Silver Thin-Film Plated Interconnected Metal Mesh Networks for Virus Detection and Prevention
Abstract
1. Introduction
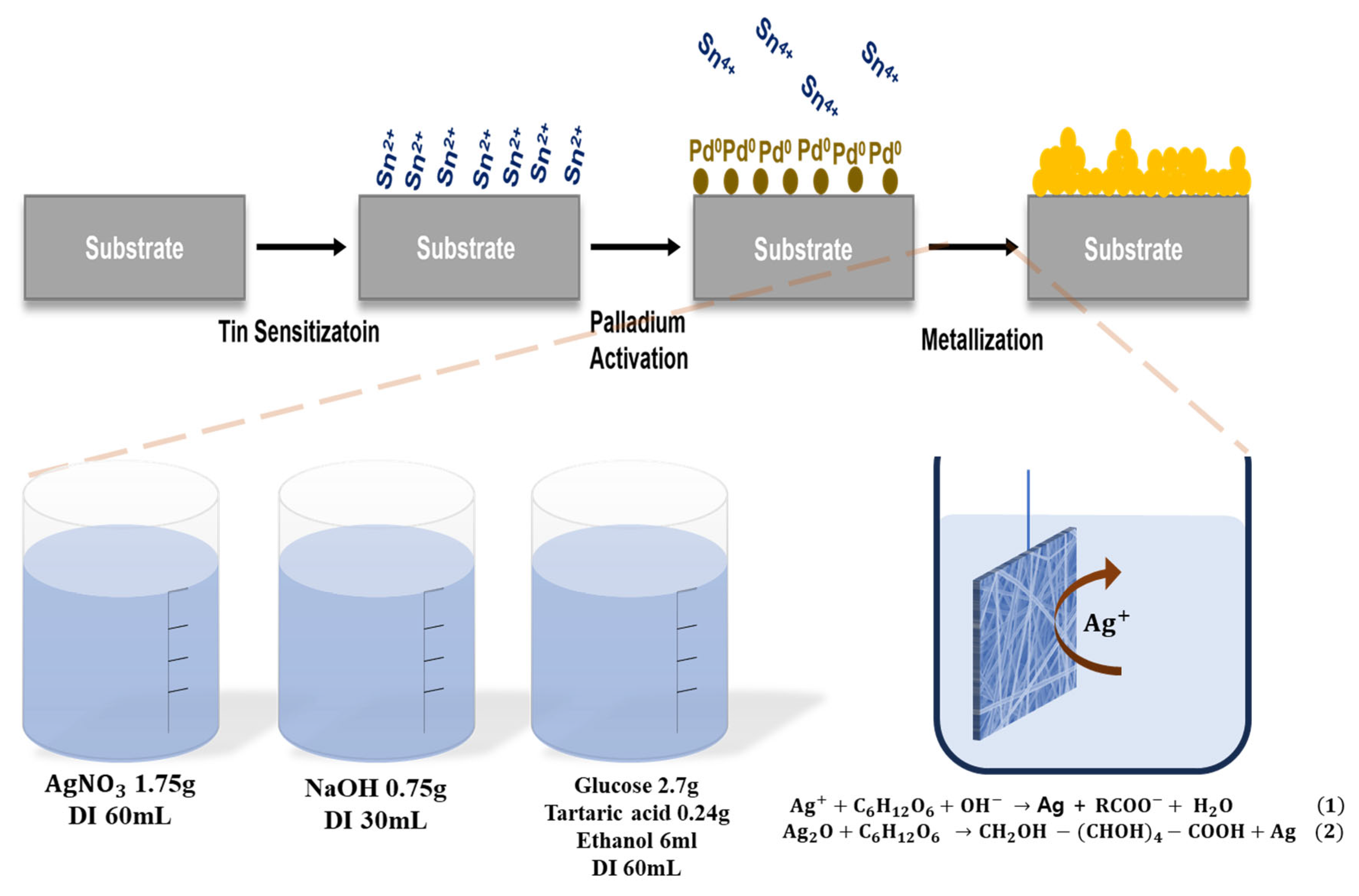
2. Materials and Methods
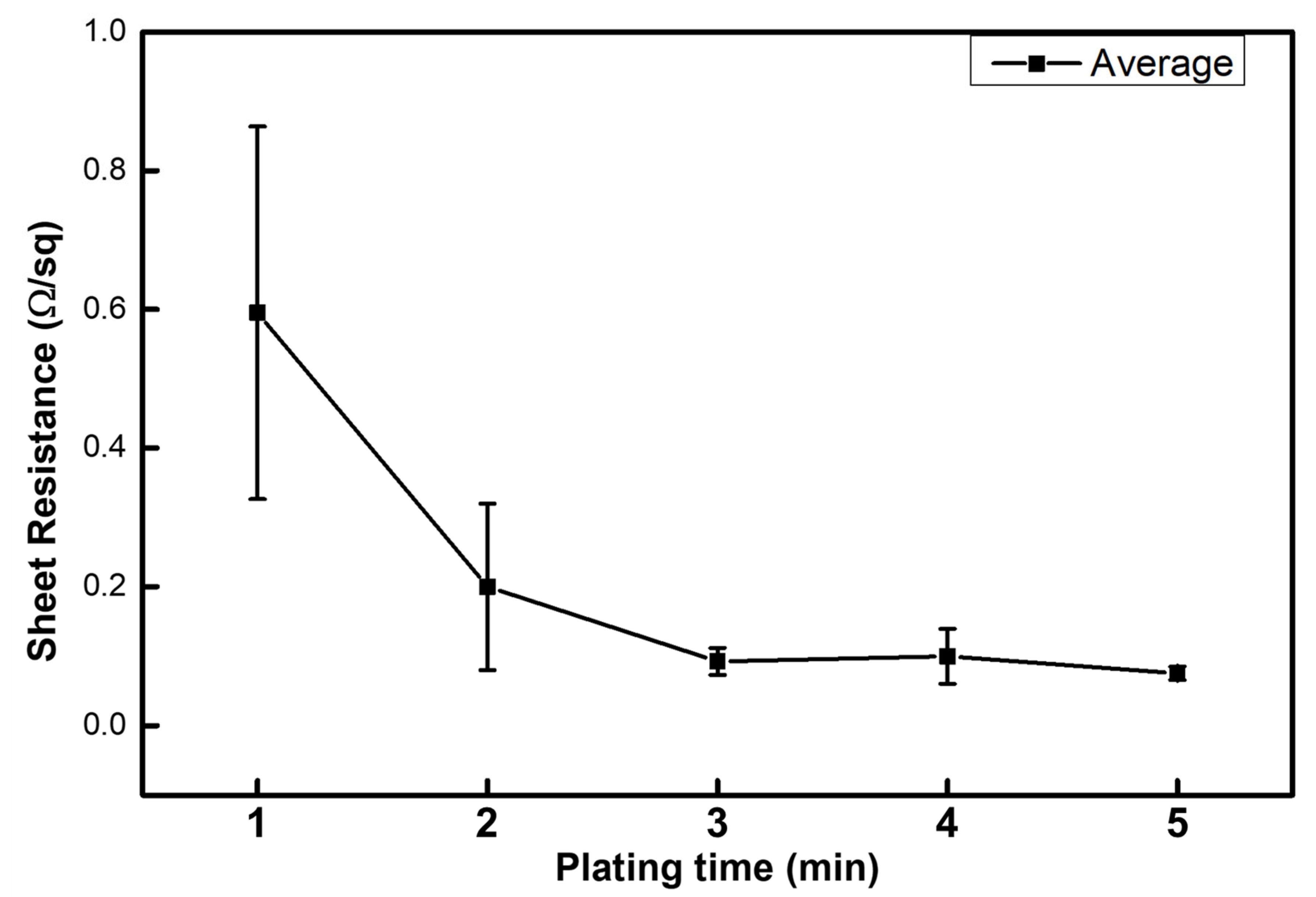
3. Results and Discussion
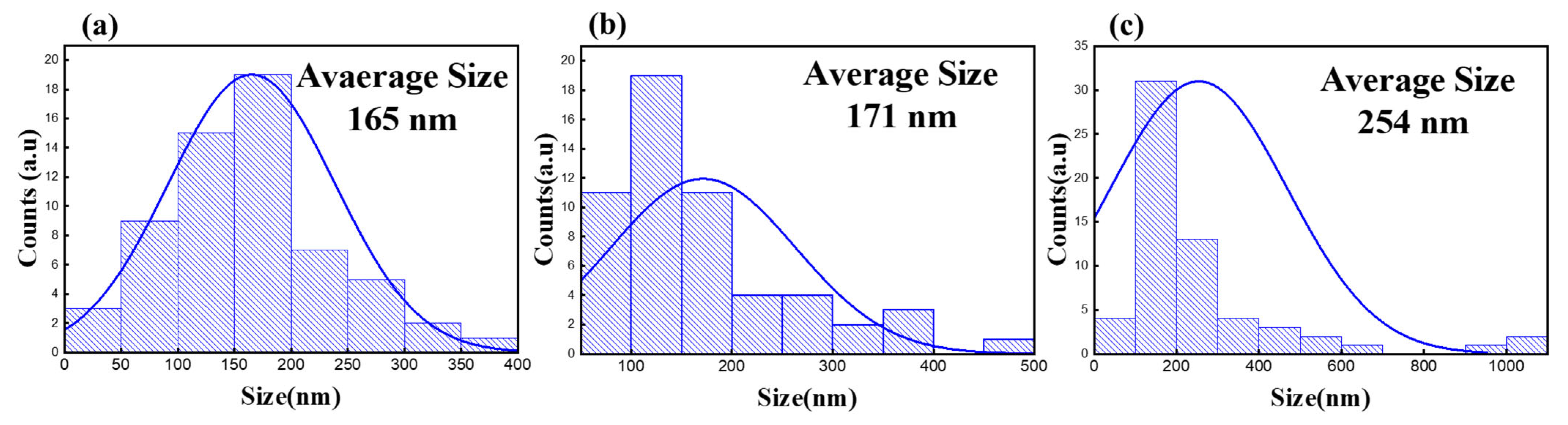
3.1. PP Filter ELP
3.2. Variation in the Pollen Surface
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- World Health Organization 2023 data.who.int, WHO Coronavirus (COVID-19) Dashboard > Cases. Available online: https://data.who.int/dashboards/covid19/cases (accessed on 15 October 2025).
- Wang, J.; Du, G. COVID-19 may transmit through aerosol. Ir. J. Med. Sci. 2020, 189, 1143–1144. [Google Scholar] [CrossRef] [PubMed]
- Pardi, N.; Hogan, M.J.; Porter, F.W.; Weissman, D. mRNA vaccines—A new era in vaccinology. Nat. Rev. Drug Discov. 2018, 17, 261–279. [Google Scholar] [CrossRef] [PubMed]
- Cagigi, A.; Douradinha, B. Have mRNA vaccines sentenced DNA vaccines to death? Expert Rev. Vaccines 2023, 22, 1154–1167. [Google Scholar] [CrossRef] [PubMed]
- Brown, H.M.; Irving, K.R. The size and weight of common allergenic pollens. Allergy 1973, 28, 132–137. [Google Scholar] [CrossRef]
- Taylor, P.E.; Flagan, R.C.; Valenta, R.; Glovsky, M.M. Release of allergens as respirable aerosols: A link between grass pollen and asthma. J. Allergy Clin. Immunol. 2002, 109, 51–56. [Google Scholar] [CrossRef]
- Corren, J. Allergic rhinitis and asthma: How important is the link? J. Allergy Clin. Immunol. 1997, 99, S781–S786. [Google Scholar] [CrossRef]
- D’amato, G.; Liccardi, G.; D’amato, M.; Holgate, S. Environmental risk factors and allergic bronchial asthma. Clin. Exp. Allergy 2005, 35, 1113–1124. [Google Scholar] [CrossRef]
