DEMIGOD: A Low-Cost Microcontroller-Based Closed-Loop System Integrating Nanoengineered Sweat-Based Glucose Monitoring and Controlled Transdermal Nanoemulsion Release of Hypoglycemic Treatment with a Software Application for Noninvasive Personalized Diabetes Care
Abstract
1. Introduction
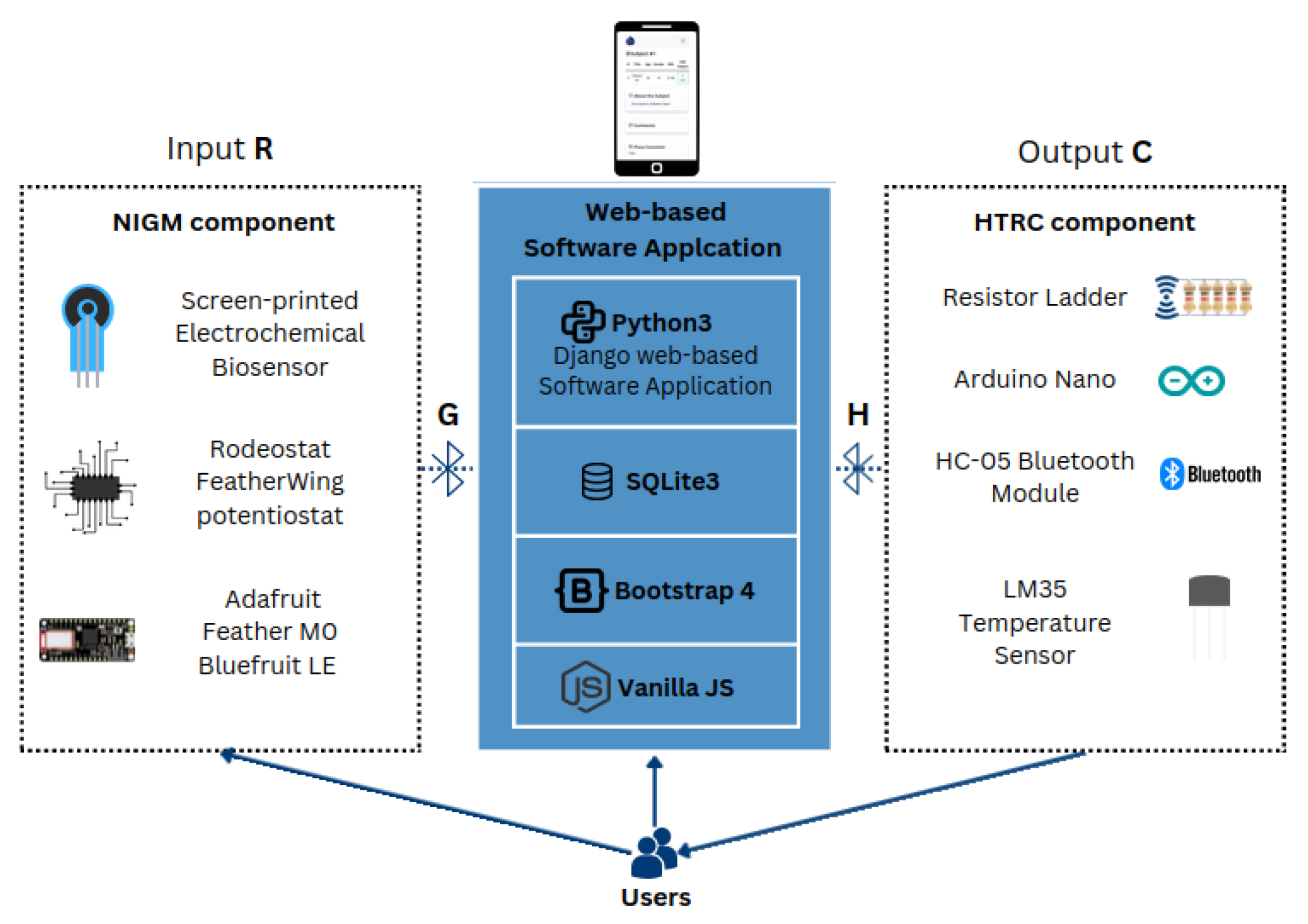
2. Materials and Methods
2.1. Noninvasive Sweat-Based Glucose Monitoring
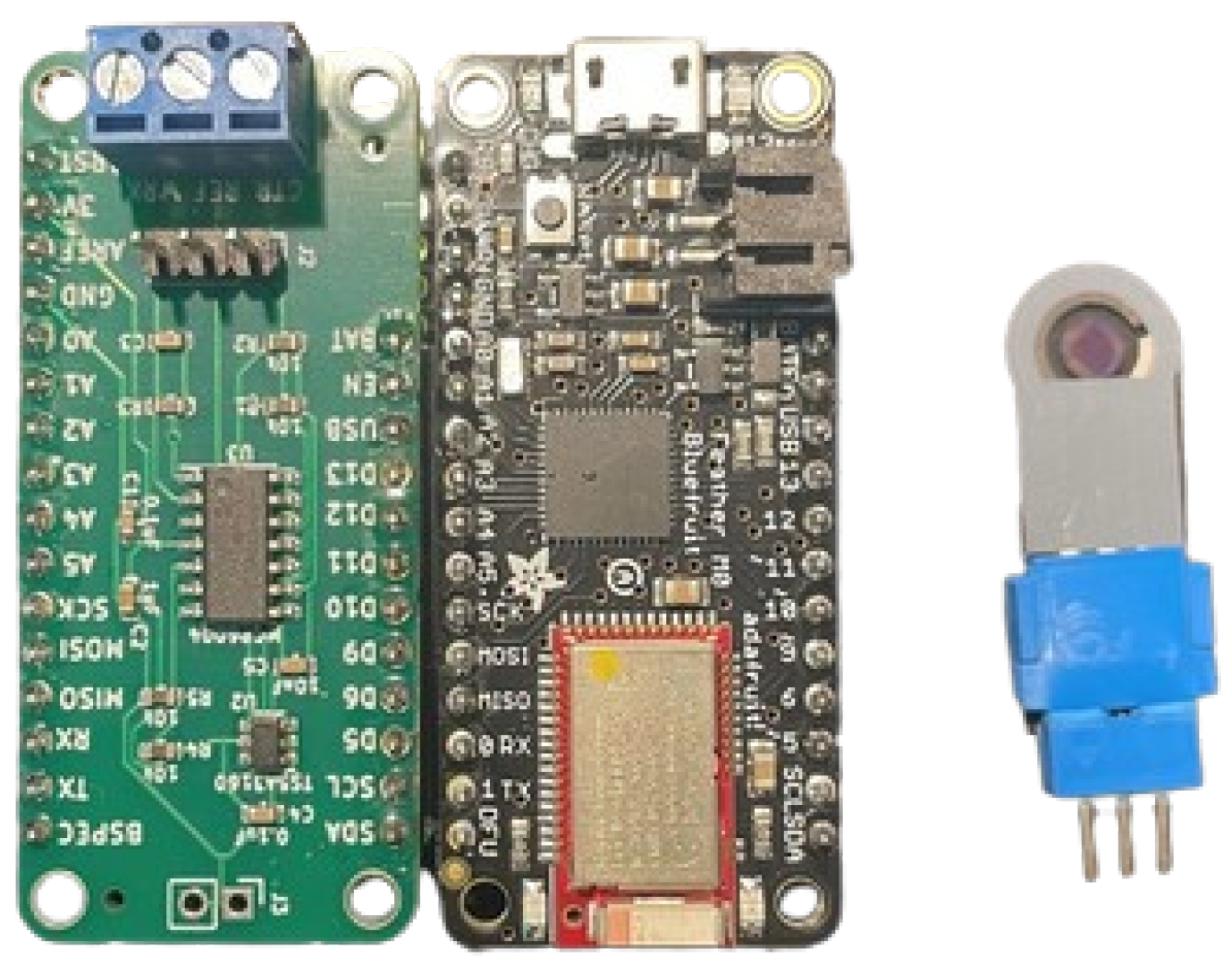
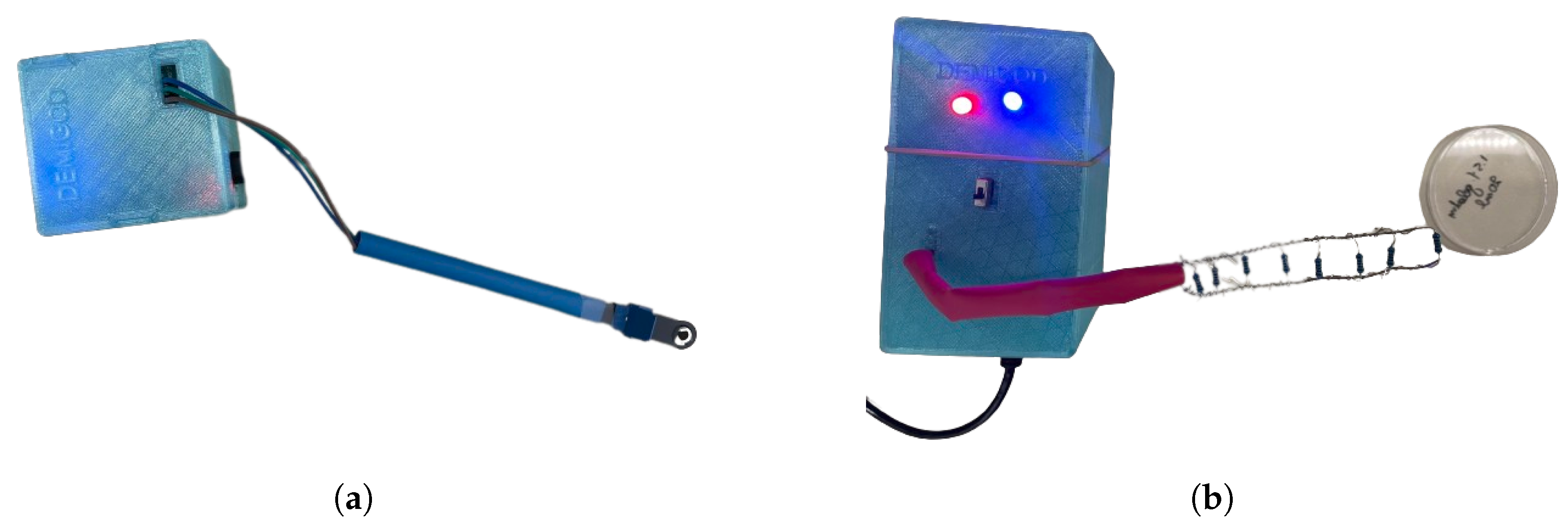
2.1.1. Screen-Printed Electrochemical Biosensor
2.1.2. Electronic Layout of the NIGM Component
- The voltage regulation between the working and reference electrodes is achieved through the utilization of analog output A0.
