One-Step Fabrication of Microfluidic Channels in Polydimethylsiloxane: Influence of Laser Power on Channel Formation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
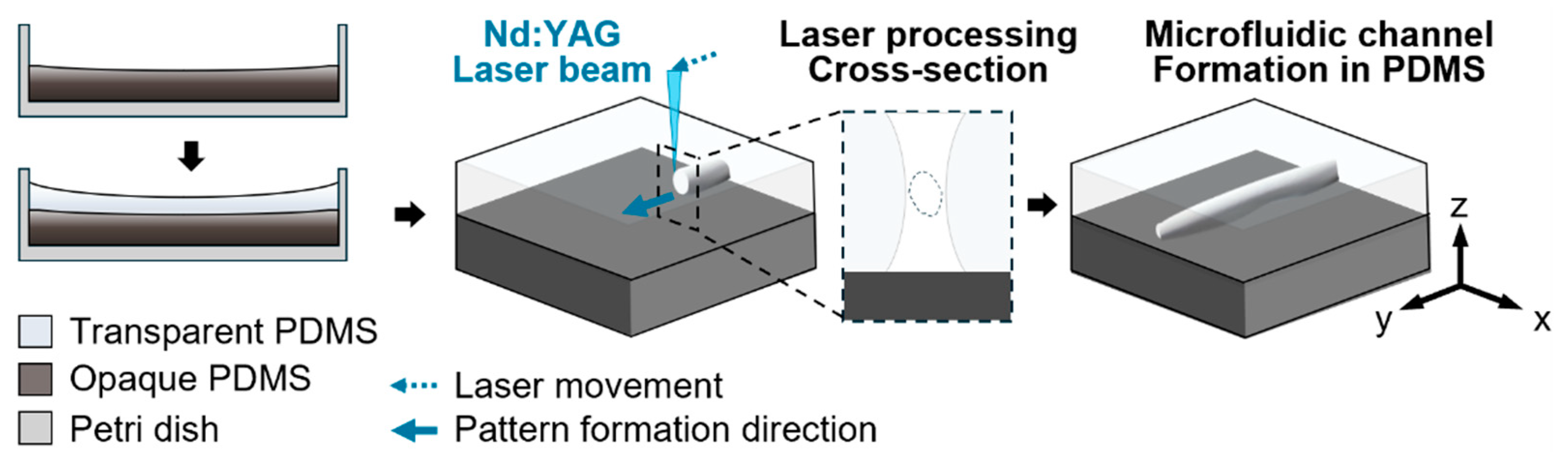
2.2. Channel Fabrication
2.3. Characterization
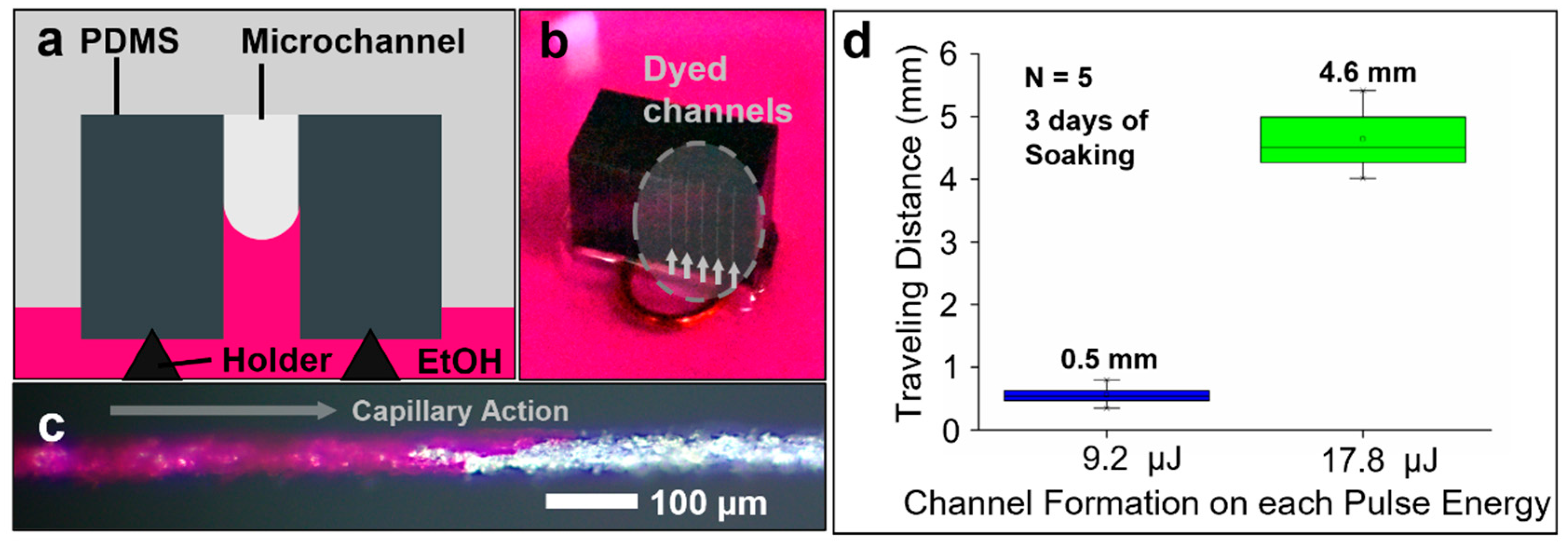
2.4. Capillary-Driven Flow Test
3. Results
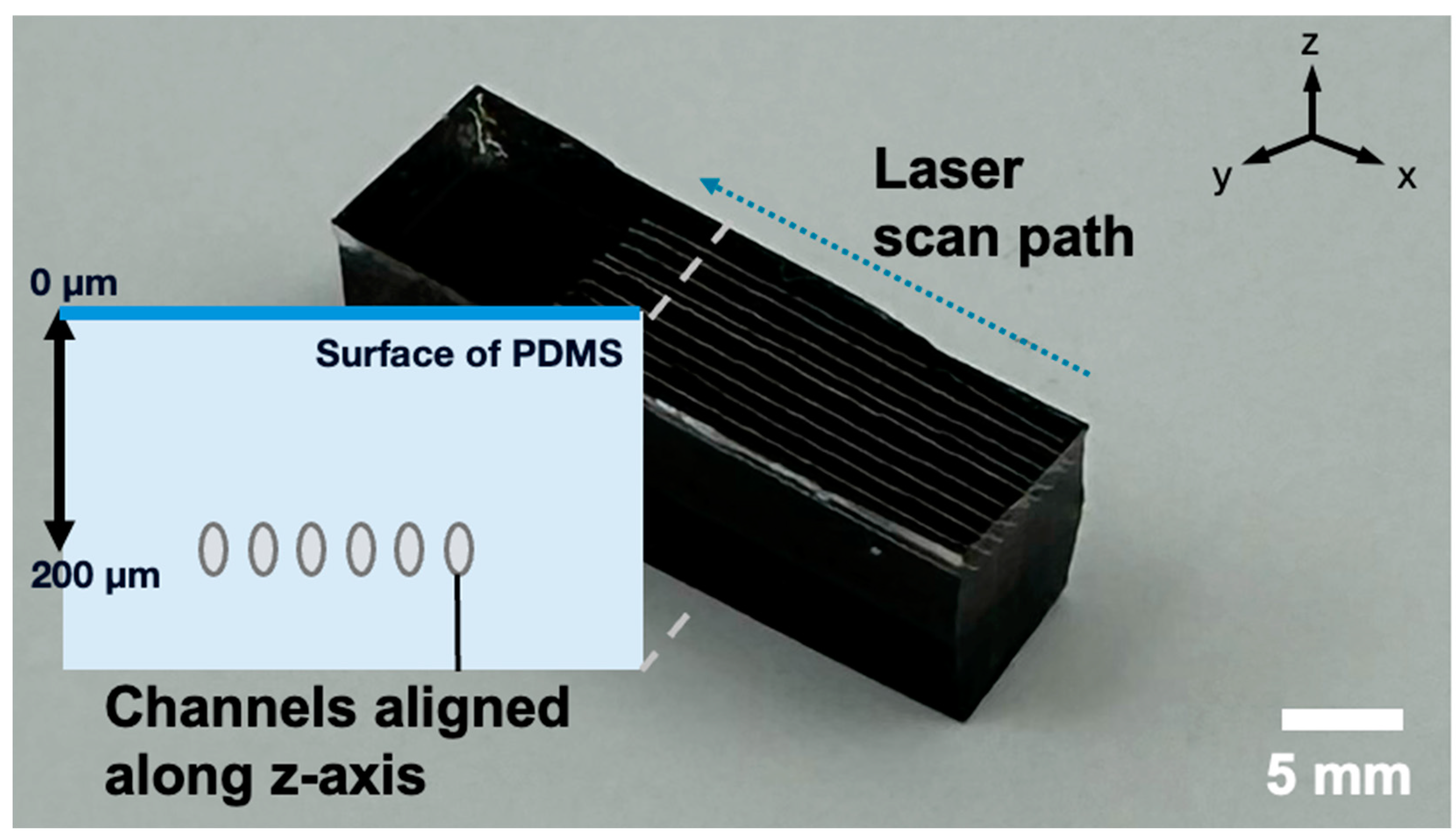
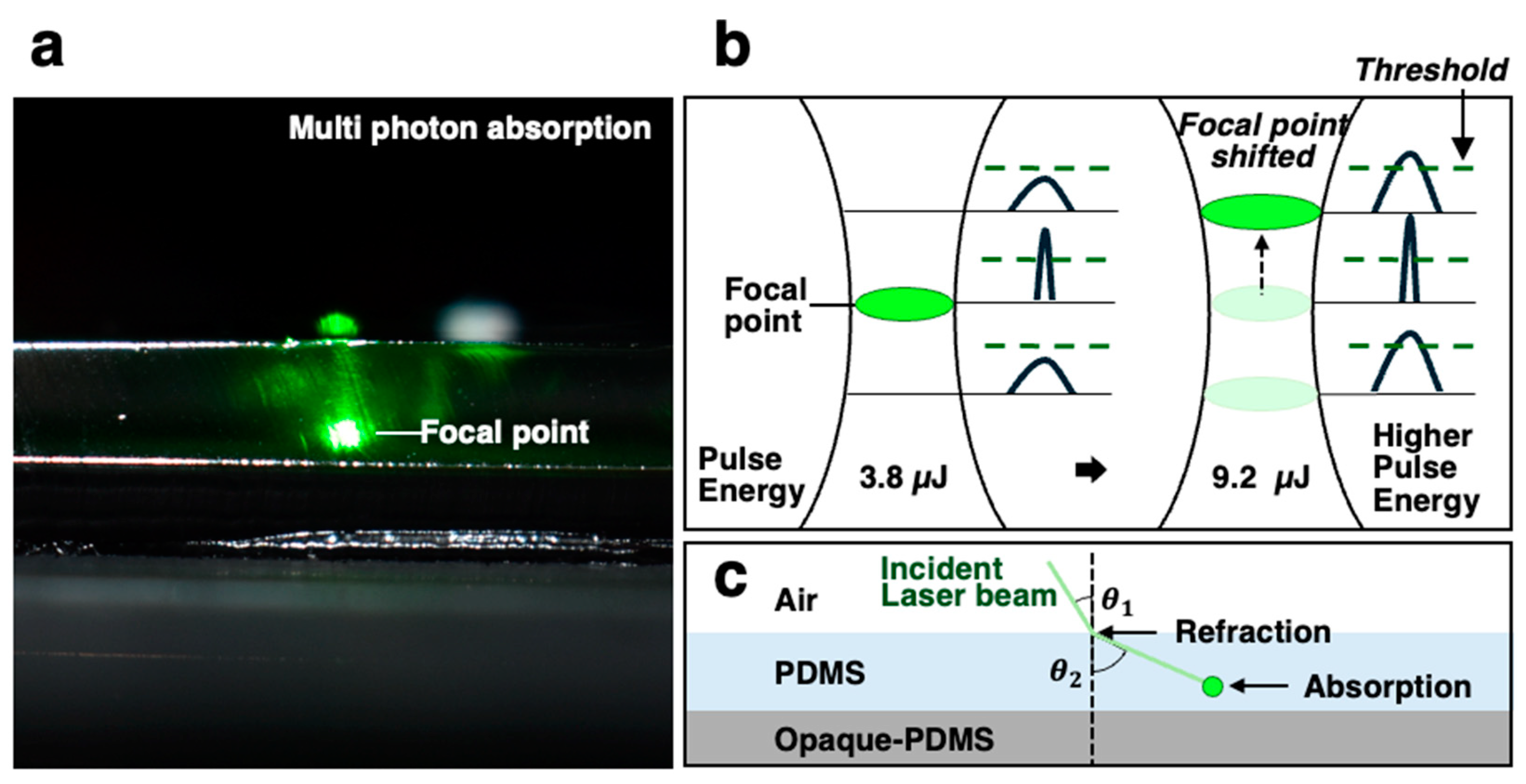
3.1. Channel Fabrication
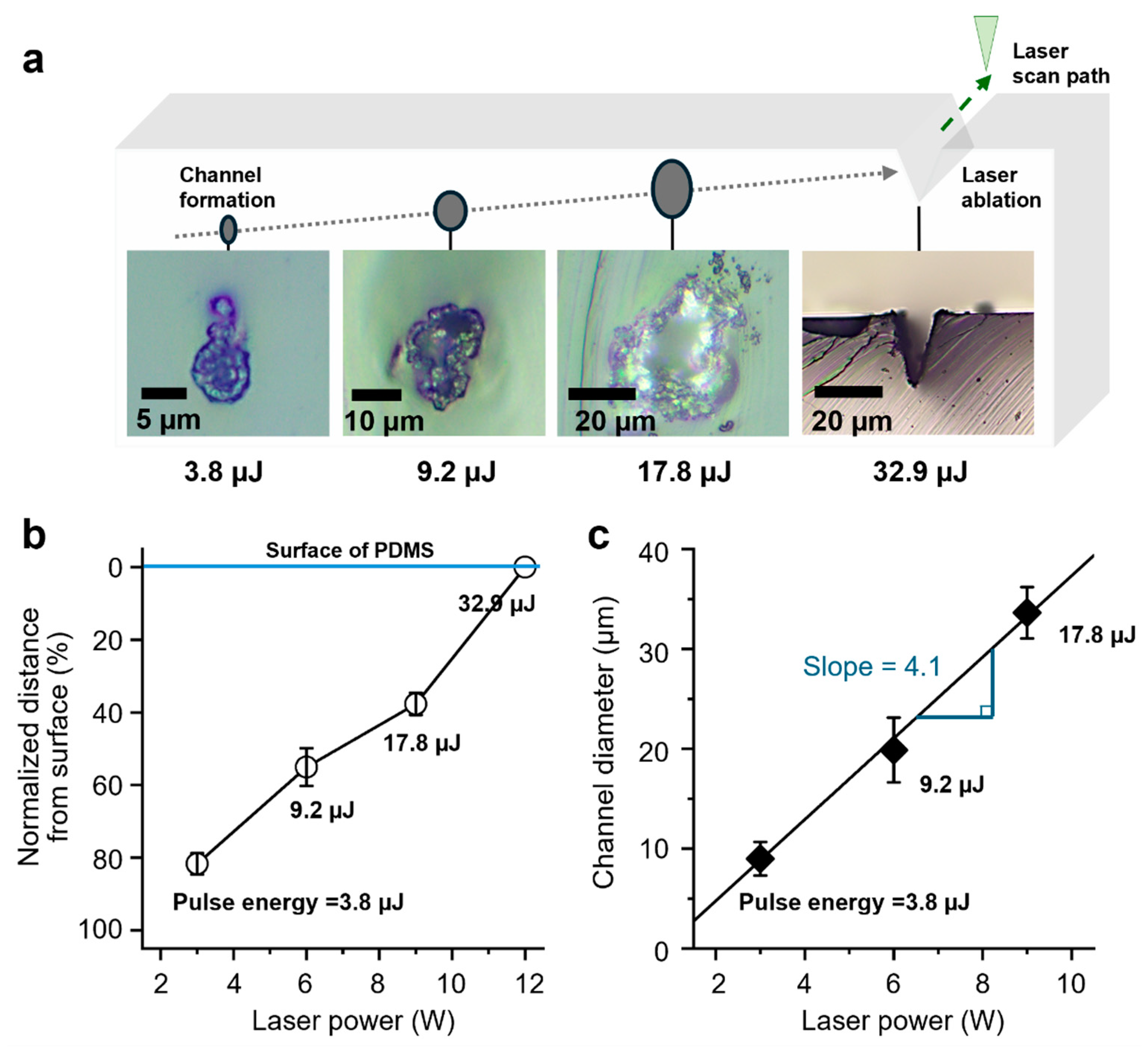
3.2. Channel Formation and Influence of Laser Power
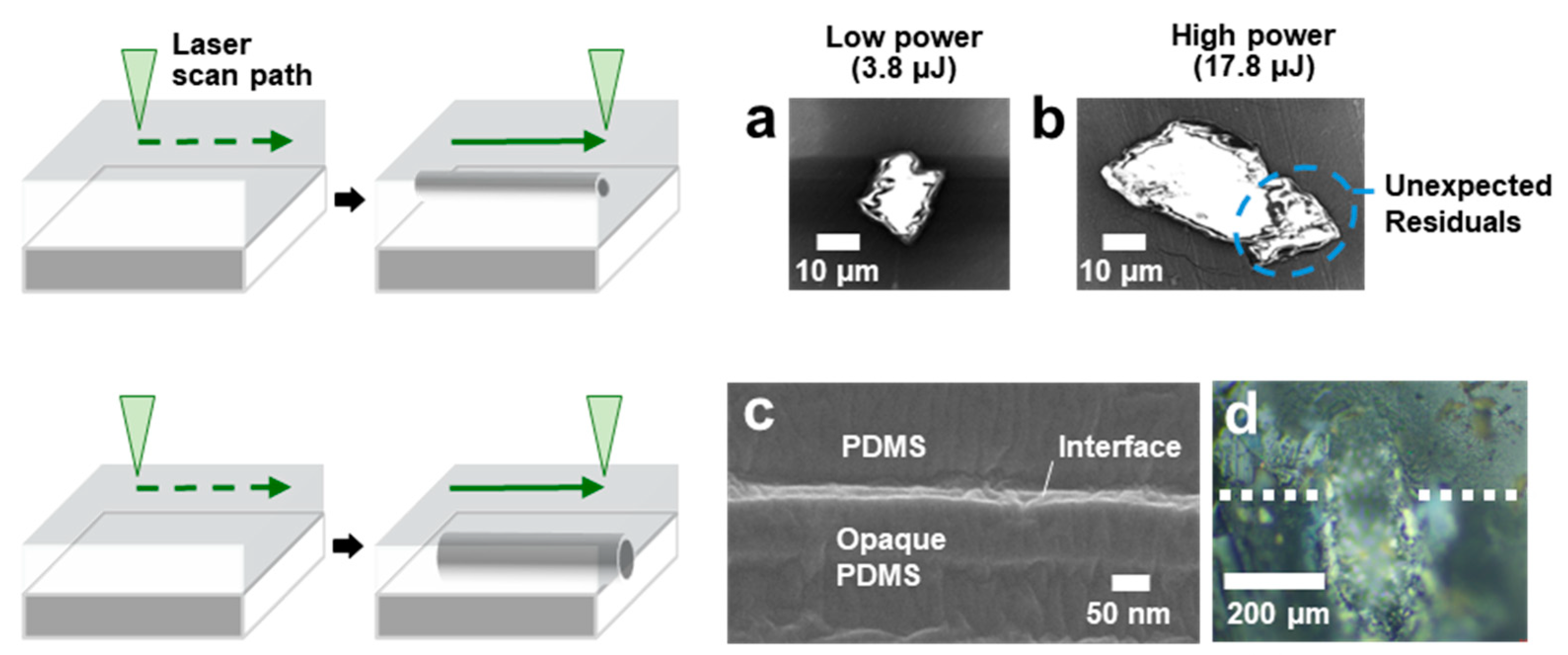
3.3. Channel Formation at PDMS-PDMS Interface
3.4. Mechanism of Channel Formation in PDMS
3.5. Fabricated Channel Connectivity Test
4. Discussion
| Parameters | Symbol | Value | Units | Reference |
|---|---|---|---|---|
| Linear refractive index | n1 | 1.4348 ± 0.0006 | - | [17] |
| Nonlinear refractive index | n2 | N.A * | cm2/W | [21] |
| Linear absorption coefficient | α | 3.58 | cm−1 | [25] |
| Nonlinear absorption coefficient ** | β | N.A * | cm/GW | [21] |
| Damping coefficient | γ | 9-12 | cm−1 | [17] |
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mashaghi, S.; Abbaspourrad, A.; Weitz, D.A.; van Oijen, A.M. Droplet microfluidics: A tool for biology, chemistry and nanotechnology. TrAC Trends Anal. Chem. 2016, 82, 118–125. [Google Scholar] [CrossRef]
