A Route to the Colorimetric Detection of Alpha-Fetoprotein Based on a Smartphone
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Instruments
2.2. Conjugation of Colloidal Gold with Antibodies
- (1)
- Firstly, the pipette gun was used to absorb citrate-stabilized AuNPs (diameter = 60 nm, 1 mg/mL) of 200 μL into the centrifuge tube, and then the centrifuge tube was centrifuged with a high-speed centrifuge (parameter: 9300 g, 10 min). Secondly, 150 μL supernatant was removed with a pipette; after this step, 150 μL MPA was added into the centrifuge tube, and then a carboxyl mercaptan layer was introduced with a high-speed centrifuge. Lastly, the sample was left to stand for more than 1 h at 23 °C.
- (2)
- The excess MPA was removed with a high-speed centrifuge, 150 μL of the supernatant was removed after each centrifugation, and the same volume of SPB was put into the centrifuge tube. This step was repeated 2–3 times.
- (3)
- After the last centrifugation, 50 μL EDC and 100 μL NHS were added to the original volume of 200 μL, mixed in, and left for about 30 min.
- (4)
- The excess EDC and NHS were removed with a pipette after centrifugation. This step was repeated 2–3 times. Then, 150 µL of supernatant was absorbed out, and SPB of the same volume was added each time. Then, 2 µL of configured AFP antibody was added after centrifugation, and the centrifuge tube with the sample was placed in a shaking table at 23 °C for 1.5 h.
- (5)