- Cox, C.S. Airborne bacteria and viruses. Sci. Prog. 1989, 73, 469–499. [Google Scholar]
- Hindmarch, I.; Shamsi, Z. Antihistamines: Models to assess sedative properties, assessment of sedation, safety and other side-effects. Clin. Exp. Allergy 1999, 29, 133–142. [Google Scholar] [CrossRef]
- Cheng, K.K.; Lam, T.H.; Leung, C.C. Wearing face masks in the community during the COVID-19 pandemic: Altruism and solidarity. Lancet 2022, 399, e39–e40. [Google Scholar] [CrossRef]
- Pasanen, A.-L.; Keinänen, J.; Kalliokoski, P.; Martikainen, P.I.; Ruuskanen, J. Microbial growth on respirator filters from improper storage. Scand. J. Work Environ. Health 1993, 19, 421–425. [Google Scholar] [CrossRef] [PubMed]
- First, M.W. HEPA filters. J. Am. Biol. Saf. Assoc. 1998, 3, 33–42. [Google Scholar] [CrossRef]
- Agrawal, S.R.; Kim, H.-J.; Lee, Y.W.; Sohn, J.-H.; Lee, J.H.; Kim, Y.-J.; Lee, S.-H.; Hong, C.-S.; Park, J.-W. Effect of an air cleaner with electrostatic filter on the removal of airborne house dust mite allergens. Yonsei Med. J. 2010, 51, 918–923. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Min, K.D.; Park, W.H.; Youk, J.H.; Kwark, Y.-J. Controlling size and distribution of silver nanoparticles generated in inorganic silica nanofibers using poly(vinyl pyrrolidone). Macromol. Res. 2008, 16, 626–630. [Google Scholar] [CrossRef]
- Isaeva, V.I.; Aizenshtein, é.M.; Soboleva, O.N. World production and use of polypropylene fibres and thread. A review. Fibre Chem. 1997, 29, 269–281. [Google Scholar] [CrossRef]
- Zhukovskii, V.A.; Voronova, I.G.; Khokhlova, V.A.; Gridneva, A.V.; Filipenko, T.S. Technological developments in making polypropylene surgical monofilaments. Fibre Chem. 2008, 40, 322–329. [Google Scholar] [CrossRef]