- Reverté, L.; Prieto-Simón, B.; Campàs, M. New advances in electrochemical biosensors for the detection of toxins: Nanomaterials, magnetic beads and microfluidics systems. A review. Anal. Chim. Acta 2016, 908, 8–21. [Google Scholar] [CrossRef] [PubMed]
- Radhakrishnan, S.; Mathew, M.; Rout, C.S. Microfluidic sensors based on two-dimensional materials for chemical and biological assessments. Mater. Adv. 2022, 3, 1874–1904. [Google Scholar] [CrossRef]
- Whitesides, G.M. The origins and the future of microfluidics. Nature 2006, 442, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Lin, X.; Li, H.; Sun, W.; Li, W.; Zhang, Q.; Bian, F.; Lin, J. Assessment of drug treatment response using primary human colon cancer cell spheroids cultivated in a microfluidic mixer chip. Biosen. Bioelectron. 2025, 269, 116944. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Song, Y.; Bo, X.; Min, J.; Pak, O.S.; Zhu, L.; Wang, M.; Tu, J.; Kogan, A.; Zhang, H.; et al. A laser-engraved wearable sensor for sensitive detection of uric acid and tyrosine in sweat. Nat. Biotechnol. 2020, 38, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Nightingale, A.M.; Leong, C.L.; Burnish, R.A.; Hassan, S.-U.; Zhang, Y.; Clough, G.F.; Boutelle, M.G.; Voegeli, D.; Niu, X. Monitoring biomolecule concentrations in tissue using a wearable droplet microfluidic-based sensor. Nat. Commun. 2019, 10, 2741. [Google Scholar] [CrossRef] [PubMed]
