The Intrinsic Relationship between Photoluminescence and Photocatalysis of MMoO4/MWO4 (M = Mg, Ca, Sr and Ba) Heterojunctions: Heterojunction Construction, Mechanism Insight and Development Tendency
Abstract
1. Introduction
2. MMoO4/MWO4 (M = Mg, Ca, Sr and Ba) Heterostructure Construction
2.1. Synthesis of MMo(W)O4 and Ion-Doped MMo(W)O4
2.2. Synthesis of MMoO4/MWO4 (M = Mg, Ca, Sr and Ba) Heterojunctions
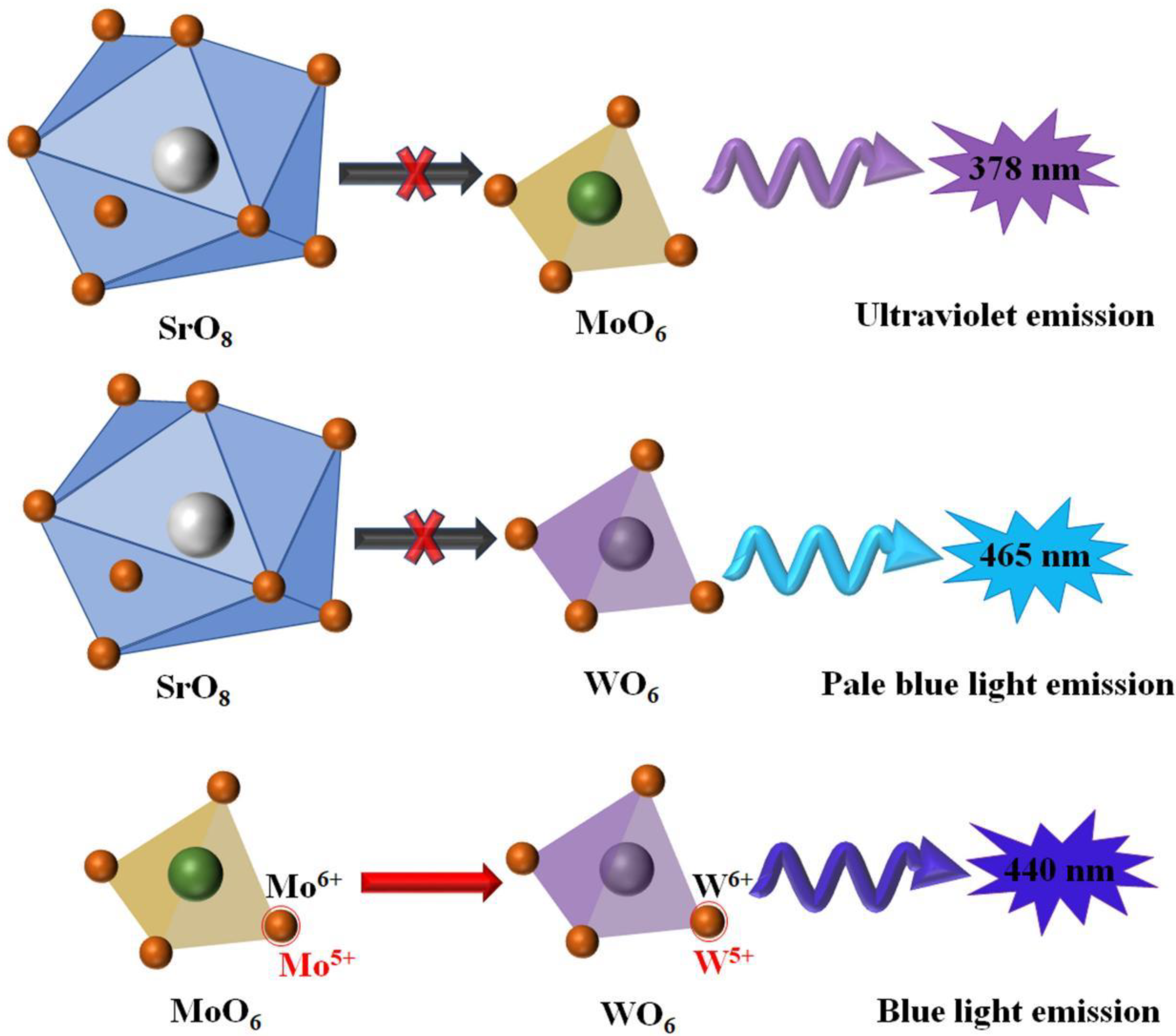
3. Photoluminescence Properties of MMoO4/MWO4 (M = Mg, Ca, Sr and Ba) Heterojunctions
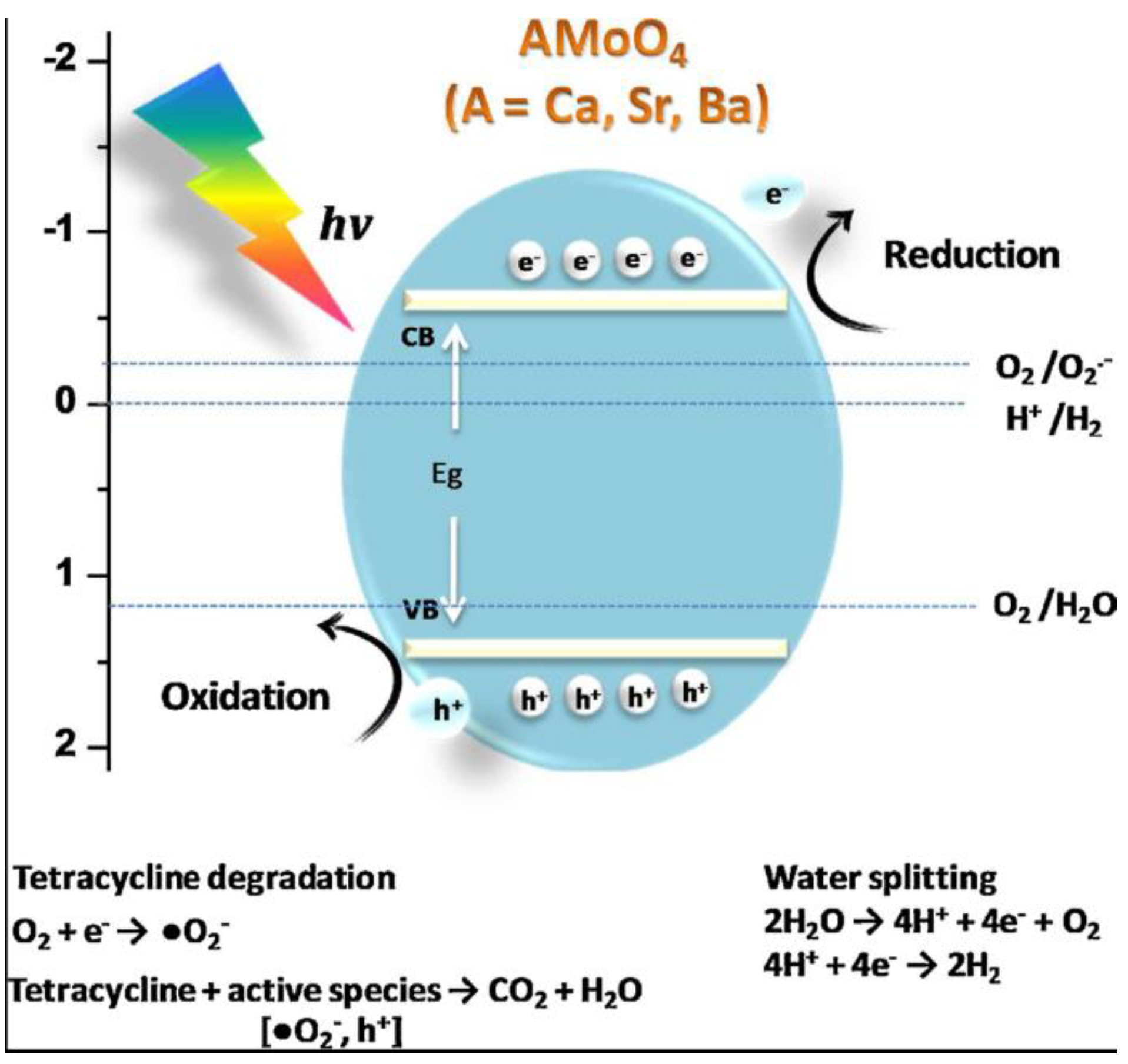
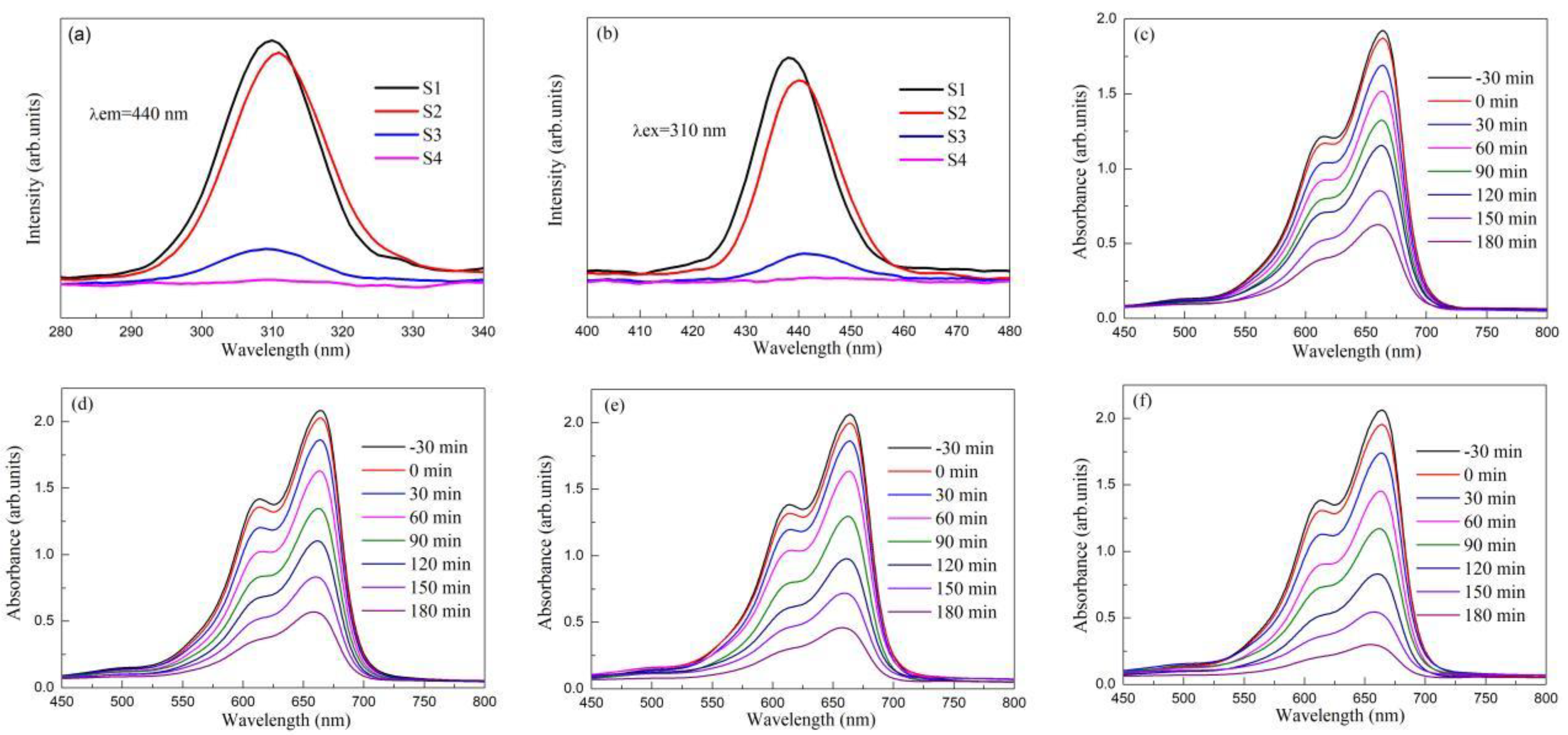
4. Photocatalytic Activity of MMoO4/MWO4 (M = Mg, Ca, Sr and Ba) Heterojunctions
- (1)
- Charge carrier generation:
- (2)
- Hydroxyl free radical and superoxide free radical production in valence band:
- (3)
- Hydroxyl free radical production in conduction band:
- (4)
- Pollutant degradation:
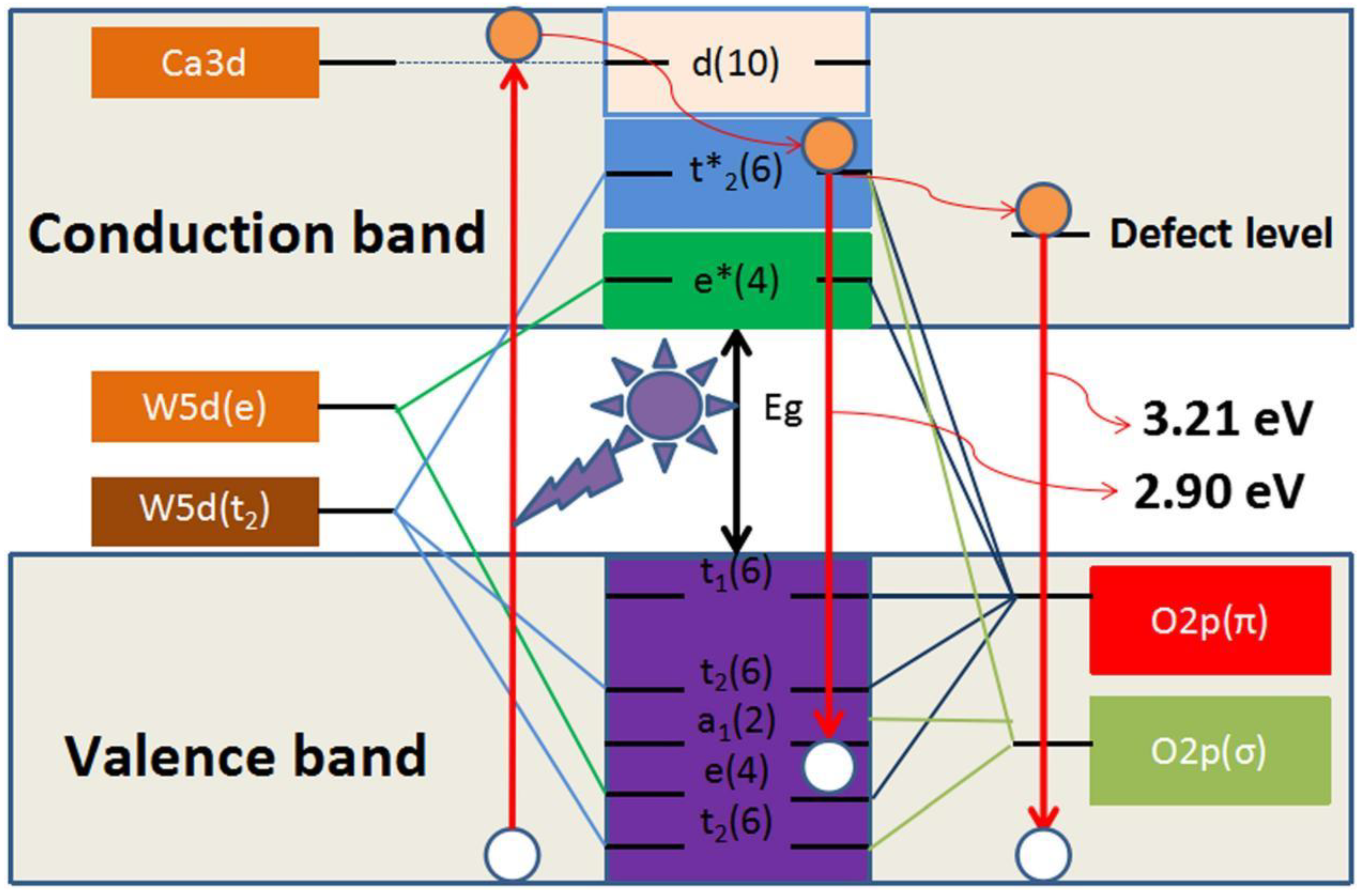
5. The Intrinsic Correlation Mechanism between Photoluminescence and Photocatalytic Activity
6. Conclusions and Prospects
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nair, G.B.; Swart, H.C.; Dhoble, S.J. A review on the advancements in phosphor-converted light emitting diodes (pc-LEDs): Phosphor synthesis, device fabrication and characterization. Prog. Mater. Sci. 2020, 109, 100622. [Google Scholar] [CrossRef]
- Polikarpov, E.; Catalini, D.; Padmaperuma, A.; Das, P.; Lemmon, T.; Arey, B.; Fernandez, C.A. A high efficiency rare earth-free orange emitting phosphor. Opt. Mater. 2015, 46, 614–618. [Google Scholar] [CrossRef]
- Fang, M.H.; Bao, Z.; Huang, W.T.; Liu, R.S. Evolutionary generation of phosphor materials and their progress in future applications for light-emitting diodes. Chem. Rev. 2022, 122, 11474–11513. [Google Scholar] [CrossRef] [PubMed]
- Harrison, D.E.; Melamed, N.T.; Subbarao, E.C. A new family of self-activated phosphors. J. Electrochem. Soc. 1963, 110, 23. [Google Scholar] [CrossRef]
- Ryu, J.H.; Koo, S.M.; Yoon, J.W.; Lim, C.S.; Shim, K.B. Synthesis of nanocrystalline MMoO4 (M = Ni, Zn) phosphors via a citrate complex route assisted by microwave irradiation and their photoluminescence. Mater. Lett. 2006, 60, 1702–1705. [Google Scholar] [CrossRef]
- Li, L.; Li, R.; Zi, W.; Gan, S. Hydrothermal synthesis and luminescent properties of color-tunable Dy3+ doped and Eu3+/Tb3+ co-doped MMoO4 (M = Ca, Sr, Ba) phosphors. Phys. B Cond. Matt. 2015, 458, 8–17. [Google Scholar] [CrossRef]
- Zhang, W.; Yin, J.; Min, F.; Jia, L.; Zhang, D.; Zhang, Q.; Xie, J. Preparation and photoluminescence properties of MMoO4 (M = Cu, Ni, Zn) nano-particles synthesized via electrolysis. J. Mol. Struct. 2017, 1127, 777–783. [Google Scholar] [CrossRef]
- Yadav, P.; Sinha, E. Structural, photophysical and microwave dielectric properties of α-ZnMoO4 phosphor. J. Alloys Compd. 2019, 795, 446–452. [Google Scholar] [CrossRef]
- Dabre, K.V.; Dhoble, S.J.; Lochab, J. Synthesis and luminescence properties of Ce3+ doped MWO4 (M = Ca, Sr and Ba) microcrystalline phosphors. J. Lumin. 2014, 149, 348–352. [Google Scholar] [CrossRef]
- Wang, X.Z. Hydrothermal synthesis and luminescence of MWO4: Tb3+ (M = Ca, Sr, Ba) microsphere phosphors. J. Mater. Sci. Mater. Electron. 2014, 25, 3271–3275. [Google Scholar] [CrossRef]
- Ryu, J.H.; Yoon, J.W.; Shim, K.B. Blue-luminescence of nanocrystalline MWO4 (M = Ca, Sr, Ba, Pb) phosphors synthesized via a citrate complex route assisted by microwave irradiation. Electrochem. Solid-State Lett. 2005, 8, D15. [Google Scholar] [CrossRef]
- Ambast, A.K.; Sharma, S.K. Calculation of spectral parameters for doped/codoped MWO4 (M = Ba2+/Ca2+) phosphors. J. Electron. Mater. 2017, 46, 4883–4890. [Google Scholar] [CrossRef]
- Wang, W.S.; Zhen, L.; Xu, C.Y.; Shao, W.Z.; Chen, Z.L. Eu3+-doped CdMoO4 red phosphor synthesized through an aqueous solution route at room temperature. J. Alloys Compd. 2012, 529, 17–20. [Google Scholar] [CrossRef]
- Kumar, M.; Singh, R.; Khajuria, H.; Sheikh, H.N. Facile hydrothermal synthesis of nanocomposites of nitrogen doped graphene with metal molybdates (NG-MMoO4) (M = Mn, Co, and Ni) for enhanced photodegradation of methylene blue. J. Mater. Sci. Mater. Electron. 2017, 28, 9423–9434. [Google Scholar] [CrossRef]
- Feizpoor, S.; Habibi-Yangjeh, A. Ternary TiO2/Fe3O4/CoWO4 nanocomposites: Novel magnetic visible-light-driven photocatalysts with substantially enhanced activity through pn heterojunction. J. Colloid Interface Sci. 2018, 524, 325–336. [Google Scholar] [CrossRef] [PubMed]
- Pirhashemi, M.; Habibi-Yangjeh, A. Facile fabrication of novel ZnO/CoMoO4 nanocomposites: Highly efficient visible-light-responsive photocatalysts in degradations of different contaminants. J. Photochem. Photobiol. A Chem. 2018, 363, 31–43. [Google Scholar] [CrossRef]