- The excess antibodies were removed after centrifugation twice. Each time, 150 μL of supernatant was taken out. The first time, 150 μL of SPB was added. The second time, 50 μL of SPB was added. The last time, only 50 μL of SPB was added to improve the colloidal gold concentration, since colloidal gold will have a trace loss each time; otherwise, the experimental phenomenon is not obvious.
- (6)
- Finally, the functionalized AuNPs were resuspended in SPB after centrifugation and stored at 4 °C until use.
2.3. AuNPs Surface Immune Reaction
3. Result and Discussion
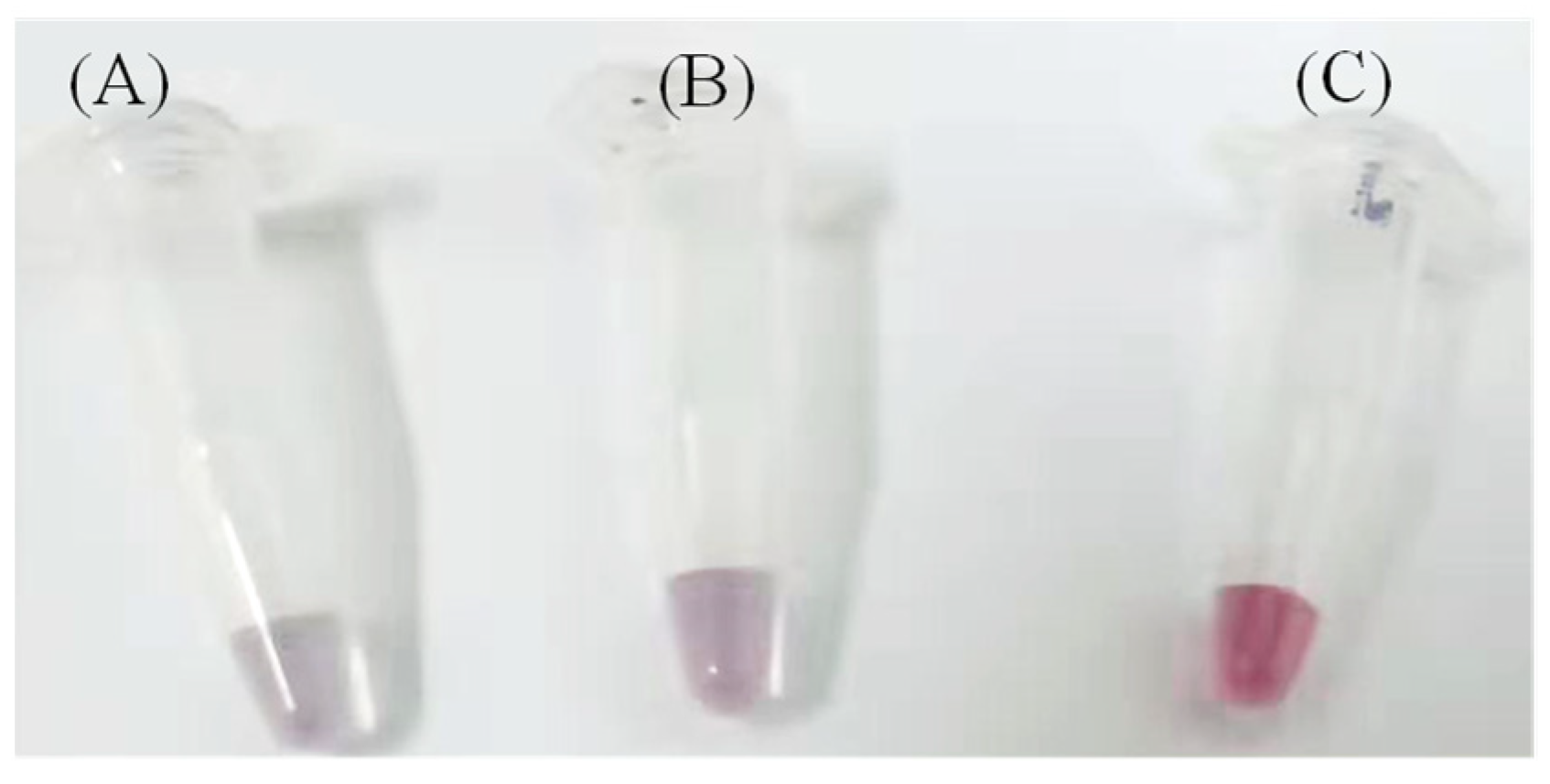
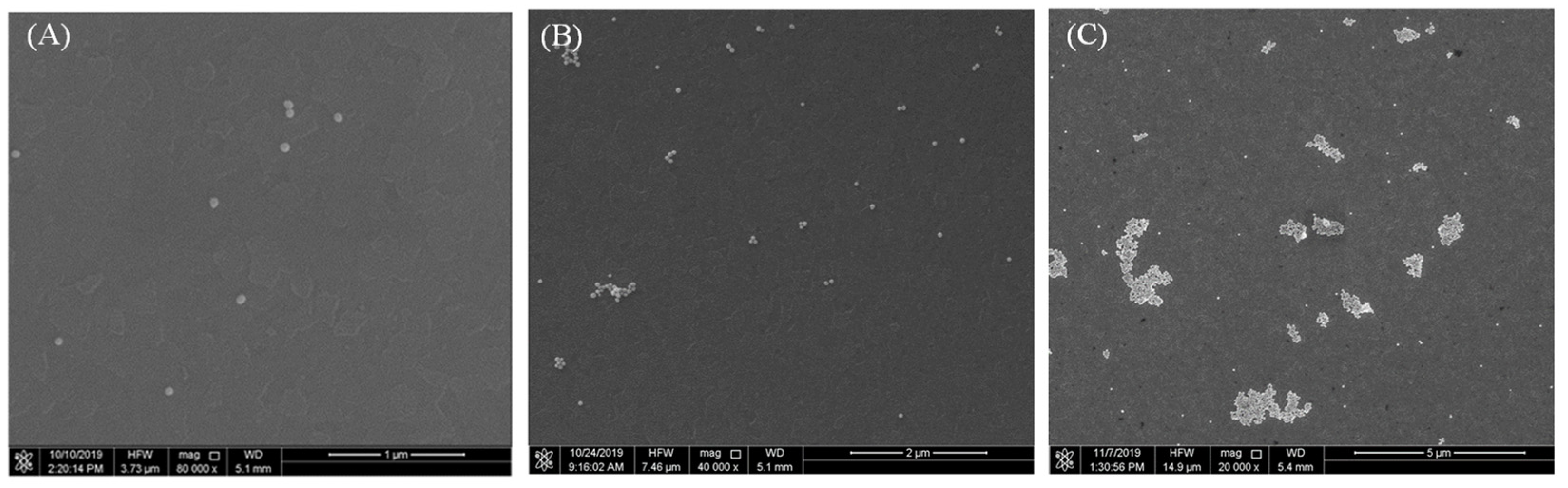
3.1. AuNPs as Colorimetric Agent
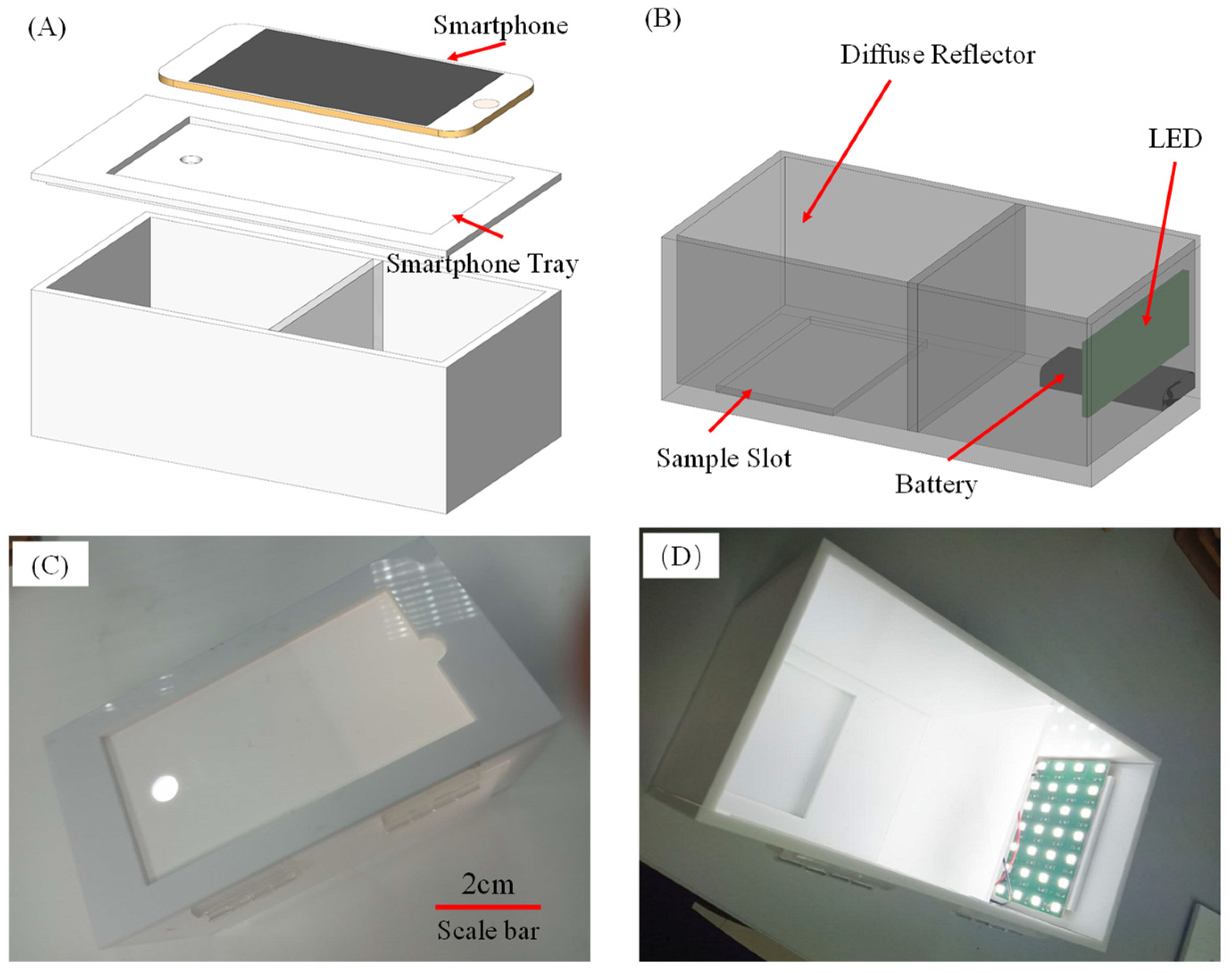
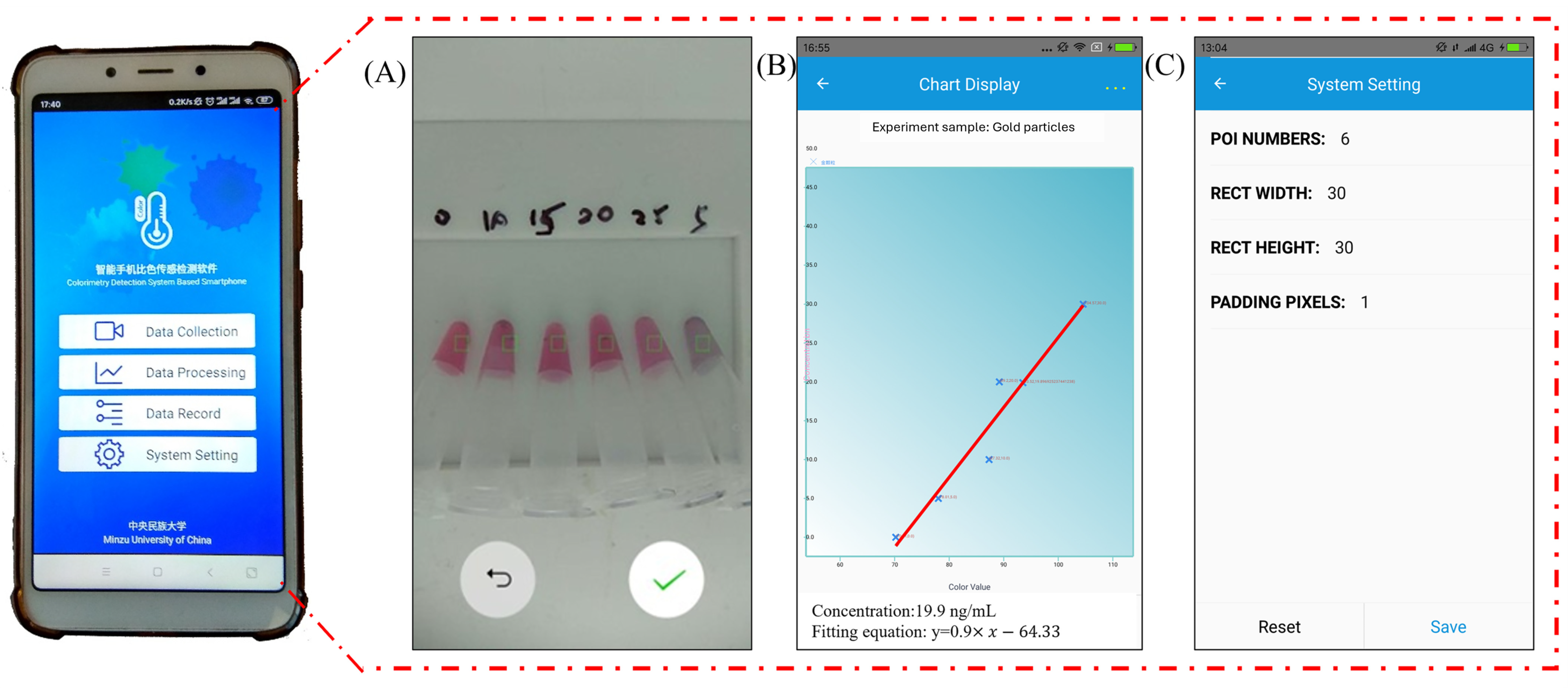
3.2. Improving the Sensitivity and Portability of Digital Image-Based Colorimetry
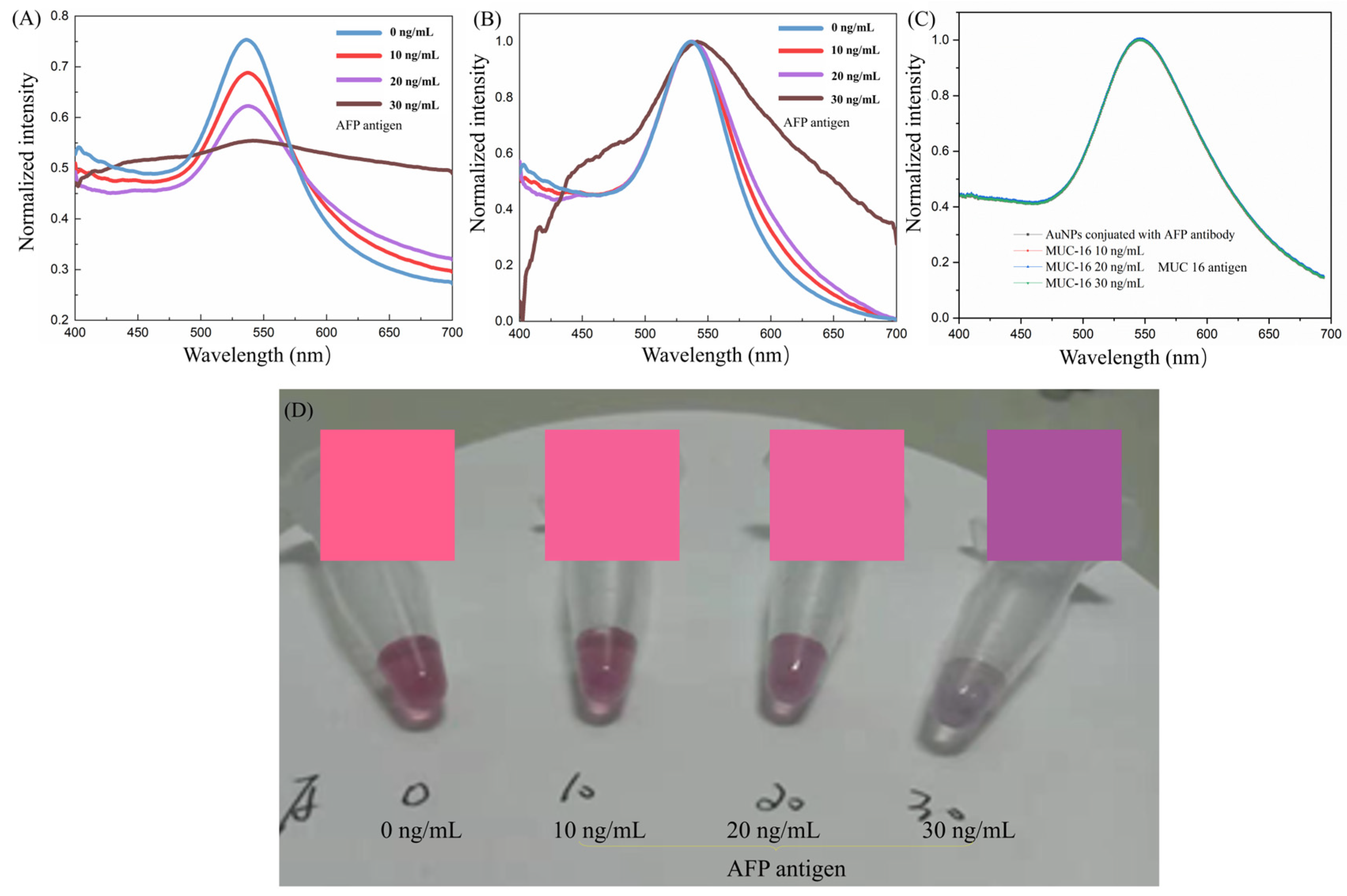
3.3. Sample Testing
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, J.T.; Book, L.; Sudar, K. Serum Alpha Fetoprotein (AFP) Levels in Normal Infants. Pediatr. Res. 1981, 15, 50–52. [Google Scholar] [CrossRef] [PubMed]
- Yeo, Y.H.; Lee, Y.-T.; Tseng, H.-R.; Zhu, Y.; You, S.; Agopian, V.G.; Yang, J.D. Alpha-Fetoprotein: Past, Present, and Future. Hepatol. Commun. 2024, 8, e0422. [Google Scholar] [CrossRef]
- Smith, C.J.P.; Kelleher, P.C. Alpha-Fetoprotein Molecular Heterogeneity: Physiologic Correlations with Normal Growth, Carcinogenesis and Tumor Growth. Biochim. Biophys. Acta (BBA)-Rev. Cancer 1980, 605, 1–32. [Google Scholar] [CrossRef]
