Fabrication of Two-Layer Microfluidic Devices with Porous Electrodes Using Printed Sacrificial Layers
Abstract
:1. Introduction
2. Materials and Methods
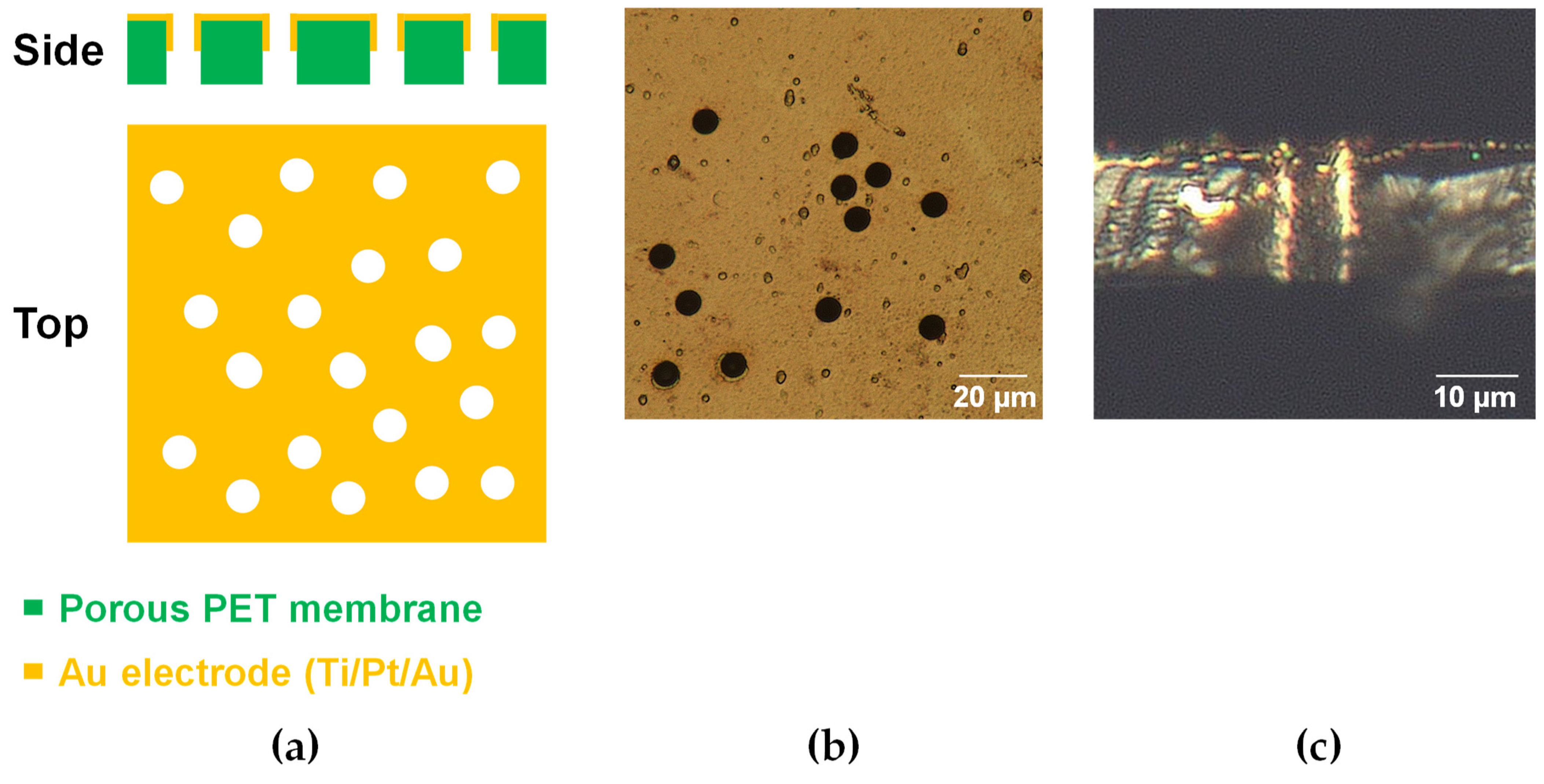
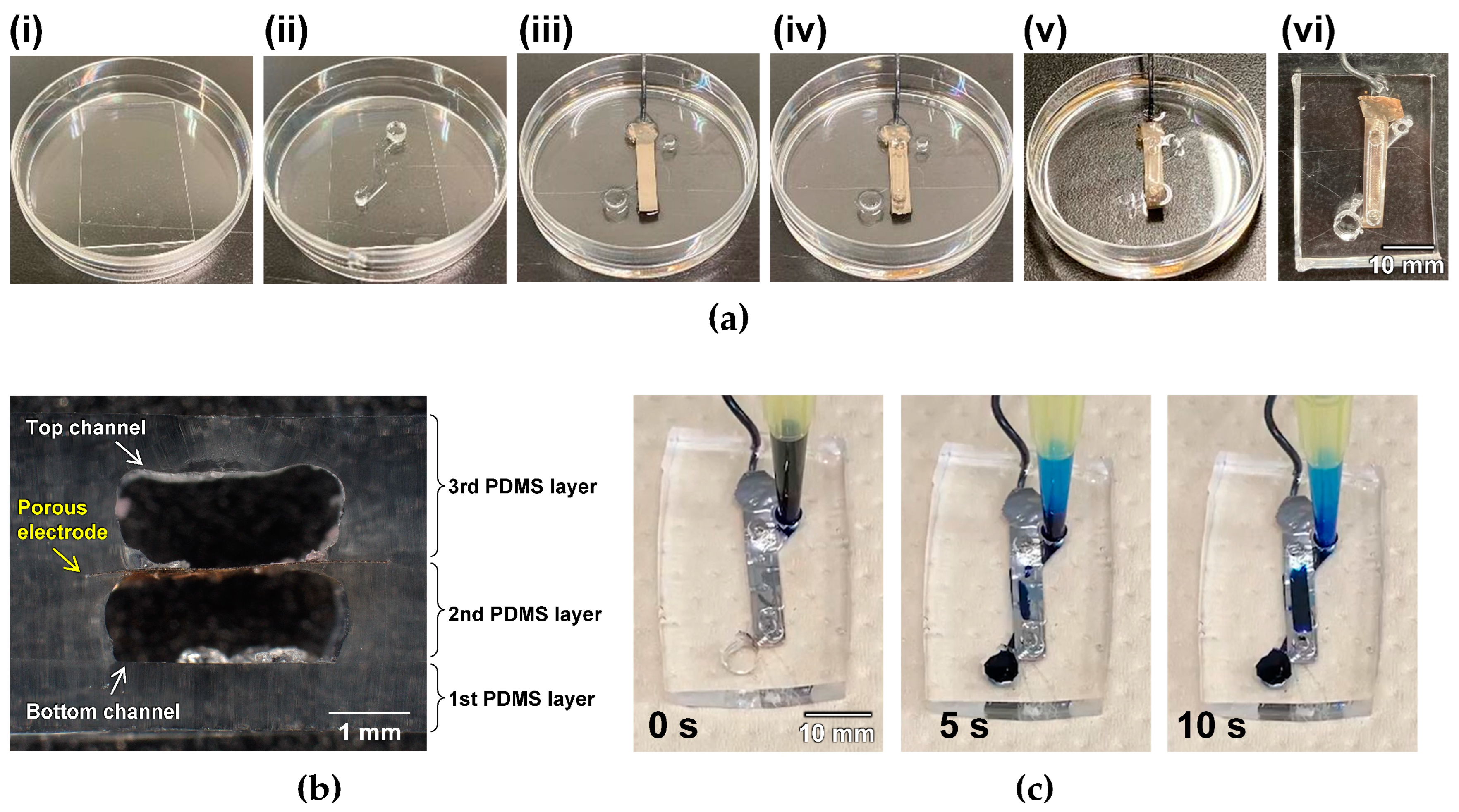
2.1. Device Fabrication
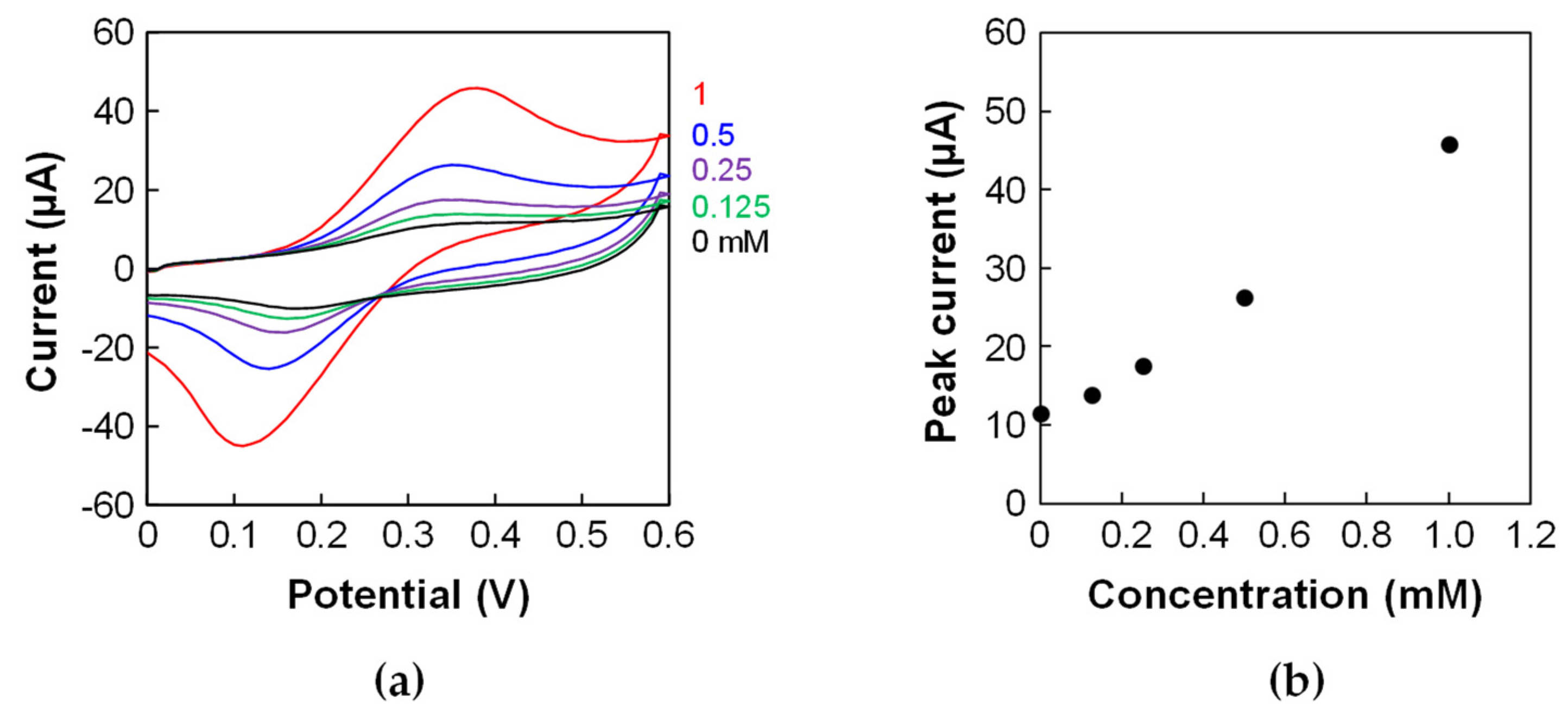
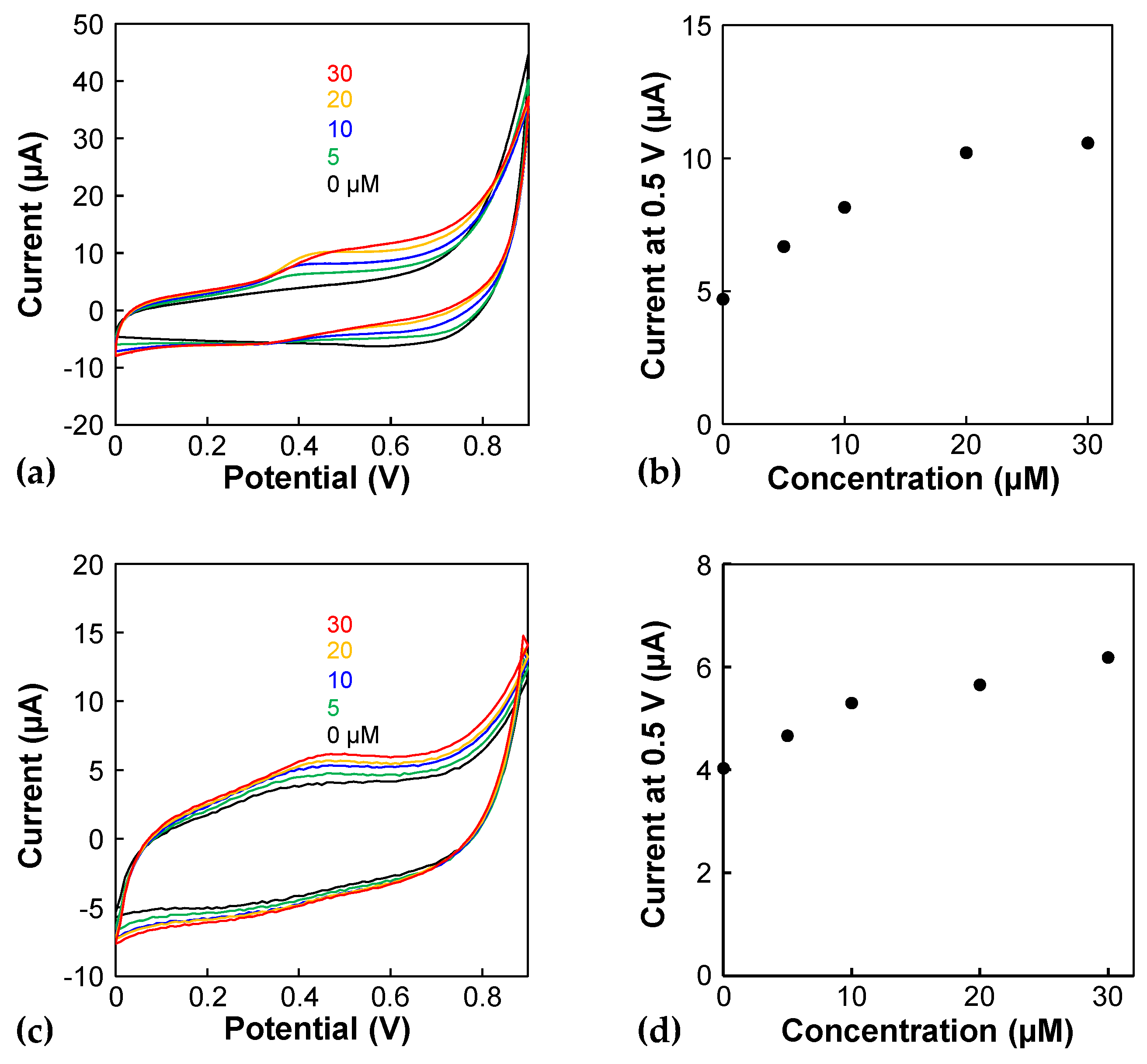
2.2. Cyclic Voltammetry
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ingber, D.E. Human organs-on-chips for disease modelling, drug development and personalized medicine. Nat. Rev. Genet. 2022, 23, 467–491. [Google Scholar] [CrossRef]
- Utagawa, Y.; Ino, K.; Hiramoto, K.; Iwase, K.; Nashimoto, Y.; Honma, I.; Shiku, H. Vasculature-on-a-chip with a porous membrane electrode for in situ electrochemical detection of nitric oxide released from endothelial cells. Anal. Chem. 2023, 95, 18158–18165. [Google Scholar] [CrossRef] [PubMed]
- Chapin, A.A.; Rajasekaran, P.R.; Quan, D.N.; Hu, L.; Herberholz, J.; Bentley, W.E.; Ghodssi, R. Electrochemical measurement of serotonin by Au-CNT electrodes fabricated on microporous cell culture membranes. Microsyst. Nanoeng. 2020, 6, 90. [Google Scholar] [CrossRef]
- Ramiah Rajasekaran, P.; Chapin, A.A.; Quan, D.N.; Herberholz, J.; Bentley, W.E.; Ghodssi, R. 3D-Printed electrochemical sensor-integrated transwell systems. Microsyst. Nanoeng. 2020, 6, 100. [Google Scholar] [CrossRef]
- McDonald, J.C.; Duffy, D.C.; Anderson, J.R.; Chiu, D.T.; Wu, H.K.; Schueller, O.J.A.; Whitesides, G.M. Fabrication of microfluidic systems in poly(dimethylsiloxane). Electrophoresis 2000, 21, 27–40. [Google Scholar] [CrossRef]