- Darnell, M.E.; Subbarao, K.; Feinstone, S.M.; Taylor, D.R. Inactivation of the coronavirus that induces severe acute respiratory syndrome, SARS-CoV. J. Virol. Methods 2004, 121, 85–91. [Google Scholar] [CrossRef]
- Pullangott, G.; Kannan, U.; Gayathri, S.; Kiran, D.V.; Maliyekkal, S.M. A comprehensive review on antimicrobial face masks: An emerging weapon in fighting pandemics. RSC Adv. 2021, 11, 6544–6576. [Google Scholar] [CrossRef]
- Liao, L.; Xiao, W.; Zhao, M.; Yu, X.; Wang, H.; Wang, Q.; Chu, S.; Cui, Y. Can N95 Respirators Be Reused after Disinfection? How Many Times? ACS Nano 2020, 14, 6348–6356. [Google Scholar] [CrossRef]
- Shan, X.; Zhang, H.; Liu, C.; Yu, L.; Di, Y.; Zhang, X.; Dong, L.; Gan, Z. Reusable self-sterilization masks based on electrothermal graphene filters. ACS Appl. Mater. Interfaces 2020, 12, 56579–56586. [Google Scholar] [CrossRef]
- Owens, M.U.; Deal, D.R.; Shoemaker, M.O.; Knudson, G.B.; Meszaros, J.E.; Deal, J.L. High-Dose Ultraviolet C Light Inactivates Spores of Bacillus Atrophaeus and Bacillus Anthracis Sterne on Nonreflective Surfaces. Appl. Biosaf. 2005, 10, 240–247. [Google Scholar] [CrossRef]
- Bentancor, M.; Fernández, S.; Viera, F.; Etcheverry, S.; Poradosú, C.; D’Angelo, P.; Montemuiño, H.; Mirazo, S.; Irigoyen, Á.; Sanabria, A.; et al. LUCIA: An open source device for disinfection of N95 masks using UV-C radiation. HardwareX 2021, 9, e00181. [Google Scholar] [CrossRef] [PubMed]
- Ullah, S.; Ullah, A.; Lee, J.; Jeong, Y.; Hashmi, M.; Zhu, C.; Joo, K.I.; Cha, H.J.; Kim, I.S. Reusability Comparison of Melt-Blown vs Nanofiber Face Mask Filters for Use in the Coronavirus Pandemic. ACS Appl. Nano Mater. 2020, 3, 7231–7241. [Google Scholar] [CrossRef] [PubMed]
