Y.; Huang, P.-S.; Huang, K.-L.; Liao, C.-J.; Hsieh, C.-H.; Wu, M.-H. The Utilization of Optically Induced Dielectrophoresis (Odep)-Based Cell Manipulation in a Microfluidic System for the Purification and Sorting of Circulating Tumor Cells (Ctcs) with Different Sizes. Micromachines 2023, 14, 2170. [Google Scholar] [CrossRef]
- Valle, M.; O’Brien, B.; Green, T.D.; Reiner, J.E.; Seashols-Williams, S. Droplet-based Optical Trapping for Cell Separation in Mock Forensic Samples. J. Forensic Sci. 2023, 69, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lu, Y.; Sun, Y.; Dong, T.; Zhou, Y.; Li, D.; Yan, L.; Sun, J.; Jiang, C.L. Compact Single Fiber Optical Tweezer–Micropipette System for Completely Noninvasive Cell Sorting. Appl. Phys. Lett. 2023, 122, 233701. [Google Scholar] [CrossRef]
- García-Hernández, L.; Martínez-Martínez, E.; Pazos-Solís, D.; Aguado-Preciado, J.; Dutt, A.; Chávez-Ramírez, A.; Korgel, B.; Sharma, A.; Oza, G. Optical Detection of Cancer Cells Using Lab-on-a-Chip. Biosensors 2023, 13, 439. [Google Scholar] [CrossRef] [PubMed]
- Qiu, H.; Wang, H.; Yang, X.; Huo, F. High Performance Isolation of Circulating Tumor Cells by ACOUSTOFLUIDIC Chip Coupled with Ultrasonic Concentrated Energy Transducer. Colloids Surf. B Biointerfaces 2023, 222, 113138. [Google Scholar] [CrossRef]
- Xue, S.; Xu, Q.; Xu, Z.; Zhang, X.; Zhang, H.; Zhang, X.; He, F.; Chen, Y.; Xue, Y.; Hao, P. Manipulation of Particle/Cell Based on Compressibility in a Divergent Microchannel by Surface Acoustic Wave. Anal. Chem. 2023, 95, 4282–4290. [Google Scholar] [CrossRef] [PubMed]
- Rezayati Charan, M.; Berg, F.; Augustsson, P. Acoustofluidic Three-Dimensional Motion of Suspended Cells at near-Zero Acoustic Contrast in Homogeneous Media. Phys. Rev. Appl. 2023, 19, 014046. [Google Scholar] [CrossRef]
- Qi, M.; Dang, D.; Yang, X.; Wang, J.; Zhang, H.; Liang, W. Surface Acoustic Wave Manipulation of Bioparticles. Soft Matter 2023, 19, 4166–4187. [Google Scholar] [CrossRef] [PubMed]
- Civelekoglu, O.; Frazier, A.B.; Sarioglu, A.F. The Origins and the Current Applications of Microfluidics-Based Magnetic Cell Separation Technologies. Magnetochemistry 2022, 8, 10. [Google Scholar] [CrossRef]
- Piao, J.; Liu, L.; Cai, L.; Ri, H.C.; Jin, X.; Sun, H.; Piao, X.; Shang, H.; Jin, X.; Pu, Q.; et al. High-Resolution Micro-Object Separation by Rotating Magnetic Chromatography. Anal. Chem. 2022, 94, 11500–11507. [Google Scholar] [CrossRef] [PubMed]
- Hewlin, R.L.; Edwards, M.; Schultz, C. Design and Development of a Traveling Wave Ferro-Microfluidic Device and System Rig for Potential Magnetophoretic Cell Separation and Sorting in a Water-Based Ferrofluid. Micromachines 2023, 14, 889. [Google Scholar] [CrossRef]
- Nasiri, R.; Shamloo, A.; Akbari, J. Design of Two Inertial-Based Microfluidic Devices for Cancer Cell Separation from Blood: A Serpentine Inertial Device and an Integrated Inertial and Magnetophoretic Device. Chem. Eng. Sci. 2022, 252, 117283. [Google Scholar] [CrossRef]
- Cha, H.; Amiri, H.A.; Moshafi, S.; Karimi, A.; Nikkhah, A.; Chen, X.; Ta, H.T.; Nguyen, N.-T.; Zhang, J. Effects of Obstacles on Inertial Focusing and Separation in Sinusoidal Channels: An Experimental and Numerical Study. Chem. Eng. Sci. 2023, 276, 118826. [Google Scholar] [CrossRef]
- Ni, C.; Wu, D.; Chen, Y.; Wang, S.; Xiang, N. Cascaded Elasto-Inertial Separation of Malignant Tumor Cells from Untreated Malignant Pleural and Peritoneal Effusions. Lab A Chip 2024, 24, 697–706. [Google Scholar] [CrossRef]