- The working electrode current (obtained from the transimpedance amplifier output) is acquired through the analog input A2.
- The voltage difference between the working electrode and the reference electrode is acquired at analog input A4.
- The counter electrode connect/disconnect switch is controlled using digital output D13. This can optionally be changed to D10, D11, or D12.
2.1.3. NIGM Component Software Development
2.2. Controlled Transdermal Nanoemulsion Release of Hypoglycemic Treatment
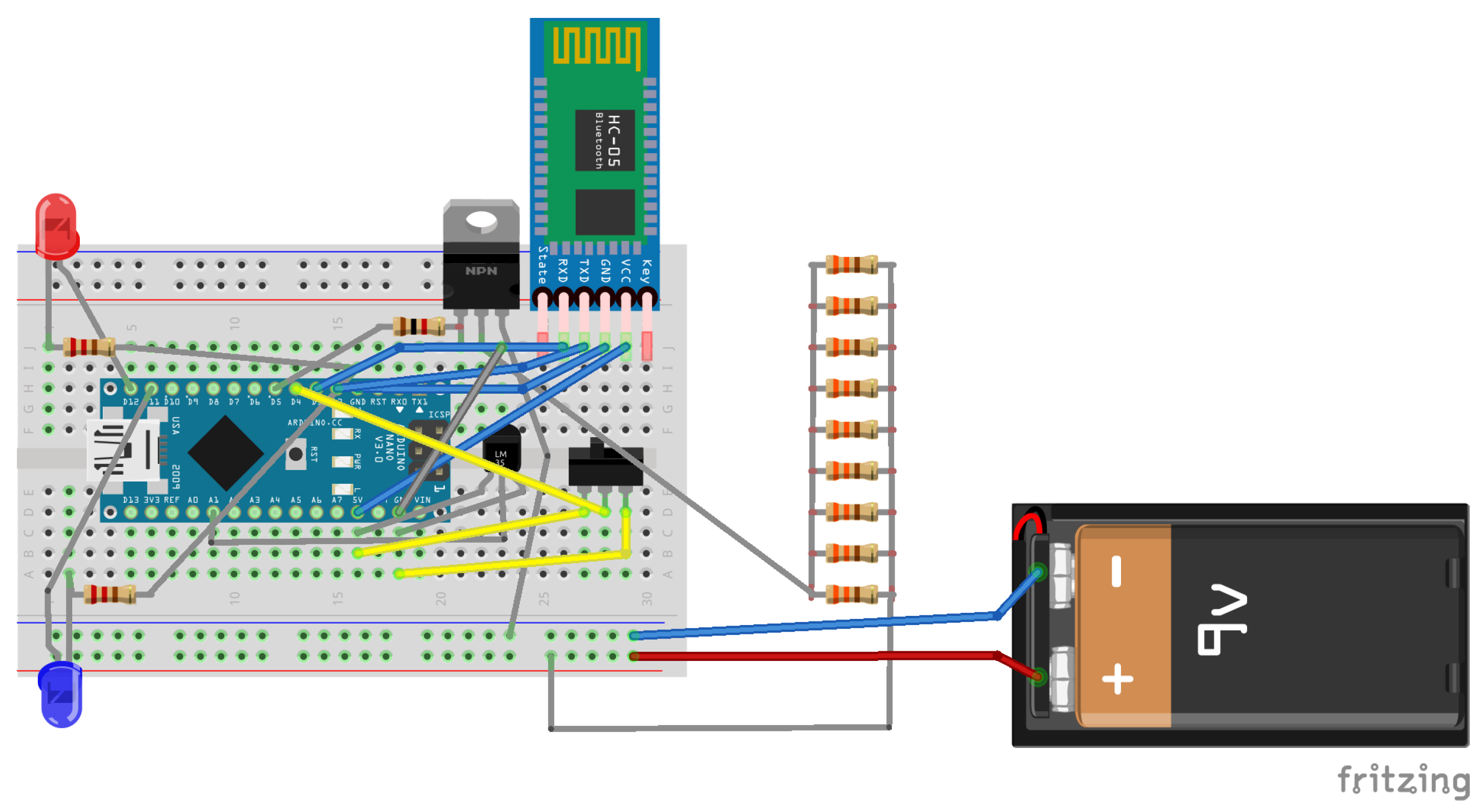
2.2.1. Electronic Layout of the HTRC Component
- Arduino Nano: A compact, comprehensive, and breadboard-compatible board, the Arduino Nano is built upon the ATmega328 microcontroller architecture (Arduino Nano 3.x).
