Three-Dimensionally Printed Microsystems to Facilitate Flow-Based Study of Cells from Neurovascular Barriers of the Retina
Abstract
:1. Introduction
2. Materials and Methods
2.1. Established Glial Line (gLL) Design
2.2. Device Reagents
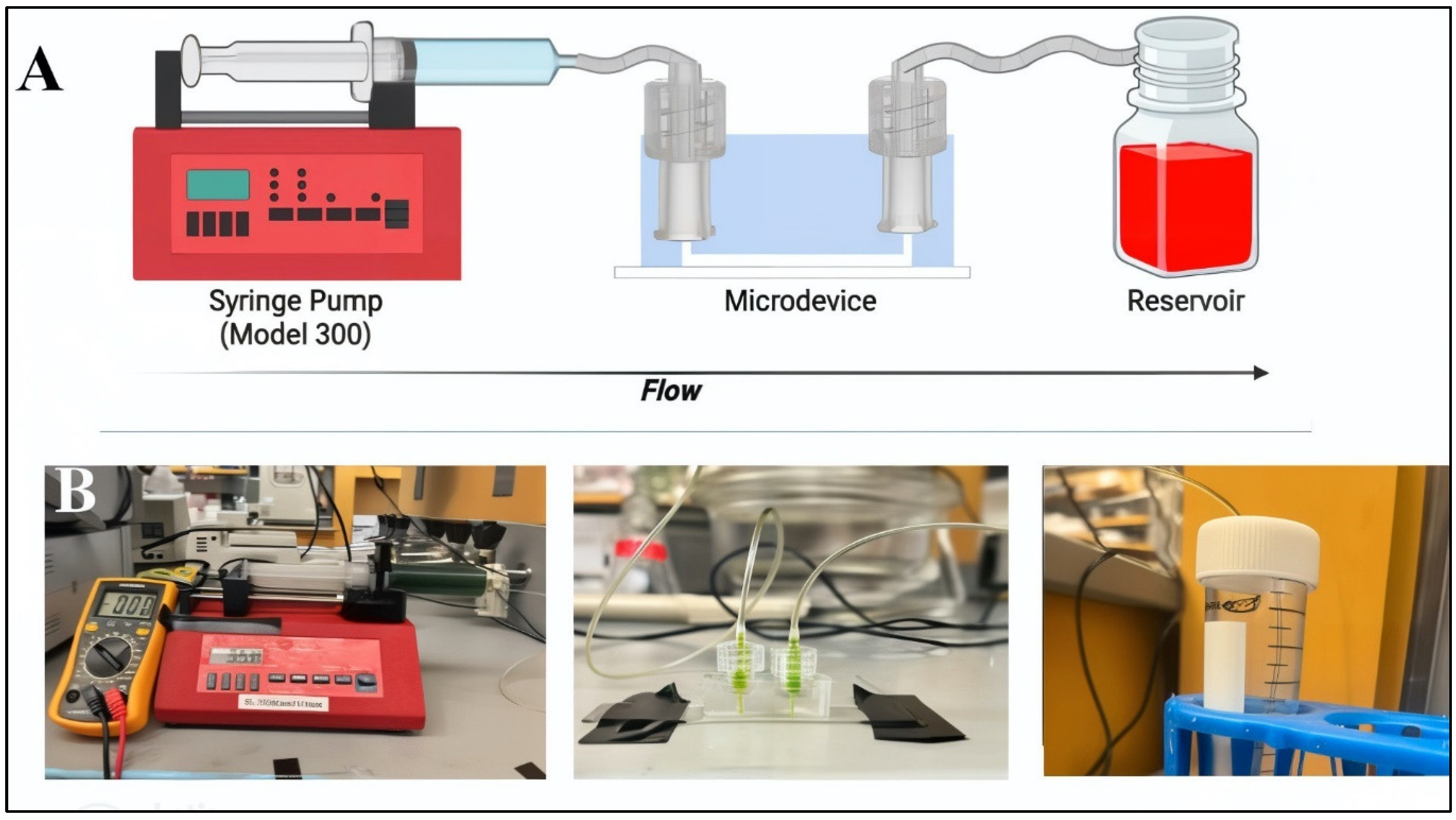
2.3. Modeling and Validation of Flow within Microdevices
2.4. Cell Culture
2.5. Measurement of Cell Viability and Morphology
2.6. Fluorescence, Imaging, and Analysis
2.7. Statistical Analysis
3. Results
3.1. Elastomeric Devices Produced from 3D-Printed Molds Exhibited Greater Variance than Devices Produced from Original Metal Molds
3.2. Elastomers Produced Using 3D-Printed Molds Exhibited Wide Variance in the Average Roughness of Microchannel Inner Surfaces
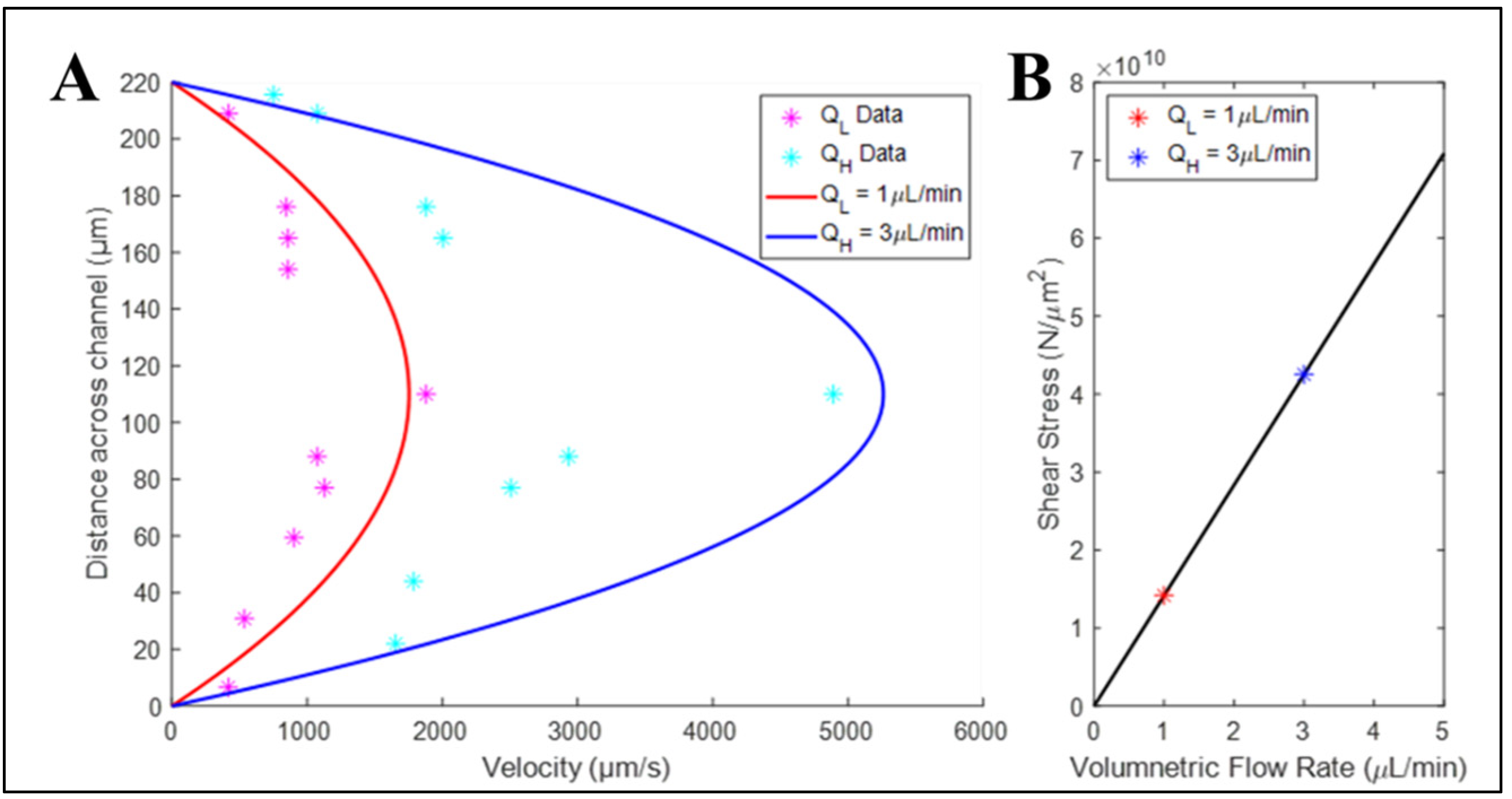
3.3. Bulk Flow within Elastomeric Devices Approached Analytical Flow Solution
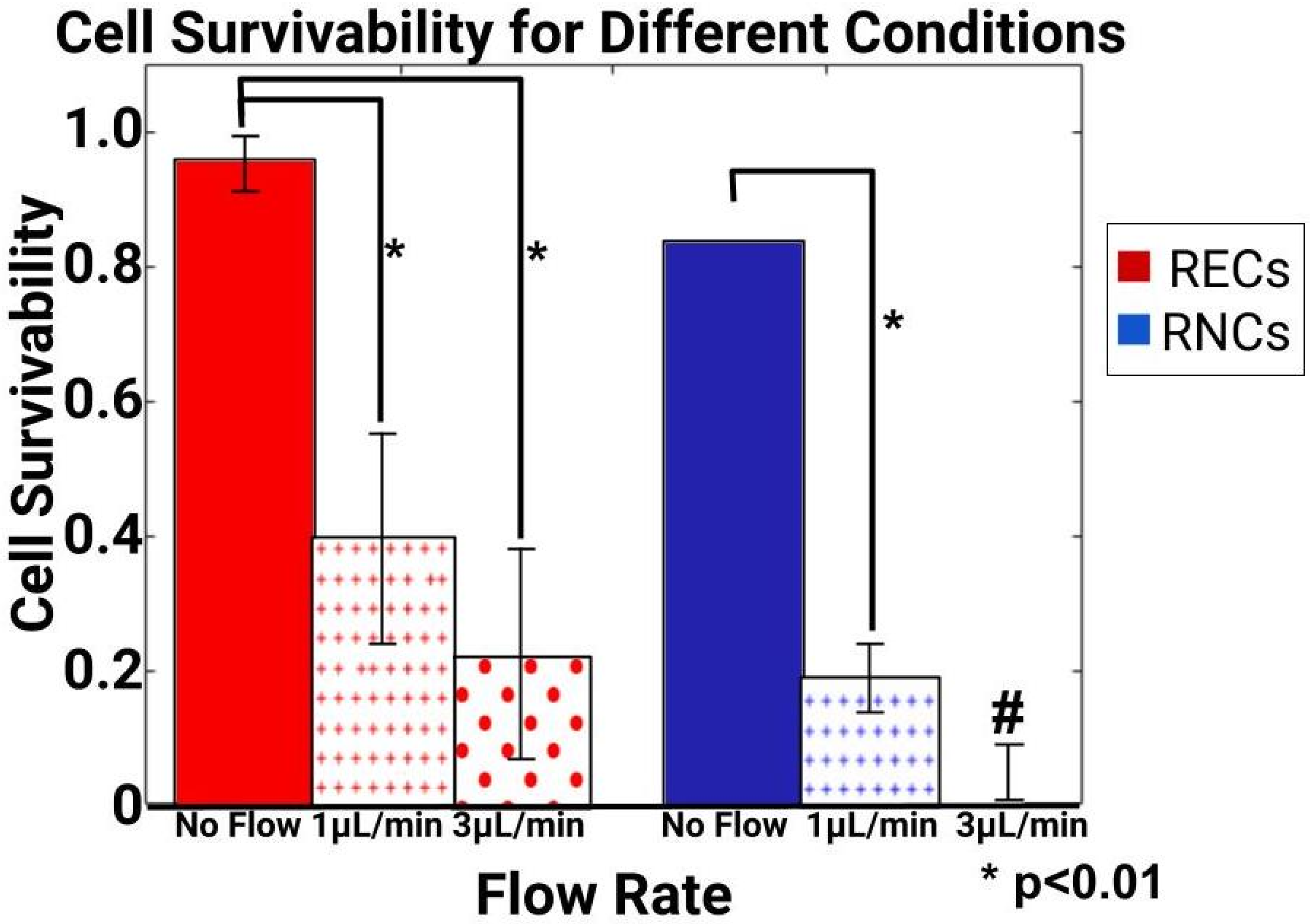
3.4. Volume Flow Rates Produced Different Survival Rates for Retinal Endothelial and Retinal Neural Cells
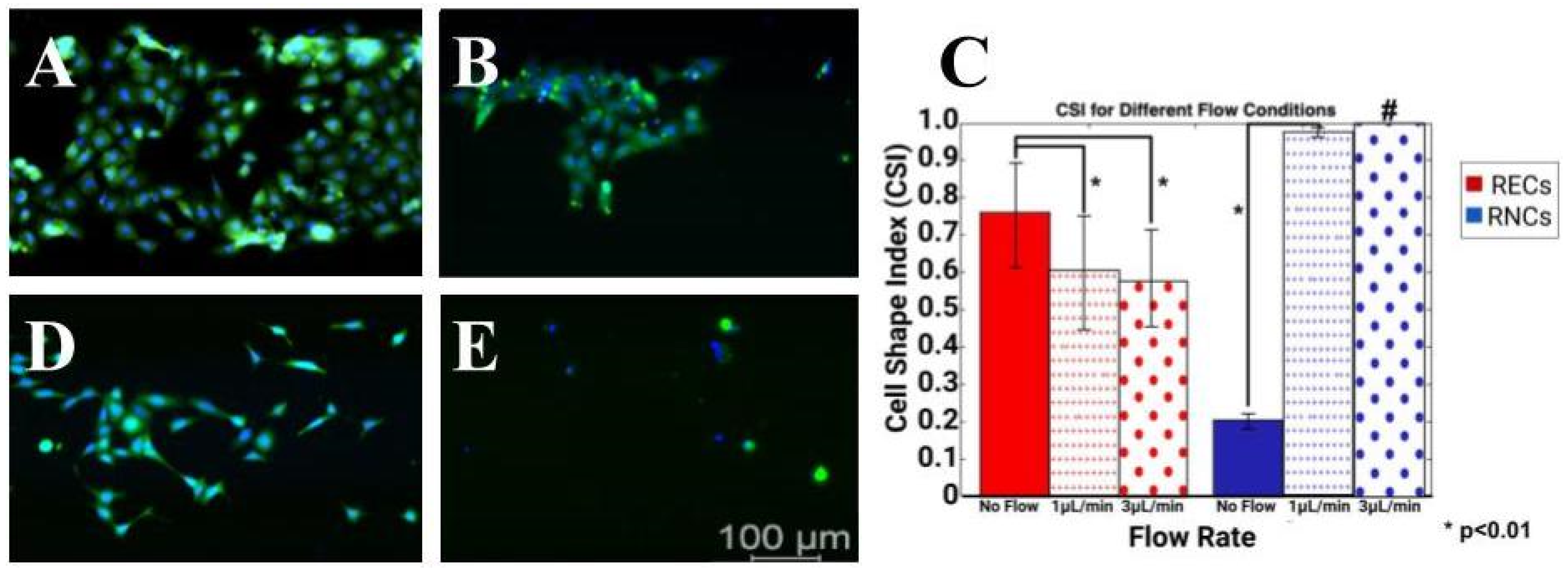
3.5. Induced Flow Rates Produced Distinct Cell Morphology Changes
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ayuso, J.M.; Virumbrales-Munoz, M.; Lang, J.M.; Beebe, D.J. A role for microfluidic systems in precision medicine. Nat. Commun. 2022, 13, 3086. [Google Scholar] [CrossRef] [PubMed]
- Leal-Alves, C.; Deng, Z.; Kermeci, N.; Shih, S.C.C. Integrating microfluidics and synthetic biology: Advancements and diverse applications across organisms. Lab Chip 2024, 24, 2834–2860. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, J.; Chaturvedi, D.; Mishra, S.; Jain, R.; Dandekar, P. Microfluidic technology for cell biology–related applications: A review. J. Biol. Phys. 2023, 50, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Patel, B.B.; Sharifi, F.; Stroud, D.P.; Montazami, R.; Hashemi, N.N.; Sakaguchi, D.S. 3D Microfibrous Scaffolds Selectively Promotes Proliferation and Glial Differentiation of Adult Neural Stem Cells: A Platform to Tune Cellular Behavior in Neural Tissue Engineering. Macromol. Biosci. 2019, 19, e1800236. [Google Scholar] [CrossRef]
- Singh, I.; Lacko, C.S.; Zhao, Z.; Schmidt, C.E.; Rinaldi, C. Preparation and evaluation of microfluidic magnetic alginate microparticles for magnetically templated hydrogels. J. Colloid Interface Sci. 2020, 561, 647–658. [Google Scholar] [CrossRef] [PubMed]
- Vazquez, M. Microfluidic and Microscale Assays to Examine Regenerative Strategies in the Neuro Retina. Micromachines 2020, 11, 1089. [Google Scholar] [CrossRef]
- Babatunde, K.A.; Ayuso, J.M.; Kerr, S.C.; Huttenlocher, A.; Beebe, D.J. Microfluidic Systems to Study Neutrophil Forward and Reverse Migration. Front. Immunol. 2021, 12, 781535. [Google Scholar] [CrossRef]
- Peña, J.S.; Vazquez, M. Harnessing the Neuroprotective Behaviors of Muller Glia for Retinal Repair. Front. Biosci. 2022, 27, 169. [Google Scholar] [CrossRef]
- Ren, J.; Wang, N.; Guo, P.; Fan, Y.; Lin, F.; Wu, J. Recent advances in microfluidics-based cell migration research. Lab Chip 2022, 22, 3361–3376. [Google Scholar] [CrossRef]
- Aydin, O.; Passaro, A.P.; Raman, R.; Spellicy, S.E.; Weinberg, R.P.; Kamm, R.D.; Sample, M.; Truskey, G.A.; Zartman, J.; Dar, R.D.; et al. Principles for the design of multicellular engineered living systems. APL Bioeng. 2022, 6, 010903. [Google Scholar] [CrossRef]