- Huh, D.; Hamilton, G.A.; Ingber, D.E. From 3D cell culture to organs-on-chips. Trends Cell Biol. 2011, 21, 745–754. [Google Scholar] [CrossRef]
- Wyatt Shields Iv, C.; Reyes, C.D.; López, G.P. Microfluidic cell sorting: A review of the advances in the separation of cells from debulking to rare cell isolation. Lab Chip 2015, 15, 1230–1249. [Google Scholar] [CrossRef]
- Zhang, Y.S.; Haghiashtiani, G.; Hübscher, T.; Kelly, D.J.; Lee, J.M.; Lutolf, M.; McAlpine, M.C.; Yeong, W.Y.; Zenobi-Wong, M.; Malda, J. 3D extrusion bioprinting. Nat. Rev. Method. Prim. 2021, 1, 75. [Google Scholar] [CrossRef]
- Ino, K.; Fukuda, M.T.; Hiramoto, K.; Taira, N.; Nashimoto, Y.; Shiku, H. Fabrication of three-dimensional calcium alginate hydrogels using sacrificial templates of sugar. J. Biosci. Bioeng. 2020, 130, 539–544. [Google Scholar] [CrossRef]
- Moeun, B.N.; Fernandez, S.A.; Collin, S.; Gauvin-Rossignol, G.; Lescot, T.; Fortin, M.-A.; Ruel, J.; Bégin-Drolet, A.; Leask, R.L.; Hoesli, C.A. Improving the 3D printability of sugar glass to engineer sacrificial vascular templates. 3D Print. Addit. Manuf. 2022, 10, 869–886. [Google Scholar] [CrossRef]
- Homan, K.A.; Kolesky, D.B.; Skylar-Scott, M.A.; Herrmann, J.; Obuobi, H.; Moisan, A.; Lewis, J.A. Bioprinting of 3D convoluted renal proximal tubules on perfusable chips. Sci. Rep. 2016, 6, 34845. [Google Scholar] [CrossRef] [PubMed]