- HC-05 Bluetooth Wireless Serial Port Module: Facilitating wireless communication, the HC-05 Bluetooth module enables seamless serial data transmission between devices.
- LM35 Temperature Sensor: The LM35 sensor provides accurate and reliable temperature measurements, essential for monitoring thermal conditions within the system. In the context of the hypoglycemic release system circuit, the LM35 sensor was chosen for its suitability and efficacy.
- Resistor Ladder: The resistor ladder comprises a network of resistors designed to create a predefined thin-layer grid, ensuring uniform heat distribution across the gelatin substrate, thereby facilitating consistent thermal response.
2.2.2. HRTC Component Software Development
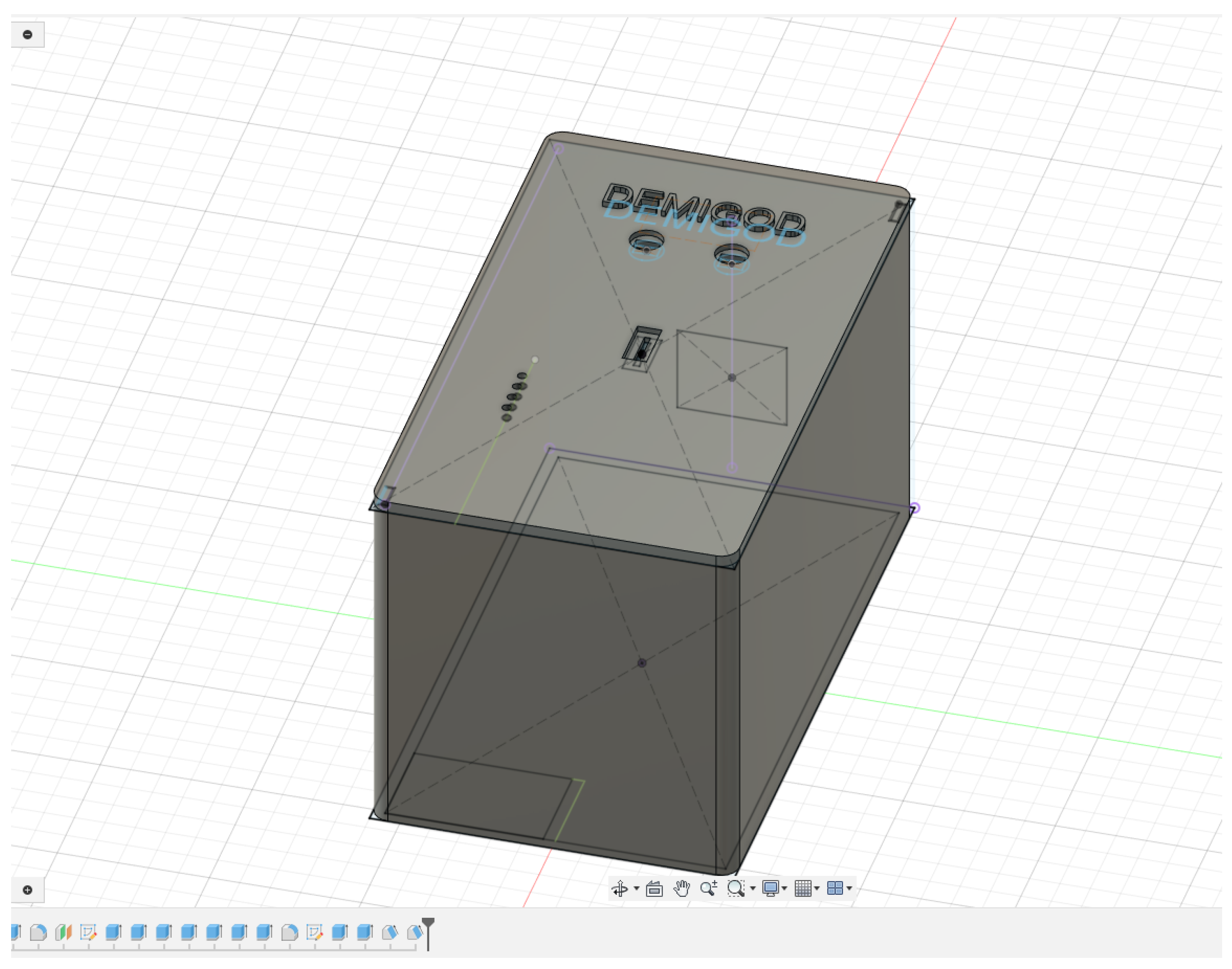
2.2.3. Design and Fabrication of 3D-Printed Cases for DEMIGOD System Components
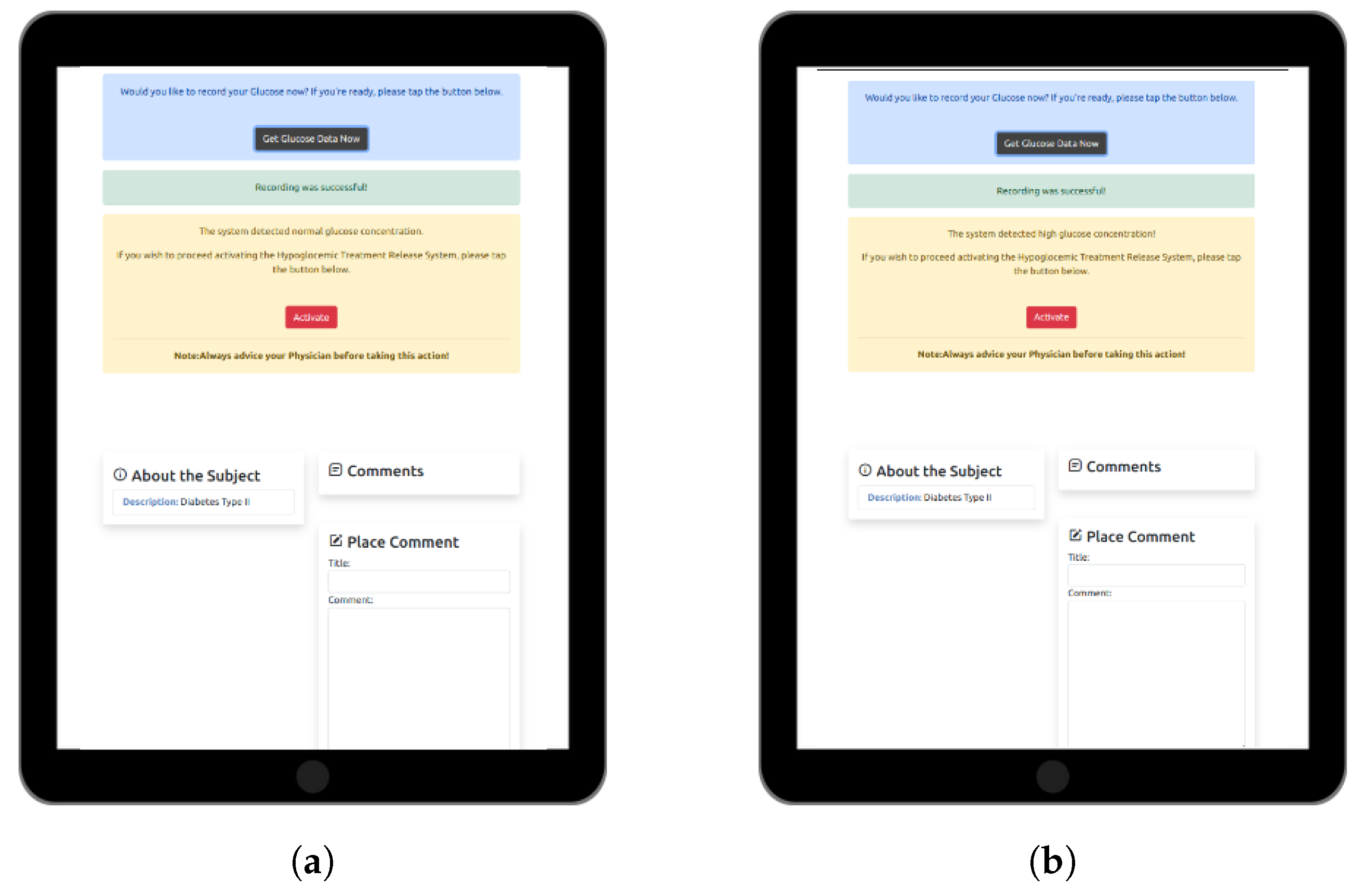
2.3. Web-Based Software Application for Personalized DM Care
2.4. Experiment Setup
2.4.1. Chronoamperometry Test and Response
2.4.2. In Vivo Hypoglycemic Activity of Nanoemulsion Formulations
Synthesis of Sitagliptin and Dapagliflozin Nanoemulsions
Glucose Challenge in SV129 Mice
Statistical Analysis
3. Results
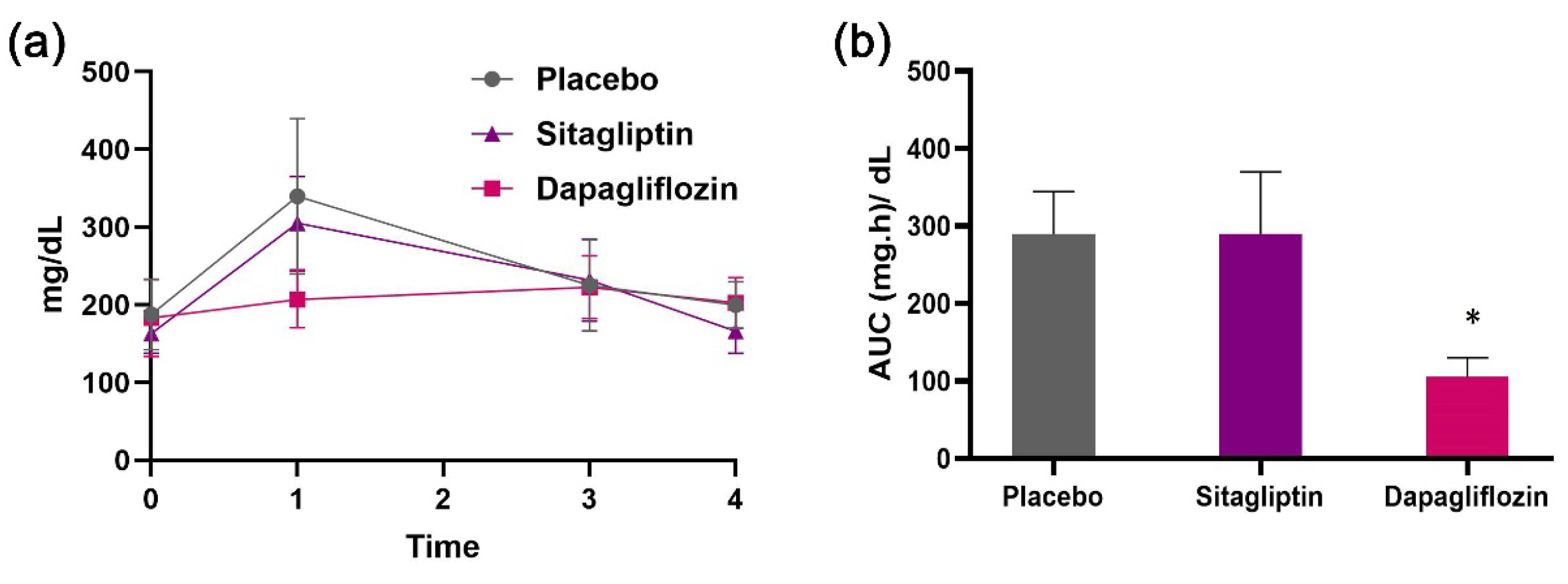
Efficacy of Sitagliptin and Dapagliflozin Nanoemulsions
4. Related Work
- Non-invasive monitoring: Unlike many commercial devices that require blood samples, our system uses a noninvasive sweat-based method, making it more user-friendly and suitable for continuous monitoring.
- Integrated decision making: The inclusion of a web-based application that interacts with the sensor system provides real-time monitoring and automated decision-making capabilities, which many commercial systems lack.
- Cost efficiency: As demonstrated in the cost analysis, our system offers a cost-effective alternative while maintaining high accuracy and reliability.
- Enhanced user experience: The web-based application offers a seamless user interface, remote monitoring capabilities, and integration with other health management systems, providing a comprehensive solution for glycemic control.
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DM | Diabetes Mellitus |
| T1DM | Type 1 DM |
| T2DM | Type 2 DM |
| GM | Glucose Monitoring |
| SGM | Sweat-based Glucose Monitoring |
| CGM | Continuous Glucose Monitoring |
| SPE | Screen printed electrode |
| AID | Automated Insulin Delivery |
| NIGM | Non-Invasive Glucose Monitoring |
| HTRC | Hypoglycemic Treatment Release Circuit |
| WE | Working Electrode |
| RE | Reference Electrode |
| IDE | Integrated Development Environment |
| CA | Chronoamperometry |
| OGTT | Oral Glucose Tolerance Test |
| SD | Standard Deviation |
References
- American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2009, 32 (Suppl. 1), S62–S67. [Google Scholar] [CrossRef] [PubMed]
- Wilcox, G. Insulin and insulin resistance. Clin. Biochem. Rev. 2005, 26, 19–39. [Google Scholar] [PubMed]
- Banday, M.Z.; Sameer, A.S.; Nissar, S. Pathophysiology of diabetes: An overview. Avicenna J. Med. 2020, 10, 174–188. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; An, X.; Yang, C.; Sun, W.; Ji, H.; Lian, F. The crucial role and mechanism of insulin resistance in metabolic disease. Front. Endocrinol. 2023, 14, 1149239. [Google Scholar] [CrossRef] [PubMed]
- Dilworth, L.; Facey, A.; Omoruyi, F. Diabetes Mellitus and Its Metabolic Complications: The Role of Adipose Tissues. Int. J. Mol. Sci. 2021, 22, 7644. [Google Scholar] [CrossRef] [PubMed]
- Solis-Herrera, C.; Triplitt, C.; Reasner, C.; DeFronzo, R.A.; Cersosimo, E.; World Health Organization. Classification of diabetes mellitus. In Endotext; Feingold, K.R., Anawalt, B., Blackman, M.R., Boyce, A., Chrousos, G., Corpas, E., de Herder, W.W., Dhatariya, K., Dungan, K., Hofland, J., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar] [PubMed]
- Hossain, M.J.; Al-Mamun, M.; Islam, M.R. Diabetes mellitus, the fastest growing global public health concern: Early detection should be focused. Health Sci. Rep. 2024, 7, 4. [Google Scholar] [CrossRef] [PubMed]