- Apoorva, S.; Nguyen, N.-T.; Rajan, S.K. Recent developments and future perspectives of microfluidics and smart technologies in wearable devices. Lab A Chip 2024, 24, 1833–1866. [Google Scholar] [CrossRef]
- Jia, W.; Bandodkar, A.J.; Valdés-Ramírez, G.; Windmiller, J.R.; Yang, Z.; Ramírez, J.; Chan, G.; Wang, J. Electrochemical Tattoo Biosensors for Real-Time Noninvasive Lactate Monitoring in Human Perspiration. Anal. Chem. 2013, 85, 6553–6560. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Mehl, B.T.; Munshi, A.S.; Townsend, A.D.; Spence, D.M.; Martin, R.S. 3D-printed microfluidic devices: Fabrication, advantages and limitations—A mini review. Anal. Methods 2016, 8, 6005–6012. [Google Scholar] [CrossRef]
- Ng, J.M.K.; Gitlin, I.; Stroock, A.D.; Whitesides, G.M. Components for integrated poly(dimethylsiloxane) microfluidic systems. Electrophoresis 2002, 23, 3461–3473. [Google Scholar] [CrossRef] [PubMed]
- Morbioli, G.G.; Speller, N.C.; Stockton, A.M. A practical guide to rapid-prototyping of PDMS-based microfluidic devices: A tutorial. Anal. Chim. Acta 2020, 1135, 150–174. [Google Scholar] [CrossRef] [PubMed]
- Shakeri, A.; Khan, S.; Didar, T.F. Conventional and emerging strategies for the fabrication and functionalization of PDMS-based microfluidic devices. Lab Chip 2021, 21, 3053–3075. [Google Scholar] [CrossRef]