- Kotera, Y. Luminescence of solid solutions CaWO4-PbWO4 activated by samarium. J. Phys. Chem. Solids 1964, 25, 353–354. [Google Scholar] [CrossRef]
- Ansari, A.A.; Alam, M. Optical and structural studies of CaMoO4:Sm, CaMoO4:Sm@ CaMoO4 and CaMoO4:Sm@ CaMoO4@SiO2 core–shell nanoparticles. J. Lumin. 2015, 157, 257–263. [Google Scholar] [CrossRef]
- Kim, I.J.; Do Han, S.; Lee, H.D.; Wang, J.S.; Singh, I.; Kornilov, S.V. Fabrication and characterization of humidity sensor based on (Li2MoO4)x(CaMoO4)1−x system. Mater. Sci. Eng. B 2005, 116, 226–230. [Google Scholar] [CrossRef]
- Sukharenko, M.A.; Garkushin, I.K.; Osipov, V.T.; Radchenko, A.V. Phase tree and investigation of the NaBr–BaMoO4–BaWO4 stable triangle and the NaBr–BaBr2–BaMoO4–BaWO4 stable tetrahedron of the Na+, Ba2+||Br–, System. Russ. J. Inorg. Chem. 2022, 67, 2030–2039. [Google Scholar] [CrossRef]
- Liu, J.; Kaczmarek, A.M.; Billet, J.; Van Driessche, I.; Van Deun, R. Upconversion luminescence of lanthanide-doped mixed CaMoO4–CaWO4 micro-/nano-materials. Dalton Transact. 2016, 45, 12094–12102. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Kaczmarek, A.; Van Deun, R. Upconversion luminescence of lanthanide-doped CaMoO4/CaWO4 3D microstructure materials. In Proceedings of the 1st Symposium’ Chemical Research in Flanders’ (CRF 2016), Blankenberge, Belgium, 24–26 October 2016; Available online: https://www.xueshufan.com/publication/2544311607 (accessed on 26 October 2016).
- Nayak, P.; Nanda, S.S.; Dash, S. Enhanced luminescence and Judd-Ofelt analysis in SiO2 encapsulated CaWO4@ CaWO4: Eu: Bi for visualization of latent fingerprints and anti-counterfeiting. Appl. Surf. Sci. 2023, 609, 155426. [Google Scholar] [CrossRef]
- Patil, P.N.; Subramanian, U.; Hyam, R.S. Enhanced electrical and thermoelectric power properties of BaWO4/CaWO4 nanocomposites. Appl. Phys. A 2021, 127, 731. [Google Scholar] [CrossRef]
- Dutta, S.; Som, S.; Sharma, S.K. Optimization and characterization of trap level distribution in Γ-irradiated doped/codoped CaMoO4 phosphors. Phys. B 2013, 417, 39–45. [Google Scholar] [CrossRef]
- Wu, H.; Hu, Y.; Zhang, W.; Kang, F.; Li, N.; Ju, G. Sol–gel synthesis of Eu3+ incorporated CaMoO4: The enhanced luminescence performance. J. Sol-Gel Sci. Technol. 2012, 62, 227–233. [Google Scholar] [CrossRef]
- Ming, X.; Meng, Q.; Xiong, J.; Sun, W. Study on the color tunability and energy transfer mechanism in Tb3+, Sm3+ co-doped CaMoO4 phosphors. J. Alloys Compd. 2017, 695, 1691–1698. [Google Scholar] [CrossRef]
- Xiong, J.; Meng, Q.; Sun, W. Luminescent properties and energy transfer mechanism from Tb3+ to Eu3+ in CaMoO4: Tb3+, Eu3+ phosphors. J. Rare Earths 2016, 34, 251–258. [Google Scholar] [CrossRef]
- Chung, J.H.; Ryu, J.H.; Mhin, S.W.; Kim, K.M.; Shim, K.B. Controllable white upconversion luminescence in Ho3+/Tm3+/Yb3+ co-doped CaMoO4. J. Mater. Chem. 2012, 22, 3997–4002. [Google Scholar] [CrossRef]
- Wang, X.F.; Peng, G.H.; Li, N.; Liang, Z.H.; Wang, X.; Wu, J.L. Hydrothermal synthesis and luminescence properties of 3d walnut-like CaMoO4:Eu3+ red phosphors. J. Alloys Compd. 2014, 599, 102–107. [Google Scholar] [CrossRef]
- Li, J.; Zhang, T.; Zhu, G.; Hairong, Z. Up-conversion photoluminescence emissions of CaMoO4:Pr3+/Yb3+ powder. J. Rare Earths 2017, 35, 645–651. [Google Scholar] [CrossRef]
- Li, S.; Yu, L.; Sun, J.; Man, X. Synthesis and photoluminescent characteristics of Eu3+-doped MMoO4 (M = Sr, Ba) nanophosphors by a hydrothermal method. J. Rare Earths 2017, 35, 347–3355. [Google Scholar] [CrossRef]
- Wu, J.; Li, M.; Jia, H.; Wang, M.; Liu, Z. Influences of calcination temperature and charge compensators on the properties of SrMoO4: Sm3+ red phosphor prepared via the sol–gel method. J. Lumin. 2019, 214, 116607. [Google Scholar] [CrossRef]
- de Azevedo Marques, A.P.; Umisedo, N.K.; Costa, J.A.; Yoshimura, E.M.; Okuno, E.; Künzel, R. The role of capping agents on the trapping levels structure and luminescent emission of SrMoO4 phosphors. J. Lumin. 2023, 257, 119662. [Google Scholar] [CrossRef]
- Wang, D.; Liang, Y.; Wang, Z.; Hu, S.; Yang, J. Hydrothermal synthesis of Eu3+-doped BaMoO4 fluorescent probe for the selective detection of Fe3+ ions. New J. Chem. 2022, 46, 16951–16958. [Google Scholar] [CrossRef]
- Thomas, J.K.; Vidya, S.; Solomon, S.; Joy, K. Structural, dielectric and optical characterization of BaMoO4 nano powder synthesized through an auto-igniting combustion technique. IOP Conf. Ser. Mater. Sci. Eng. 2011, 23, 012031. [Google Scholar] [CrossRef]
- Bosacka, M.; Kurzawa, M.; Jakubus, P. Reactivity of FeVO4 towards MgMoO4 in the solid-state. J. Therm. Anal. Calor. 2004, 77, 33–40. [Google Scholar] [CrossRef]
- Tranquilin, R.L.; Lovisa, L.X.; Almeida, C.R.R.; Paskocimas, C.A.; Li, M.S.; Oliveira, M.C.; Gracia, L.; Andres, J.; Longo, E.; Motta, F.V.; et al. Understanding the white-emitting CaMoO4 Co-doped Eu3+, Tb3+, and Tm3+ phosphor through experiment and computation. J. Phys. Chem. C 2019, 123, 18536–18550. [Google Scholar] [CrossRef]