- Lansdown, A.B. Silver in health care: Antimicrobial effects and safety in use. Curr. Probl. Dermatol. 2006, 33, 17–34. [Google Scholar] [CrossRef]
- Marambio-Jones, C.; Hoek, E.M. A review of the antibacterial effects of silver nanomaterials and potential implications for human health and the environment. J. Nanopart. Res. 2010, 12, 1531–1551. [Google Scholar] [CrossRef]
- Mijnendonckx, K.; Leys, N.; Mahillon, J.; Silver, S.; Van Houdt, R. Antimicrobial silver: Uses, toxicity and potential for resistance. Biometals 2013, 26, 609–621. [Google Scholar] [CrossRef]
- Saeed, N.; Atiq, A.; Rafiq, F.; Khan, I.; Atiq, M.; Saleem, M.; Anjum, D.H.; Usman, Z.; Abbas, M. Engineering of self-assembled silver-peptide colloidal nanohybrids with enhanced biocompatibility and antibacterial activity. Sci. Rep. 2024, 14, 26398. [Google Scholar] [CrossRef]
- Le Ouay, B.; Stellacci, F. Antibacterial activity of silver nanoparticles: A surface science insight. Nano Today 2015, 10, 339–354. [Google Scholar] [CrossRef]
- Sotiriou, G.A.; Pratsinis, S.E. Antibacterial activity of nanosilver ions and particles. Environ. Sci. Technol. 2010, 44, 5649–5654. [Google Scholar] [CrossRef]
- Sun, Z.; Kong, Y.; Lan, L.; Meng, Y.; You, T.; Pauer, R.; Wang, H.; Zhang, Y.; Tang, M.; Demello, A. A High Efficiency, Low Resistance Antibacterial Filter Formed by Dopamine-Mediated In Situ Deposition of Silver onto Glass Fibers. Small 2024, 20, 2301074. [Google Scholar] [CrossRef]
- Tarimala, S.; Kothari, N.; Abidi, N.; Hequet, E.; Fralick, J.; Dai, L.L. New approach to antibacterial treatment of cotton fabric with silver nanoparticle–doped silica using sol–gel process. J. Appl. Polym. Sci. 2006, 101, 2938–2943. [Google Scholar] [CrossRef]