- Doost, N.F.; Srivastava, S.K. A Comprehensive Review of Organ-on-a-Chip Technology and Its Applications. Biosensors 2024, 14, 225. [Google Scholar] [CrossRef]
- Agarwal, A.; Salahuddin, A.; Ahamed, M.J. Demonstration of a Transparent and Adhesive Sealing Top for Microfluidic Lab-Chip Applications. Sensors 2024, 24, 1797. [Google Scholar] [CrossRef] [PubMed]
- Naderi, A.; Bhattacharjee, N.; Folch, A. Digital Manufacturing for Microfluidics. Annu. Rev. Biomed. Eng. 2019, 21, 325–364. [Google Scholar] [CrossRef]
- Yarali, E.; Mirzaali, M.J.; Ghalayaniesfahani, A.; Accardo, A.; Diaz-Payno, P.J.; Zadpoor, A.A. 4D Printing for Biomedical Applications. Adv. Mater. 2024, 36, e2402301. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Kaushal, J.B.; Lee, H.P. Sustainable Sensing with Paper Microfluidics: Applications in Health, Environment, and Food Safety. Biosensors 2024, 14, 300. [Google Scholar] [CrossRef] [PubMed]
- Noviana, E.; Ozer, T.; Carrell, C.S.; Link, J.S.; McMahon, C.; Jang, I.; Henry, C.S. Microfluidic Paper-Based Analytical Devices: From Design to Applications. Chem. Rev. 2021, 121, 11835–11885. [Google Scholar] [CrossRef] [PubMed]
- Hoang, T.; Truong, H.; Han, J.; Lee, S.; Lee, J.; Parajuli, S.; Lee, J.; Cho, G. Room temperature roll-to-roll additive manufacturing of polydimethylsiloxane-based centrifugal microfluidic device for on-site isolation of ribonucleic acid from whole blood. Mater. Today Bio 2023, 23, 100838. [Google Scholar] [CrossRef]
- Zhang, N.; Liu, J.; Zhang, H.; Kent, N.J.; Diamond, D.; Gilchrist, M.D. 3D Printing of Metallic Microstructured Mould Using Selective Laser Melting for Injection Moulding of Plastic Microfluidic Devices. Micromachines 2019, 10, 595. [Google Scholar] [CrossRef]
- Bhattacharjee, N.; Urrios, A.; Kang, S.; Folch, A. The upcoming 3D-printing revolution in microfluidics. Lab Chip 2016, 16, 1720–1742. [Google Scholar] [CrossRef]
- Fleck, E.; Sunshine, A.; DeNatale, E.; Keck, C.; McCann, A.; Potkay, J. Advancing 3D-Printed Microfluidics: Characterization of a Gas-Permeable, High-Resolution PDMS Resin for Stereolithography. Micromachines 2021, 12, 1266. [Google Scholar] [CrossRef] [PubMed]
- Jamal, M.A.; Shah, O.R.; Ghafoor, U.; Qureshi, Y.; Bhutta, M.R. Additive Manufacturing of Continuous Fiber-Reinforced Polymer Composites via Fused Deposition Modelling: A Comprehensive Review. Polymers 2024, 16, 1622. [Google Scholar] [CrossRef] [PubMed]
- Parvanda, R.; Kala, P.; Sharma, V. Bibliometric Analysis-Based Review of Fused Deposition Modeling 3D Printing Method (1994–2020). 3D Print. Addit. Manuf. 2024, 11, 383–405. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, F.A.M.M.; Fonseca, A.C.; Cordeiro, R.; Piedade, A.P.; Faneca, H.; Serra, A.; Coelho, J.F.J. Fabrication of 3D scaffolds based on fully biobased unsaturated polyester resins by microstereo-lithography. Biomed. Mater. 2022, 17, 025010. [Google Scholar] [CrossRef] [PubMed]
- Grygier, D.; Kurzawa, A.; Stachowicz, M.; Krawiec, K.; Stępczak, M.; Roszak, M.; Kazimierczak, M.; Aniszewska, D.; Pyka, D. Investigations into the Material Characteristics of Selected Plastics Manufactured Using SLA-Type Additive Methods. Polymers 2024, 16, 1607. [Google Scholar] [CrossRef]