- Zhang, M.; Yang, H.; Xian, T.; Wei, Z.; Feng, W. Preparation of bismuth ferrite nanoparticles and their photocatalytic activity for MO degradation. In Proceedings of the 2011 International Conference on Remote Sensing, Environment and Transportation Engineering, Nanjing, China, 24–26 June 2011; pp. 6177–6180. [Google Scholar]
- Yu, X.X.; Wu, Y.; Dong, B.; Dong, Z.F.; Yang, X. Enhanced solar light photocatalytic properties of ZnO nanocrystals by Mg-doping via polyacrylamide polymer method. J. Photochem. Photobio. A Chem. 2018, 356, 681–688. [Google Scholar] [CrossRef]
- Fan, X.; Liang, X.; Guo, G.; Wang, R.; Li, J. Preparation of nano-TiO2 powder by polyacrylamide gel method. Second. Int. Conf. Smart Mater. Nanotechnol. Eng. 2009, 7493, 1615–1620. [Google Scholar]
- Chen, Y.; Wang, Y.; Shi, J.; Guo, C.; Qi, L.; Zhao, J.; Luo, S.; Zhou, H.; Lu, X.; Fan, Q. Highly effective pyroelectric catalysis for simultaneous tumor-targeted dynamic therapy and gentle photothermal therapy by oxygen-vacancy-rich CeO2-BaTiO3 nanorods. Adv. Health Mater. 2024, 2400781. [Google Scholar] [CrossRef]
- Quintanar, C.; Caballero, R.; Barreto, J.; Chavira, E.; Marinero, E.E. Structural and electronic properties of cubic CeO2: Unpaired electrons in CeO2. Int. J. Quant. Chem. 2010, 110, 2949–2954. [Google Scholar] [CrossRef]
- Amirshaghaghi, A.; Kokabi, M.; Keschtkar, H.A. Al2O3-ZrO2 nanopowder preparation by polymer gel-net at low temperature. Synth. React. Inorg. Met.-Organ. Nano-Metal Chem. 2010, 40, 576–580. [Google Scholar] [CrossRef]
- Wang, H.; Gao, L. Preparation of nanoscale α-Al2O3 powder by the polyacrylamide gel method. Nanostruct. Mater. 1999, 11, 1263–1267. [Google Scholar] [CrossRef]
- Tahmasebpour, M.; Babaluo, A.A.; Shafiei, S.; Pipelzadeh, E. Studies on the synthesis of α-Al2O3 nanopowders by the polyacrylamide gel method. Powder Technol. 2009, 191, 91–97. [Google Scholar] [CrossRef]
- Su, X.; Zhou, J.; Bai, G.; Zhang, J.; Zhao, P. Low temperature synthesis and characterization of YAG nanopowders by polyacrylamide gel method. Ceram. Int. 2016, 42, 17497–17502. [Google Scholar] [CrossRef]
- Lin, J.; Guo, Z.; Li, M.; Lin, Q.; Huang, K.; He, Y. Magnetic and dielectric properties of Ca-substituted BiFeO3 nanoferrites by the sol-gel method. J. Appl. Biomater. Funct. Mater. 2018, 16, 93–100. [Google Scholar] [PubMed]
- Guilhaume, N.; Primet, M. Three-way catalytic activity and oxygen storage capacity of perovskite LaMn0.976Rh0.024O3+δ. J. Catal. 1997, 165, 197–204. [Google Scholar] [CrossRef]
- Ning, W.; Li, Y.; Fang, Y.; Li, F.; Pournajaf, R.; Hamawandi, B. Characterization and photocatalytic activity of CoCr2O4/g-C3N4 nanocomposite for water treatment. Environ. Sci. Pollut. Res. 2023, 30, 76515–76527. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Yang, H.; Xian, T.; Wei, Z.Q.; Jiang, J.L.; Feng, Y.C.; Liu, X.Q. Polyacrylamide gel synthesis and photocatalytic performance of Bi2Fe4O9 nanoparticles. J. Alloys Compd. 2011, 509, 809–812. [Google Scholar] [CrossRef]
- Wu, Q.; Jiang, F.; Feng, G.; Wang, S.; Miao, L.; Jiang, W.; Liang, J.; Liu, J. Nonhydrolytic sol-gel in-situ synthesis of high performance MgAl2O4/C adsorbent materials. Arab. J. Chem. 2023, 16, 104393. [Google Scholar] [CrossRef]
- Jafari, M.; Hassanzadeh-Tabrizi, S.A. Preparation of CoAl2O4 nanoblue pigment via polyacrylamide gel method. Powder Ttechnol. 2014, 266, 236–239. [Google Scholar] [CrossRef]
- Xiong, G.; Guo, R.; Zhang, W.; Pournajaf, R.; Tayebi, M. Effect of adding silver on photocatalytic properties of ZnAl2O4 nanopowder synthesized using microemulsion and polyacrylamide gel methods for recycled water. Mater. Chem. Phys. 2024, 315, 128898. [Google Scholar] [CrossRef]
- Sankaranarayanan, R.; Shailajha, S.; Seema, S.; Kairon Mubina, M.S. Enhanced magneto-electric properties of ZnAl2O4@ NiFe2O4 nanocomposites in magnetic sensor applications. J. Supercond. Novel Magn. 2023, 36, 693–709. [Google Scholar] [CrossRef]
- Zhou, N.; Li, Y.; Zhang, Y.; Shu, Y.; Nian, S.; Cao, W.; Wu, Z. Synthesis and characterization of Co1-xCaxAl2O4 composite blue nano-pigments by the polyacrylamide gel method. Dyes Pigments 2018, 148, 25–30. [Google Scholar] [CrossRef]
- Han, X.; Sun, M.; Chai, X.; Li, J.; Wu, Y.; Sun, W. Progress in synthesis and photocatalytic activity of MAl2O4 (M= Mg, Sr, Ba) based photocatalysts. Front. Mater. 2022, 9, 845664. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, M. Preparation and study of the adsorption performances of (Mg, Ca, Ba)Fe2O4 magnetic porous materials for removal of Cd (II) heavy metal ion from water environment. Russ. J. Phys. Chem. A 2022, 96, 1761–1767. [Google Scholar] [CrossRef]
- Tian, X.; Zhu, X. Photocatalytic activity of magnesium ferrite synthesized by microwave sintering assisted polyacrylamide gel method in degradation of methyl orange dye in wastewater. Russ. J. Phys. Chem. A 2021, 95, 2163–2170. [Google Scholar] [CrossRef]