- van der Elst, L.A.; Gokce, M.; Coulter, J.R.; Cavdar, Z.B.; Koraganji, V.N.; Ozturk, M.; Ghatak, S.; Sen, C.K.; Gumennik, A. Microstructured Electroceutical Fiber-Device for Inhibition of Bacterial Proliferation in Wounds. Adv. Mater. Interfaces 2023, 10, 2201854. [Google Scholar] [CrossRef]
- Pinto, N.J.; Carrión, P.; Quiñones, J.X. Electroless deposition of nickel on electrospun fibers of 2-acrylamido-2-methyl-1-propanesulfonic acid doped polyaniline. Mater. Sci. Eng. A 2004, 366, 1–5. [Google Scholar] [CrossRef]
- Drew, C.; Liu, X.; Ziegler, D.; Wang, X.; Bruno, F.F.; Whitten, J.; Samuelson, L.A.; Kumar, J. Metal Oxide-Coated Polymer Nanofibers. Nano Lett. 2003, 3, 143–147. [Google Scholar] [CrossRef]
- Kim, N.K.; Kim, K.; Jang, H.; An, T.; Shin, H.-J.; Kim, G.H. Microheater with copper nanofiber network via electrospinning and electroless deposition. Sci. Rep. 2023, 13, 22248. [Google Scholar] [CrossRef]
- Lin, M.-F.; Chang, K.-W.; Lee, C.-H.; Wu, X.-X.; Huang, Y.-C. Electrospun P3HT/PVDF-HFP semiconductive nanofibers for triboelectric nanogenerators. Sci. Rep. 2022, 12, 14842. [Google Scholar] [CrossRef]
- Montazer, M.; Allahyarzadeh, V. Electroless plating of silver nanoparticles/nanolayer on polyester fabric using AgNO3/NaOH and ammonia. Ind. Eng. Chem. Res. 2013, 52, 8436–8444. [Google Scholar] [CrossRef]
- Montazer, M.; Alimohammadi, F.; Shamei, A.; Rahimi, M.K. In situ synthesis of nano silver on cotton using Tollens’ reagent. Carbohydr. Polym. 2012, 87, 1706–1712. [Google Scholar] [CrossRef]
- Tollens, B. Ueber ammon-alkalische Silberlösung als Reagens auf Aldehyd. Berichte Dtsch. Chem. Ges. 1882, 15, 1635–1639. [Google Scholar] [CrossRef]