- Raj, M.K.; Chakraborty, S. PDMS microfluidics: A mini review. J. Appl. Polym. Sci. 2020, 137, 48958. [Google Scholar] [CrossRef]
- Eddings, M.A.; Johnson, M.A.; Gale, B.K. Determining the optimal PDMS–PDMS bonding technique for microfluidic devices. J. Micromech. Microeng. 2008, 18, 067001. [Google Scholar] [CrossRef]
- Saliminia, A.; Bérubé, J.-P.; Vallée, R. Refractive index-modified structures in glass written by 266 nm fs laser pulses. Opt. Express 2012, 20, 27410–27419. [Google Scholar] [CrossRef]
- Schneider, F.; Draheim, J.; Kamberger, R.; Wallrabe, U. Process and material properties of polydimethylsiloxane (PDMS) for Optical MEMS. Sens. Actuators A Phys. 2009, 151, 95–99. [Google Scholar] [CrossRef]
- Olbrich, M.; Viertel, T.; Pflug, T.; Ebert, R.; Fischer, A.; Horn, A. Simulation of the spherical aberration of focused laser radiation in transparent materials: Comparison of different simulation approaches. Appl. Phys. A 2016, 122, 482. [Google Scholar] [CrossRef]
- Viertel, T.; Pabst, L.; Olbrich, M.; Ebert, R.; Horn, A.; Exner, H. Generation of nano-voids inside polylactide using femtosecond laser radiation. Appl. Phys. A 2017, 123, 789. [Google Scholar] [CrossRef]
- Mkrtychev, O.V.; Privalov, V.E.; Fotiadi, A.E.; Shemanin, V.G. Laser ablation studies of nanocomposites. St. Petersburg Polytech. Univ. J. Phys. Math. 2015, 1, 82–86. [Google Scholar] [CrossRef]
