ZnMn2O4/V2CTx Composites Prepared as an Anode Material via High-Temperature Calcination Method for Optimized Li-Ion Batteries
Abstract
1. Introduction
2. Experimental Methods
2.1. Materials
2.2. Preparation Methods
2.2.1. Preparation of ZnMn2O4 Micron Rods
2.2.2. Preparation of V2CTx MXene by Hydrochloric Acid and Ammonium Fluoride Etching Method
2.2.3. Preparation of ZnMn2O4/V2CTx Composite by High Temperature Calcination Method
2.3. Characterization
2.4. Electrochemical Tests
3. Results and Discussion
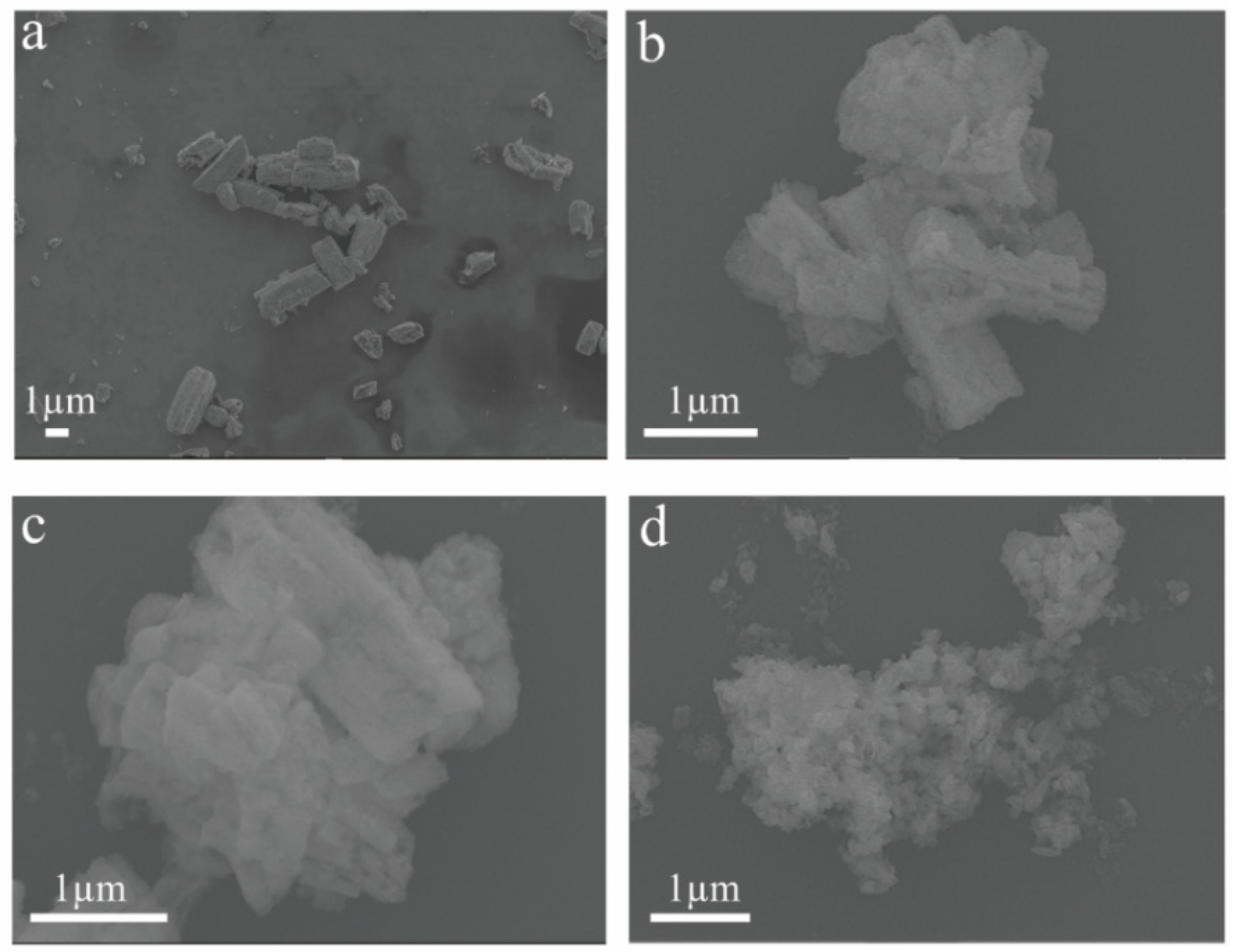
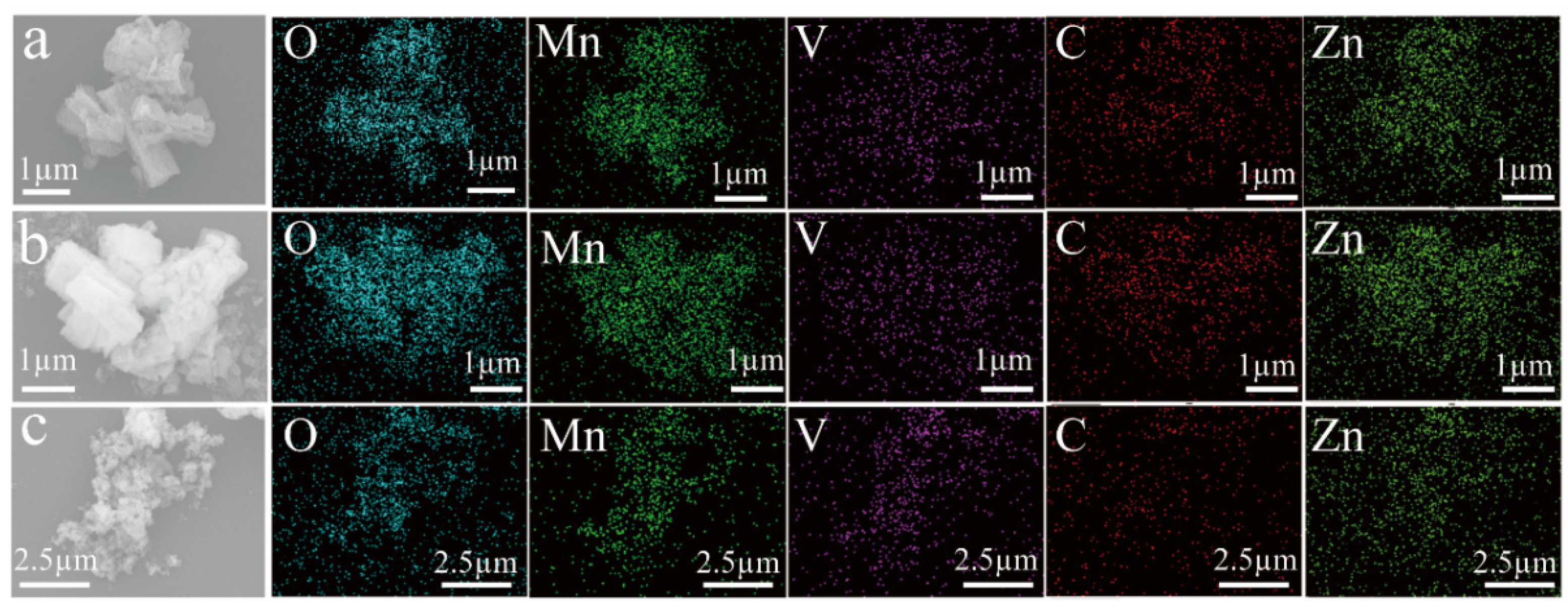
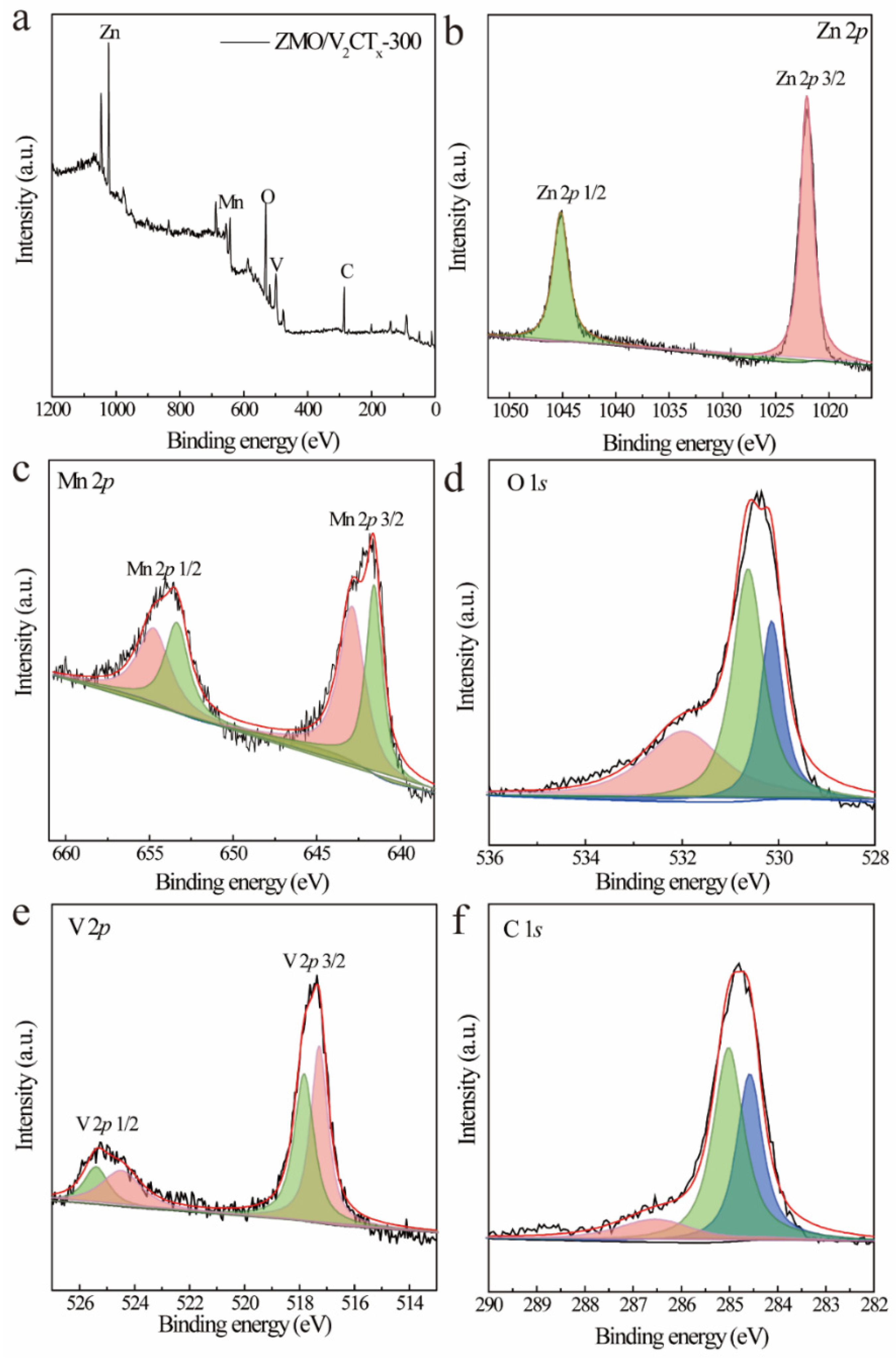
3.1. Structural Characterization
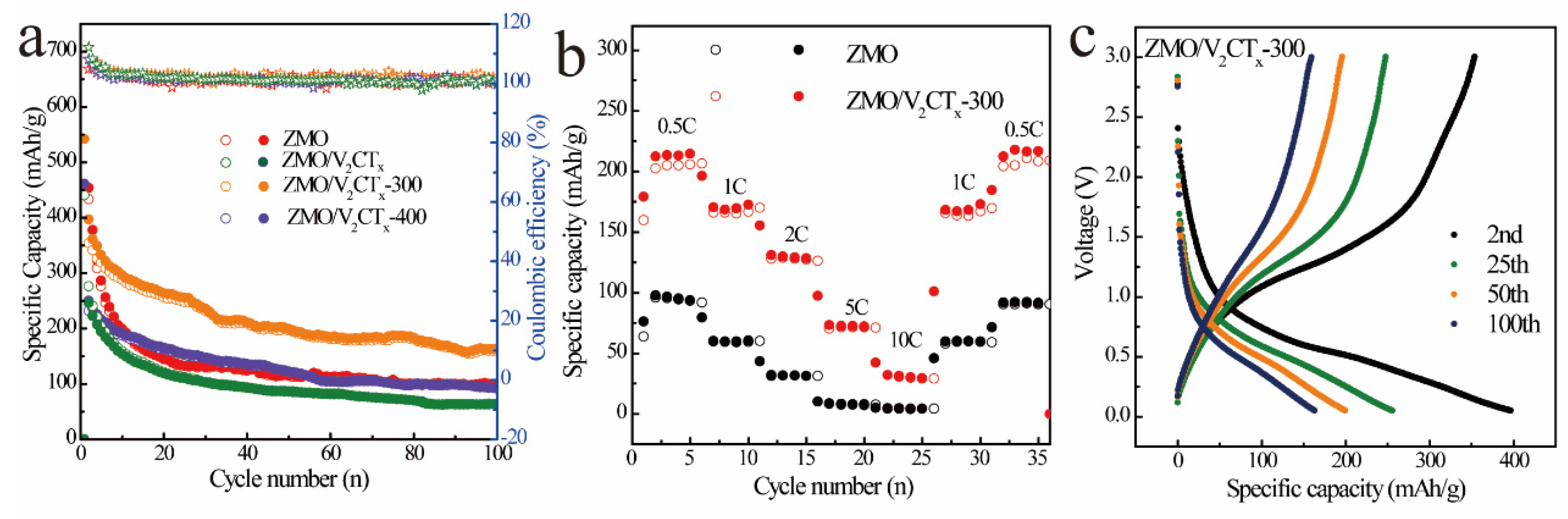
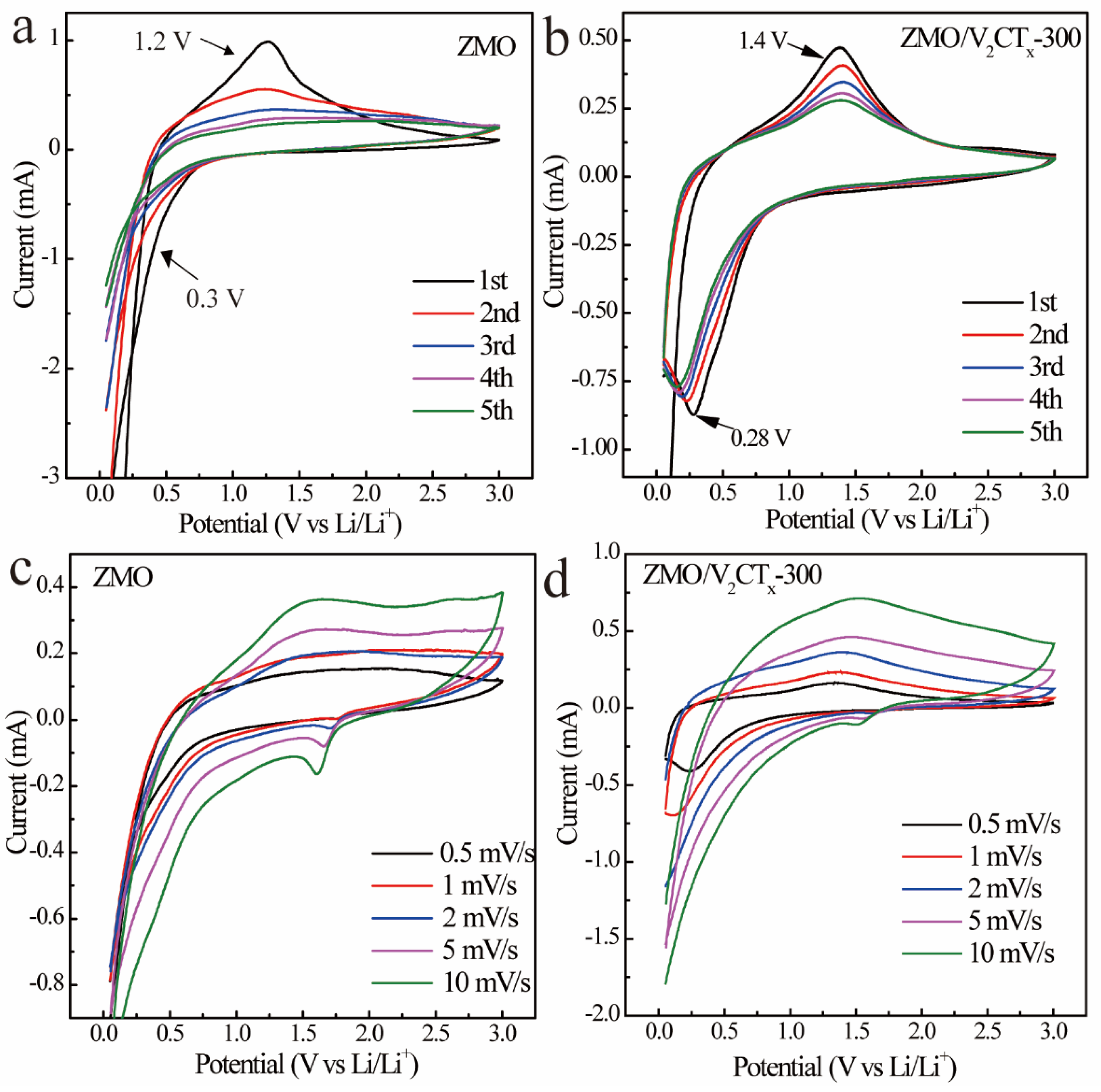
3.2. Electrochemical Performance
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhu, L.; Li, F.; Yao, T.; Liu, T.; Wang, J.; Li, Y.; Lu, H.; Qian, R.; Liu, Y.; Wang, H. Electrospun MnCo2O4 Nanotubes as High-Performance Anode Materials for Lithium-Ion Batteries. Energy Fuels 2020, 34, 11574–11580. [Google Scholar] [CrossRef]
- Deng, Z.; He, M.; Feng, Y.; Xiong, D. A fluorine-doped MnFe2O4 nanorod/carbon composite as an anode material for high-performance lithium-ion batteries. Int. J. Electrochem. Sci. 2020, 15, 4203–4217. [Google Scholar] [CrossRef]
