Innovative Micro- and Nano-Architectures in Biomedical Engineering for Therapeutic and Diagnostic Applications
Abstract
1. Introduction
1.1. Background on Micro- and Nano-Architectures in Biomedical Engineering
1.2. Importance in Healthcare Applications
1.3. Scope and Objectives of This Review
2. Advances in Fabrication Techniques
2.1. Lithography-Based Methods
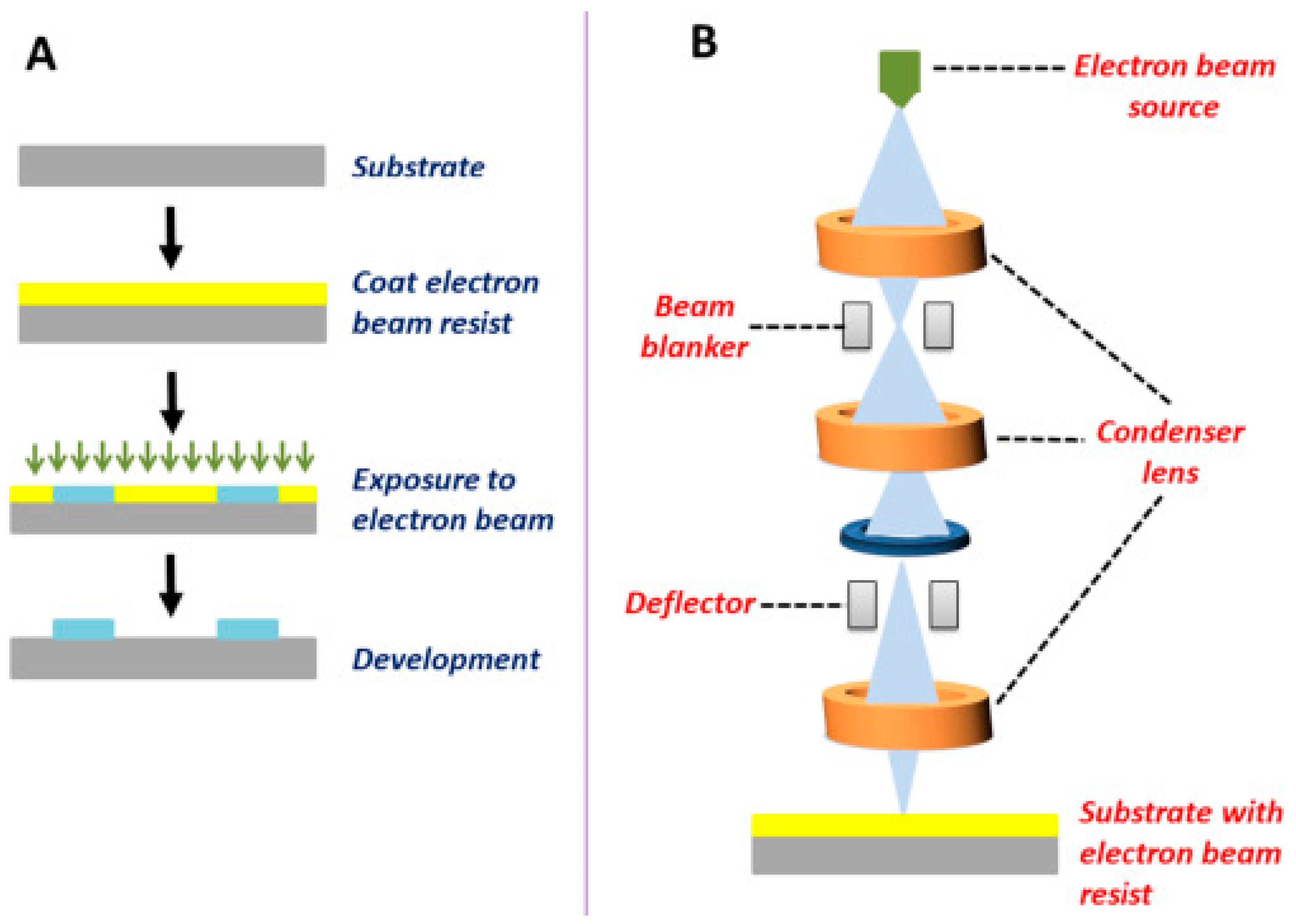
2.1.1. Photolithography
2.1.2. Electron Beam Lithography
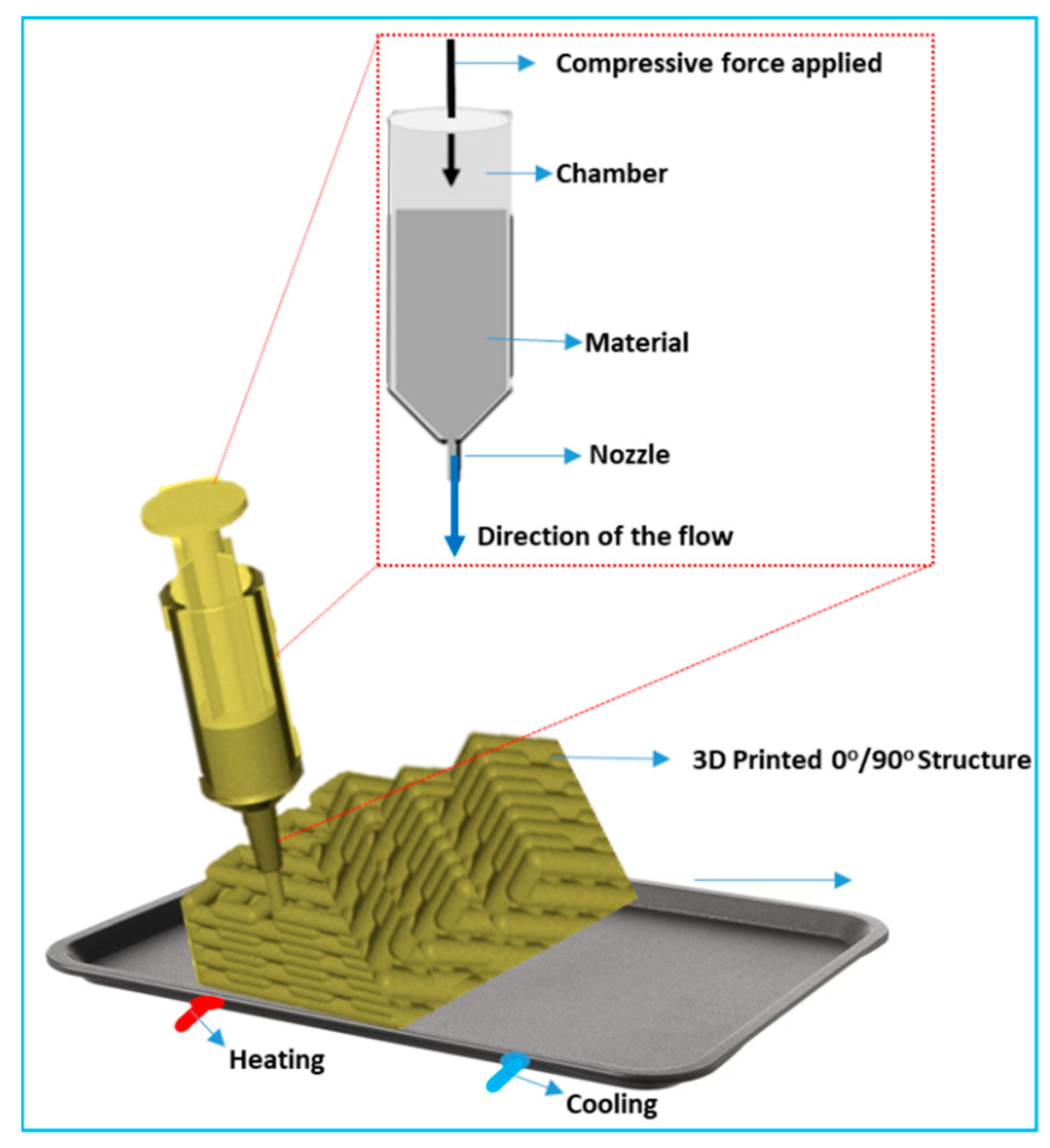
2.1.3. Three-Dimensional Printing Technologies
2.1.4. Stereolithography (SLA)
2.1.5. Two-Photon Polymerization