- Glowska-Ciemny, J.; Pankiewicz, J.; Malewski, Z.; Von Kaisenberg, C.; Kocylowski, R. Alpha-Fetoprotein (AFP)—New Aspects of a Well-Known Marker in Perinatology. Ginekol. Pol. 2022, 93, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Sauzay, C.; Petit, A.; Bourgeois, A.-M.; Barbare, J.-C.; Chauffert, B.; Galmiche, A.; Houessinon, A. Alpha-Foetoprotein (AFP): A Multi-Purpose Marker in Hepatocellular Carcinoma. Clin. Chim. Acta 2016, 463, 39–44. [Google Scholar] [CrossRef]
- Mizejewski, G.J. Physiology of Alpha-Fetoprotein as a Biomarker for Perinatal Distress: Relevance to Adverse Pregnancy Outcome. Exp. Biol. Med. 2007, 232, 993–1004. [Google Scholar] [CrossRef]
- Matonóg, A.; Drosdzol-Cop, A. Alpha-Fetoprotein Level in Fetuses, Infants, and Children with Ovarian Masses: A Literature Review. Front. Endocrinol. 2024, 15, 1307619. [Google Scholar] [CrossRef]
- Kew, M. Alpha-Fetoprotein in Primary Liver Cancer and Other Diseases. Gut 1974, 15, 814–821. [Google Scholar] [CrossRef]
- Warsinke, A. Point-of-Care Testing of Proteins. Anal. Bioanal. Chem. 2009, 393, 1393–1405. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, P.; Arora, B.; Saxena, S.; Singh, S.; Palkar, P.; Goda, J.S.; Banerjee, R. Paper-Based Point of Care Diagnostics for Cancer Biomarkers. Sens. Diagn. 2024, 3, 504–535. [Google Scholar] [CrossRef]