- Liang, C.; Chen, J.; Yu, K.; Jin, W. ZnMn2O4 spheres anchored on jute porous carbon for use as a high-performance anode material in lithium-ion batteries. J. Alloys Compd. 2021, 878, 160445. [Google Scholar] [CrossRef]
- Rajoba, S.J.; Kale, R.D.; Kulkarni, S.B.; Parale, V.G.; Patil, R.; Olin, H.; Park, H.-H.; Dhavale, R.P.; Phadatare, M. Synthesis and Electrochemical Performance of Mesoporous NiMn2O4 Nanoparticles as an Anode for Lithium-Ion Battery. J. Compos. Sci. 2021, 5, 69. [Google Scholar] [CrossRef]
- Lu, H.; Qian, R.; Zhu, L.; Yao, T.; Li, C.; Li, L.; Wang, H. Phase structure engineering of MnCo2Ox within electrospun carbon nanofibers towards high-performance lithium-ion batteries. J. Colloid Interface Sci. 2022, 607, 171–180. [Google Scholar] [CrossRef]
- Zhang, F.; Jia, Z.; Wang, C.; Feng, A.; Wang, K.; Hou, T.; Liu, J.; Zhang, Y.; Wu, G. Sandwich-like silicon/Ti3C2Tx MXene composite by electrostatic self-assembly for high performance lithium ion battery. Energy 2020, 195, 117047. [Google Scholar] [CrossRef]