- Utagawa, Y.; Ino, K.; Hiramoto, K.; Shiku, H. Simple, rapid, and large-scale fabrication of multi-branched hydrogels based on viscous fingering for cell culture applications. Macromol. Biosci. 2023, 23, 2300069. [Google Scholar] [CrossRef]
- Abe, H.; Yabu, H. Bio-inspired incrustation interfacial polymerization of dopamine and cross-linking with gelatin toward robust, biodegradable three-dimensional hydrogels. Langmuir 2021, 37, 6201–6207. [Google Scholar] [CrossRef] [PubMed]
- Pan, B.; Shao, L.; Jiang, J.; Zou, S.; Kong, H.; Hou, R.; Yao, Y.; Du, J.; Jin, Y. 3D printing sacrificial templates for manufacturing hydrogel constructs with channel networks. Mater. Des. 2022, 222, 111012. [Google Scholar] [CrossRef]
- Brossard, R.; Brouchet, T.; Malloggi, F. Replication of a printed volatile mold: A novel microfabrication method for advanced microfluidic systems. Sci. Rep. 2019, 9, 17473. [Google Scholar] [CrossRef]
- Li, S.; Li, H.; Shang, X.; He, J.; Hu, Y. Recent advances in 3D printing sacrificial templates for fabricating engineered vasculature. MedComm: Biomater. Appl. 2023, 2, e46. [Google Scholar] [CrossRef]
- Diniz, I.M.; Chen, C.; Xu, X.; Ansari, S.; Zadeh, H.H.; Marques, M.M.; Shi, S.; Moshaverinia, A. Pluronic F-127 hydrogel as a promising scaffold for encapsulation of dental-derived mesenchymal stem cells. J. Mater. Sci. Mater. Med. 2015, 26, 153. [Google Scholar] [CrossRef]