- Sugandh, F.; Chandio, M.; Raveena, F.; Kumar, L.; Karishma, F.; Khuwaja, S.; Memon, U.A.; Bai, K.; Kashif, M.; Varrassi, G.; et al. Advances in the Management of Diabetes Mellitus: A Focus on Personalized Medicine. Cureus 2023, 15, e43697. [Google Scholar] [CrossRef] [PubMed]
- Abubeker, K.M.; Ramani, R.; Krishnamoorthy, R.; Gogula, S.; Baskar, S.; Muthu, S.; Chellamuthu, G.; Subramaniam, K. Internet of Things enabled open source assisted real-time blood glucose monitoring framework. Sci. Rep. 2024, 14, 6151. [Google Scholar] [CrossRef]
- Di Filippo, D.; Sunstrum, F.; Khan, J.; Welsh, A. Non-Invasive Glucose Sensing Technologies and Products: A Comprehensive Review for Researchers and Clinicians. Sensors 2023, 23, 9130. [Google Scholar] [CrossRef]
- Sankhala, D.; Sardesai, A.U.; Pali, M.; Lin, K.C.; Jagannath, B.; Muthukumar, S.; Prasad, S. A machine learning-based on-demand sweat glucose reporting platform. Sci. Rep. 2022, 12, 434. [Google Scholar] [CrossRef]
- Zafar, H.; Channa, A.; Jeoti, V.; Stojanović, G.M. Comprehensive Review on Wearable Sweat-Glucose Sensors for Continuous Glucose Monitoring. Sensors 2022, 22, 638. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Liu, C.; Zhang, L.; Liu, T.; Wang, Z.; Song, Z.; Cai, H.; Fang, Z.; Chen, J.; Wang, J.; et al. Wearable and flexible electrochemical sensors for sweat analysis: A review. Microsyst. Nanoeng. 2023, 9, 6. [Google Scholar] [CrossRef] [PubMed]
- Moyer, J.; Wilson, D.; Finkelshtein, I.; Wong, B.; Potts, R. Correlation between Sweat Glucose and Blood Glucose in Subjects with Diabetes. Diabetes Technol. Ther. 2012, 14, 398–402. [Google Scholar] [CrossRef] [PubMed]
- Tovar-Lopez, F.J. Recent Progress in Micro- and Nanotechnology-Enabled Sensors for Biomedical and Environmental Challenges. Sensors 2023, 23, 5406. [Google Scholar] [CrossRef] [PubMed]
- Safarkhani, M.; Aldhaher, A.; Heidari, G.; Zare, E.N.; Warkiani, M.E.; Akhavan, O.; Huh, Y.; Rabiee, N. Nanomaterial-assisted wearable glucose biosensors for noninvasive real-time monitoring: Pioneering point-of-care and beyond. Nano Mater. Sci. 2023. [Google Scholar] [CrossRef]
- Yáñez-Sedeño, P.; Campuzano, S.; Pingarrón, J.M. Screen-Printed Electrodes: Promising Paper and Wearable Transducers for (Bio)Sensing. Biosensors 2020, 10, 76. [Google Scholar] [CrossRef]
- Singh, S.; Wang, J.; Cinti, S. Review—An Overview on Recent Progress in Screen-Printed Electroanalytical (Bio)Sensors. ECS Sensors Plus 2022, 1, 023401. [Google Scholar] [CrossRef]
- Alam, M.M.; Howlader, M.M.R. High performance nonenzymatic electrochemical sensors via thermally grown Cu native oxides (CuNOx) towards sweat glucose monitoring. Analist 2024, 149, 712–728. [Google Scholar] [CrossRef]
- Chung, M.; Fortunato, G.; Radacsi, N. Wearable flexible sweat sensors for healthcare monitoring: A review. J. R. Soc. Interface 2019, 16, 20190217. [Google Scholar] [CrossRef]
- Ng, L.C.; Gupta, M. Transdermal drug delivery systems in diabetes management: A review. Asian J. Pharm. Sci. 2020, 15, 13–25. [Google Scholar] [CrossRef]
- Leong, M.Y.; Kong, Y.L.; Burgess, K.; Wong, W.F.; Sethi, G.; Looi, C.Y. Recent Development of Nanomaterials for Transdermal Drug Delivery. Biomedicines 2023, 11, 1124. [Google Scholar] [CrossRef]
- Souto, E.B.; Souto, S.B.; Campos, J.R.; Severino, P.; Pashirova, T.N.; Zakharova, L.Y.; Silva, A.M.; Durazzo, A.; Lucarini, M.; Izzo, A.A.; et al. Nanoparticle Delivery Systems in the Treatment of Diabetes Complications. Molecules 2019, 24, 4209. [Google Scholar] [CrossRef] [PubMed]
- Aloke, C.; Egwu, C.O.; Aja, P.M.; Obasi, N.A.; Chukwu, J.; Akumadu, B.O.; Ogbu, P.N.; Achilonu, I. Current Advances in the Management of Diabetes Mellitus. Biomedicines 2022, 10, 2436. [Google Scholar] [CrossRef] [PubMed]
- Stoumpos, A.I.; Kitsios, F.; Talias, M.A. Digital Transformation in Healthcare: Technology Acceptance and Its Applications. Int. J. Environ. Res. Public Health 2023, 20, 3407. [Google Scholar] [CrossRef] [PubMed]
- Allioui, H.; Mourdi, Y. Exploring the Full Potentials of IoT for Better Financial Growth and Stability: A Comprehensive Survey. Sensors 2023, 23, 8015. [Google Scholar] [CrossRef] [PubMed]
