Zweifach–Fung Microfluidic Device for Efficient Microparticle Separation: Cost-Effective Fabrication Using CO2 Laser-Ablated PMMA
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Computational Modeling of Microparticle Separation Dynamics
2.3. Simulation Specifics and Particle Properties
2.4. Discretization and Solvers
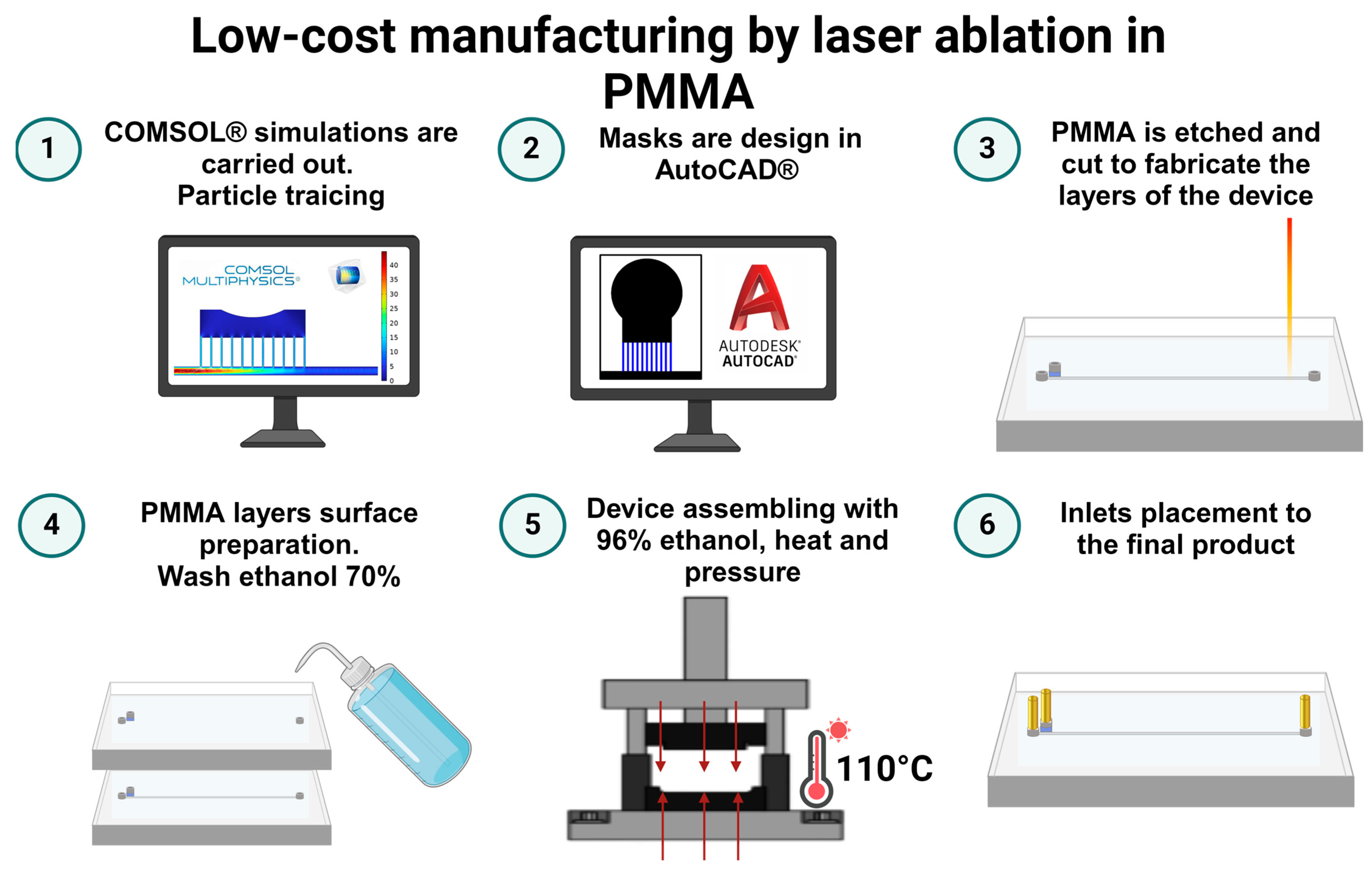
2.5. Low-Cost Manufacture
2.6. Particle Synthesis for Assessing Device Performance
Microparticles of Chitosan
2.7. Experimental Separation Tests
2.7.1. Sensitivity Evaluation of the Passive Zweifach–Fung Microfluidic Devices
2.7.2. Passive Chitosan Microparticle Separation
3. Results and Discussion
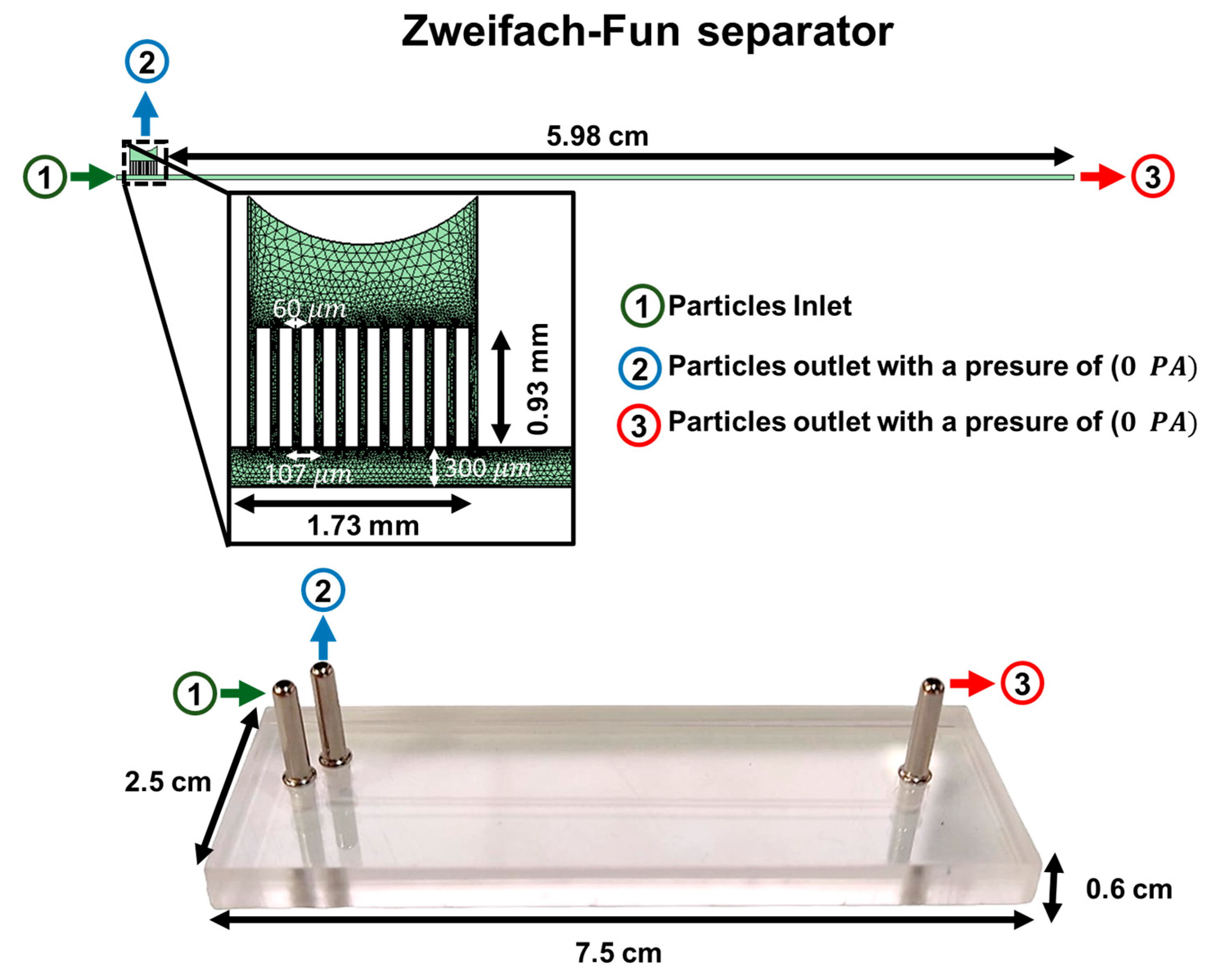
3.1. Geometric Optimization of Passive Microfluidic Devices
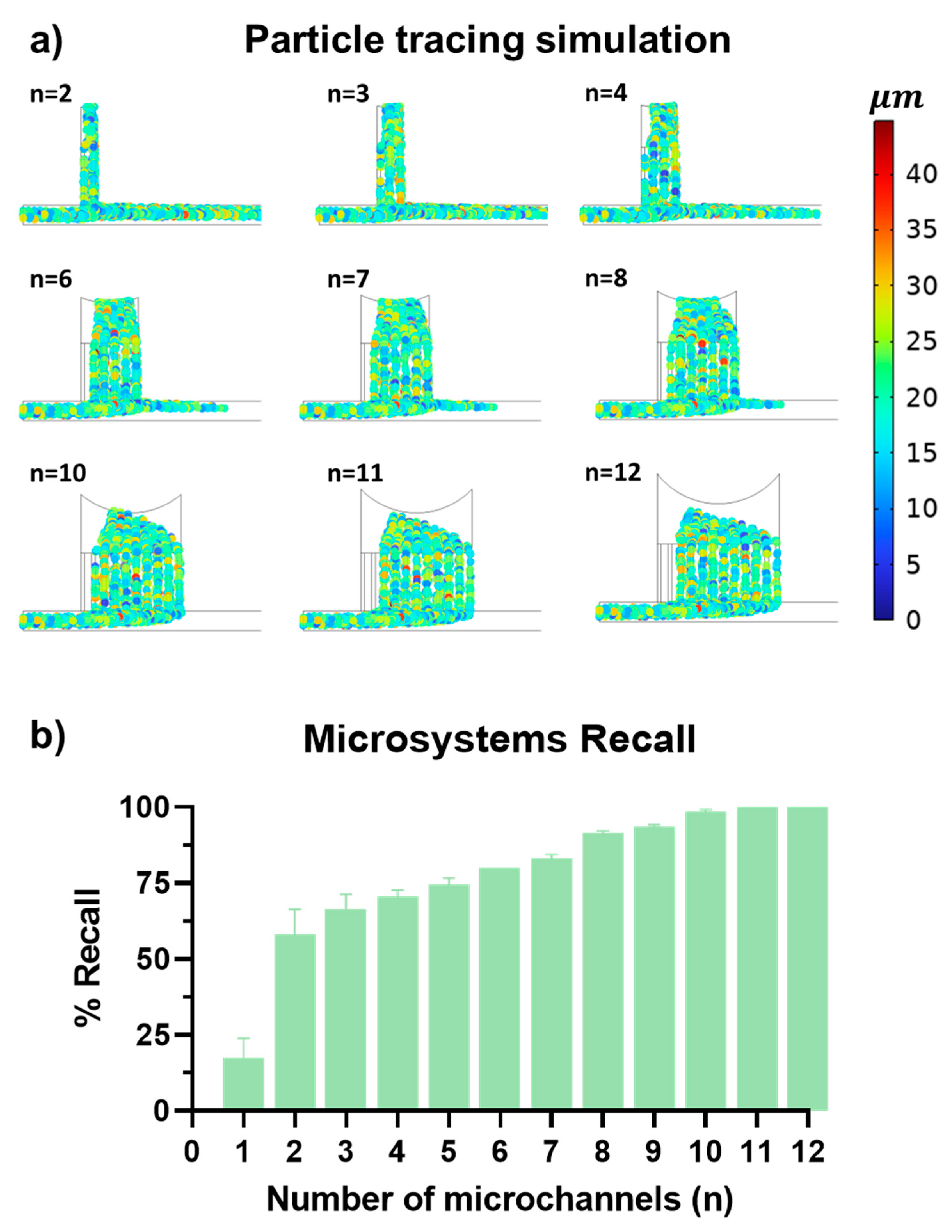
3.2. Intrinsic Errors and Dimensional Reductions in PMMA Microfluidic Devices Fabricated by Laser Ablation
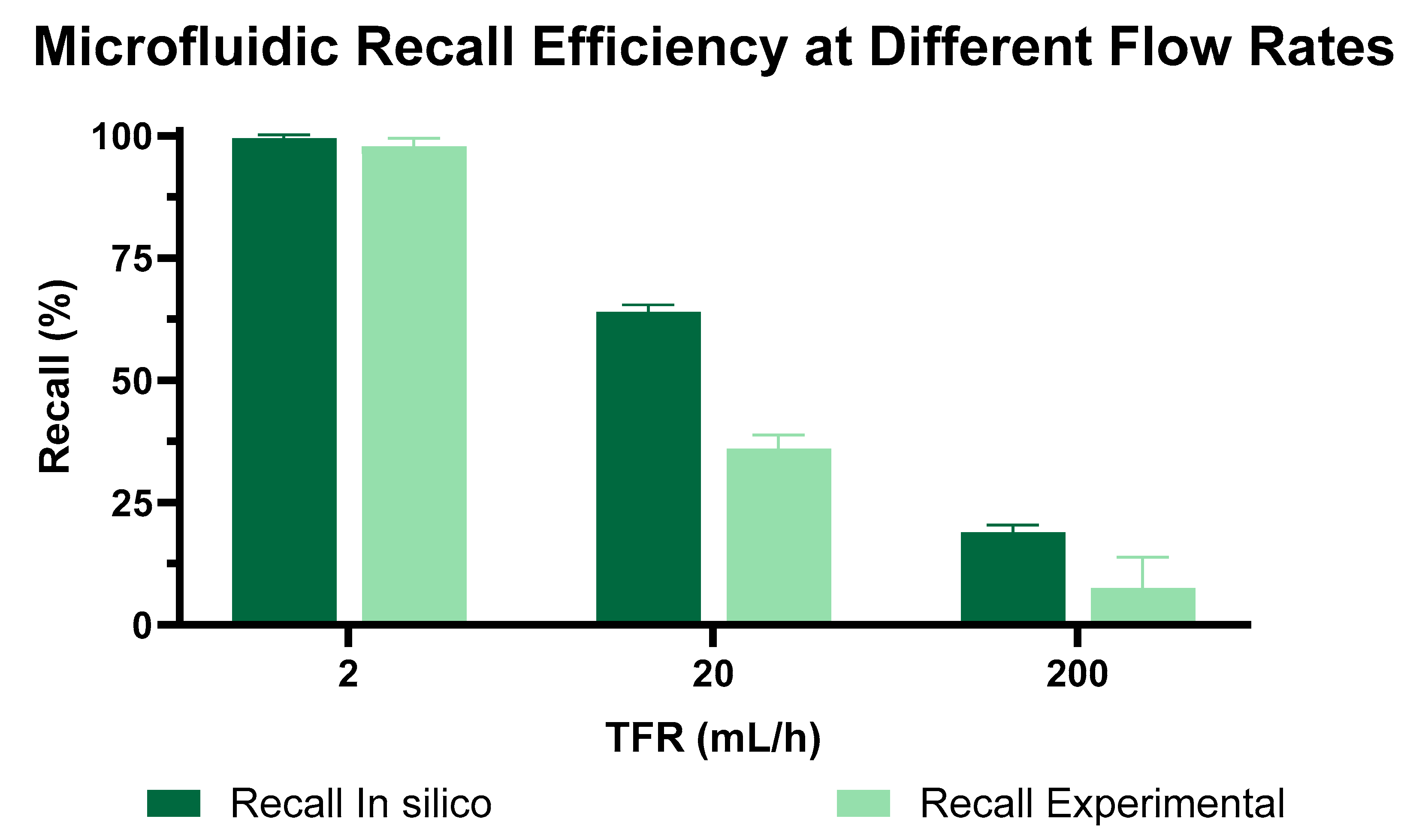
3.3. TFR Impact on Zweifach–Fung (ZF) Microfluidic Device Performance
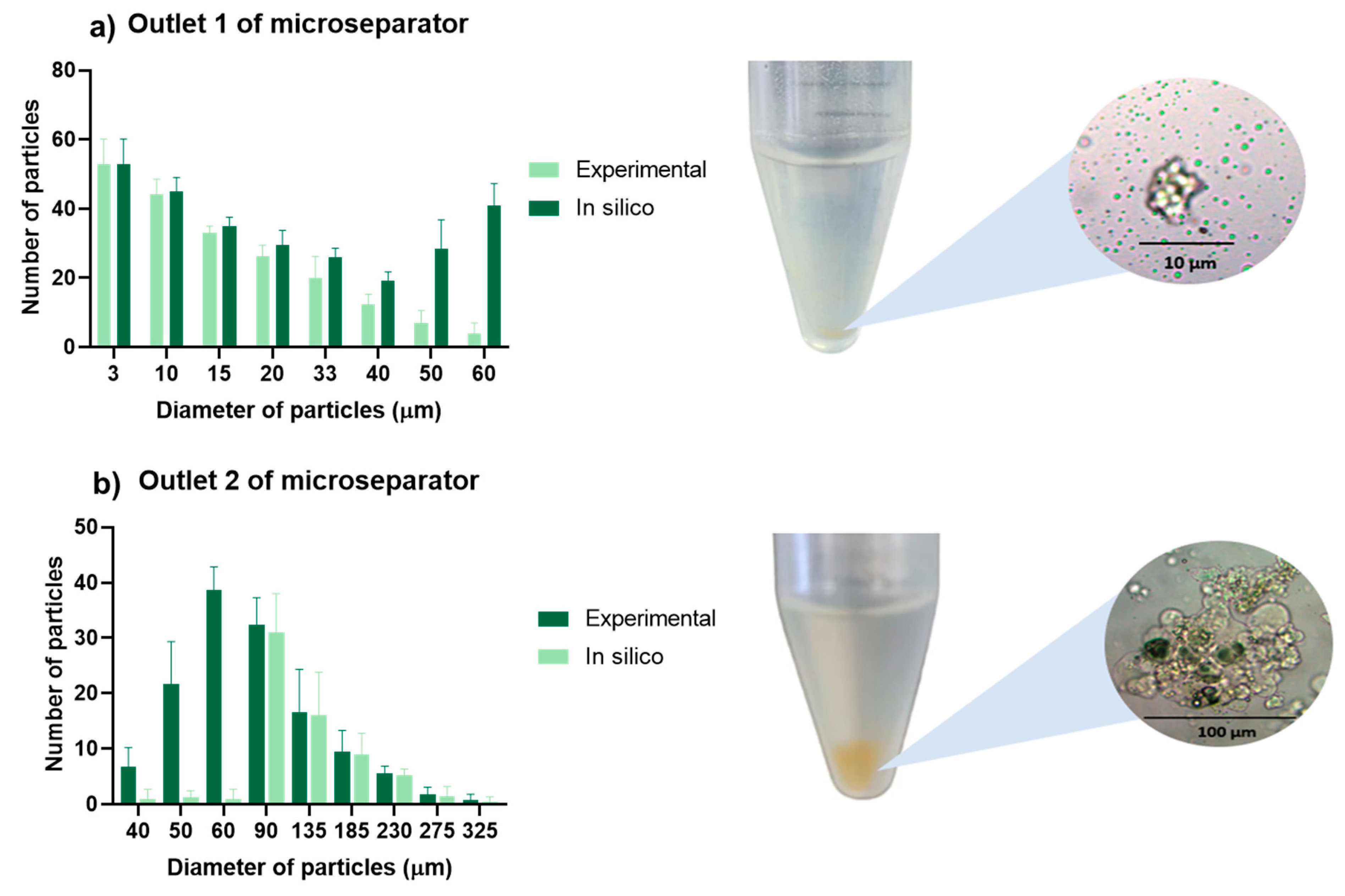
3.4. Chitosan Particle Separation
3.5. SWOT Analysis (Strengths, Weaknesses, Opportunities, and Threats)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wong, W.D.; Majnis, M.F.; Lai, C.W.; Sagadevan, S.; Muhd Julkapli, N. Enhancement of Mixing and Reaction Efficiency of Various Fluids Applications at Different Microfluidic Configuration and Design. In Chemical Engineering and Processing—Process Intensification; Elsevier: Amsterdam, The Netherlands, 2024. [Google Scholar] [CrossRef]
- Saxena, A.; Kumar, M.; Mishra, D.; Singh, K. Optimization of Newtonian Fluid Pressure in Microcantilever Integrated Flexible Microfluidic Channel for Healthcare Application. Biomed. Phys. Eng. Express 2024, 10, 035015. [Google Scholar] [CrossRef] [PubMed]
- Gurkan, U.A.; Wood, D.K.; Carranza, D.; Herbertson, L.H.; Diamond, S.L.; Du, E.; Guha, S.; Di Paola, J.; Hines, P.C.; Papautsky, I.; et al. Next Generation Microfluidics: Fulfilling the Promise of Lab-on-a-Chip Technologies. Lab Chip 2024, 24, 1867–1874. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Ma, X.; Zhang, Z.; Wang, X.; Yang, B.; Yang, J.; Zeng, Y.; Yuan, X.; Zhang, D.; Yamaguchi, Y. A Rapid and Low-Cost Platform for Detection of Bacterial Based on Microchamber PCR Microfluidic Chip. Biomed. Microdevices 2024, 26, 20. [Google Scholar] [CrossRef] [PubMed]
- Mc Veigh, M.; Bellan, L.M. Microfluidic Synthesis of Radiotracers: Recent Developments and Commercialization Prospects. Lab Chip 2024, 24, 1226–1243. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.E.; Li, W.; Chandrasekhar, M.; Corazza, C.M.O.W.; Deijs, G.S.; Djoko, L.; Govind, B.; Jose, E.; Kwon, Y.J.; Lowe, T.; et al. Lab on a Bead with Oscillatory Centrifugal Microfluidics for Fast and Complete Mixing Enables Fast and Accurate Biomedical Assays. Sci. Rep. 2024, 14, 8637. [Google Scholar] [CrossRef] [PubMed]