- Chen, H.; Liao, F.; Yuan, Z.; Han, X.; Xu, C. Simple and fast fabrication of conductive silver coatings on carbon fabrics via an electroless plating technique. Mater. Lett. 2017, 196, 205–208. [Google Scholar] [CrossRef]
- Lin, F.; Zhu, X.; Li, X.; Guo, W.; Lu, Y.; Sun, L.; Zhu, G. Low-intensity direct current electric field-induced structural and functional modulation of key denitrifying enzyme: In vitro experimental and molecular dynamics simulation insights. J. Environ. Chem. Eng. 2025, 13, 117536. [Google Scholar] [CrossRef]
- Calzia, D.; Panfoli, I.; Ravera, S.; Dazzi, E.; Gandolfo, S.; Pepe, I.M.; Vergani, L.; Morelli, A.M. Structural modification of proteins by direct electric current from low voltage. J. Biochem. Mol. Toxicol. 2009, 23, 309–317. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, T.M.; Lee, H.R.; Pyo, S.G. Silver Thin-Film Plated Interconnected Metal Mesh Networks for Virus Detection and Prevention. Micromachines 2025, 16, 1177. https://doi.org/10.3390/mi16101177
Choi TM, Lee HR, Pyo SG. Silver Thin-Film Plated Interconnected Metal Mesh Networks for Virus Detection and Prevention. Micromachines. 2025; 16(10):1177. https://doi.org/10.3390/mi16101177
Chicago/Turabian StyleChoi, Tae Min, Hwa Rim Lee, and Sung Gyu Pyo. 2025. "Silver Thin-Film Plated Interconnected Metal Mesh Networks for Virus Detection and Prevention" Micromachines 16, no. 10: 1177. https://doi.org/10.3390/mi16101177
APA StyleChoi, T. M., Lee, H. R., & Pyo, S. G. (2025). Silver Thin-Film Plated Interconnected Metal Mesh Networks for Virus Detection and Prevention. Micromachines, 16(10), 1177. https://doi.org/10.3390/mi16101177