- Jin, B.; Zhang, C.; Ma, C.; Yin, H.; Li, S.; Du, Z.; Zhao, G.; Huang, H.; Li, Z. Innovative Strategies and Approaches for Enhancing Performance in Optical Probe-Based Biosensors for Point-of-Care Testing. TrAC Trends Anal. Chem. 2024, 176, 117775. [Google Scholar] [CrossRef]
- Yang, S.-M.; Lv, S.; Zhang, W.; Cui, Y. Microfluidic Point-of-Care (POC) Devices in Early Diagnosis: A Review of Opportunities and Challenges. Sensors 2022, 22, 1620. [Google Scholar] [CrossRef] [PubMed]
- Antabe, R.; Ziegler, B.R. Diseases, Emerging and Infectious. Int. Encycl. Hum. Geogr. 2020, 389–391. [Google Scholar] [CrossRef]
- Zhang, Y.-P.; Bu, J.-W.; Shu, R.-X.; Liu, S.-L. Advances in Rapid Point-of-Care Virus Testing. Analyst 2024, 149, 2507–2525. [Google Scholar] [CrossRef] [PubMed]
- Stranieri, A.; Venkatraman, S.; Minicz, J.; Zarnegar, A.; Firmin, S.; Balasubramanian, V.; Jelinek, H.F. Emerging Point of Care Devices and Artificial Intelligence: Prospects and Challenges for Public Health. Smart Health 2022, 24, 100279. [Google Scholar] [CrossRef]
- Sachdeva, A. Point-of-Care Diagnosis in Pediatric Practice. Indian Pediatr. 2017, 54, 539–540. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-J.; Chang, Y.-C.; Sun, R.; Li, L. A Multichannel Smartphone Optical Biosensor for High-Throughput Point-of-Care Diagnostics. Biosens. Bioelectron. 2017, 87, 686–692. [Google Scholar] [CrossRef]
- Son, M.H.; Park, S.W.; Sagong, H.Y.; Jung, Y.K. Recent Advances in Electrochemical and Optical Biosensors for Cancer Biomarker Detection. BioChip J. 2023, 17, 44–67. [Google Scholar] [CrossRef]