- Bagi, M.; Amjad, F.; Ghoreishian, S.M.; Sohrabi Shahsavari, S.; Huh, Y.S.; Moraveji, M.K.; Shimpalee, S. Advances in Technical Assessment of Spiral Inertial Microfluidic Devices toward Bioparticle Separation and Profiling: A Critical Review. BioChip J. 2024, 18, 45–67. [Google Scholar] [CrossRef]
- Grier, D.G. A Revolution in Optical Manipulation. Nature 2003, 424, 810–816. [Google Scholar] [CrossRef] [PubMed]
- Chiou, P.Y.; Ohta, A.T.; Wu, M.C. Massively Parallel Manipulation of Single Cells and Microparticles Using Optical Images. Nature 2005, 436, 370–372. [Google Scholar] [CrossRef]
- Yang, A.H.; Moore, S.D.; Schmidt, B.S.; Klug, M.; Lipson, M.; Erickson, D. Optical Manipulation of Nanoparticles and Biomolecules in Sub-Wavelength Slot Waveguides. Nature 2009, 457, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Guo, C.; Yang, J.; Yuan, L. Tapered Fiber Optical Tweezers for Microscopic Particle Trapping: Fabrication and Application. Opt. Express 2006, 14, 12510. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, K.; Ueno, H.; Hiramatsu, T.; Ikeda, M. Optical Trapping of Dielectric Particle and Biological Cell Using Optical Fibre. Electron. Lett. 1997, 33, 413. [Google Scholar] [CrossRef]
- Sakata, H.; Imada, A. Lensed Plastic Optical Fiber Employing Concave End Filled with High-Index Resin. J. Light. Technol. 2002, 20, 638–642. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, S.; Yuan, L. Optical-Fiber-Based Powerful Tools for Living Cell Manipulation [Invited]. Chin. Opt. Lett. 2019, 17, 090603. [Google Scholar] [CrossRef]
- Rodrigues Ribeiro, R.S.; Soppera, O.; Oliva, A.G.; Guerreiro, A.; Jorge, P.A. New Trends on Optical Fiber Tweezers. J. Light. Technol. 2015, 33, 3394–3405. [Google Scholar] [CrossRef]
- Ismaeel, R.; Lee, T.; Ding, M.; Belal, M.; Brambilla, G. Optical Microfiber Passive Components. Laser Photonics Rev. 2012, 7, 350–384. [Google Scholar] [CrossRef]
- Zhao, X.; Zhao, N.; Shi, Y.; Xin, H.; Li, B. Optical Fiber Tweezers: A Versatile Tool for Optical Trapping and Manipulation. Micromachines 2020, 11, 114. [Google Scholar] [CrossRef]
- Liu, X.; Wu, Y.; Xu, X.; Li, Y.; Zhang, Y.; Li, B. Bio-conveyor Belts: Bidirectional Transport of Nanoparticles and Cells with a Bio-conveyor Belt (Small 50/2019). Small 2019, 15, 1970274. [Google Scholar] [CrossRef]
- Mamuti, R.; Fuji, T.; Kudo, T. Opto-Thermophoretic Trapping of Micro and Nanoparticles with a 2 Μm TM-Doped Fiber Laser. Opt. Express 2021, 29, 38314. [Google Scholar] [CrossRef]
- Zhao, H.; Farrell, G.; Wang, P.; Yuan, L. Investigation of Particle Harmonic Oscillation Using Four-Core Fiber Integrated Twin-Tweezers. IEEE Photonics Technol. Lett. 2016, 28, 461–464. [Google Scholar] [CrossRef]
- Tang, X.; Zhang, Y.; Zhang, Y.; Liu, Z.; Yang, X.; Zhang, J.; Yang, J.; Yuan, L. All-Fiber Active Tractor Beam Generator and Its Application. J. Light. Technol. 2020, 38, 1420–1426. [Google Scholar] [CrossRef]
- Deng, H.; Zhang, Y.; Yuan, T.; Zhang, X.; Zhang, Y.; Liu, Z.; Yuan, L. Fiber-Based Optical Gun for Particle Shooting. ACS Photonics 2017, 4, 642–648. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, T.; Zhang, Y.; Tang, X.; Liu, P.; Zhang, Y.; Yang, X.; Zhang, J.; Yang, J.; Yuan, L. Single Fiber Dual-Functionality Optical Tweezers Based on Graded-Index Multimode Fiber. Chin. Opt. Lett. 2018, 16, 053501. [Google Scholar]
- Zhang, Y.; Tang, X.; Zhang, Y.; Liu, Z.; Zhao, E.; Yang, X.; Zhang, J.; Yang, J.; Yuan, L. Multiple Particles 3-D Trap Based on All-Fiber Bessel Optical Probe. J. Light. Technol. 2017, 35, 3849–3853. [Google Scholar] [CrossRef]