- Guan, Z.; Liu, Q.; Ma, C.-B.; Du, Y. Electrochemical Microfluidic Sensing Platforms for Biosecurity Analysis. Anal. Bioanal. Chem. 2024, 1, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Rashidi, N.; Slater, A.; Peregrino, G.; Santin, M. A Novel, Microfluidic High-Throughput Single-Cell Encapsulation of Human Bone Marrow Mesenchymal Stromal Cells. J. Mater. Sci. Mater. Med. 2024, 35, 19. [Google Scholar] [CrossRef] [PubMed]
- Duarte, L.C.; Figueredo, F.; Chagas, C.L.S.; Cortón, E.; Coltro, W.K.T. A Review of the Recent Achievements and Future Trends on 3D Printed Microfluidic Devices for Bioanalytical Applications. Anal. Chim. Acta 2024, 1299, 342429. [Google Scholar] [CrossRef] [PubMed]
- Chavez-Pineda, O.G.; Rodriguez-Moncayo, R.; Gonzalez-Suarez, A.M.; Guevara-Pantoja, P.E.; Maravillas-Montero, J.L.; Garcia-Cordero, J.L. Portable Platform for Leukocyte Extraction from Blood Using Sheath-Free Microfluidic DLD. Lab Chip 2024, 24, 2575–2589. [Google Scholar] [CrossRef]
- Young, O.M.; Xu, X.; Sarker, S.; Sochol, R.D. Direct Laser Writing-Enabled 3D Printing Strategies for Microfluidic Applications. Lab Chip 2024, 24, 2371–2396. [Google Scholar] [CrossRef]
- Ortega Quesada, B.A.; Cuccia, J.; Coates, R.; Nassar, B.; Littlefield, E.; Martin, E.C.; Melvin, A.T. A Modular Microfluidic Platform to Study How Fluid Shear Stress Alters Estrogen Receptor Phenotype in ER + Breast Cancer Cells. Microsyst. Nanoeng. 2024, 10, 25. [Google Scholar] [CrossRef] [PubMed]
- Pan, P.; Qin, Z.; Sun, W.; Zhou, Y.; Wang, S.; Song, P.; Wang, Y.; Ru, C.; Wang, X.; Calarco, J.; et al. Correction: A Spiral Microfluidic Device for Rapid Sorting, Trapping, and Long-Term Live Imaging of Caenorhabditis Elegans Embryos. Microsyst. Nanoeng. 2023, 9, 147. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Mohan, A.; Mishra, A.; Nair, A.; Chowdhury, N.; Balekai, D.; Rai, K.; Prabhakar, A.; Saiyed, T. Multiplexed Fluorescence and Scatter Detection with Single Cell Resolution Using On-Chip Fiber Optics for Droplet Microfluidic Applications. Microsyst. Nanoeng. 2024, 10, 35. [Google Scholar] [CrossRef] [PubMed]
- Shan, H.; Sun, Q.; Xie, Y.; Liu, X.; Chen, X.; Zhao, S.; Chen, Z. Dialysis-Functionalized Microfluidic Platform for in Situ Formation of Purified Liposomes. Colloids Surf. B Biointerfaces 2024, 236, 113829. [Google Scholar] [CrossRef] [PubMed]
- He, G.; Pan, Y.; Zeng, F.; Qin, S.; Luan, X.; Lu, Q.; Xie, C.; Hu, P.; Gao, Y.; Yang, J.; et al. Microfluidic Synthesis of CuH Nanoparticles for Antitumor Therapy through Hydrogen-Enhanced Apoptosis and Cuproptosis. ACS Nano 2024, 18, 9031–9042. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, Y.; Zhang, D.; Guo, G.; Wang, L.; Wang, X. Kinetically Controlled Nucleation Enabled by Tunable Microfluidic Mixing for the Synthesis of Dendritic Au@Pt Core/Shell Nanomaterials. Small 2024, 20, 2302589. [Google Scholar] [CrossRef] [PubMed]