- Zong, H.; Zhang, Y.; Liu, X.; Xu, Z.; Ye, J.; Lu, S.; Guo, X.; Yang, Z.; Zhang, X.; Chai, M.; et al. Recent Trends in Smartphone-Based Optical Imaging Biosensors for Genetic Testing: A Review. VIEW 2023, 4, 20220062. [Google Scholar] [CrossRef]
- Pour, S.R.S.; Calabria, D.; Emamiamin, A.; Lazzarini, E.; Pace, A.; Guardigli, M.; Zangheri, M.; Mirasoli, M. Electrochemical vs. Optical Biosensors for Point-of-Care Applications: A Critical Review. Chemosensors 2023, 11, 546. [Google Scholar] [CrossRef]
- Geng, Z.; Zhang, X.; Fan, Z.; Lv, X.; Su, Y.; Chen, H. Recent Progress in Optical Biosensors Based on Smartphone Platforms. Sensors 2017, 17, 2449. [Google Scholar] [CrossRef] [PubMed]
- Chu, S.-L.; Sudo, K.; Yokota, H.; Abe, K.; Nakamura, Y.; Tsai, M.-D. Human Induced Pluripotent Stem Cell Formation and Morphology Prediction during Reprogramming with Time-Lapse Bright-Field Microscopy Images Using Deep Learning Methods. Comput. Methods Programs Biomed. 2023, 229, 107264. [Google Scholar] [CrossRef]
- Jia, X.; Ma, P.; Tarwa, K.; Wang, Q. Machine Vision-Based Colorimetric Sensor Systems for Food Applications. J. Agric. Food Res. 2023, 11, 100503. [Google Scholar] [CrossRef]
- Singh, R.; Mehra, R.; Walia, A.; Gupta, S.; Chawla, P.; Kumar, H.; Thakur, A.; Kaushik, R.; Kumar, N. Colorimetric Sensing Approaches Based on Silver Nanoparticles Aggregation for Determination of Toxic Metal Ions in Water Sample: A Review. Int. J. Environ. Anal. Chem. 2023, 103, 1361–1376. [Google Scholar] [CrossRef]