- Ma, R.T.; Shang, H.Y.; Wang, X.; Jiang, D. Dielectric, magnetic and microwave absorbing properties of polyaniline-Co0.7Cr0.1Zn0.2Fe2O4 composites. Rare Met. 2017, 36, 118–122. [Google Scholar] [CrossRef]
- Pierre, A.C.; Favre, A.; Guilhaume, N. Variations in the synthesis of barium hexaferrite doped with iridium and its effect in the catalytic combustion of hydrocarbons. J. Mater. Res. 1998, 13, 1637–1643. [Google Scholar] [CrossRef]
- Shi, J.; Wang, J.; He, H.; Lu, Y.; Shi, Z. Rodlike YMn2O5 powders derived from hydrothermal process using oxygen as oxidant. Materials 2020, 13, 805. [Google Scholar] [CrossRef]
- Li, S.H.; Lei, G.P.; Peng, H. Synthesized and magnetic and ferroelectric phase transitions of single phase multiferroic YMn2O5 nanoparticles through microwave assisted polyacrylamide gel method. Ferroelectrics 2017, 520, 135–143. [Google Scholar] [CrossRef]
- Wang, S.; Gao, H.; Sun, G.; Zhang, J.; Xia, Y.; Xie, C.; Yang, G.; Yong, W.; Fang, L. M-type barium hexaferrite nanoparticles synthesized by γ-ray irradiation assisted polyacrylamide gel method and its optical, magnetic and supercapacitive performances. J. Cluster Sci. 2021, 32, 569–578. [Google Scholar] [CrossRef]
- Feng, W.; Liu, H.; Hui, P.; Yang, H.; Li, J.; Wang, J.S. Preparation and properties of SrFe12O19/ZnFe2O4 core/shell nano-powder microwave absorber. Integr. Ferroelectr. 2014, 152, 120–126. [Google Scholar] [CrossRef]
- Balamurugan, S.; Brightlin, B.C.; Arun, T.; Jainshaa, J. Morphological structure, optical and microbial findings of ferrites. J. Nanosci. Nanotechnol. 2019, 19, 5667–5673. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.F.; Zhang, C.; Sun, G.; Chen, B.; Liu, W.; Xiang, X.; Wang, H.; Fang, L.; Tian, Q.; Ding, Q.; et al. Effect of carbon and sintering temperature on the structural and magnetic properties of SrFe12O19 nanoparticles. J. Sol-Gel Sci. Technol. 2015, 73, 371–378. [Google Scholar] [CrossRef]
- Chen, X.; Wang, S.; Gao, H.; Yang, H.; Fang, L.; Chen, X.; Tang, S.; Yu, C.; Li, D. A novel lead hexagonal ferrite (PbFe12O19) magnetic separation catalyst with excellent ultrasonic catalytic activity. J. Sol-Gel Sci. Technol. 2024, 110, 578–593. [Google Scholar]
- Chen, X.; Liu, H.; Li, M.; Wang, S. Hexagonal lead ferrite magnetic separation catalysts: Synthesis, optical characterization, ultrasonic catalytic activity and performance prediction. J. Mod. Polym. Chem. Mater. 2022, 1, 11. [Google Scholar]
- Gu, H.; Zhang, H.; Ma, C.; Sun, H.; Liu, C.; Dai, K.; Zhang, J.; Wei, R.; Ding, T.; Guo, Z. Smart strain sensing organic–inorganic hybrid hydrogels with nano barium ferrite as the cross-linker. J. Mater. Chem. C 2019, 7, 2353–2360. [Google Scholar] [CrossRef]
- Douy, A. Polyacrylamide gel: An efficient tool for easy synthesis of multicomponent oxide precursors of ceramics and glasses. Int. J. Inorg. Mater. 2001, 3, 699–707. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, K.; Wang, Y.; Wang, C.; Wang, Y.; Chang, Q. Low temperature synthesis of Cu0.1Co0.9Al2O4 blue ceramic pigment via the combustion method. Ceram. Mater. 2019, 71, 389–396. [Google Scholar]
- Wu, Y.; Yun, J.; Wang, L.; Yang, X. Structure and optical properties of Mg-doped ZnO nanoparticles by polyacrylamide method. Cryst. Res. Technol. 2013, 48, 145–152. [Google Scholar] [CrossRef]
- Yang, J.; Luo, L.; Chen, Z.; Chen, X.; Tang, X. Tb-doped YPO4 phosphors: Polyacrylamide gel synthesis and optical properties. J. Alloys Compd. 2016, 689, 837–842. [Google Scholar] [CrossRef]
- Ling, R.; Yang, X.; Li, Y.; Huan, L.; Cai, Y.; Wang, A.; Tan, X.; Sun, G. Synthesis and influence of Zn substitution on optical and magnetic properties of cobalt ferrite nanoparticles by a polyacrylamide gel route. J. Mater. Sci. Mater. Electron. 2024, 35, 543. [Google Scholar] [CrossRef]
- Munisha, B.; Nanda, J.; Mishra, B.; Parida, C. Exploring the structural, optical, and dielectric characteristics of polyacrylamide gel synthesized CeMnO3 nanoparticles with Co & Ni ion doing. Mater. Sci. Eng. B 2024, 304, 117371. [Google Scholar] [CrossRef]
- Fu, X.; Zhang, H.; Niu, S.; Xin, Q. Synthesis and luminescent properties of SnO2: Eu nanopowder via polyacrylamide gel method. J. Solid State Chem. 2005, 178, 603–607. [Google Scholar] [CrossRef]
- Wang, S.; Gao, H.; Chen, C.; Li, Q.; Li, C.; Wei, Y.; Fang, L. Effect of phase transition on optical and photoluminescence properties of nano-MgWO4 phosphor prepared by a gamma-ray irradiation assisted polyacrylamide gel method. J. Mater. Sci. Mater. Electron. 2019, 30, 15744–15753. [Google Scholar] [CrossRef]
- Wang, S.; Gao, H.; Sun, G.; Li, Y.; Wang, Y.; Liu, H.; Chen, C.; Yang, L. Structure characterization, optical and photoluminescence properties of scheelite-type CaWO4 nanophosphors: Effects of calcination temperature and carbon skeleton. Opt. Mater. 2020, 99, 109562. [Google Scholar] [CrossRef]