2.2. Self-Assembly Techniques
2.2.1. Molecular Self-Assembly
2.2.2. Block Copolymer Self-Assembly
3. Applications in Therapeutics
3.1. Drug Delivery Systems
3.1.1. Controlled Release Mechanisms
Mechanisms of Controlled Release
3.1.2. Targeted Delivery Strategies
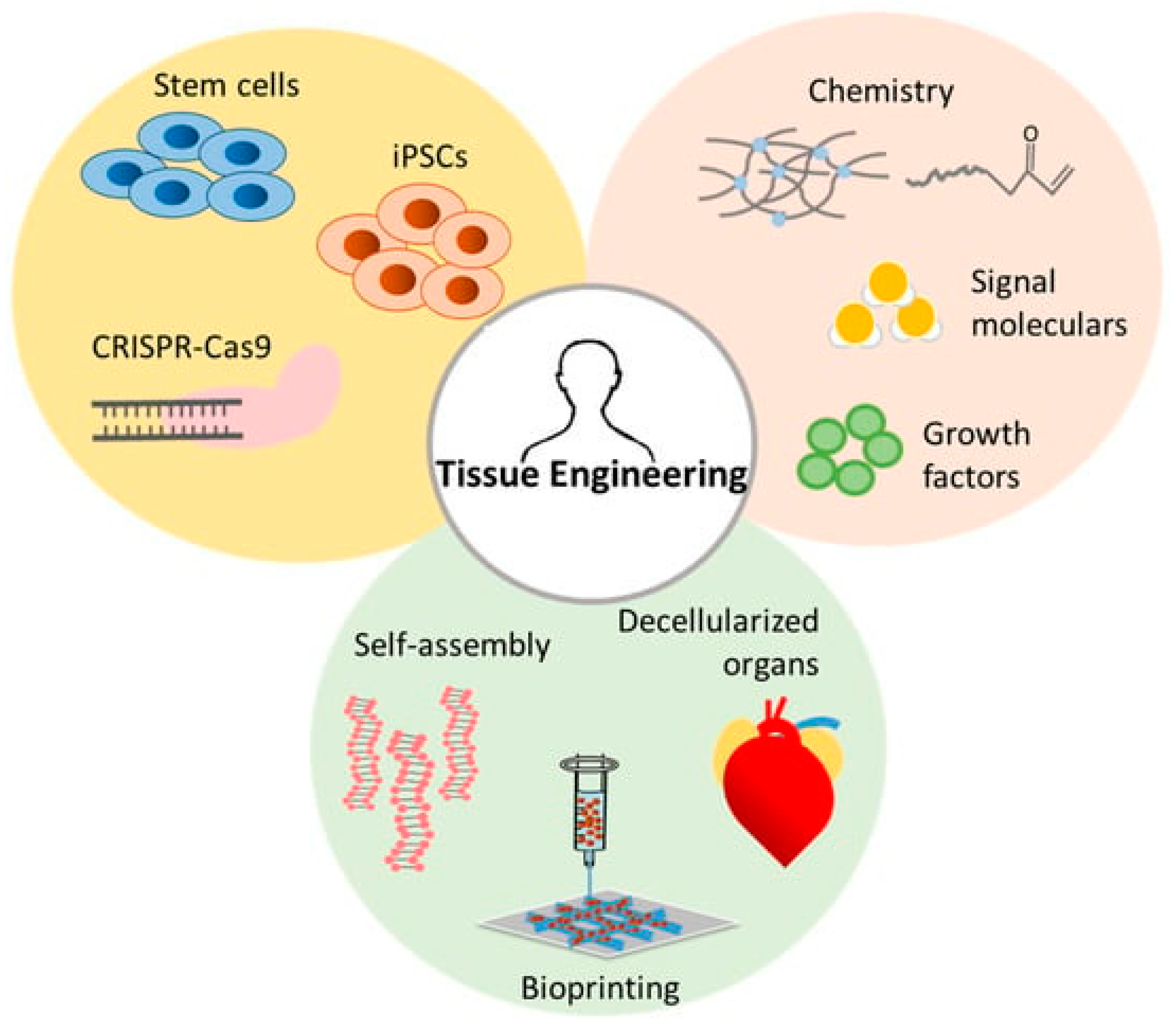
3.2. Tissue Engineering and Regenerative Medicine
3.2.1. Scaffolds for Cellular Interactions
3.2.2. Stimuli-Responsive Materials
3.3. Therapeutic Implants and Devices
4. Applications in Diagnostics
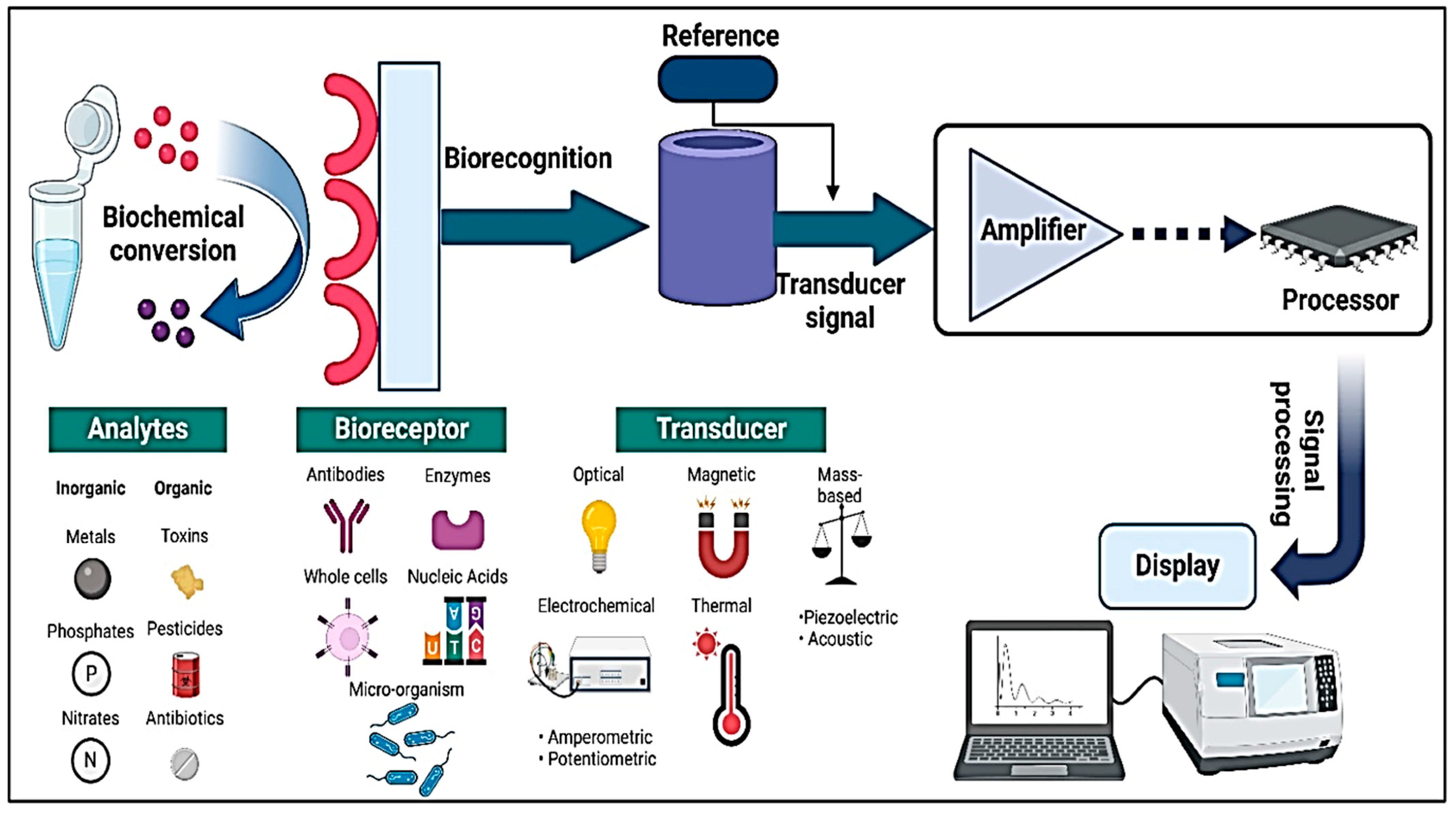
4.1. Biosensing and Detection Technologies
4.1.1. Nano-Biosensors for Disease Detection