- Bongu, S.R.; Buchmüller, M.; Neumaier, D.; Görrn, P. Introducing Optical Nonlinearity in PDMS Using Organic Solvent Swelling. Optics 2024, 5, 66–75. [Google Scholar] [CrossRef]
- Stankova, N.E.; Atanasov, P.A.; Nedyalkov, N.N.; Stoyanchov, T.R.; Kolev, K.N.; Valova, E.I.; Georgieva, J.S.; Armyanov, S.A.; Amoruso, S.; Wang, X.; et al. fs- and ns-laser processing of polydimethylsiloxane (PDMS) elastomer: Comparative study. Appl. Surf. Sci. 2015, 336, 321–328. [Google Scholar] [CrossRef]
- Wu, C.; Xu, J.; Zhang, T.; Xin, G.; Li, M.; Rong, Y.; Zhang, G.; Huang, Y. Precision cutting of PDMS film with UV-nanosecond laser based on heat generation-diffusion regulation. Opt. Laser Technol. 2022, 145, 107462. [Google Scholar] [CrossRef]
- Shank, C.V.; Yen, R.; Hirlimann, C. Time-Resolved Reflectivity Measurements of Femtosecond-Optical-Pulse-Induced Phase Transitions in Silicon. Phys. Rev. Lett. 1983, 50, 454–457. [Google Scholar] [CrossRef]
- Stankova, N.E.; Atanasov, P.A.; Nikov, R.G.; Nikov, R.G.; Nedyalkov, N.N.; Stoyanchov, T.R.; Fukata, N.; Kolev, K.N.; Valova, E.I.; Georgieva, J.S.; et al. Optical properties of polydimethylsiloxane (PDMS) during nanosecond laser processing. Appl. Surf. Sci. 2016, 374, 96–103. [Google Scholar] [CrossRef]