- Mehraji, S.; DeVoe, D.L. Microfluidic Synthesis of Lipid-Based Nanoparticles for Drug Delivery: Recent Advances and Opportunities. Lab Chip 2024, 24, 1154–1174. [Google Scholar] [CrossRef]
- Kulkarni, M.B.; Ayachit, N.H.; Aminabhavi, T.M.; Pogue, B.W. Recent Advances in Microfluidics-Based Paper Analytical Devices (ΜPADs) for Biochemical Sensors: From Fabrication to Detection Techniques. Biochem. Eng. J. 2023, 198, 109027. [Google Scholar] [CrossRef]
- Kang, K.; Lee, S.Y.; Kim, C.S.; Park, C.H. Hybrid Magnetic Field System with Helmholtz Coils and Magnets for Real-Time Circulating Tumor Cell Separation. Sens. Actuators A Phys. 2024, 370, 115229. [Google Scholar] [CrossRef]
- Xuan, Y.; Yin, M.; Sun, Y.; Liu, M.; Bai, G.; Diao, Z.; Ma, B. Magnetic Nanoparticle-Mediated Enrichment Technology Combined with Microfluidic Single Cell Separation Technology: A Technology for Efficient Separation and Degradation of Functional Bacteria in Single Cell Liquid Phase. Bioresour. Technol. 2024, 401, 130686. [Google Scholar] [CrossRef]
- Kutluk, H.; Viefhues, M.; Constantinou, I. Integrated Microfluidics for Single-Cell Separation and On-Chip Analysis: Novel Applications and Recent Advances. Small Sci. 2024, 4, 2300206. [Google Scholar] [CrossRef]
- Peng, T.; Lin, X.; Li, L.; Huang, L.; Jiang, B.; Jia, Y. Investigation on Submicron Particle Separation and Deflection Using Tilted-Angle Standing Surface Acoustic Wave Microfluidics. Heliyon 2024, 10, e25042. [Google Scholar] [CrossRef]
- Zhang, T.; Di Carlo, D.; Lim, C.T.; Zhou, T.; Tian, G.; Tang, T.; Shen, A.Q.; Li, W.; Li, M.; Yang, Y.; et al. Passive Microfluidic Devices for Cell Separation. Biotechnol. Adv. 2024, 71, 108317. [Google Scholar] [CrossRef] [PubMed]
- Bagi, M.; Amjad, F.; Ghoreishian, S.M.; Sohrabi Shahsavari, S.; Huh, Y.S.; Moraveji, M.K.; Shimpalee, S. Advances in Technical Assessment of Spiral Inertial Microfluidic Devices Toward Bioparticle Separation and Profiling: A Critical Review. Biochip J. 2024, 18, 45–67. [Google Scholar] [CrossRef]
- Ngum, L.F.; Matsushita, Y.; El-Mashtoly, S.F.; Fath El-Bab, A.M.R.; Abdel-Mawgood, A.L. Separation of Microalgae from Bacterial Contaminants Using Spiral Microchannel in the Presence of a Chemoattractant. Bioresour. Bioprocess. 2024, 11, 36. [Google Scholar] [CrossRef] [PubMed]
- Karimi, A.; Sattari-Najafabadi, M. Numerical Study of Bacteria Removal from Microalgae Solution Using an Asymmetric Contraction-Expansion Microfluidic Device: A Parametric Analysis Approach. Heliyon 2023, 9, e20380. [Google Scholar] [CrossRef] [PubMed]
- Fung, Y.C.; Zweifach, B.W. Microcirculation: Mechanics of Blood Flow in Capillaries. Annu. Rev. Fluid Mech. 1971, 3, 189–210. [Google Scholar] [CrossRef]