- Nietzold, C.; Lisdat, F. Fast Protein Detection Using Absorption Properties of Gold Nanoparticles. Analyst 2012, 137, 2821. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Qin, L.; Liu, D.; Liu, C.; Sun, Y.; Duan, Y. Fast Detection of Alpha-Fetoprotein-L3 Using Lens Culinaris Agglutinin Immobilized Gold Nanoparticles. J. Nanosci. Nanotechnol. 2014, 14, 4078–4081. [Google Scholar] [CrossRef]
- Xiao, Q.; Zheng, Y.; Liu, J.; Wang, S.; Feng, B. Enzyme–Antibody Dual-Film Modified Gold Nanoparticle Probe for Ultrasensitive Detection of Alpha Fetoprotein. Biologicals 2017, 47, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Aydindogan, E.; Ceylan, A.E.; Timur, S. Paper-Based Colorimetric Spot Test Utilizing Smartphone Sensing for Detection of Biomarkers. Talanta 2020, 208, 120446. [Google Scholar]
- Lu, X.; Mei, T.; Guo, Q.; Zhou, W.; Li, X.; Chen, J.; Zhou, X.; Sun, N.; Fang, Z. Improved Performance of Lateral Flow Immunoassays for Alpha-Fetoprotein and Vanillin by Using Silica Shell-Stabilized Gold Nanoparticles. Microchim. Acta 2018, 186, 2. [Google Scholar] [CrossRef]
- Zare, I.; Yaraki, M.T.; Speranza, G.; Najafabadi, A.H.; Shourangiz-Haghighi, A.; Nik, A.B.; Manshian, B.B.; Saraiva, C.; Soenen, S.J.; Kogan, M.J.; et al. Gold Nanostructures: Synthesis, Properties, and Neurological Applications. Chem. Soc. Rev. 2022, 51, 2601–2680. [Google Scholar] [CrossRef]