- Ogo, A.; Okayama, S.; Nakatani, M.; Hashimoto, M. CO2-Laser-Micromachined, Polymer Microchannels with a Degassed PDMS slab for the Automatic Production of Monodispersed Water-in-Oil Droplets. Micromachines 2022, 13, 1389. [Google Scholar] [CrossRef] [PubMed]
- Konari, P.R.; Clayton, Y.-D.; Vaughan, M.B.; Khandaker, M.; Hossan, M.R. Experimental Analysis of Laser Micromachining of Microchannels in Common Microfluidic Substrates. Micromachines 2021, 12, 138. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Cedillo, L.M.; Vázquez-Cuevas, F.G.; Quintero-Torres, R.; Aragón, J.L.; Ocampo Mortera, M.A.; Ordóñez-Romero, C.L.; Domínguez-Juárez, J.L. Microfabrication with Very Low-Average Power of Green Light to Produce PDMS Microchips. Polymers 2021, 13, 607. [Google Scholar] [CrossRef] [PubMed]
- Yong, J.; Zhan, Z.; Singh, S.C.; Chen, F.; Guo, C. Microfluidic Channels Fabrication Based on Underwater Superpolymphobic Microgrooves Produced by Femtosecond Laser Direct Writing. ACS Appl. Polym. Mater. 2019, 1, 2819–2825. [Google Scholar] [CrossRef]
- Joanni, E.; Peressinotto, J.; Domingues, P.S.; Setti, G.d.O.; Jesus, D.P.d. Fabrication of molds for PDMS microfluidic devices by laser swelling of PMMA. RSC Adv. 2015, 5, 25089–25096. [Google Scholar] [CrossRef]
- Hsieh, Y.-K.; Chen, S.-C.; Huang, W.-L.; Hsu, K.-P.; Gorday, K.A.V.; Wang, T.; Wang, J. Direct Micromachining of Microfluidic Channels on Biodegradable Materials Using Laser Ablation. Polymers 2017, 9, 242. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Value | Unit |