- Zhou, K.; Dey, M.; Ayan, B.; Zhang, Z.; Ozbolat, V.; Kim, M.H.; Khristov, V.; Ozbolat, I.T. Fabrication of PDMS microfluidic devices using nanoclay-reinforced Pluronic F-127 as a sacrificial ink. Biomed. Mater. 2021, 16, 045005. [Google Scholar] [CrossRef]
- Császár, N.; Bókkon, I. Gut serotonin as a general membrane permeability regulator. Curr. Neuropharmacol. 2022, 20, 269–271. [Google Scholar] [CrossRef]
- Wang, Y.; Sims, C.E.; Allbritton, N.L. Enterochromaffin cell-enriched monolayer platform for assaying serotonin release from human primary intestinal cells. Anal. Chem. 2020, 92, 12330–12337. [Google Scholar] [CrossRef]
- Han, J.; Stine, J.M.; Chapin, A.A.; Ghodssi, R. A portable electrochemical sensing platform for serotonin detection based on surface-modified carbon fiber microelectrodes. Anal. Methods 2023, 15, 1096–1104. [Google Scholar] [CrossRef]
- Yasukawa, T.; Nagamine, K.; Horiguchi, Y.; Shiku, H.; Koide, M.; Itayama, T.; Shiraishi, F.; Matsue, T. Electrophoretic cell manipulation and electrochemical gene-function analysis based on a yeast two-hybrid system in a microfluidic device. Anal. Chem. 2008, 80, 3722–3727. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, R.; Suzuki, M.; Yasukawa, T. Electrorotation rates of K562 cells accompanied by erythroid differentiation induced by sodium butyrate. Anal. Sci. 2021, 37, 229–232. [Google Scholar] [CrossRef]
- Onohara, I.; Suzuki, M.; Isozaki, Y.; Tsumoto, K.; Tomita, M.; Yasukawa, T. Electrofusion of cells with different diameters by generating asymmetrical electric field in the microwell array. Anal. Sci. 2022, 38, 235–239. [Google Scholar] [CrossRef] [PubMed]