4.1.2. Integration with Point-of-Care Devices
4.2. Imaging and Contrast-Enhancing Agents
4.3. Lab-on-a-Chip and Microfluidic Platforms
5. Design Parameters for Micro- and Nano-Architectures
5.1. Biocompatibility and Safety Considerations
5.2. Mechanical Properties and Resilience
5.3. Scalability and Cost-Effectiveness
6. Challenges and Limitations
6.1. Barriers to Clinical Translation
6.2. Long-Term Stability and Performance
6.3. Regulatory and Ethical Considerations
7. Future Perspectives
7.1. Emerging Trends and Innovations
7.2. Integration with Personalized Medicine
7.3. Roadmap for Translational Research
8. Conclusions
8.1. Summary of Key Insights
- Advancements in Fabrication: Techniques such as lithography, 3D printing, and self-assembly enable precise control over micro- and nano-architectures, enhancing material functionalities.
- Therapeutic Applications: Engineered micro- and nano-architectures facilitate targeted drug delivery, regenerative medicine, and therapeutic implants, improving treatment efficiency and reducing side effects.
- Diagnostic Innovations: Integration into biosensors, lab-on-a-chip devices, and imaging agents enhances disease detection with improved sensitivity, specificity, and real-time monitoring.
- Key Design Parameters: Biocompatibility, mechanical resilience, and scalability are crucial for clinical translation and long-term stability of these materials.
- Regulatory and Ethical Considerations: Progress is being made in standardizing the guidelines for safety, efficacy, and ethical transparency in the application of micro- and nano-architectures.
- Future Prospects: The integration of these materials into personalized and precision medicine holds immense potential for transforming healthcare through tailored treatments and cost-effective solutions.