|---|---|---|
| Wavelength | 532 | [nm] |
| Beam shape | Gaussian profile | -- |
| Beam quality (M2) | 1.11 | -- |
| Spot size | 12-15 | [µm] |
| Laser mode | TEM00 | -- |
| Focal length | 240 | mm |
| Rayleigh length | 192 | [µm] |
| Divergence angle | 0.54 | [mRad] |
| Scanner type | Galvanometer | -- |
| Pulse duration | 12 | [ps] |
| Laser Parameters | Unit | Level 1 | Level 2 | Level 3 | Level 4 | |
|---|---|---|---|---|---|---|
| Applied variables | Power | [W] | 4 | 6 | 9 | 12 |
| Repetition rate | [kHz] | 300 | 300 | 300 | 300 | |
| Scanning speed | [mm/s] | 50 | 50 | 50 | 50 | |
| Measured parameters | Pulse energy | [µJ] | 3.8 | 9.2 | 17.8 | 32.9 |
| Fluence | [J/cm2] | 1.2 | 2.9 | 5.7 | 10.5 | |
| L.S.O | [%] | 99.2 | 99.2 | 99.2 | 99.2 |
| Reference | Laser Source | Pulse Duration | Wavelength [nm] | Materials | Channel Width [µm] | Controlled Axis | Performance |
|---|---|---|---|---|---|---|---|
| This study | Nd:YAG | Picosecond | 532 | PDMS | 10–150 | x-y | Microchannels fabrication within PDMS |
| [26] | CO2 | CW | 9300 | PDMS, PMMA | ~80 | x-y-z | Uniform microchannels with controlled depth and width |
| [27] | CO2 | Nanosecond | 9300 | PDMS, PMMA, Glass | 77–85 | x-y-z | Microchannels with controlled width/depth |
| [28] | Nd:YAG | CW | 532 | PS/PDMS | 4.7–12 | x-y-z | 3D microchannels in glass |