- Zhang, T.; Shan, W.; Le Dot, M.; Xiao, P. Structural Functions of 3D-Printed Polymer Scaffolds in Regulating cell Fates and Behaviors for Repairing Bone and Nerve Injuries. Macromol. Rapid Commun. 2024, e2400293. [Google Scholar] [CrossRef]
- Azad, M.A.; Olawuni, D.; Kimbell, G.; Badruddoza, A.Z.M.; Hossain, S.; Sultana, T. Polymers for Extrusion-Based 3D Printing of Pharmaceuticals: A Holistic Materials–Process Perspective. Pharmaceutics 2020, 12, 124. [Google Scholar] [CrossRef] [PubMed]
- Del Rosario, M.; Heil, H.S.; Mendes, A.; Saggiomo, V.; Henriques, R. The Field Guide to 3D Printing in Optical Microscopy for Life Sciences. Adv. Biol. 2021, 6, e2100994. [Google Scholar] [CrossRef]
- Saggiomo, V. A 3D Printer in the Lab: Not Only a Toy. Adv. Sci. 2022, 9, e2202610. [Google Scholar] [CrossRef]
- Guo, Y.H.; Lee, C.H.; Yang, Y.T. A Versatile Kit Based on Digital Microfluidics Droplet Actuation for Science Education. J. Vis. Exp. 2021, 14, e61978. [Google Scholar] [CrossRef]
- Lagally, E.T.; Fox, J.A. Jell-O((R)) microfluidics and synthetic biology: Combining science outreach with basic research. Bioanalysis 2010, 2, 1671–1672. [Google Scholar] [CrossRef]
- Sun, M.; Li, Z.; Yang, Q. mudroPi: A Hand-Held Microfluidic Droplet Imager and Analyzer Built on Raspberry Pi. J. Chem. Educ. 2019, 96, 1152–1156. [Google Scholar] [CrossRef]
- Lei, X.; Ye, W.; Safdarin, F.; Baghaei, S. Microfluidics devices for sports: A review on technology for biomedical application used in fields such as biomedicine, drug encapsulation, preparation of nanoparticles, cell targeting, analysis, diagnosis, and cell culture. Tissue Cell 2024, 87, 102339. [Google Scholar] [CrossRef] [PubMed]
- Sonmez, U.M.; Frey, N.; LeDuc, P.R.; Minden, J.S. Fly Me to the Micron: Microtechnologies for Drosophila Research. Annu. Rev. Biomed. Eng. 2024, 26, 441–473. [Google Scholar] [CrossRef]
- Whitesides, G.M.; Ostuni, E.; Takayama, S.; Jiang, X.; Ingber, D.E. Soft lithography in biology and biochemistry. Annu. Rev. Biomed. Eng. 2001, 3, 335–373. [Google Scholar] [CrossRef] [PubMed]
- Ghaznavi, A.; Xu, J.; Hara, S.A. A Non-Sacrificial 3D Printing Process for Fabricating Integrated Micro/Mesoscale Molds. Micromachines 2023, 14, 1363. [Google Scholar] [CrossRef] [PubMed]
- Hosic, S.; Bindas, A.J.; Puzan, M.L.; Lake, W.; Soucy, J.R.; Zhou, F.; Koppes, R.A.; Breault, D.T.; Murthy, S.K.; Koppes, A.N. Rapid Prototyping of Multilayer Microphysiological Systems. ACS Biomater. Sci. Eng. 2020, 7, 2949–2963. [Google Scholar] [CrossRef] [PubMed]
- Morbioli, G.G.; Speller, N.C.; Stockton, A.M. A practical guide to rapid-prototyping of PDMS-based microfluidic devices: A tutorial. Anal. Chim. Acta 2020, 1135, 150–174. [Google Scholar] [CrossRef]
- Rein, C.; Toner, M.; Sevenler, D. Rapid prototyping for high-pressure microfluidics. Sci. Rep. 2023, 13, 1232. [Google Scholar] [CrossRef]
- Zhu, W.; Ma, X.; Gou, M.; Mei, D.; Zhang, K.; Chen, S. 3D printing of functional biomaterials for tissue engineering. Curr. Opin. Biotechnol. 2016, 40, 103–112. [Google Scholar] [CrossRef]
- Herreros-Pomares, A.; Zhou, X.; Calabuig-Fariñas, S.; Lee, S.-J.; Torres, S.; Esworthy, T.; Hann, S.Y.; Jantus-Lewintre, E.; Camps, C.; Zhang, L.G. 3D printing novel in vitro cancer cell culture model systems for lung cancer stem cell study. Mater. Sci. Eng. C 2021, 122, 111914. [Google Scholar] [CrossRef]