8.2. Final Remarks on Potential Impact
Author Contributions
Funding
Conflicts of Interest
References
- Rijns, L.; Rutten, M.G.T.A.; Vrehen, A.F.; Aldana, A.A.; Baker, M.B.; Dankers, P.Y.W. Mimicking the Extracellular World: From Natural to Fully Synthetic Matrices Utilizing Supramolecular Biomaterials. Nanoscale 2024, 16, 16290–16312. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, E.; Thomas, J.; Oppenheimer, R.; Rickard, J.J.S.; Goldberg, P. Collagen-Electrohydrodynamic Hierarchical Lithography for Biomimetic Photonic Micro-Nanomaterials. Small 2024, 20, e2402565. [Google Scholar] [CrossRef]
- Karthik, V.; Poornima, S.; Vigneshwaran, A.; Raj, D.P.R.D.D.; Subbaiya, R.; Manikandan, S.; Saravanan, M. Nanoarchitectonics Is an Emerging Drug/Gene Delivery and Targeting Strategy—A Critical Review. J. Mol. Struct. 2021, 1243, 130844. [Google Scholar] [CrossRef]
- Wu, D.; Chen, Q.; Chen, X.; Han, F.; Chen, Z.; Wang, Y. The Blood–Brain Barrier: Structure, Regulation and Drug Delivery. Signal Transduct. Target. Ther. 2023, 8, 217. [Google Scholar] [CrossRef] [PubMed]
- Parvin, N.; Mandal, T.K.; Nagajyothi, P.C.; Reddy, P.M.; Reddy, N.R.; Joo, S.W. Highly Fluorescent Doped Fe3O4@C Nanoparticles Cross the Blood–Brain Barrier: Help in Brain Imaging and Blocking the Life Cycle of Mosquitoes. J. Clust. Sci. 2021, 32, 1761–1767. [Google Scholar] [CrossRef]
- Visan, A.I.; Negut, I. Integrating Artificial Intelligence for Drug Discovery in the Context of Revolutionizing Drug Delivery. Life 2024, 14, 233. [Google Scholar] [CrossRef]
- Maghsoudi, S.; Taghavi Shahraki, B.; Rabiee, N.; Fatahi, Y.; Dinarvand, R.; Tavakolizadeh, M.; Ahmadi, S.; Rabiee, M.; Bagherzadeh, M.; Pourjavadi, A.; et al. Burgeoning Polymer Nano Blends for Improved Controlled Drug Release: A Review. Int. J. Nanomedicine 2020, 15, 4363–4392. [Google Scholar] [CrossRef]
- Mhlanga, N.; Mphuthi, N.; Van der Walt, H.; Nyembe, S.; Mokhena, T.; Sikhwivhilu, L. Nanostructures and Nanoparticles as Medical Diagnostic Imaging Contrast Agents: A Review. Mater. Today Chem. 2024, 40, 102233. [Google Scholar] [CrossRef]
- Nur, M.G.; Rahman, M.; Dip, T.M.; Hossain, M.H.; Hossain, N.B.; Baratchi, S.; Padhye, R.; Houshyar, S. Recent Advances in Bioactive Wound Dressings. Wound Repair Regen. 2025, 33, e13233. [Google Scholar] [CrossRef]
- Gu, X.G.; Su, I.; Sharma, S.; Voros, J.L.; Qin, Z.; Buehler, M.J. Three-Dimensional-Printing of Bio-Inspired Composites. J. Biomech. Eng. 2016, 138, 021006. [Google Scholar] [CrossRef]
- Paramasivam, G.; Palem, V.V.; Meenakshy, S.; Suresh, L.K.; Gangopadhyay, M.; Antherjanam, S.; Sundramoorthy, A.K. Advances on Carbon Nanomaterials and Their Applications in Medical Diagnosis and Drug Delivery. Colloids Surfaces B Biointerfaces 2024, 241, 114032. [Google Scholar] [CrossRef]
- Reddy, K.T.K.; Reddy, A.S. Recent Breakthroughs in Drug Delivery Systems for Targeted Cancer Therapy: An Overview. Cell. Mol. Biomed. Reports 2025, 5, 13–27. [Google Scholar] [CrossRef]