- Fan, P.; Liu, H.; Marosz, V.; Samuels, N.T.; Suib, S.L.; Sun, L.; Liao, L. High Performance Composite Polymer Electrolytes for Lithium-Ion Batteries. Adv. Funct. Mater. 2021, 31, 2101380. [Google Scholar] [CrossRef]
- Li, P.; Kim, H.; Myung, S.-T.; Sun, Y.-K. Diverting Exploration of Silicon Anode into Practical Way: A Review Focused on Silicon-Graphite Composite for Lithium Ion Batteries. Energy Storage Mater. 2021, 35, 550–576. [Google Scholar] [CrossRef]
- Dong, L.; Hao, J.; Liu, H.; Shi, W.; Yang, J.; Lian, J. Three-Dimensional ZnMn2O4 Nanoparticles/Carbon Cloth Anodes for High-Performance Flexible Lithium-Ion Batteries. ChemistrySelect 2020, 5, 2372–2378. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, Y.; Li, Z.; Zhang, W.; Zheng, M.; Zhang, H. Biomass-mediated synthesis of carbon-supported ZnMn2O4 nanoparticles as high-performance anode materials for lithium-ion batteries. Colloids Surf. A Physicochem. Eng. Asp. 2020, 600, 2738–2745. [Google Scholar] [CrossRef]
- Min, X.; Zhang, Y.; Yu, M.; Wang, Y.; Yuan, A.; Xu, J. A hierarchical dual-carbon supported ZnMn2O4/C composite as an anode material for Li-ion batteries. J. Alloys Compd. 2021, 877, 160242. [Google Scholar] [CrossRef]