- Zaman, M.A.; Padhy, P.; Wu, M.; Ren, W.; Jensen, M.A.; Davis, R.W.; Hesselink, L. Controlled transport of individual microparticles using dielectrophoresis. Langmuir 2023, 39, 101–110. [Google Scholar] [CrossRef]
- Ramón-Azcón, J.; Ahadian, S.; Obregón, R.; Camci-Unal, G.; Ostrovidov, S.; Hosseini, V.; Kaji, H.; Ino, K.; Shiku, H.; Khademhosseini, A.; et al. Gelatin methacrylate as a promising hydrogel for 3D microscale organization and proliferation of dielectrophoretically patterned cells. Lab Chip 2012, 12, 2959–2969. [Google Scholar] [CrossRef]
- Taff, B.M.; Voldman, J. A scalable addressable positive-dielectrophoretic cell-sorting array. Anal. Chem. 2005, 77, 7976–7983. [Google Scholar] [CrossRef] [PubMed]
- Yasukawa, T.; Yamada, J.; Shiku, H.; Matsue, T.; Suzuki, M. Microfluidic separation of blood cells based on the negative dielectrophoresis operated by three dimensional microband electrodes. Micromachines 2020, 11, 833. [Google Scholar] [CrossRef]
- Hata, M.; Suzuki, M.; Yasukawa, T. Selective trapping and retrieval of single cells using microwell array devices combined with dielectrophoresis. Anal. Sci. 2021, 37, 803–806. [Google Scholar] [CrossRef]
- Suzuki, M.; Minakuchi, Y.; Mizutani, F.; Yasukawa, T. Discrimination of cell-differentiation using a cell-binding assay based on the conversion of cell-patterns with dielectrophoresis. Biosens. Bioelectron. 2021, 175, 112892. [Google Scholar] [CrossRef]