- Ouyang, X.; Zhang, K.; Wu, J.; Wong, D.S.; Feng, Q.; Bian, L.; Zhang, A.P. Optical micro-Printing of Cellular-Scale Microscaffold Arrays for 3D Cell Culture. Sci. Rep. 2017, 7, 8880. [Google Scholar] [CrossRef] [PubMed]
- Xue, D.; Wang, Y.; Zhang, J.; Mei, D.; Wang, Y.; Chen, S. Projection-Based 3D Printing of Cell Patterning Scaffolds with Multiscale Channels. ACS Appl. Mater. Interfaces 2018, 10, 19428–19435. [Google Scholar] [CrossRef] [PubMed]
- Winkler, S.; Menke, J.; Meyer, K.V.; Kortmann, C.; Bahnemann, J. Automation of cell culture assays using a 3D-printed servomotor-controlled microfluidic valve system. Lab Chip 2022, 22, 4656–4665. [Google Scholar] [CrossRef]
- Satzer, P.; Achleitner, L. 3D printing: Economical and supply chain independent single-use plasticware for cell culture. New Biotechnol. 2022, 69, 55–61. [Google Scholar] [CrossRef]
- Miller, M.I.; Brightman, A.O.; Epstein, F.H.; Grande-Allen, K.J.; Green, J.J.; Haase, E.; Laurencin, C.T.; Logsdon, E.; Mac Gabhann, F.; Ogle, B.; et al. BME 2.0: Engineering the Future of Medicine. BME Front. 2023, 4, 0001. [Google Scholar] [CrossRef] [PubMed]
- Dhamodaran, K.; Subramani, M.; Ponnalagu, M.; Shetty, R.; Das, D. Ocular stem cells: A status update! Stem Cell Res. Ther. 2014, 5, 56. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.; Ji, J.; Yao, K.; Fu, Q. Regenerative treatment of ophthalmic diseases with stem cells: Principles, progress, and challenges. Adv. Ophthalmol. Pr. Res. 2024, 4, 52–64. [Google Scholar] [CrossRef]
- Dodson, K.H.; Echevarria, F.D.; Li, D.; Sappington, R.M.; Edd, J.F. Retina-on-a-chip: A microfluidic platform for point access signaling studies. Biomed. Microdevices 2015, 17, 114. [Google Scholar] [CrossRef]
- Mut, S.R.; Mishra, S.; Vazquez, M. A Microfluidic Eye Facsimile System to Examine the Migration of Stem-like Cells. Micromachines 2022, 13, 406. [Google Scholar] [CrossRef]
- Pena, C.D.; Zhang, S.; Majeska, R.; Venkatesh, T.; Vazquez, M. Invertebrate Retinal Progenitors as Regenerative Models in a Microfluidic System. Cells 2019, 8, 1301. [Google Scholar] [CrossRef]
- Unachukwu, U.J.; Warren, A.; Li, Z.; Mishra, S.; Zhou, J.; Sauane, M.; Lim, H.; Vazquez, M.; Redenti, S. Predicted molecular signaling guiding photoreceptor cell migration following transplantation into damaged retina. Sci. Rep. 2016, 6, 22392. [Google Scholar] [CrossRef]
- Ottonelli, I.; Caraffi, R.; Tosi, G.; Vandelli, M.A.; Duskey, J.T.; Ruozi, B. Tunneling Nanotubes: A New Target for Nanomedicine? Int. J. Mol. Sci. 2022, 23, 2237. [Google Scholar] [CrossRef]
- Hui, J.; Nie, X.; Wei, P.; Deng, J.; Kang, Y.; Tang, K.; Han, G.; Wang, L.; Liu, W.; Han, Q. 3D printed fibroblast-loaded hydrogel for scleral remodeling to prevent the progression of myopia. J. Mater. Chem. B 2024, 12, 2559–2570. [Google Scholar] [CrossRef]
- Shechter, Y.; Cohen, R.; Namestnikov, M.; Shapira, A.; Barak, A.; Barzelay, A.; Dvir, T. Sequential Fabrication of a Three-Layer Retina-like Structure. Gels 2024, 10, 336. [Google Scholar] [CrossRef]
- Peña, J.S.; Robles, D.; Zhang, S.; Vazquez, M. A Milled Microdevice to Advance Glia-Mediated Therapies in the Adult Nervous System. Micromachines 2019, 10, 513. [Google Scholar] [CrossRef]