- Chen, S.; Yao, M.; Wang, F.; Wang, J.; Zhang, Y.; Wang, Y. Facile microemulsion synthesis of mesoporous ZnMn2O4 submicrocubes as high-rate and long-life anodes for lithium ion batteries. Ceram. Int. 2019, 45, 5594–5600. [Google Scholar] [CrossRef]
- Pang, F.; Hou, S.; Wang, P.; Liu, M.; Luo, Y.; Zhao, L. β-MnO2/Metal-Organic Framework Derived Nanoporous ZnMn2O4 Nanorods as Lithium-Ion Battery Anodes with Superior Lithium-Storage Performance. Chem. Eur. J. 2019, 25, 5043–5050. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Li, S.; Zou, J.; Cui, Z.; Zhang, Q.; Schiettekatte, F.; Barba, D.; Liu, B.; Wang, Y. Graphene-Wrapped ZnMn2O4 Nanoparticles with Enhanced Performance as Lithium-Ion Battery Anode Materials. Nano 2020, 15, 2050117. [Google Scholar] [CrossRef]
- Tang, Q.; Shi, Y.; Ding, Z.; Wu, T.; Wu, J.; Mattick, V.; Yuan, Q.; Yu, H.; Huang, K. Three-dimensional hierarchical graphene and CNT-coated spinel ZnMn2O4 as a high-stability anode for lithium-ion batteries. Electrochim. Acta 2020, 338, 135853. [Google Scholar] [CrossRef]
- Kommu, P.; Singh, G.; Chakra, C.S.; Jana, S.; Kumar, V.; Bhattacharyya, A. Preparation of ZnMn2O4 and ZnMn2O4/graphene nano composites by combustion synthesis for their electrochemical properties. Mater. Sci. Eng. B 2020, 261, 114647. [Google Scholar] [CrossRef]