- Alaslawi, H.; Berrou, I.; Al Hamid, A.; Alhuwail, D.; Aslanpour, Z. Diabetes Self-management Apps: Systematic Review of Adoption Determinants and Future Research Agenda. JMIR Diabetes 2022, 7, e28153. [Google Scholar] [CrossRef] [PubMed]
- Peyser, T.; Dassau, E.; Breton, M.; Skyler, J.S. The artificial pancreas: Current status and future prospects in the management of diabetes. Ann. N. Y. Acad. Sci. 2014, 1311, 102–123. [Google Scholar] [CrossRef] [PubMed]
- Phillip, M.; Nimri, R.; Bergenstal, R.M.; Barnard-Kelly, K.; Danne, T.; Hovorka, R.; Kovatchev, B.P.; Messer, L.H.; Parkin, C.G.; Ambler-Osborn, L.; et al. Consensus Recommendations for the Use of Automated Insulin Delivery Technologies in Clinical Practice. Endocr. Rev. 2022, 44, 254–280. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Rodríguez, I.; Rodríguez, J.V.; Campo-Valera, M. Applications of the Internet of Medical Things to Type 1 Diabetes Mellitus. Electronics 2023, 12, 756. [Google Scholar] [CrossRef]
- Kovatchev, B.; Clarke, W. Peculiarities of the Continuous Glucose Monitoring Data Stream and Their Impact on Developing Closed-Loop Control Technology. J. Diabetes Sci. Technol. 2008, 2, 158–163. [Google Scholar] [CrossRef]
- Haidar, A. Insulin-and-Glucagon Artificial Pancreas Versus Insulin-Alone Artificial Pancreas: A Short Review. Diabetes Spectr. 2019, 32, 215–221. [Google Scholar] [CrossRef]
- Fiska, V.; Evangelou, S.; Giannakeas, N.; Tzallas, A.T.; Angelidis, P.; Tsipouras, M.G. A non-invasive closed-loop diagnostic and therapeutic wearable device for diabetic hyperglycemia prevention: System architecture. In Proceedings of the 2022 IEEE 7th South-East Europe Design Automation, Computer Engineering, Computer Networks and Social Media Conference (SEEDA-CECNSM), Ioannina, Greece, 10–12 September 2022. [Google Scholar] [CrossRef]
- Baker, L.B. Physiology of sweat gland function: The roles of sweating and sweat composition in human health. Temperature 2019, 6, 211–259. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Sun, J. Sweat detection theory and fluid driven methods: A review. Nanotechnol. Precis. Eng. 2020, 3, 126–140. [Google Scholar] [CrossRef]
- Mansour, M.; Saeed Darweesh, M.; Soltan, A. Wearable devices for glucose monitoring: A review of state-of-the-art technologies and emerging trends. Alex. Eng. J. 2024, 89, 224–243. [Google Scholar] [CrossRef]
- Kim, J.; Campbell, A.S.; Wang, J. Wearable non-invasive epidermal glucose sensors: A review. Talanta 2018, 177, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Khosravi Ardakani, H.; Gerami, M.; Chashmpoosh, M.; Omidifar, N.; Gholami, A. Recent Progress in Nanobiosensors for Precise Detection of Blood Glucose Level. Biochem. Res. Int. 2022, 2022, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.S.; Jeong, J.W.; Kim, Y.A.; Chang, M. Carbon Nanomaterials as Versatile Platforms for Biosensing Applications. Micromachines 2020, 11, 814. [Google Scholar] [CrossRef] [PubMed]
- Goodrum, R.; Weldekidan, H.; Li, H.; Mohanty, A.K.; Misra, M. Graphene-based nanostructures from green processes and their applications in biomedical sensors. Adv. Ind. Eng. Polym. Res. 2024, 7, 37–53. [Google Scholar] [CrossRef]
- Fritea, L.; Banica, F.; Costea, T.; Moldovan, L.; Dobjanschi, L.; Muresan, M.; Cavalu, S. Metal Nanoparticles and Carbon-Based Nanomaterials for Improved Performances of Electrochemical (Bio)Sensors with Biomedical Applications. Materials 2021, 14, 6319. [Google Scholar] [CrossRef] [PubMed]
- Juska, V.B.; Pemble, M.E. A Critical Review of Electrochemical Glucose Sensing: Evolution of Biosensor Platforms Based on Advanced Nanosystems. Sensors 2020, 20, 6013. [Google Scholar] [CrossRef]
- Milton, R.D.; Giroud, F.; Thumser, A.E.; Minteer, S.D.; Slade, R.C.T. Hydrogen peroxide produced by glucose oxidase affects the performance of laccase cathodes in glucose/oxygen fuel cells: FAD-dependent glucose dehydrogenase as a replacement. Phys. Chem. Chem. Phys. 2013, 15, 19371. [Google Scholar] [CrossRef]
- Alatzoglou, C.; Tzianni, E.I.; Patila, M.; Trachioti, M.G.; Prodromidis, M.I.; Stamatis, H. Structure-Function Studies of Glucose Oxidase in the Presence of Carbon Nanotubes and Bio-Graphene for the Development of Electrochemical Glucose Biosensors. Nanomaterials 2023, 14, 85. [Google Scholar] [CrossRef] [PubMed]