- Su, P.-J.; Liu, Z.; Zhang, K.; Han, X.; Saito, Y.; Xia, X.; Yokoi, K.; Shen, H.; Qin, L. Retinal synaptic regeneration via microfluidic guiding channels. Sci. Rep. 2015, 5, 13591. [Google Scholar] [CrossRef]
- Mishra, S.; Thakur, A.; Redenti, S.; Vazquez, M. A model microfluidics-based system for the human and mouse retina. Biomed. Microdevices 2015, 17, 107. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.K.; Sy, K.H.S.; Wong, C.Y.; Man, P.K.; Wong, D.; Shum, H.C. In Vitro Modeling of Emulsification of Silicone Oil as Intraocular Tamponade Using Microengineered Eye-on-a-Chip. Investig. Opthalmol. Vis. Sci. 2015, 56, 3314–3319. [Google Scholar] [CrossRef]
- McCutcheon, S.; Unachukwu, U.; Thakur, A.; Majeska, R.; Redenti, S.; Vazquez, M. In vitro formation of neuroclusters in microfluidic devices and cell migration as a function of stromal-derived growth factor 1 gradients. Cell Adh. Migr. 2017, 11, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lu, Y.; Chen, Q.; Kang, Y.; Yu, L. Probing of peripheral blood mononuclear cells anchoring on TNF-alpha challenged-vascular endothelia in an in vitro model of the retinal microvascular. Biomed. Microdevices 2017, 19, 54. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Vazquez, M. A Gal-MmicroS Device to Evaluate Cell Migratory Response to Combined Galvano-Chemotactic Fields. Biosensors 2017, 7, 54. [Google Scholar] [CrossRef] [PubMed]
- Thakur, A.; Mishra, S.; Pena, J.; Zhou, J.; Redenti, S.; Majeska, R.; Vazquez, M. Collective adhesion and displacement of retinal progenitor cells upon extracellular matrix substrates of transplantable biomaterials. J. Tissue Eng. 2018, 9, 2041731417751286. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Mak, H.K.; Chan, Y.K.; Lin, C.; Kong, C.; Leung, C.K.S.; Shum, H.C. An in vitro pressure model towards studying the response of primary retinal ganglion cells to elevated hydrostatic pressures. Sci. Rep. 2019, 9, 9057. [Google Scholar] [CrossRef]
- Xue, Y.; Seiler, M.J.; Tang, W.C.; Wang, J.Y.; Delgado, J.; McLelland, B.T.; Nistor, G.; Keirstead, H.S.; Browne, A.W. Retinal organoids on-a-chip: A micro-millifluidic bioreactor for long-term organoid maintenance. Lab Chip 2021, 21, 3361–3377. [Google Scholar] [CrossRef]
- Jahagirdar, D.; Yadav, S.; Gore, M.; Korpale, V.; Mathpati, C.S.; Chidambaram, S.; Majumder, A.; Jain, R.; Dandekar, P. Compartmentalized microfluidic device for in vitro co-culture of retinal cells. Biotechnol. J. 2022, 17, e2100530. [Google Scholar] [CrossRef]
- Sun, X.; Cui, Z.; Liang, Y.; Duan, C.; Chan, H.F.; Mao, S.; Gu, J.; Ding, C.; Yang, X.; Wang, Q.; et al. One-stop assembly of adherent 3D retinal organoids from hiPSCs based on 3D-printed derived PDMS microwell platform. Biofabrication 2023, 15, 035005. [Google Scholar] [CrossRef]
- Goodsell, D.S.; Olson, A.J.; Forli, S. Art and Science of the Cellular Mesoscale. Trends Biochem. Sci. 2020, 45, 472–483. [Google Scholar] [CrossRef] [PubMed]
- Tolstik, E.; Lehnart, S.E.; Soeller, C.; Lorenz, K.; Sacconi, L. Cardiac multiscale bioimaging: From nano- through micro- to mesoscales. Trends Biotechnol. 2024, 42, 212–227. [Google Scholar] [CrossRef] [PubMed]