- Hata, M.; Suzuki, M.; Yasukawa, T. Selective retrieval of antibody-secreting hybridomas in cell arrays based on the dielectrophoresis. Biosens. Bioelectron. 2022, 209, 114250. [Google Scholar] [CrossRef] [PubMed]
- Ino, K.; Nishijo, T.; Arai, T.; Kanno, Y.; Takahashi, Y.; Shiku, H.; Matsue, T. Local redox-cycling-based electrochemical chip device with deep microwells for evaluation of embryoid bodies. Angew. Chem. Int. Ed. 2012, 51, 6648–6652. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ino, K.; Konno, A.; Utagawa, Y.; Kanno, T.; Iwase, K.; Abe, H.; Shiku, H. Fabrication of Two-Layer Microfluidic Devices with Porous Electrodes Using Printed Sacrificial Layers. Micromachines 2024, 15, 1054. https://doi.org/10.3390/mi15081054
Ino K, Konno A, Utagawa Y, Kanno T, Iwase K, Abe H, Shiku H. Fabrication of Two-Layer Microfluidic Devices with Porous Electrodes Using Printed Sacrificial Layers. Micromachines. 2024; 15(8):1054. https://doi.org/10.3390/mi15081054
Chicago/Turabian StyleIno, Kosuke, An Konno, Yoshinobu Utagawa, Taiyo Kanno, Kazuyuki Iwase, Hiroya Abe, and Hitoshi Shiku. 2024. "Fabrication of Two-Layer Microfluidic Devices with Porous Electrodes Using Printed Sacrificial Layers" Micromachines 15, no. 8: 1054. https://doi.org/10.3390/mi15081054
APA StyleIno, K., Konno, A., Utagawa, Y., Kanno, T., Iwase, K., Abe, H., & Shiku, H. (2024). Fabrication of Two-Layer Microfluidic Devices with Porous Electrodes Using Printed Sacrificial Layers. Micromachines, 15(8), 1054. https://doi.org/10.3390/mi15081054