- Papanikolaou, E.; Simos, Y.V.; Spyrou, K.; Patila, M.; Alatzoglou, C.; Tsamis, K.; Vezyraki, P.; Stamatis, H.; Gournis, D.P.; Peschos, D.; et al. Does Green Exfoliation of Graphene Produce More Biocompatible Structures? Pharmaceutics 2023, 15, 993. [Google Scholar] [CrossRef] [PubMed]
- Hill, A.; Tait, S.; Harris, P.; Baillie, C.; Virdis, B.; McCabe, B.K. Design and analysis of a low-cost potentiostat for application with microbial electrochemical sensors. Electrochim. Acta 2023, 468, 143201. [Google Scholar] [CrossRef]
- Hoilett, O.S.; Walker, J.F.; Balash, B.M.; Jaras, N.J.; Boppana, S.; Linnes, J.C. KickStat: A Coin-Sized Potentiostat for High-Resolution Electrochemical Analysis. Sensors 2020, 20, 2407. [Google Scholar] [CrossRef] [PubMed]
- Meloni, G.N. Building a Microcontroller Based Potentiostat: A Inexpensive and Versatile Platform for Teaching Electrochemistry and Instrumentation. J. Chem. Educ. 2016, 93, 1320–1322. [Google Scholar] [CrossRef]
- Li, Y.C.; Melenbrink, E.L.; Cordonier, G.J.; Boggs, C.; Khan, A.; Isaac, M.K.; Nkhonjera, L.K.; Bahati, D.; Billinge, S.J.; Haile, S.M.; et al. An Easily Fabricated Low-Cost Potentiostat Coupled with User-Friendly Software for Introducing Students to Electrochemical Reactions and Electroanalytical Techniques. J. Chem. Educ. 2018, 95, 1658–1661. [Google Scholar] [CrossRef]
- Chakrabarty, A.; Healey, E.; Shi, D.; Zavitsanou, S.; Doyle, F.J.; Dassau, E. Embedded Model Predictive Control for a Wearable Artificial Pancreas. IEEE Trans. Control Syst. Technol. 2020, 28, 2600–2607. [Google Scholar] [CrossRef] [PubMed]
- Griffith, J.; Hamilton, A.; Long, G.; Mujezinovic, A.; Warren, D.; Vij, K. Human skin temperature response to absorbed thermal power. In Proceedings of the Medical Imaging 1997: Ultrasonic Transducer Engineering; Shung, K.K., Ed.; International Society for Optics and Photonics, SPIE: Bellingham, WA, USA, 1997; Volume 3037, pp. 129–134. [Google Scholar] [CrossRef]
- Patila, M.; Bakratsas, G.; Simos, Y.V.; Polydera, A.C.; Dounousi, E.; Peschos, D.; Stamatis, H. Development of nanoemulsion systems for the encapsulation and delivery of hypoglycemic drugs against Diabetes mellitus. In Proceedings of the 35th Conference of the European Colloid & Interface Society, Athens, Greece, 5 September 2021. [Google Scholar]
- Knörig, A.; Wettach, R.; Cohen, J. Fritzing: A tool for advancing electronic prototyping for designers. In Proceedings of the 3rd International Conference on Tangible and Embedded Interaction, New York, NY, USA, 16–18 February 2009; TEI ’09. pp. 351–358. [Google Scholar] [CrossRef]
- Guy, O.J.; Walker, K.A.D. Chapter 4—Graphene functionalization for biosensor applications. In Silicon Carbide Biotechnology, 2nd ed.; Saddow, S.E., Ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 85–141. [Google Scholar] [CrossRef]
- Simos, Y.V.; Spyrou, K.; Patila, M.; Karouta, N.; Stamatis, H.; Gournis, D.; Dounousi, E.; Peschos, D. Trends of nanotechnology in type 2 diabetes mellitus treatment. Asian J. Pharm. Sci. 2021, 16, 62–76. [Google Scholar] [CrossRef] [PubMed]
- Villena Gonzales, W.; Mobashsher, A.; Abbosh, A. The Progress of Glucose Monitoring—A Review of Invasive to Minimally and Non-Invasive Techniques, Devices and Sensors. Sensors 2019, 19, 800. [Google Scholar] [CrossRef]
- Accu-Chek Guide. Available online: https://www.accu-chek.gr/systimata-metrisis/guide (accessed on 7 June 2024).
- OneTouch Verio Reflect. Available online: https://shop.onetouch.com/verio-reflect-meter/product/OTSUS05_0003 (accessed on 7 June 2024).
- Contour Next. Available online: https://www.diabetes.ascensia.gr/products/contour-next/ (accessed on 7 June 2024).
- FreeStyle Lite. Available online: https://www.freestyle.abbott/gr-el/freestyle-libre-system/freestyle-freedom-lite.html (accessed on 7 June 2024).
- iHealth Gluco+. Available online: https://ihealthlabs.com/products/ihealth-gluco-wirelss-smart-glucose-monitor (accessed on 7 June 2024).
- Dario. Available online: https://shop.mydario.com/product/dario-blood-glucose-monitoring-starter-kit/ (accessed on 7 June 2024).