- Sun, G.; Gao, Q.; Tang, S.; Chen, X.; Liu, H.; Gao, H.; Zhao, X.; Wang, A.; Yu, X.; Wang, S. Facile synthesis, optical and photoluminescence properties of copper tungstate phosphors with strong near-infrared photoabsorption. Russ. J. Phys. Chem. A 2022, 96, 1348–1355. [Google Scholar] [CrossRef]
- Tang, S.; Wang, S.; Yu, X.; Gao, H.; Niu, X.; Wang, Y.; Zhao, X.; Sun, G.; Li, D. Gamma-ray irradiation assisted polyacrylamide gel synthesis of scheelite type BaWO4 phosphors and its colorimetric, optical and photoluminescence properties. ChemistrySelect 2020, 5, 10599–10606. [Google Scholar] [CrossRef]
- Xuan, Z.; Xuan, C. Regulating the synthesis, optical and photocatalytic activity of MgMoO4 nanoparticles. Russ. J. Phys. Chem. A 2023, 97, 2060–2069. [Google Scholar] [CrossRef]
- Balu, S.; Sivaganesh, D.; Saravanakumar, S.; Sivakumar, V.; Kim, J.M.; Kannan, P.K.; Ganesh, V. Influence of surfactant on the structural, morphological and optical properties of SrWO4: Insights through electron density distribution analysis. Bull. Mater. Sci. 2023, 46, 62. [Google Scholar] [CrossRef]
- Gao, H.; Wang, Y.; Gao, Q.; Pan, X.; Wang, S.; Yang, H.; Chen, C.; Wang, Y.; Fang, L.; Yi, Z. Phase evolution and photoluminescence behavior of MMoO4 (M = Mg, Ca, Sr) phosphors. Optik 2021, 241, 167040. [Google Scholar] [CrossRef]
- Song, Y.; Liang, S.; Li, F.; Wang, X.; You, C.; Yang, Y. The self-assembly mechanism of CaWO4@ CaWO4:Dy3+ core/shell microspheres via a simple surfactant-free hydrothermal route. Mater. Lett. 2015, 161, 100–103. [Google Scholar] [CrossRef]
- Guan, S.; Li, R.; Sun, X.; Xian, T.; Yang, H. Construction of novel ternary Au/LaFeO3/Cu2O composite photocatalysts for RhB degradation via photo-Fenton catalysis. Mater. Technol. 2021, 36, 603–615. [Google Scholar] [CrossRef]
- Zhao, H.; Sun, X.; Mao, C.; Du, J. Preparation and microwave–absorbing properties of NiFe2O4-polystyrene composites. Phys. B Cond. Matt. 2009, 404, 69–72. [Google Scholar] [CrossRef]
- Li, M.; Wang, S.; Gao, H.; Yin, Z.; Chen, C.; Yang, H.; Fang, L.; Veerabhadrappa, J.A.; Yi, Z.; Li, D. Selective removal of antibiotics over MgAl2O4/C3N4/YMnO3 photocatalysts: Performance prediction and mechanism insight. J. Am. Ceram. Soc. 2023, 106, 2420–2442. [Google Scholar] [CrossRef]
- Su, X.; Zhou, J.; Wang, B.; Zhao, P. Synthesis and electric field-assisted sintering behavior of Al2O3–ZrO2 composite nanopowders by polyacrylamide gel method. J. Sol-Gel Sci. Technol. 2016, 80, 126–132. [Google Scholar] [CrossRef]
- Wang, S.; Li, M.; Gao, H.; Yin, Z.; Chen, C.; Yang, H.; Fang, L.; Angadi, V.J.; Yi, Z.; Li, D. Construction of CeO2/YMnO3 and CeO2/MgAl2O4/YMnO3 photocatalysts and adsorption of dyes and photocatalytic oxidation of antibiotics: Performance prediction, degradation pathway and mechanism insight. Appl. Surf. Sci. 2023, 608, 154977. [Google Scholar] [CrossRef]
- Wang, S.; Li, M.; Yin, Z.; Gao, H.; Liu, H.; Yang, H.; Fang, L.; Angadi, V.J.; Hu, L.; Li, D. Skillfully grafted C-O functional group to enhance the adsorption/photocatalytic mechanism of YMnO3/MgAl2O4 heterojunction photocatalysts. Adv. Powder Technol. 2022, 33, 103771. [Google Scholar] [CrossRef]
- Wang, S.; Chen, X.; Fang, L.; Gao, H.; Han, M.; Chen, X.; Xia, Y.; Xie, L.; Yang, H. Double heterojunction CQDs/CeO2/BaFe12O19 magnetic separation photocatalysts: Construction, structural characterization, dye and POPs removal, and the interrelationships between magnetism and photocatalysis. Nucl. Anal. 2022, 1, 100026. [Google Scholar] [CrossRef]
- Sankaranarayanan, R.; Shailajha, S.; Mubina, M.K.; Anilkumar, C.P. Effect of Zn2+ ions on structural, optical, magnetic, and impedance response of Znx@ Ni1−xFe2O4 core materials prepared by two-step polyacrylamide gel method. J. Mater. Sci. Mater. Electron. 2020, 31, 11833–11846. [Google Scholar] [CrossRef]
- Wang, S.; Gao, H.; Li, J.; Wang, Y.; Chen, C.; Yu, X.; Tang, S.; Zhao, X.; Sun, G.; ad Li, D. Comparative study of the photoluminescence performance and photocatalytic activity of CeO2/MgAl2O4 composite materials with an n-n heterojunction prepared by one-step synthesis and two-step synthesis methods. J. Phys. Chem. Solid. 2021, 150, 109891. [Google Scholar] [CrossRef]
- Li, J.; Wang, S.; Sun, G.; Gao, H.; Yu, X.; Tang, S.; Zhao, X.; Yi, Z.; Wang, Y.; Wei, Y. Facile preparation of MgAl2O4/CeO2/Mn3O4 heterojunction photocatalyst and enhanced photocatalytic activity. Mater. Today Chem. 2021, 19, 100390. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, H.; Wang, S.; Fang, L.; Chen, X.; Yu, C.; Tang, S.; Liu, H.; Yi, Z.; Yang, H. Facile synthesis of BaMoO4 and BaMoO4/BaWO4 heterostructures with type-I band arrangement and enhanced photoluminescence properties. Adv. Powder Technol. 2021, 32, 4186–4197. [Google Scholar] [CrossRef]