- Lu, M.; Zhang, Y.; Chen, J.; Han, W.; Zhang, W.; Li, H.; Zhang, X.; Zhang, B. K+ alkalization promoted Ca2+ intercalation in V2CT MXene for enhanced Li storage. J. Energy Chem. 2020, 49, 358–364. [Google Scholar] [CrossRef]
- Liu, F.; Liu, Y.; Zhao, X.; Liu, K.; Yin, H.; Fan, L. Prelithiated V2C MXene: A High-Performance Electrode for Hybrid Magnesium/Lithium-Ion Batteries by Ion Cointercalation. Small 2020, 16, 1906076. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Xue, Z.; Qin, J.; Sawangphruk, M.; Zhang, X.; Liu, R. NiCo-LDH/Ti3C2 MXene hybrid materials for lithium ion battery with high-rate capability and long cycle life. J. Energy Chem. 2020, 50, 143–153. [Google Scholar] [CrossRef]
- Feng, C.; Wang, W.; Chen, X.; Wang, S.; Guo, Z. Synthesis and electrochemical properties of ZnMn2O4 anode for lithium-ion batteries. Electrochim. Acta 2015, 178, 847–855. [Google Scholar] [CrossRef]
- Hu, J.; Xu, B.; Ouyang, C.; Yang, S.A.; Yao, Y. Investigations on V2C and V2CX2 (X = F, OH) Monolayer as a Promising Anode Material for Li Ion Batteries from First-Principles Calculations. J. Phys. Chem. C 2014, 118, 24274–24281. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Wang, Y.; Pei, X.; Zhou, C.; Zhao, Q.; Lu, M.; Han, W.; Wang, L. ZnMn2O4/V2CTx Composites Prepared as an Anode Material via High-Temperature Calcination Method for Optimized Li-Ion Batteries. Micromachines 2024, 15, 828. https://doi.org/10.3390/mi15070828
Li J, Wang Y, Pei X, Zhou C, Zhao Q, Lu M, Han W, Wang L. ZnMn2O4/V2CTx Composites Prepared as an Anode Material via High-Temperature Calcination Method for Optimized Li-Ion Batteries. Micromachines. 2024; 15(7):828. https://doi.org/10.3390/mi15070828
Chicago/Turabian StyleLi, Ji, Yu Wang, Xinyuan Pei, Chunhe Zhou, Qing Zhao, Ming Lu, Wenjuan Han, and Li Wang. 2024. "ZnMn2O4/V2CTx Composites Prepared as an Anode Material via High-Temperature Calcination Method for Optimized Li-Ion Batteries" Micromachines 15, no. 7: 828. https://doi.org/10.3390/mi15070828
APA StyleLi, J., Wang, Y., Pei, X., Zhou, C., Zhao, Q., Lu, M., Han, W., & Wang, L. (2024). ZnMn2O4/V2CTx Composites Prepared as an Anode Material via High-Temperature Calcination Method for Optimized Li-Ion Batteries. Micromachines, 15(7), 828. https://doi.org/10.3390/mi15070828