| [29] | Nd:YAG | Femtosecond | 800 | Glass/PDMS | 53 | x-y | Underwater superpolymphobic microchannels (PCA 155.5°) |
| [30] | Nd:YVO4 | N/A | 355 | PMMA | 20–200 | x-y-z | High-quality PDMS mold fabrication with smooth surface finish |
| [31] | Excimer | Nanosecond | 193 | PDMS, PGS, APS | 10–150 | x-y | Precise microchannel fabrication via surface ablation |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.-Y.; Son, H.-B.; Lim, H.-R. One-Step Fabrication of Microfluidic Channels in Polydimethylsiloxane: Influence of Laser Power on Channel Formation. Micromachines 2025, 16, 282. https://doi.org/10.3390/mi16030282
Kim S-Y, Son H-B, Lim H-R. One-Step Fabrication of Microfluidic Channels in Polydimethylsiloxane: Influence of Laser Power on Channel Formation. Micromachines. 2025; 16(3):282. https://doi.org/10.3390/mi16030282
Chicago/Turabian StyleKim, Seong-Yeop, Han-Byeol Son, and Hyo-Ryoung Lim. 2025. "One-Step Fabrication of Microfluidic Channels in Polydimethylsiloxane: Influence of Laser Power on Channel Formation" Micromachines 16, no. 3: 282. https://doi.org/10.3390/mi16030282
APA StyleKim, S.-Y., Son, H.-B., & Lim, H.-R. (2025). One-Step Fabrication of Microfluidic Channels in Polydimethylsiloxane: Influence of Laser Power on Channel Formation. Micromachines, 16(3), 282. https://doi.org/10.3390/mi16030282