- Keto-Mojo. Available online: https://ketoabout.gr/product/keto-mojo-metritis/ (accessed on 7 June 2024).
- ReliOn. Available online: https://relionbgm.com/relion-meters/relion-platinum/ (accessed on 7 June 2024).
- CareSens. Available online: https://frontalpmd.gr/en/product/caresens-n-premier-glucose-meter/ (accessed on 7 June 2024).
- On Call. Available online: https://www.aconlabs.com/brands/on-call/on-call-express-family/ (accessed on 7 June 2024).
- Medtronic MiniMed 630G. Available online: https://www.medtronicdiabetes.com/products/minimed-630g-insulin-pump-system (accessed on 7 June 2024).
- Medtronic MiniMed 770 G. Available online: https://www.medtronicdiabetes.com/products/minimed-770g-insulin-pump-system (accessed on 7 June 2024).
- Tandem. Available online: https://www.tandemdiabetes.com/products/insulin-pumps/t-slim-x2-insulin-pump (accessed on 7 June 2024).
- Insulet. Available online: https://www.omnipod.com/ (accessed on 7 June 2024).
- Beta Bionics. Available online: https://www.betabionics.com/ilet-bionic-pancreas/ilet-adults/ (accessed on 7 June 2024).
- Silva, R.M.; da Silva, A.D.; Camargo, J.R.; de Castro, B.S.; Meireles, L.M.; Silva, P.S.; Janegitz, B.C.; Silva, T.A. Carbon Nanomaterials-Based Screen-Printed Electrodes for Sensing Applications. Biosensors 2023, 13, 453. [Google Scholar] [CrossRef]



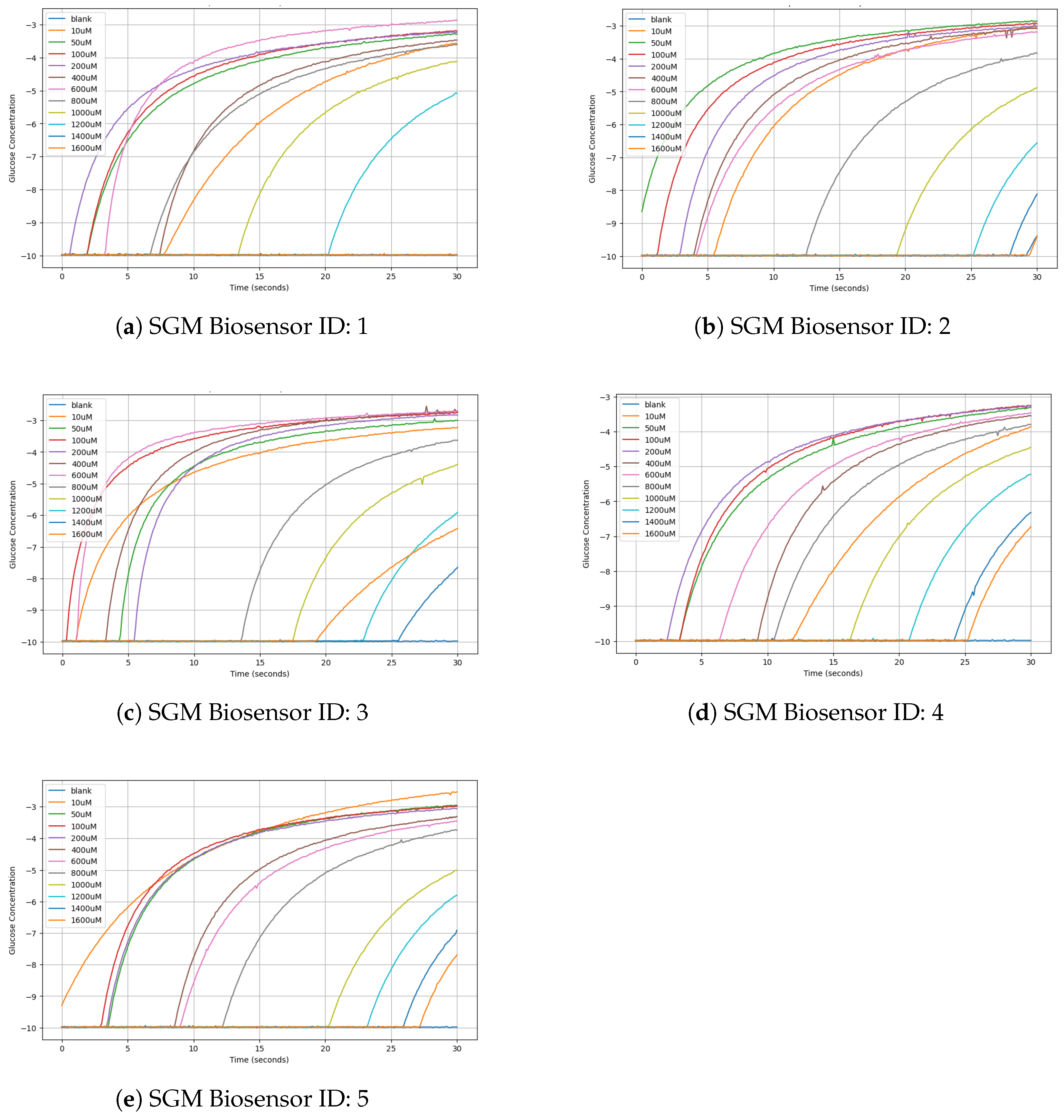
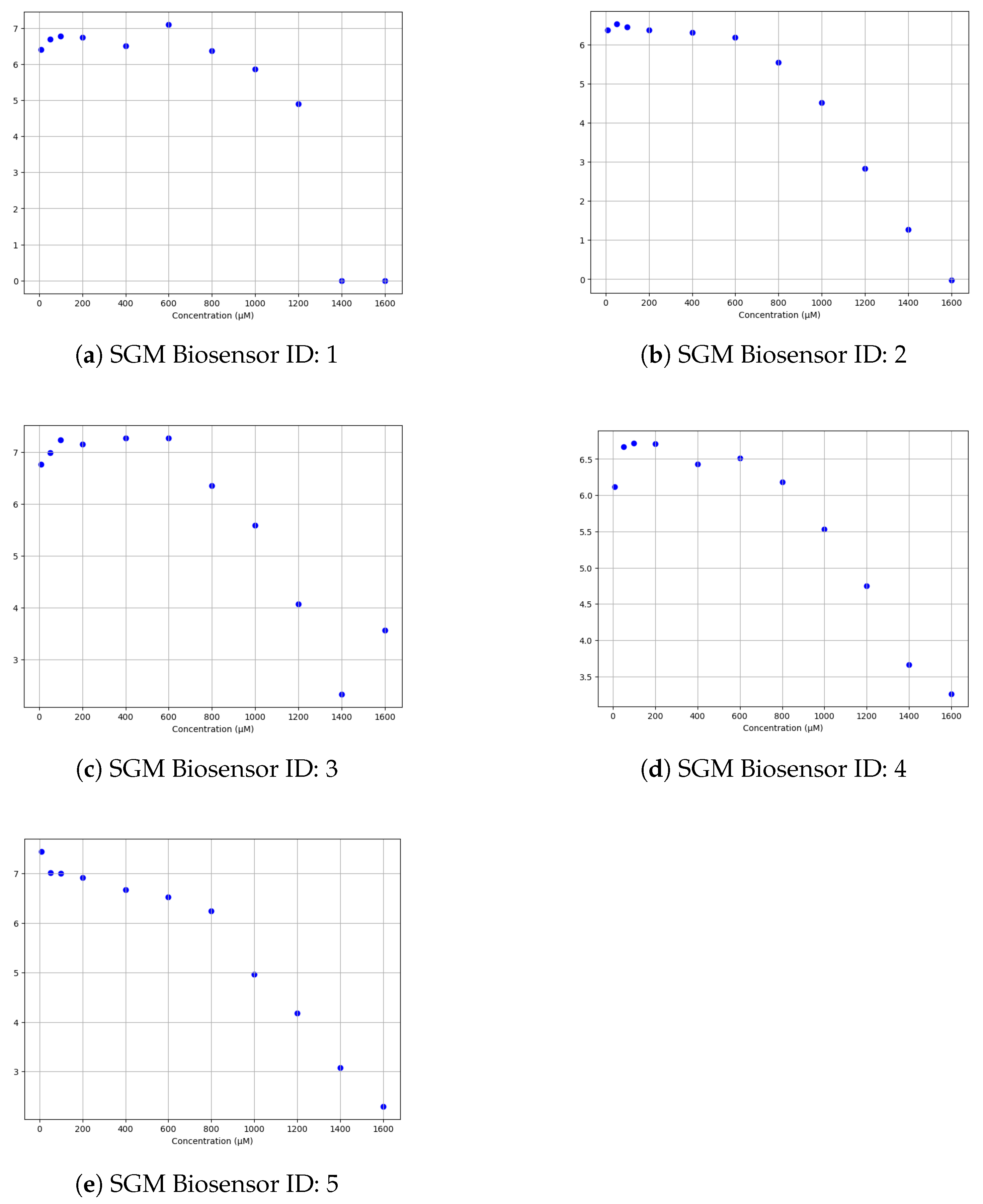

| SGM Biosensor ID | Blank | 10 μM | 50 μM | 100 μM | 200 μM | 400 μM | 600 μM | 800 μM | 100 μM | 1200 μM | 1400 μM | 1600 μM |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | −9.98 | −3.57 | −3.29 | −3.2 | −3.24 | −3.47 | −2.88 | −3.61 | −4.12 | −5.09 | −9.98 | −9.98 |
| 2 | −9.39 | −3.02 | −2.86 | −2.94 | −3.02 | −3.08 | −3.2 | −3.84 | −4.88 | −6.56 | −8.12 | −9.41 |
| 3 | −9.98 | −3.22 | 3 | −2.75 | −2.83 | −2.71 | −2.71 | −3.63 | −4.39 | −5.91 | −7.65 | −6.42 |
| 4 | −9.98 | −3.86 | −3.31 | −3.26 | −3.27 | −3.55 | −3.47 | −3.8 | −4.45 | −5.23 | −6.32 | −6.72 |
| 5 | −9.98 | −2.53 | −2.96 | −2.98 | −3.06 | −3.31 | −3.45 | −3.74 | −5.01 | −5.8 | −6.91 | −7.69 |
| SGM Biosensor ID | 10 μM | 50 μM | 100 μM | 200 μM | 400 μM | 600 μM | 800 μM | 1000 μM | 1200 μM | 1400 μM | 1600 μM |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 6.41 | 6.69 | 6.78 | 6.74 | 6.51 | 7.1 | 6.37 | 5.86 | 4.89 | 0.0 | 0.0 |
| 2 | 6.37 | 6.53 | 6.45 | 6.37 | 6.31 | 6.19 | 5.55 | 4.51 | 2.83 | 1.27 | −0.02 |
| 3 | 6.76 | 6.98 | 7.23 | 7.15 | 7.27 | 7.27 | 6.35 | 5.59 | 4.07 | 2.33 | 3.56 |
| 4 | 6.12 | 6.67 | 6.72 | 6.71 | 6.43 | 6.51 | 6.18 | 5.53 | 4.75 | 3.66 | 3.26 |
| 5 | 7.45 | 7.02 | 7.0 | 6.92 | 6.67 | 6.53 | 6.24 | 4.97 | 4.18 | 3.07 | 2.29 |
| Brand | Model | Invasive | Provides Therapy | Cost (EUR) |
|---|---|---|---|---|
| Accu-Chek [57] | Guide | Yes | No | 9.00 |