- Petit, N. Control of a Microfluidic Separation Process Governed by the Zweifach-Fung Effect. IFAC-Pap. 2022, 55, 975–980. [Google Scholar] [CrossRef]
- Bekiaris-Liberis, N.; Bresch-Pietri, D.; Petit, N. Compensation of Input-Dependent Hydraulic Input Delay for a Model of a Microfluidic Process under Zweifach–Fung Effect. Automatica 2024, 160, 111428. [Google Scholar] [CrossRef]
- Lu, Y.; Shi, Z.; Yu, L.; Li, C.M. Fast Prototyping of a Customized Microfluidic Device in a Non-Clean-Room Setting by Cutting and Laminating Parafilm®. RSC Adv. 2016, 6, 85468–85472. [Google Scholar] [CrossRef]
- Zhou, W.; Dou, M.; Timilsina, S.S.; Xu, F.; Li, X. Recent Innovations in Cost-Effective Polymer and Paper Hybrid Microfluidic Devices. Lab Chip 2021, 21, 2658–2683. [Google Scholar] [CrossRef]
- Thomas, T.; Agrawal, A. Design and Fabrication of Microfluidic Devices: A Cost-Effective Approach for High Throughput Production. J. Micromech. Microeng. 2024, 34, 015008. [Google Scholar] [CrossRef]
- Li, Y.; Wang, X.; Wang, Y.; Fan, Y. Low-Cost Hybrid Bonding between Thermoplastics and PDMS with Differential Adhesive Tape for Microfluidic Devices. J. Mater. Sci. Mater. Electron. 2023, 34, 565. [Google Scholar] [CrossRef]
- Li, Y.; Wang, X.; Yang, S.; Liu, J.; Zhang, Q.; Fan, Y. Reversible Bonding of Thermoplastic-Based Microfluidics with Freeze-Release Adhesive. Microfluid. Nanofluidics 2023, 27, 33. [Google Scholar] [CrossRef]
- Tsao, C.-W. Polymer Microfluidics: Simple, Low-Cost Fabrication Process Bridging Academic Lab Research to Commercialized Production. Micromachines 2016, 7, 225. [Google Scholar] [CrossRef] [PubMed]
- Cong, H.; Zhang, N. Perspectives in Translating Microfluidic Devices from Laboratory Prototyping into Scale-up Production. Biomicrofluidics 2022, 16, 021301. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, F.; Fan, Y. Thermoplastic-Based Microfluidic Chip Bonding with PES Hot Melt Adhesive Film. J. Adhes 2024, 100, 178–185. [Google Scholar] [CrossRef]
- Kaba, A.M.; Jeon, H.; Park, A.; Yi, K.; Baek, S.; Park, A.; Kim, D. Cavitation-Microstreaming-Based Lysis and DNA Extraction Using a Laser-Machined Polycarbonate Microfluidic Chip. Sens. Actuators B Chem. 2021, 346, 130511. [Google Scholar] [CrossRef]
- Zhang, D.; Gao, R.; Huang, S.; Huang, Y.; Zhang, J.; Su, X.; Zhang, S.; Ge, S.; Zhang, J.; Xia, N. All-in-One Microfluidic Chip for 30-Min Quantitative Point-of-Care-Testing of Nucleic Acids. Sens. Actuators B Chem. 2023, 390, 133939. [Google Scholar] [CrossRef]
- Madadi, M.; Madadi, A.; Zareifar, R.; Nikfarjam, A. A Simple Solvent-Assisted Method for Thermal Bonding of Large-Surface, Multilayer PMMA Microfluidic Devices. Sens. Actuators A Phys. 2023, 349, 114077. [Google Scholar] [CrossRef]
- Persson, H.; Park, S.; Mohan, M.; Cheung, K.K.; Simmons, C.A.; Young, E.W.K. Rapid Assembly of PMMA Microfluidic Devices with PETE Membranes for Studying the Endothelium. Sens. Actuators B Chem. 2022, 356, 131342. [Google Scholar] [CrossRef]