- Tang, X.; Zhang, Y.; Su, W.; Zhang, Y.; Liu, Z.; Yang, X.; Zhang, J.; Yang, J.; Yuan, L. Super-Low-Power Optical Trapping of a Single Nanoparticle. Opt. Lett. 2019, 44, 5165. [Google Scholar] [CrossRef]
- Zhang, Y.; Tang, X.; Zhang, Y.; Liu, Z.; Yang, X.; Zhang, J.; Yang, J.; Yuan, L. Optical Attraction of Strongly Absorbing Particles in Liquids. Opt. Express 2019, 27, 12414. [Google Scholar] [CrossRef]
- MacDonald, M.P.; Spalding, G.C.; Dholakia, K. Microfluidic Sorting in an Optical Lattice. Nature 2003, 426, 421–424. [Google Scholar] [CrossRef]
- Shi, Y.; Xiong, S.; Chin, L.K.; Zhang, J.; Ser, W.; Wu, J.; Chen, T.; Yang, Z.; Hao, Y.; Liedberg, B.; et al. Nanometer-Precision Linear Sorting with Synchronized Optofluidic Dual Barriers. Sci. Adv. 2018, 4, eaao0773. [Google Scholar] [CrossRef]
- Shi, Y.Z.; Xiong, S.; Zhang, Y.; Chin, L.K.; Chen, Y.-Y.; Zhang, J.B.; Zhang, T.H.; Ser, W.; Larrson, A.; Lim, S.H.; et al. Sculpting Nanoparticle Dynamics for Single-Bacteria-Level Screening and Direct Binding-Efficiency Measurement. Nat. Commun. 2018, 9, 815. [Google Scholar] [CrossRef] [PubMed]
- Nan, F.; Yan, Z. Creating Multifunctional Optofluidic Potential Wells for Nanoparticle Manipulation. Nano Lett. 2018, 18, 7400–7406. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Zhao, H.; Chin, L.K.; Zhang, Y.; Yap, P.H.; Ser, W.; Qiu, C.-W.; Liu, A.Q. Optical Potential-Well Array for High-Selectivity, Massive Trapping and Sorting at Nanoscale. Nano Lett. 2020, 20, 5193–5200. [Google Scholar] [CrossRef] [PubMed]
- Nie, Y.; Xie, C.; Zhu, G.; Fang, Y. Trapping and Sorting of Nanoparticles by Bowtie-Nanohole Plasmonic Tweezers. J. Phys. B At. Mol. Opt. Phys. 2023, 56, 175401. [Google Scholar] [CrossRef]
- Wang, J.; Ren, H.; Zhou, Y. Applications of T-Matrix Method in Optical Tweezers and Its Progress. Laser Optoelectron. Prog. 2014, 51, 120007. [Google Scholar] [CrossRef]
- Mi, X.; Te, L.; La, Q.; Wang, J. Scattering, Absorption, and Emission of Light by Small Particles; Guo Fang Gong Ye Chu Ban She: Beijing, China, 2013. [Google Scholar]
- Jones, P.H.; Maragò, O.M.; Volpe, G. Optical Tweezers: Principles and Applications; Cambridge University Press: Cambridge, UK, 2015. [Google Scholar]
- Constable, A.; Kim, J.; Mervis, J.; Zarinetchi, F.; Prentiss, M. Demonstration of a Fiber-Optical Light-Force Trap. Opt. Lett. 1993, 18, 1867. [Google Scholar] [CrossRef]
- Poyraz, B.; Tozluoğlu, A.; Candan, Z.; Demir, A.; Yavuz, M. Influence of PVA and Silica on Chemical, Thermo-Mechanical and Electrical Properties of Celluclast-Treated Nanofibrillated Cellulose Composites. Int. J. Biol. Macromol. 2017, 104, 384–392. [Google Scholar] [CrossRef]


| Parameter | Value |
|---|---|
| Particle diameter 1 d1/µm | 10 |
| Particle diameter 2 d2/µm | 20 |
| Laser power/mW | 180 |
| Laser wavelength/[nm] | 980 |
| Fiber core diameter df/µm | 8 |
| Main channel inlet velocity/µm/min | 1 |
| Sheath flow upper inlet velocity/µm/min | 1.2 |
| Sheath flow lower inlet velocity/µm/min | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Zhang, T.; Zhang, X.; Cheng, J.; Zhang, S. Label-Free Continuous Cell Sorting Using Optofluidic Chip. Micromachines 2024, 15, 818. https://doi.org/10.3390/mi15070818
Zhang Y, Zhang T, Zhang X, Cheng J, Zhang S. Label-Free Continuous Cell Sorting Using Optofluidic Chip. Micromachines. 2024; 15(7):818. https://doi.org/10.3390/mi15070818
Chicago/Turabian StyleZhang, Yingjie, Tao Zhang, Xinchun Zhang, Jingmeng Cheng, and Sixiang Zhang. 2024. "Label-Free Continuous Cell Sorting Using Optofluidic Chip" Micromachines 15, no. 7: 818. https://doi.org/10.3390/mi15070818