| OneTouch [58] | Verio Reflect | Yes | No | 25.89 |
| Contour [59] | Next | Yes | No | 13.79 |
| FreeStyle [60] | Lite | Yes | No | 23.91 |
| iHealth [61] | Gluco+ | Yes | No | 36.79 |
| Dario [62] | Blood Glucose Monitoring System | Yes | No | 45.99 |
| Keto-Mojo [63] | Blood Glucose & Ketone | Yes | No | 56.85 |
| ReliOn [64] | Platinum | Yes | No | 18.48 |
| CareSens [65] | N Premier | Yes | No | 15.00 |
| On Call [66] | Express II | Yes | No | 18.39 |
| Medtronic [67] | MiniMed 630G | Yes | Yes | 7500 |
| Medtronic [68] | MiniMed 770G | Yes | Yes | 8000 |
| Tandem [69] | t X2 with Control-IQ | Yes | Yes | 7000 |
| Insulet [70] | Omnipod 5 | Yes | Yes | 5000 |
| Beta Bionics [71] | iLet Bionic Pancreas | Yes | Yes | 9000 |
| DEMIGOD (proposed system) | initial prototype | No | Yes | 300 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fiska, V.; Papanikolaou, E.; Patila, M.; Prodromidis, M.I.; Trachioti, M.G.; Tzianni, E.I.; Spyrou, K.; Angelidis, P.; Tsipouras, M.G. DEMIGOD: A Low-Cost Microcontroller-Based Closed-Loop System Integrating Nanoengineered Sweat-Based Glucose Monitoring and Controlled Transdermal Nanoemulsion Release of Hypoglycemic Treatment with a Software Application for Noninvasive Personalized Diabetes Care. Micromachines 2024, 15, 887. https://doi.org/10.3390/mi15070887
Fiska V, Papanikolaou E, Patila M, Prodromidis MI, Trachioti MG, Tzianni EI, Spyrou K, Angelidis P, Tsipouras MG. DEMIGOD: A Low-Cost Microcontroller-Based Closed-Loop System Integrating Nanoengineered Sweat-Based Glucose Monitoring and Controlled Transdermal Nanoemulsion Release of Hypoglycemic Treatment with a Software Application for Noninvasive Personalized Diabetes Care. Micromachines. 2024; 15(7):887. https://doi.org/10.3390/mi15070887
Chicago/Turabian StyleFiska, Vasiliki, Eirini Papanikolaou, Michaela Patila, Mamas I. Prodromidis, Maria G. Trachioti, Eleni I. Tzianni, Konstantinos Spyrou, Pantelis Angelidis, and Markos G. Tsipouras. 2024. "DEMIGOD: A Low-Cost Microcontroller-Based Closed-Loop System Integrating Nanoengineered Sweat-Based Glucose Monitoring and Controlled Transdermal Nanoemulsion Release of Hypoglycemic Treatment with a Software Application for Noninvasive Personalized Diabetes Care" Micromachines 15, no. 7: 887. https://doi.org/10.3390/mi15070887
APA StyleFiska, V., Papanikolaou, E., Patila, M., Prodromidis, M. I., Trachioti, M. G., Tzianni, E. I., Spyrou, K., Angelidis, P., & Tsipouras, M. G. (2024). DEMIGOD: A Low-Cost Microcontroller-Based Closed-Loop System Integrating Nanoengineered Sweat-Based Glucose Monitoring and Controlled Transdermal Nanoemulsion Release of Hypoglycemic Treatment with a Software Application for Noninvasive Personalized Diabetes Care. Micromachines, 15(7), 887. https://doi.org/10.3390/mi15070887