- Smith, S.; Sypabekova, M.; Kim, S. Double-Sided Tape in Microfluidics: A Cost-Effective Method in Device Fabrication. Biosensors 2024, 14, 249. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Jiang, B.; Li, X.; Zhou, M. Investigation of Solvent-Assisted In-Mold Bonding of Cyclic Olefin Copolymer (COC) Microfluidic Chips. Micromachines 2022, 13, 965. [Google Scholar] [CrossRef] [PubMed]
- Srikantaprasad, G.; Mathew, N.T.; Nagar, S.V. Laser Micromachining on PMMA: An Efficient Fabrication of Microchannels for Sustainable Microfluidic Devices. J. Braz. Soc. Mech. Sci. Eng. 2024, 46, 325. [Google Scholar] [CrossRef]
- Li, S.-C.; Chiang, C.-C.; Tsai, Y.-S.; Chen, C.-J.; Lee, T.-H. Fabrication of a Three-Dimensional Microfluidic System from Poly(Methyl Methacrylate) (PMMA) Using an Intermiscibility Vacuum Bonding Technique. Micromachines 2024, 15, 454. [Google Scholar] [CrossRef] [PubMed]
- Ecker, R.; Mitteramskogler, T.; Fuchsluger, A.; Jakoby, B. Fabrication of an Ultrathin PMMA Foil for Sensing Applications in Microfluidic Systems. In Proceedings of the XXXV EUROSENSORS Conference, Lecce, Italy, 10–13 September 2023; p. 48. [Google Scholar] [CrossRef]
- Anjum, A.; Azharuddin Ali, M.; Shaikh, A.A.; Akhtar, S.S. A Numerical and Experimental Analysis of CO2 Laser Micro-Milling on PMMA Sheet Considering a Multipass Approach for Microfluidic Devices. Opt. Laser Technol. 2024, 176, 110860. [Google Scholar] [CrossRef]
- Klank, H.; Kutter, J.P.; Geschke, O. CO2-Laser Micromachining and Back-End Processing for Rapid Production of PMMA-Based Microfluidic Systems. Lab Chip 2002, 2, 242. [Google Scholar] [CrossRef] [PubMed]
- Nasser, G.A.; Fath El-Bab, A.M.R.; Abdel-Mawgood, A.L.; Mohamed, H.; Saleh, A.M. CO2 Laser Fabrication of PMMA Microfluidic Double T-Junction Device with Modified Inlet-Angle for Cost-Effective PCR Application. Micromachines 2019, 10, 678. [Google Scholar] [CrossRef] [PubMed]
- Tweedie, M.; Maguire, P.D. Microfluidic Ratio Metering Devices Fabricated in PMMA by CO2 Laser. Microsyst. Technol. 2021, 27, 47–58. [Google Scholar] [CrossRef]
- Huang, T.-K.; Huang, C.-H.; Chen, P.-A.; Chen, C.H.; Lu, F.; Yang, W.-J.; Huang, J.Y.J.; Li, B.-R. Development of a Thermotaxis and Rheotaxis Microfluidic Device for Motile Spermatozoa Sorting. Biosens. Bioelectron. 2024, 258, 116353. [Google Scholar] [CrossRef]
- Qin, L.; Yue, J.; Zhou, D.; Yang, A.; Zheng, S. Numerical and Experimental Study on the Synergistic Effect of Vortex and Contact Surface of Fluid in the Inlet Section of Micromixer. Chem. Eng. Process.—Process Intensif. 2024, 196, 109645. [Google Scholar] [CrossRef]
- Yang, L.; Xu, F.; Chen, G. Effective Mixing in a Passive Oscillating Micromixer with Impinging Jets. Chem. Eng. J. 2024, 489, 151329. [Google Scholar] [CrossRef]