- Hajal, C.; Offeddu, G.S.; Shin, Y.; Zhang, S.; Morozova, O.; Hickman, D.; Knutson, C.G.; Kamm, R.D. Engineered human blood–brain barrier microfluidic model for vascular permeability analyses. Nat. Protoc. 2022, 17, 95–128. [Google Scholar] [CrossRef]
- Diemler, C.A.; MacLean, M.; Heuer, S.E.; Hewes, A.A.; Marola, O.J.; Libby, R.T.; Howell, G.R. Microglia depletion leads to increased susceptibility to ocular hypertension-dependent glaucoma. Front. Aging Neurosci. 2024, 16, 1396443. [Google Scholar] [CrossRef]
- Reddy, S.K.; Devi, V.; Seetharaman, A.T.M.; Shailaja, S.; Bhat, K.M.R.; Gangaraju, R.; Upadhya, D. Cell and molecular targeted therapies for diabetic retinopathy. Front. Endocrinol. 2024, 15, 1416668. [Google Scholar] [CrossRef]
- Shi, L.J.; Ge, H.; Ye, F.; Li, X.; Jiang, Q. The role of pericyte in ocular vascular diseases. J. Biomed. Res. 2024, 38, 1–10. [Google Scholar] [CrossRef]
- Albargothy, M.J.; Azizah, N.N.; Stewart, S.L.; Troendle, E.P.; Steel, D.H.W.; Curtis, T.M.; Taggart, M.J. Investigation of heterocellular features of the mouse retinal neurovascular unit by 3D electron microscopy. J. Anat. 2022, 243, 245–257. [Google Scholar] [CrossRef]
- Yoo, H.-S.; Shanmugalingam, U.; Smith, P.D. Harnessing Astrocytes and Muller Glial Cells in the Retina for Survival and Regeneration of Retinal Ganglion Cells. Cells 2021, 10, 1339. [Google Scholar] [CrossRef]
- Shetty, A.K.; Zanirati, G. The Interstitial System of the Brain in Health and Disease. Aging Dis. 2020, 11, 200–211. [Google Scholar]
- Zhou, H.L.; Jiang, X.Z.; Ventikos, Y. Role of blood flow in endothelial functionality: A review. Front. Cell Dev. Biol. 2023, 11, 1259280. [Google Scholar] [CrossRef]
- Beck, C.; Singh, T.; Farooqi, A.; Venkatesh, T.; Vazquez, M. Controlled microfluidics to examine growth-factor induced migration of neural progenitors in the Drosophila visual system. J. Neurosci. Methods 2016, 262, 32–40. [Google Scholar] [CrossRef]
- Martin, K.A.; Riveros, G.A.; Thornell, T.L.; McClelland, Z.B.; Freeman, E.L.; Stinson, J.T. Thermomechanical Material Characterization of Polyethylene Terephthalate Glycol with 30% Carbon Fiber for Large-Format Additive Manufacturing of Polymer Structures. Polymers 2024, 16, 1913. [Google Scholar] [CrossRef]
- Rivera-Lopez, F.; Pavón, M.M.L.; Correa, E.C.; Molina, M.H. Effects of Nozzle Temperature on Mechanical Properties of Polylactic Acid Specimens Fabricated by Fused Deposition Modeling. Polymers 2024, 16, 1867. [Google Scholar] [CrossRef]
- Kong, Q.; Able, R.A., Jr.; Dudu, V.; Vazquez, M. A microfluidic device to establish concentration gradients using reagent density differences. J. Biomech. Eng. 2010, 132, 121012. [Google Scholar] [CrossRef]
- Seigel, G.M. Review: R28 retinal precursor cells: The first 20 years. Mol. Vis. 2014, 20, 301–306. [Google Scholar]
- Etayo-Escanilla, M.; Campillo, N.; Avila-Fernandez, P.; Baena, J.M.; Chato-Astrain, J.; Campos, F.; Sanchez-Porras, D.; Garcia-Garcia, D.; Carriel, V. Comparison of Printable Biomaterials for Use in Neural Tissue Engineering: An In Vitro Characterization and In Vivo Biocompatibility Assessment. Polymers 2024, 16, 1426. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Liu, T.; Huang, Q.; Wang, Y. From Organ-on-a-Chip to Human-on-a-Chip: A Review of Research Progress and Latest Applications. ACS Sens. 2024, 9, 3466–3488. [Google Scholar] [CrossRef]