- Zhu, J.; Li, C.; Sun, Y.; Yang, C.; Zhao, Y.; Zhu, Z.; Wang, D.; Hu, Z.; Chou, S.; Li, L.; et al. Enhanced photoluminescence of hollow CaWO4 microspheres: The fast fabrication, structural manipulation, and exploration of the growth mechanism. Mater. Chem. Front. 2022, 6, 1046–1055. [Google Scholar] [CrossRef]
- Mikhailik, V.B.; Kraus, H.; Kapustyanyk, V.; Panasyuk, M.; Prots, Y.; Tsybulskyi, V.; Vasylechko, L. Structure, luminescence and scintillation properties of the MgWO4–MgMoO4 system. J. Phys. Cond. Matt. 2008, 20, 365219. [Google Scholar] [CrossRef]
- Mikhailik, V.B.; Vasylechko, L.; Kraus, H.; Kapustyanyk, V.; Panasyuk, M.; Prots, Y.; Tsybulskyy, V. Correlation between the peculiarities of structure and luminescence properties of MgWO4–MgMoO4 system. J. Phys. Stud. 2010, 14, 1–11. [Google Scholar] [CrossRef]
- Gao, H.; Wang, Y.; Wang, S.; Yang, H.; Yi, Z. A simple fabrication, microstructure, optical, photoluminescence and supercapacitive performances of MgMoO4/MgWO4 heterojunction micro/nanocomposites. Solid State Sci. 2022, 129, 106909. [Google Scholar] [CrossRef]
- Gao, H.; Wang, S.; Wang, Y.; Yang, H.; Wang, F.; Tang, S.; Yi, Z.; Li, D. CaMoO4/CaWO4 heterojunction micro/nanocomposites with interface defects for enhanced photocatalytic activity. Colloid Surf. A 2022, 642, 128642. [Google Scholar] [CrossRef]
- Gao, H.; Yu, C.; Wang, Y.; Wang, S.; Yang, H.; Wang, F.; Tang, S.; Yi, Z.; Li, D. A novel photoluminescence phenomenon in a SrMoO4/SrWO4 micro/nano heterojunction phosphors obtained by the polyacrylamide gel method combined with low temperature calcination technology. J. Lumin. 2022, 243, 118660. [Google Scholar] [CrossRef]
- Orhan, E.; Anicete-Santos, M.; Maurera, M.A.; Pontes, F.M.; Souza, A.G.; Andrès, J.; Beltràn, A.; Varela, J.A.; Pizani, P.S.; Taft, C.A.; et al. Towards an insight on the photoluminescence of disordered CaWO4 from a joint experimental and theoretical analysis. J. Solid State Chem. 2005, 178, 1284–1291. [Google Scholar] [CrossRef]
- Sun, L.; Cao, M.; Wang, Y.; Sun, G.; Hu, C. The synthesis and photoluminescent properties of calcium tungstate nanocrystals. J. Cryst. Growth 2006, 289, 231–235. [Google Scholar] [CrossRef]
- Cavalcante, L.S.; Sczancoski, J.C.; Lima, L.F., Jr.; Espinosa, J.W.M.; Pizani, P.S.; Varela, J.A.; Longo, E. Synthesis, characterization, anisotropic growth and photoluminescence of BaWO4. Cryst. Growth Des. 2009, 9, 1002–1012. [Google Scholar] [CrossRef]
- Cavalcante, L.S.; Sczancoski, J.C.; Tranquilin, R.L.; Joya, M.R.; Pizani, P.S.; Varela, J.A.; Longo, E. BaMoO4 powders processed in domestic microwave-hydrothermal: Synthesis, characterization and photoluminescence at room temperature. J. Phys. Chem. Solids 2008, 69, 2674–2680. [Google Scholar] [CrossRef]
- Dutta, S.; Som, S.; Kunti, A.K.; Kumar, V.; Sharma, S.K.; Swart, H.C.; Visser, H.G. Structural and luminescence responses of CaMoO4 nano phosphors synthesized by hydrothermal route to swift heavy ion irradiation: Elemental and spectral stability. Acta Mater. 2017, 124, 109–119. [Google Scholar] [CrossRef]
- Botelho, G.; Nogueira, I.C.; Moraes, E.; Longo, E. Study of structural and optical properties of CaMoO4 nanoparticles synthesized by the microwave-assisted solvothermal method. Mater. Chem. Phys. 2016, 183, 110–120. [Google Scholar] [CrossRef]
- de Azevedo Marques, A.P.; de Melo, D.M.; Paskocimas, C.A.; Pizani, P.S.; Joya, M.R.; Leite, E.R.; Longo, E. Photoluminescent BaMoO4 nanopowders prepared by complex polymerization method (CPM). J. Solid State Chem. 2006, 179, 671–678. [Google Scholar] [CrossRef]
- Vidya, S.; Solomon, S.; Thomas, J.K. Synthesis, sintering and optical properties of CaMoO4: A promising scheelite LTCC and photoluminescent material. Phys. Status Solidi-A 2012, 209, 1067–1074. [Google Scholar] [CrossRef]
- Phuruangrat, A.; Thongtem, T.; Thongtem, S. Preparation, characterization and photoluminescence of nanocrystalline calcium molybdate. J. Alloys Compd. 2009, 481, 568–572. [Google Scholar] [CrossRef]
- Zakharko, Y.; Luchechko, A.; Syvorotka, I.; Stryganyuk, G.; Solskii, I. Anisotropy of optical absorption and luminescent properties of CaMoO4. Radiat. Meas. 2010, 45, 429–431. [Google Scholar] [CrossRef]
- Sczancoski, J.C.; Cavalcante, L.S.; Joya, M.R.; Varela, J.A.; Pizani, P.S.; Longo, E. SrMoO4 powders processed in microwave-hydrothermal: Synthesis, characterization and optical properties. Chem. Eng. J. 2008, 140, 632–637. [Google Scholar] [CrossRef]
- Gao, H.; Wang, S.; Wang, Y.; Yang, H.; Fang, L.; Chen, X.; Yi, Z.; Li, D. Fabrication and characterization of BaMoO4-coupled CaWO4 heterojunction micro/nanocomposites with enhanced photocatalytic activity towards MB and CIP degradation. J. Electron. Mater. 2022, 51, 5230–5245. [Google Scholar] [CrossRef]