- Su, W.; Han, B.; Yeboah, S.; Du, D.; Wang, L. Fabrication of Monodisperse Droplets and Microcapsules Using Microfluidic Chips: A Review of Methodologies and Applications. Rev. Chem. Eng. 2024, 40, 401–434. [Google Scholar] [CrossRef]
- Ma, L.; Zhao, X.; Hou, J.; Huang, L.; Yao, Y.; Ding, Z.; Wei, J.; Hao, N. Droplet Microfluidic Devices: Working Principles, Fabrication Methods, and Scale-Up Applications. Small Methods 2024. [Google Scholar] [CrossRef] [PubMed]
- Shamsi, A.; Shamloo, A.; Mohammadaliha, N.; Hajghassem, H.; Mehrabadi, J.F.; Bazzaz, M. High Throughput Blood Plasma Separation Using a Passive PMMA Microfluidic Device. Microsyst. Technol. 2016, 22, 2447–2454. [Google Scholar] [CrossRef]
- Bazaz, S.R.; Mehrizi, A.A.; Hesari, A.Z. A Novel Microfluidic Design for Blood Plasma Separation. In Proceedings of the 2016 23rd Iranian Conference on Biomedical Engineering and 2016 1st International Iranian Conference on Biomedical Engineering (ICBME), Tehran, Iran, 24–25 November 2016; IEEE: New York, NY, USA, 2016; pp. 97–101. [Google Scholar] [CrossRef]
- Rodríguez, C.F.; Andrade-Pérez, V.; Vargas, M.C.; Mantilla-Orozco, A.; Osma, J.F.; Reyes, L.H.; Cruz, J.C. Breaking the Clean Room Barrier: Exploring Low-Cost Alternatives for Microfluidic Devices. Front. Bioeng. Biotechnol. 2023, 11, 1176557. [Google Scholar] [CrossRef] [PubMed]
- Schneider, P.J.; Christie, L.B.; Eadie, N.M.; Siskar, T.J.; Sukhotskiy, V.; Koh, D.; Wang, A.; Oh, K.W. Pysanky to Microfluidics: An Innovative Wax-Based Approach to Low Cost, Rapid Prototyping of Microfluidic Devices. Micromachines 2024, 15, 240. [Google Scholar] [CrossRef]
- Halwes, M.; Stamp, M.; Collins, D.J. A Rapid Prototyping Approach for Multi-Material, Reversibly Sealed Microfluidics. Micromachines 2023, 14, 2213. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Chen, L.; Ma, Y.; Weng, D.; Wang, Z.; Zhang, X.; Wang, J. Multi-Physics Coupling Simulation and Design of Magnetic Field-Driven Soft Microrobots in Liquid Environments. Int. J. Mech. Sci. 2024, 272, 109136. [Google Scholar] [CrossRef]
- Rodriguez, C.F.; Ortiz, C.L.; Giraldo, R.K.A.; Munoz, C.C.; Cruz, J.C. In Silico Study of Spheroids Fusion through Magnetic Field Gradients. In Proceedings of the 2021 IEEE 2nd International Congress of Biomedical Engineering and Bioengineering (CI-IB&BI), Bogota D.C., Colombia, 13–15 October 2021; IEEE: New York, NY, USA, 2021; pp. 1–9. [Google Scholar] [CrossRef]
- Rodríguez, C.F.; Guzmán-Sastoque, P.; Gantiva-Diaz, M.; Gómez, S.C.; Quezada, V.; Muñoz-Camargo, C.; Osma, J.F.; Reyes, L.H.; Cruz, J.C. Low-Cost Inertial Microfluidic Device for Microparticle Separation: A Laser-Ablated PMMA Lab-on-a-Chip Approach without a Cleanroom. HardwareX 2023, 16, e00493. [Google Scholar] [CrossRef]
- Mumps, A. MUltifrontal Massively Parallel Sparse Direct Solve. Available online: http://graal.ens-lyon.fr/MUMPS (accessed on 1 February 2024).