- Milton, L.A.; Viglione, M.S.; Ong, L.J.Y.; Nordin, G.P.; Toh, Y.-C. Vat photopolymerization 3D printed microfluidic devices for organ-on-a-chip applications. Lab Chip 2023, 23, 3537–3560. [Google Scholar] [CrossRef]
- Boylin, K.; Aquino, G.V.; Purdon, M.; Abedi, K.; Kasendra, M.; Barrile, R. Basic models to advanced systems: Harnessing the power of organoids-based microphysiological models of the human brain. Biofabrication 2024, 16, 032007. [Google Scholar] [CrossRef]
- Urciuolo, F.; Imparato, G.; Netti, P.A. Engineering Cell Instructive Microenvironments for In Vitro Replication of Functional Barrier Organs. Adv. Healthc. Mater. 2024, 3, e2400357. [Google Scholar] [CrossRef]
- Cheng, L.; Shoma, K.; Suresh, S.; He, H.; Rajput, R.S.; Feng, Q.; Ramesh, S.; Wang, Y.; Krishnan, S.; Ostrovidov, S.; et al. 3D Printing of Micro- and Nanoscale Bone Substitutes: A Review on Technical and Translational Perspectives. Int. J. Nanomed. 2021, 16, 4289–4319. [Google Scholar] [CrossRef]
- Costa, P.; Albers, H.J.; Linssen, J.E.A.; Middelkamp, H.H.T.; Van Der Hout, L.; Passier, R.; van den Berg, A.; Malda, J.; Van Der Meer, A.D. Mimicking arterial thrombosis in a 3D-printed microfluidic in vitro vascular model based on computed tomography angiography data. Lab Chip 2017, 17, 2785–2792. [Google Scholar] [CrossRef]
- Esparza, A.; Jimenez, N.; Joddar, B.; Natividad-Diaz, S. Development of in vitro cardiovascular tissue models within capillary circuit microfluidic devices fabricated with 3D Stereolithography printing. SN Appl. Sci. 2023, 5, 240. [Google Scholar] [CrossRef]
- Desai, T.A.; Eniola-Adefeso, O.; Stevens, K.R.; Vazquez, M.; Imoukhuede, P. Perspectives on Disparities in Scientific Visibility. Nat. Rev. Mater. 2021, 6, 556–559. [Google Scholar] [CrossRef]
- Vazquez, M. Teaching Tips to Enrich Remote Student Engagement in Transport Phenomena Using a Hybrid Teaching and Assessment Model. Biomed. Eng. Educ. 2020, 1, 19–24. [Google Scholar] [CrossRef]
- Deng, K.; Chen, H.; Wei, W.; Wang, X.; Sun, Y. Accuracy of tooth positioning in 3D-printing aided manufactured complete dentures: An in vitro study. J. Dent. 2023, 131, 104459. [Google Scholar] [CrossRef] [PubMed]
- Pham, Y.L.; Beauchamp, J.; Clement, A.; Wiegandt, F.; Holz, O. 3D-printed mouthpiece adapter for sampling exhaled breath in medical applications. 3D Print. Med. 2022, 8, 27. [Google Scholar] [CrossRef] [PubMed]
- Simeon, P.; Unkovskiy, A.; Sarmadi, B.S.; Nicic, R.; Koch, P.J.; Beuer, F.; Schmidt, F. Wear resistance and flexural properties of low force SLA- and DLP-printed splint materials in different printing orientations: An in vitro study. J. Mech. Behav. Biomed. Mater. 2024, 152, 106458. [Google Scholar] [CrossRef] [PubMed]
- Kumar, H.; Kim, K. Stereolithography 3D Bioprinting. Methods Mol. Biol. 2020, 2140, 93–108. [Google Scholar]
- Viray, C.M.; van Magill, B.; Zreiqat, H.; Ramaswamy, Y. Stereolithographic Visible-Light Printing of Poly(l-glutamic acid) Hydrogel Scaffolds. ACS Biomater. Sci. Eng. 2022, 8, 1115–1131. [Google Scholar] [CrossRef]