- Xu, R. Progress in Nanoparticles Characterization: Sizing and Zeta Potential Measurement. Particuology 2008, 6, 112–115. [Google Scholar] [CrossRef]
- Lunardi, C.N.; Gomes, A.J.; Rocha, F.S.; De Tommaso, J.; Patience, G.S. Experimental Methods in Chemical Engineering: Zeta Potential. Can. J. Chem. Eng. 2021, 99, 627–639. [Google Scholar] [CrossRef]
- Tantra, R.; Schulze, P.; Quincey, P. Effect of Nanoparticle Concentration on Zeta-Potential Measurement Results and Reproducibility. Particuology 2010, 8, 279–285. [Google Scholar] [CrossRef]
- Long, G.L.; Winefordner, J.D. Limit of Detection A Closer Look at the IUPAC Definition. Anal. Chem. 1983, 55, 712A–724A. [Google Scholar]
- Liu, J.; Cui, D.; Jiang, Y.; Li, Y.; Liu, Z.; Tao, L.; Zhao, Q.; Diao, A. Selection and Characterization of a Novel Affibody Peptide and Its Application in a Two-Site ELISA for the Detection of Cancer Biomarker 4Alpha-Fetoprotein. Int. J. Biol. Macromol. 2021, 166, 884–892. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Jiang, X.; Xue, J.; Zhou, Z.; Zhou, J. Antibody Modified Gold Nano-Mushroom Arrays for Rapid Detection of Alpha-Fetoprotein. Biosens. Bioelectron. 2015, 68, 468–474. [Google Scholar] [CrossRef]
- Chen, C.; Zhao, J.; Lu, Y.; Sun, J. Fluorescence Immunoassay Based on the Phosphate-Triggered Fluorescence Turn-on Detection of Alkaline Phosphatase. Anal. Chem. 2018, 90, 3505–3511. [Google Scholar] [CrossRef]