- Rodríguez-Soto, M.A.; Riveros-Cortés, A.; Orjuela-Garzón, I.C.; Fernández-Calderón, I.M.; Rodríguez, C.F.; Vargas, N.S.; Ostos, C.; Camargo, C.M.; Cruz, J.C.; Kim, S.; et al. Redefining Vascular Repair: Revealing Cellular Responses on PEUU—Gelatin Electrospun Vascular Grafts for Endothelialization and Immune Responses on in Vitro Models. Front. Bioeng. Biotechnol. 2024, 12, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Amestoy, P.R.; Duff, I.S.; L’Excellent, J.-Y.; Koster, J. MUMPS: A General Purpose Distributed Memory Sparse Solver. In Proceedings of the 5th International Workshop, PARA 2000, Bergen, Norway, 18–20 June 2000; Springer: Berlin/Heidelberg, Germany; pp. 121–130. [CrossRef]
- Ortegón, S.; Peñaranda, P.A.; Rodríguez, C.F.; Noguera, M.J.; Florez, S.L.; Cruz, J.C.; Rivas, R.E.; Osma, J.F. Magnetic Torus Microreactor as a Novel Device for Sample Treatment via Solid-Phase Microextraction Coupled to Graphite Furnace Atomic Absorption Spectroscopy: A Route for Arsenic Pre-Concentration. Molecules 2022, 27, 6198. [Google Scholar] [CrossRef] [PubMed]
- Keyes, D.E.; McInnes, L.C.; Woodward, C.; Gropp, W.; Myra, E.; Pernice, M.; Bell, J.; Brown, J.; Clo, A.; Connors, J.; et al. Multiphysics Simulations. Int. J. High Perform. Comput. Appl. 2013, 27, 4–83. [Google Scholar] [CrossRef]
- Rodríguez, C.F.; Cruz, J.C. Critique—Simulation Apps. Educ. Chem. Eng. 2023, 42, 88–89. [Google Scholar] [CrossRef]
- Zhang, J.; Guo, Q.; Liu, M.; Yang, J. A Lab-on-CD Prototype for High-Speed Blood Separation. J. Micromech. Microeng. 2008, 18, 125025. [Google Scholar] [CrossRef]
- Tripathi, S.; Prabhakar, A.; Kumar, N.; Singh, S.G.; Agrawal, A. Blood Plasma Separation in Elevated Dimension T-Shaped Microchannel. Biomed. Microdevices 2013, 15, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Chávez Ramos, K.; Cañizares Macías, M.d.P. Microdevice Based on Centrifugal Effect and Bifurcation Law for Separation of Plasma from On-Line Diluted Whole Blood. Anal. Bioanal. Chem. 2021, 413, 5361–5372. [Google Scholar] [CrossRef] [PubMed]
- Kersaudy-Kerhoas, M.; Kavanagh, D.M.; Dhariwal, R.S.; Campbell, C.J.; Desmulliez, M.P.Y. Validation of a Blood Plasma Separation System by Biomarker Detection. Lab Chip 2010, 10, 1587. [Google Scholar] [CrossRef]
- Yang, S.; Ündar, A.; Zahn, J.D. A Microfluidic Device for Continuous, Real Time Blood Plasma Separation. Lab Chip 2006, 6, 871–880. [Google Scholar] [CrossRef]
- Kersaudy-Kerhoas, M.; Dhariwal, R.; Desmulliez, M.P.Y.; Jouvet, L. Hydrodynamic Blood Plasma Separation in Microfluidic Channels. Microfluid. Nanofluidics 2010, 8, 105–114. [Google Scholar] [CrossRef]
- de Souza, H.F.; dos Santos, F.R.; Cunha, J.S.; Pacheco, F.C.; Pacheco, A.F.C.; Soutelino, M.E.M.; Martins, C.C.N.; Andressa, I.; Rocha, R.d.S.; da Cruz, A.G.; et al. Microencapsulation to Harness the Antimicrobial Potential of Essential Oils and Their Applicability in Dairy Products: A Comprehensive Review of the Literature. Foods 2024, 13, 2197. [Google Scholar] [CrossRef]
- Espinoza-Espinoza, L.A.; Muñoz-More, H.D.; Nole-Jaramillo, J.M.; Ruiz-Flores, L.A.; Arana-Torres, N.M.; Moreno-Quispe, L.A.; Valdiviezo-Marcelo, J. Microencapsulation of Vitamins: A Review and Meta-Analysis of Coating Materials, Release and Food Fortification. Food Res. Int. 2024, 187, 114420. [Google Scholar] [CrossRef] [PubMed]
- Calderón-Oliver, M.; Ponce-Alquicira, E. The Role of Microencapsulation in Food Application. Molecules 2022, 27, 1499. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Tang, Q.; Gao, Y.; Chen, S.; Yu, Y.; Qian, H.; McClements, D.J.; Cao, C.; Yuan, B. Recent Developments in the Fabrication of Food Microparticles and Nanoparticles Using Microfluidic Systems. Crit. Rev. Food Sci. Nutr. 2024, 64, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Bolanos-Barbosa, A.D.; Rodríguez, C.F.; Acuña, O.L.; Cruz, J.C.; Reyes, L.H. The Impact of Yeast Encapsulation in Wort Fermentation and Beer Flavor Profile. Polymers 2023, 15, 1742. [Google Scholar] [CrossRef]
- Ma, D.; Yang, B.; Zhao, J.; Yuan, D.; Li, Q. Advances in Protein-Based Microcapsules and Their Applications: A Review. Int. J. Biol. Macromol. 2024, 263, 129742. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Jaiswal, A.; Liu, X.; Yang, Z.; Yin, Q.; Kong, K.V.; Yong, K. Microfluidics Evolution and Surface Functionalization: A Pathway to Enhanced Heavy Metal Ion Detection. Adv. Sens. Res. 2024. [Google Scholar] [CrossRef]