- Kusuma, M.; Chandrappa, G.T. Effect of calcination temperature on characteristic properties of CaMoO4 nanoparticles. J. Sci. Adv. Mater. Dev. 2019, 4, 150–157. [Google Scholar] [CrossRef]
- Hosseinpour-Mashkani, S.S.; Hosseinpour-Mashkani, S.S.; Sobhani-Nasab, A. Synthesis and characterization of rod-like CaMoO4 nanostructure via free surfactant sonochemical route and its photocatalytic application. J. Mater. Sci. Mater. Electron. 2016, 27, 4351–4355. [Google Scholar] [CrossRef]
- Cavalcante, L.S.; Sczancoski, J.C.; Batista, N.C.; Longo, E.; Varela, J.A.; Orlandi, M.O. Growth mechanism and photocatalytic properties of SrWO4 microcrystals synthesized by injection of ions into a hot aqueous solution. Adv. Powder Technol. 2013, 24, 344–353. [Google Scholar] [CrossRef]
- Yanalak, G.; Ozen, A.; Sarılmaz, A.; Keles, A.; Aslan, E.; Ozel, F.; Patir, I.H. Scheelite-type BaMoO4 and BaWO4 based dye sensitized photocatalytic hydrogen evolution by water splitting. J. Phys. Chem. Solids 2022, 168, 110821. [Google Scholar] [CrossRef]
- Bellucci, G.V.; Gouveia, A.F.; Ribeiro, L.K.; Assis, M.; Longo, E.; San-Miguel, M.A.; Rosa, I.L.V.; Andrés, J. Surfactant effects in the morphology and the photocatalytic activity of the BaMoO4 crystals. Eclética Química J. 2022, 34, 105074. [Google Scholar] [CrossRef]
- Huerta-Flores, A.M.; Juárez-Ramírez, I.; Torres-Martínez, L.M.; Carrera-Crespo, J.E.; Gómez-Bustamante, T.; Sarabia-Ramos, O. Synthesis of AMoO4 (A = Ca, Sr, Ba) photocatalysts and their potential application for hydrogen evolution and the degradation of tetracycline in water. J. Photochem. Photobio. A Chem. 2018, 356, 29–37. [Google Scholar] [CrossRef]
- Dutta, D.P.; Singh, A.; Ramkumar, J.; Bhattacharya, K.; Tyagi, A.K.; Fulekar, M.H. Exploration of sorption properties of sonochemically synthesized BaMoO4 nanoparticles for hazardous cationic dye removal. Adv. Porous Mater. 2014, 2, 237–245. [Google Scholar] [CrossRef]
- Alencar, L.; Mesquita, A.; Feitosa, C.A.; Balzer, R.; Probst, L.F.; Batalha, D.C.; Rosmaninho, M.G.; Fajardo, H.V.; Bernardi, M.I. Preparation, characterization and catalytic application of barium molybdate (BaMoO4) and barium tungstate (BaWO4) in the gas-phase oxidation of toluene. Ceram. Int. 2017, 43, 4462–4469. [Google Scholar] [CrossRef]
- Bi, J.; Wu, L.; Zhang, Y.; Li, Z.; Li, J.; Fu, X. Solvothermal preparation, electronic structure and photocatalytic properties of PbMoO4 and SrMoO4. Appl. Catal. B Environ. 2009, 91, 135–143. [Google Scholar] [CrossRef]
- Syed, A.; Al-Shwaiman, H.A.; Al Khulaifi, M.M.; Al Zahrani, R.R.; Almajhdi, F.N.; Elgorban, A.M. Integrating plasmonic effect and nano-heterojunction formation for boosted light harvesting and photocatalytic performance using CaWO4/Ag2MoO4 and its antibacterial applications. Mater. Sci. Semicond. Proc. 2021, 133, 105921. [Google Scholar] [CrossRef]
- Li, Y.; Li, X.; Wang, S.; Tan, S.; Xia, Y.; Shi, J.; Chen, W.; Gao, L. In-situ generated SrWO4/g-C3N4 heterojunction photocatalyst for enhanced visible light degradation activity of tetracycline. Colloid Surf. A 2022, 643, 128806. [Google Scholar] [CrossRef]
- Wang, C.; Fu, M.; Cao, J.; Wu, X.; Hu, X.; Dong, F. BaWO4/g-C3N4 heterostructure with excellent bifunctional photocatalytic performance. Chem. Eng. J. 2020, 385, 123833. [Google Scholar] [CrossRef]
- Xu, H.M.; Huo, Y.Z. Study on organic dye removal in wastewater treatment and purification technology using MgWO4@ carbon quantum dot composite photocatalyst. Russ. J. Phys. Chem. A 2021, 95, 1247–1254. [Google Scholar]
- Ray, S.K.; Dhakal, D.; Lee, S.W. Insight into sulfamethoxazole degradation, mechanism, and pathways by AgBr-BaMoO4 composite photocatalyst. J. Photochem. Photobio. A Chem. 2018, 364, 686–695. [Google Scholar] [CrossRef]
- Tabatabai-Yazdi, F.S.; Pirbazari, A.E.; Saraei, F.E.K.; Gilani, N. Construction of graphene based photocatalysts for photocatalytic degradation of organic pollutant and modeling using artificial intelligence techniques. Phys. B Cond. Matt. 2021, 608, 412869. [Google Scholar] [CrossRef]
- Wang, S.; Mo, P.; Li, D.; Syed, A. Intelligent algorithms enable photocatalyst design and performance prediction. Catalysts 2024, 14, 217. [Google Scholar] [CrossRef]
- Mohammadzadeh Kakhki, R.; Zirjanizadeh, S.; Mohammadpoor, M. A review of clinoptilolite, its photocatalytic, chemical activity, structure and properties: In time of artificial intelligence. J. Mater. Sci. 2023, 58, 10555–10575. [Google Scholar] [CrossRef]
- McKeever, S.W.S.; Chen, R. Luminescence models. Radiat. Meas. 1997, 27, 625–661. [Google Scholar] [CrossRef]
- Griseri, V.; Dissado, L.A.; Fothergill, J.C.; Laurent, C.; Teyssedre, G. Photoluminescence, recombination induced luminescence and electroluminescence in epoxy resin. J. Phys. D Appl. Phys. 2001, 34, 2534. [Google Scholar] [CrossRef]
- Williams, F.E. Review of the interpretations of luminescence phenomena. J. Opt. Soc. Am. 1949, 39, 648–654. [Google Scholar] [CrossRef]