| AFP Concentration (ng/mL) | Testing Result (ng/mL) | Deviation Value (ng/mL) | Accuracy |
|---|---|---|---|
| 50 | 56.2 | 6.2 | 87.6% |
| 100 | 107.2 | 7.2 | 92.8% |
| 150 | 162.3 | 12.3 | 91.8% |
| 200 | 217.9 | 17.9 | 91.05% |
| Method | Detection Element | Linear Range | LOD | Sample | Ref |
|---|---|---|---|---|---|
| Paper-based colorimetry | Antibody | 0.1–100 ng/mL | 1.054 ng/mL | Serum | [33] |
| Two-site ELISA # | Affibody | 6–100 ng/mL | 2 ng/mL | Serum | [34] |
| LSPR combined immunoassay | Antibody | 20–200 ng/mL | 24 ng/mL | Serum | [28] |
| Fluorescence immunoassay | Antibody | 1.0–4.0 ng/mL | 41 pg/mL | Serum | [35] |
| Photoelectrochemical immunoassay | Antibody | 0.01–50 ng/mL | 1.2 pg/mL | Serum | [36] |
| Colorimetric ELISA | Antibody | 15–600 ng/mL | 0.82 ng/mL | Serum | [37] |
| Smartphone colorimetry | Antigen | 10–300 ng/mL | 0.083 ng/mL | Serum | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Geng, Q.; Geng, Z. A Route to the Colorimetric Detection of Alpha-Fetoprotein Based on a Smartphone. Micromachines 2024, 15, 1116. https://doi.org/10.3390/mi15091116
Liu J, Geng Q, Geng Z. A Route to the Colorimetric Detection of Alpha-Fetoprotein Based on a Smartphone. Micromachines. 2024; 15(9):1116. https://doi.org/10.3390/mi15091116
Chicago/Turabian StyleLiu, Junjie, Qingfubo Geng, and Zhaoxin Geng. 2024. "A Route to the Colorimetric Detection of Alpha-Fetoprotein Based on a Smartphone" Micromachines 15, no. 9: 1116. https://doi.org/10.3390/mi15091116