- Rohr, T.; Ogletree, D.F.; Svec, F.; Fréchet, J.M.J. Surface Functionalization of Thermoplastic Polymers for the Fabrication of Microfluidic Devices by Photoinitiated Grafting. Adv. Funct. Mater. 2003, 13, 264–270. [Google Scholar] [CrossRef]
- Nielsen, J.B.; Hanson, R.L.; Almughamsi, H.M.; Pang, C.; Fish, T.R.; Woolley, A.T. Microfluidics: Innovations in Materials and Their Fabrication and Functionalization. Anal. Chem. 2020, 92, 150–168. [Google Scholar] [CrossRef]
- Shakeri, A.; Jarad, N.A.; Khan, S.; F Didar, T. Bio-Functionalization of Microfluidic Platforms Made of Thermoplastic Materials: A Review. Anal. Chim. Acta 2022, 1209, 339283. [Google Scholar] [CrossRef]
- Torres, C.E.; Cifuentes, J.; Gómez, S.C.; Quezada, V.; Giraldo, K.A.; Puentes, P.R.; Rueda-Gensini, L.; Serna, J.A.; Muñoz-Camargo, C.; Reyes, L.H.; et al. Microfluidic Synthesis and Purification of Magnetoliposomes for Potential Applications in the Gastrointestinal Delivery of Difficult-to-Transport Drugs. Pharmaceutics 2022, 14, 315. [Google Scholar] [CrossRef] [PubMed]


| Material | Quantity | Cost per Unit (USD) | Total Cost (USD) |
|---|---|---|---|
| Microfluidic Connectors | 3 units | USD 0.05 | USD 0.15 |
| PMMA Sheets (7.5 cm × 2.5 cm) | 2 units | USD 0.26 | USD 0.51 |
| 1 mL | - | USD 0.071 | |
| 5 mL | - | USD 0.02 | |
| 1 mL | - | USD 0.069 | |
| Total cost | USD 0.82 |
| Literature | Our Work | [26] | [71] | [72] | [64] | [73] | [57] | [74] | [75] | [76] |
|---|---|---|---|---|---|---|---|---|---|---|
| Channel Geometry | T-shaped | U and W-shaped | Curved | T-shaped | Wave | Spiral-shaped | T-shaped | V complex-shaped | T and Y-shaped | T-shaped |
| Material | PMMA | PMMA | PDMS | PDMS | PMMA | PDMS | PMMA | PMMA | PDMS | PMMA |
| Fabrication Technique | CO2 laser | CO2 laser | Soft lithography | Photolithography | CO2 laser | Soft lithography | Soft lithography | Photolithography | Lithography | Lithography |
| Depth | 60 µm | 162–210 µm | 40 µm | 50 µm | - | - | - | 20 µm | 10 µm | 20 µm |
| Channel Width | 60 µm | 180–227 µm | 100–200 µm | 300–700 µm | 300 µm | 15–40 µm | 10–20 µm | 9.6–15 µm | 100 µm | |
| Cross Section | Gaussian | Gaussian | Gaussian | Rectangular | Gaussian | Rectangular | Rectangular | Gaussian | Rectangular | Rectangular |
| Flow Rate | 2 mL/h | 42 mL/h | - | - | 23 mL/h | 0.18–0.42 and 1.5–6 mL/h | 6 mL/h | 1–10 mL/h | 0.01 mL/h | 10 mL/h |
| Separation Technique | Zweifach–Fung effect | Hydrodynamic force | Centrifugal force, Coriolis force | Zweifach–Fung effect | Zweifach–Fung effect | Dean drag force | Zweifach–Fung effect | Plasma skimming effect | Zweifach–Fung effect | Zweifach–Fung effect |
| Cost per Chip | <USD 0.90 | <USD 1.00 | <USD 1.00 | >USD 1.00 | <USD 2.00 | >USD 1.00 | >USD 1.00 | >USD 1.00 | >USD 1.00 | >USD 1.00 |
| Fabrication Time | 15 min | - | - | >1 h | 15 min | >2 h | - | - | - | <1 h |
| Separation Efficiency | 94% | 92–96% | 99% | 99.70% | 96.14% | 8–13% | 66.6% | 65.1–100% | 0.25 | 0.4 |
| Particle Size | <40 µm | RBC size | RBC size | RBC size | 15–40 µm | RBC size | RBC size | RBC size | 8–16 µm | RBC size |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez, C.F.; Báez-Suárez, M.; Muñoz-Camargo, C.; Reyes, L.H.; Osma, J.F.; Cruz, J.C. Zweifach–Fung Microfluidic Device for Efficient Microparticle Separation: Cost-Effective Fabrication Using CO2 Laser-Ablated PMMA. Micromachines 2024, 15, 932. https://doi.org/10.3390/mi15070932
Rodríguez CF, Báez-Suárez M, Muñoz-Camargo C, Reyes LH, Osma JF, Cruz JC. Zweifach–Fung Microfluidic Device for Efficient Microparticle Separation: Cost-Effective Fabrication Using CO2 Laser-Ablated PMMA. Micromachines. 2024; 15(7):932. https://doi.org/10.3390/mi15070932
Chicago/Turabian StyleRodríguez, Cristian F., Mateo Báez-Suárez, Carolina Muñoz-Camargo, Luis H. Reyes, Johann F. Osma, and Juan C. Cruz. 2024. "Zweifach–Fung Microfluidic Device for Efficient Microparticle Separation: Cost-Effective Fabrication Using CO2 Laser-Ablated PMMA" Micromachines 15, no. 7: 932. https://doi.org/10.3390/mi15070932