- Jing, L.Q.; Sun, X.J.; Xin, B.F.; Cai, W.M.; Fu, H.G. The preparation and characterization of La doped TiO2 nanoparticles and their photocatalytic activity. J. Solid State Chem. 2004, 177, 3375. [Google Scholar]
- Jing, L.Q.; Yuan, F.L.; Hou, H.G.; Xin, B.F.; Cai, W.M.; Fu, H.G. Relationships of surface oxygen vacancies with photoluminescence and photocatalytic performance of ZnO nanoparticles. Sci. Chin. B 2005, 48, 25. [Google Scholar] [CrossRef]
- Li, H.; Sun, B.; Gao, T.; Li, H.; Ren, Y.; Zhou, G. Ti3C2 MXene co-catalyst assembled with mesoporous TiO2 for boosting photocatalytic activity of methyl orange degradation and hydrogen production. Chin. J. Catal. 2022, 43, 461–471. [Google Scholar] [CrossRef]
- Zhang, B.; Sun, B.; Liu, F.; Gao, T.; Zhou, G. TiO2-based S-scheme photocatalysts for solar energy conversion and environmental remediation. Sci. China Mater. 2024, 67, 424–443. [Google Scholar] [CrossRef]
- Zhang, B.; Liu, F.; Sun, B.; Gao, T.; Zhou, G. Hierarchical S-scheme heterojunctions of ZnIn2S4-decorated TiO2 for enhancing photocatalytic H2 evolution. Chin. J. Catal. 2024, 59, 334–345. [Google Scholar] [CrossRef]
- Ghosh, S.K.; Rout, S.K.; Tiwari, A.; Yadav, P.; Sczancoski, J.C.; Filho, M.G.R.; Cavalcante, L.S. Structural refinement, Raman spectroscopy, optical and electrical properties of (Ba1−xSrx)MoO4 ceramics. J. Mater. Sci. Mater. Electron. 2015, 26, 8319–8335. [Google Scholar] [CrossRef]
- Ke, J.; Adnan Younis, M.; Kong, Y.; Zhou, H.; Liu, J.; Lei, L.; Hou, Y. Nanostructured ternary metal tungstate-based photocatalysts for environmental purification and solar water splitting: A review. Nano-Micro Lett. 2018, 10, 69. [Google Scholar] [CrossRef]

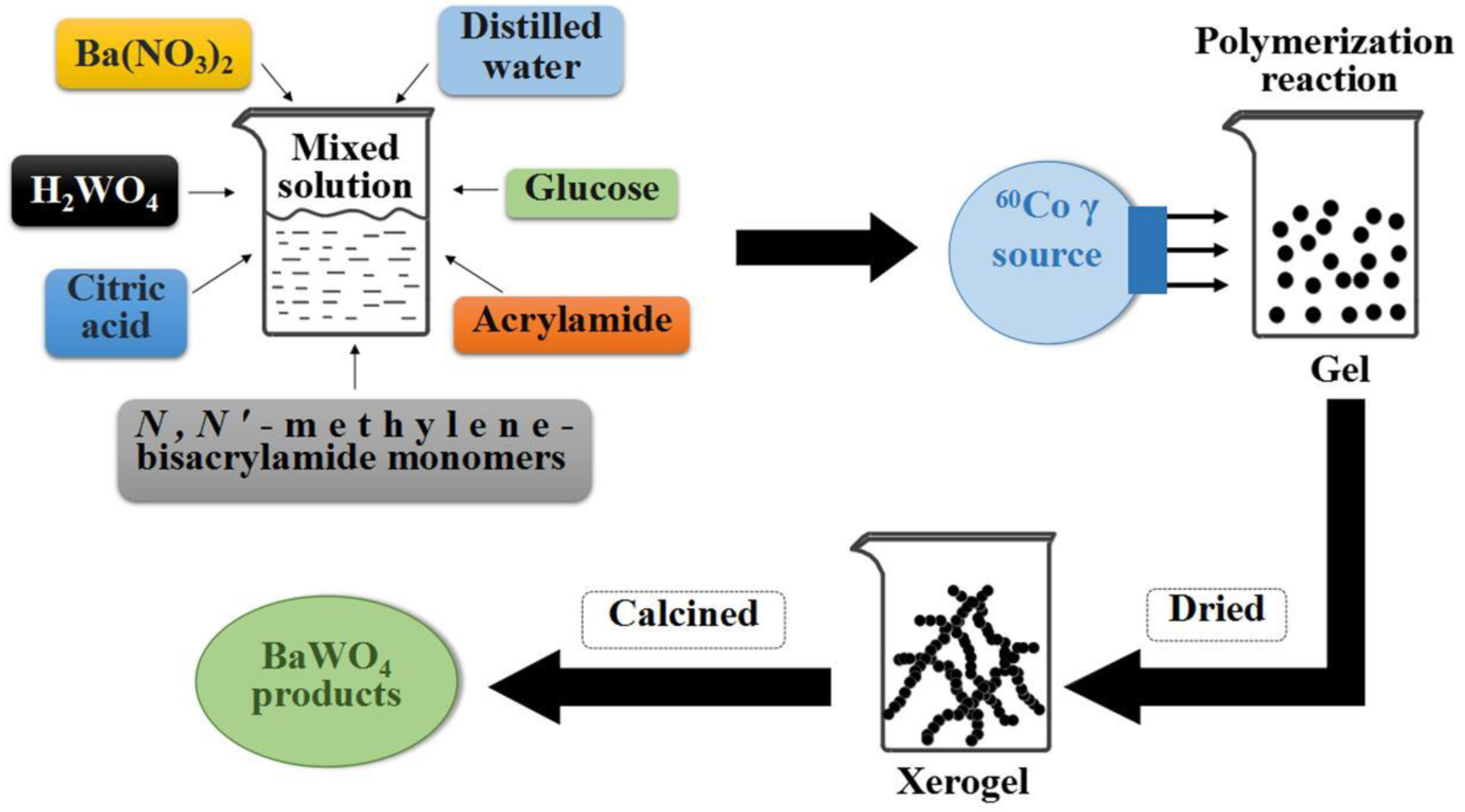

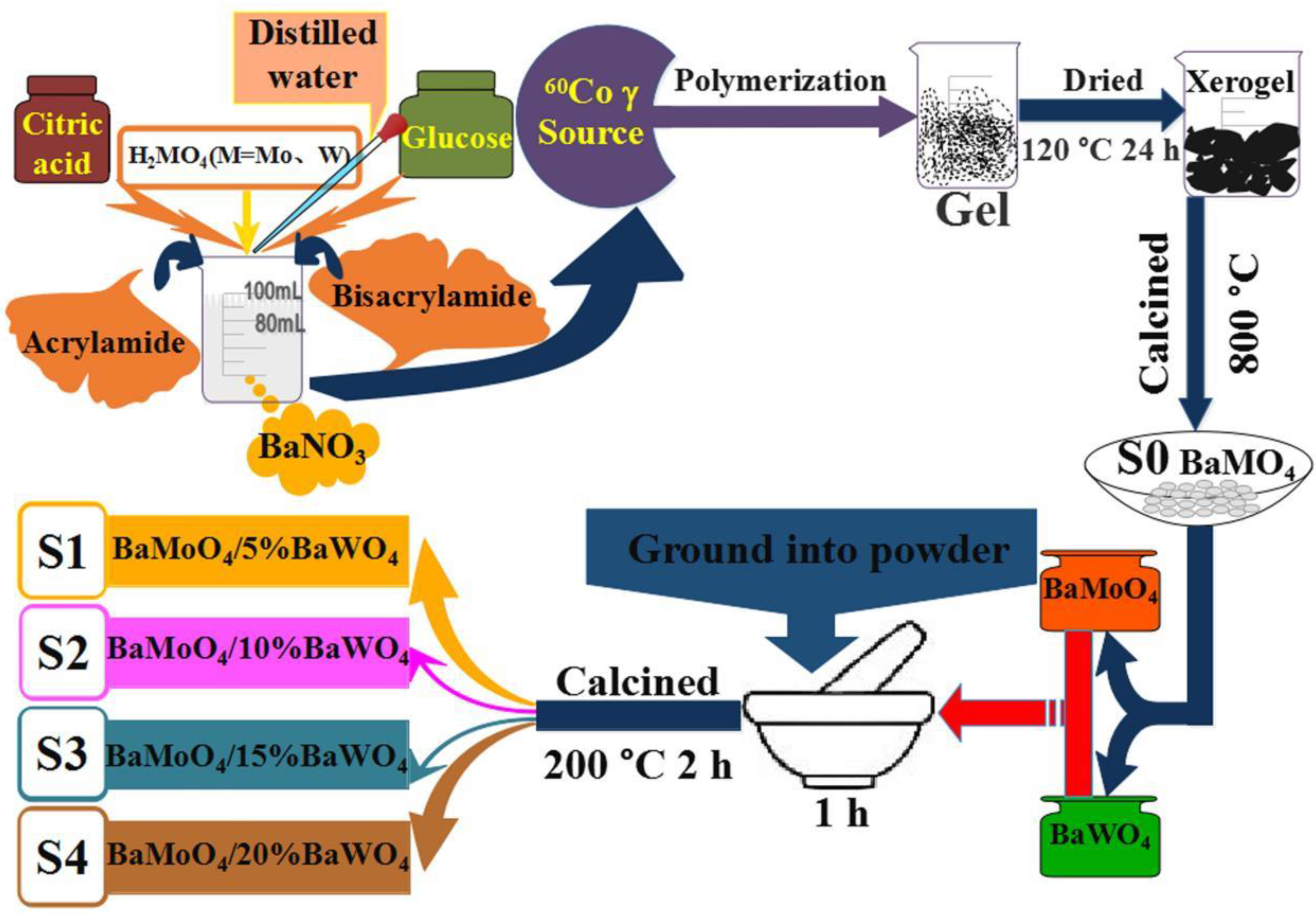



| Sample | Synthesis Method | Starting Chemicals | Reaction Conditions | Refs. |
|---|---|---|---|---|
| CaMoO4 | The solid-state diffusion method | Calcium carbonate (CaCO3), ammonium molybdate([NH4]6Mo7O24·4H2O) and polyethylene glycol [PEG] HO(CH2-CH2O)nH | Sintered at 900 °C for 1.5 h | [25] |
| CaMoO4:Eu | The sol–gel method | CaCl2 (96%), (NH4)6Mo7O24·4H2O (99%), CaCO3 (99%), MoO3 (99%) and Eu2O3 (99.99%) | Sintered at 800 °C for 3 h | [26] |
| CaMoO4 | Chemical precipitation method | Ca(NO3)3, Na2MoO4 | Sintered at 800 °C for 1 h | [27] |
| CaMoO4 | Hydrothermal method | Ethylene glycol, Ca(NO3)2·4H2O, Na2MoO4·2H2O, HNO3 | 160 °C for 16 h | [30] |
| BaMoO4 | Hydrothermal method | Ba(NO3)2, Na2MoO4·2H2O, HNO3 | 220 °C for 24 h | [35] |
| BaMoO4 | Auto-igniting combustion technique | Ba(NO3)2, MoO3, HNO3 | Sintered at 750 °C for 3 h | [36] |
| CaWO4 | Polyacrylamide gel method | Ca(NO3)2·4H2O (99%), H2WO4, citric acid, glucose, acrylamide and N, N’-methylene-bisacrylamide | Sintered at 800 °C for 5 h | [79] |
| BaWO4 | Polyacrylamide gel method | Ba(NO3)2, H2WO4, citric acid, glucose, acrylamide and N, N’-methylene-bisacrylamide | Sintered at 800 °C for 5 h | [81] |
| MMoO4 (M = Mg, Ca, Sr) | Polyacrylamide gel method | Mg2(OH)2CO3, CaCO3, SrCO3, H2MoO4, citric acid, glucose, acrylamide and N, N’-methylene- bisacrylamide | Sintered at 800 °C for 5 h | [84] |
| CaWO4@ CaWO4: Dy3+ | A simple surfactant-free hydrothermal route | Ca(NO3)2·4H2O, Na2WO4·2H2O and Dy(NO3)3·4H2O | 120 °C for 12 h | [85] |
| BaMoO4/BaWO4 | Polyacrylamide gel method combined with low temperature sintering technology | BaMoO4 and BaWO4 | 200 °C for 2 h | [96] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, M.; Veerabhadrappa, J.A.; Shaikh, S.F.; Kumar, A. The Intrinsic Relationship between Photoluminescence and Photocatalysis of MMoO4/MWO4 (M = Mg, Ca, Sr and Ba) Heterojunctions: Heterojunction Construction, Mechanism Insight and Development Tendency. Micromachines 2024, 15, 878. https://doi.org/10.3390/mi15070878
Zhang M, Veerabhadrappa JA, Shaikh SF, Kumar A. The Intrinsic Relationship between Photoluminescence and Photocatalysis of MMoO4/MWO4 (M = Mg, Ca, Sr and Ba) Heterojunctions: Heterojunction Construction, Mechanism Insight and Development Tendency. Micromachines. 2024; 15(7):878. https://doi.org/10.3390/mi15070878
Chicago/Turabian StyleZhang, Man, Jagadeesha Angadi Veerabhadrappa, Shoyebmohamad Fattemohamad Shaikh, and Ashok Kumar. 2024. "The Intrinsic Relationship between Photoluminescence and Photocatalysis of MMoO4/MWO4 (M = Mg, Ca, Sr and Ba) Heterojunctions: Heterojunction Construction, Mechanism Insight and Development Tendency" Micromachines 15, no. 7: 878. https://doi.org/10.3390/mi15070878
APA StyleZhang, M., Veerabhadrappa, J. A., Shaikh, S. F., & Kumar, A. (2024). The Intrinsic Relationship between Photoluminescence and Photocatalysis of MMoO4/MWO4 (M = Mg, Ca, Sr and Ba) Heterojunctions: Heterojunction Construction, Mechanism Insight and Development Tendency. Micromachines, 15(7), 878. https://doi.org/10.3390/mi15070878