- Pena, J.; Dulger, N.; Singh, T.; Zhou, J.; Majeska, R.; Redenti, S.; Vazquez, M. Controlled microenvironments to evaluate chemotactic properties of cultured Müller glia. Exp. Eye Res. 2018, 173, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Pena, J.S.; Ramanujam, R.K.; Risman, R.A.; Tutwiler, V.; Berthiaume, F.; Vazquez, M. Neurovascular Relationships in AGEs-Based Models of Proliferative Diabetic Retinopathy. Bioengineering 2024, 11, 63. [Google Scholar] [CrossRef] [PubMed]




| Author (Year) | Fabrication and Material | Cell Type | Application | Ref |
|---|---|---|---|---|
| Su et al. (2015) | Photolithography, PDMS | Retinal Neural Cells (RNCs) | Synaptic Guiding | [56] |
| Mishra et al. (2015) | Photolithography, PDMS | RNCs | Chemotaxis | [57] |
| Chan et al. (2015) 26024114 | Laser Engraving, PMMA | Retinal Ganglion Cells | Drop Delivery | [58] |
| McCutcheon et al. (2017) | Photolithography, PDMS | RNCs | Adhesion, Migration | [59] |
| Li et al. (2017) 28612282 | Photolithography, PDMS | Endothelial Cells | Microvascular | [60] |
| Mishra et al. (2017) | Photolithography, PDMS | RNCs | Electrotaxis | [61] |
| Thakur et al. (2018) | Photolithography, PDMS | RNCs | Adhesion, clustering | [62] |
| Wu et al. (2019) 31227762 | PMMA, Engraving | Retinal Ganglion Cells | Dendritic branching | [63] |
| Pena et al. (2019) | Metal Milling | Muller Glia | Hypertrophy, migration | [55] |
| Xue et al. (2021) 34236056 | Resin stereolithography | Retinal Stem Cells | micro-millifluidic bioreactor | [64] |
| Jahagirdar et al. (2022) 35652558 | PDMS layers, Punching | RNCs | Cell-Cell interactions | [65] |
| Sun et al. (2023) 36963105 | Resin stereolithography | Retinal Stem Cells | Differentiation | [66] |
| Mold Type | Device Microchannel | Device Reservoirs | |||||
|---|---|---|---|---|---|---|---|
| Length (cm) | Height (µm) | Width (µm) | Height-Width Ratio | Hydraulic Diameter (µm) | Height (mm) | Diameter (mm) | |
| Metal | 1.48 ± 0.05 | 239.1 ± 6.75 | 180.7 ± 4.6 | 1.32, Rect. | 205.7 ± 3.07 | 4.10 ± 0.05 | 1.00 ± 0.05 |
| Resin (PLA) | 1.21 ± 0.05 | 250.9 ± 32.8 | 255.2 ± 8.1 | 0.98, Square | 251.9 ± 14.7 | 6.73 ± 0.05 | 1.67 ± 0.05 |
| Plastic (PTEG) | 1.19 ± 0.05 | 398.9 ± 10.8 | 207.8 ± 4.0 | 1.92, Rect. | 273.3 ± 6.0 | 6.21 ± 0.05 | 1.66 ± 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leverant, A.; Oprysk, L.; Dabrowski, A.; Kyker-Snowman, K.; Vazquez, M. Three-Dimensionally Printed Microsystems to Facilitate Flow-Based Study of Cells from Neurovascular Barriers of the Retina. Micromachines 2024, 15, 1103. https://doi.org/10.3390/mi15091103
Leverant A, Oprysk L, Dabrowski A, Kyker-Snowman K, Vazquez M. Three-Dimensionally Printed Microsystems to Facilitate Flow-Based Study of Cells from Neurovascular Barriers of the Retina. Micromachines. 2024; 15(9):1103. https://doi.org/10.3390/mi15091103
Chicago/Turabian StyleLeverant, Adam, Larissa Oprysk, Alexandra Dabrowski, Kelly Kyker-Snowman, and Maribel Vazquez. 2024. "Three-Dimensionally Printed Microsystems to Facilitate Flow-Based Study of Cells from Neurovascular Barriers of the Retina" Micromachines 15, no. 9: 1103. https://doi.org/10.3390/mi15091103
APA StyleLeverant, A., Oprysk, L., Dabrowski, A., Kyker-Snowman, K., & Vazquez, M. (2024). Three-Dimensionally Printed Microsystems to Facilitate Flow-Based Study of Cells from Neurovascular Barriers of the Retina. Micromachines, 15(9), 1103. https://doi.org/10.3390/mi15091103