- Wang, S.; Cheng, K.; Chen, K.; Xu, C.; Ma, P.; Dang, G.; Yang, Y.; Lei, Q.; Huang, H.; Yu, Y.; et al. Nanoparticle-Based Medicines in Clinical Cancer Therapy. Nano Today 2022, 45, 101512. [Google Scholar] [CrossRef]
- Parvin, N.; Mandal, T.K. RSC Advances Synthesis of a Highly Fl Uorescence Nitrogen-Doped Carbon Quantum Dots Bioimaging Probe and Its In. RSC Adv. 2016, 6, 18134–18140. [Google Scholar] [CrossRef]
- Parvin, N.; Mandal, T.K. Dually Emissive P,N-Co-Doped Carbon Dots for Fluorescent and Photoacoustic Tissue Imaging in Living Mice. Microchim. Acta 2017, 184, 1117–1125. [Google Scholar] [CrossRef]
- Ciftci, F.; Özarslan, A.C.; Kantarci, İ.C.; Yelkenci, A.; Tavukcuoglu, O.; Ghorbanpour, M. Advances in Drug Targeting, Drug Delivery, and Nanotechnology Applications: Therapeutic Significance in Cancer Treatment. Pharmaceutics 2025, 17, 121. [Google Scholar] [CrossRef]
- Mittal, P.; Namratha, M.P.; Kapoor, R.; Ajmal, G.; Mishra, B. Fabrication of Micro- and Nanodevices for Tissue Engineering. In Marine Biopolymers; Elsevier: Amsterdam, The Netherlands, 2025; pp. 147–161. [Google Scholar]
- Fatima Balderrama, I.; Schafer, S.; El Shatanofy, M.; Bergamo, E.T.P.; Mirsky, N.A.; Nayak, V.V.; Marcantonio Junior, E.; Alifarag, A.M.; Coelho, P.G.; Witek, L. Biomimetic Tissue Engineering Strategies for Craniofacial Applications. Biomimetics 2024, 9, 636. [Google Scholar] [CrossRef]
- Parvin, N.; Kumar, V.; Joo, S.W.; Mandal, T.K. Cutting-Edge Hydrogel Technologies in Tissue Engineering and Biosensing: An Updated Review. Materials 2024, 17, 4792. [Google Scholar] [CrossRef]
- Kapoor, A.; Chaudhari, P.; Awasthi, A.; Nayak, J.; Kannan, D. Advances in Microfluidics for Detection of Infectious Diseases. In Advances in Separation Sciences; Elsevier: Amsterdam, The Netherlands, 2025; pp. 345–365. [Google Scholar]
- Parvin, N.; Kumar, V.; Joo, S.W.; Mandal, T.K. Emerging Trends in Nanomedicine: Carbon-Based Nanomaterials for Healthcare. Nanomaterials 2024, 14, 1085. [Google Scholar] [CrossRef]
- Zhu, Y.; Tang, T.; Zhao, S.; Joralmon, D.; Poit, Z.; Ahire, B.; Keshav, S.; Raje, A.R.; Blair, J.; Zhang, Z.; et al. Recent Advancements and Applications in 3D Printing of Functional Optics. Addit. Manuf. 2022, 52, 102682. [Google Scholar] [CrossRef]
- Akhtar, Z. Bin Advancements within Molecular Engineering for Regenerative Medicine and Biomedical Applications an Investigation Analysis towards A Computing Retrospective. J. Electron. Electromed. Eng. Med. Inform. 2024, 6, 54–72. [Google Scholar] [CrossRef]
- Udegbe, F.C.; Ebulue, O.R.; Ebulue, C.C.; Ekesiobi, C.S. Synthetic Biology and Its Potential in U.S. Medical Therapeutics: A Comprehensive Review: Exploring the Cutting-Edge Intersections of Biology and Engineering in Drug Development and Treatments. Eng. Sci. Technol. J. 2024, 5, 1395–1414. [Google Scholar] [CrossRef]
- Wood, M. Colloidal Lithography and Current Fabrication Techniques Producing In-Plane Nanotopography for Biological Applications. J. R. Soc. Interface 2007, 4, 1–17. [Google Scholar] [CrossRef]
- Khonina, S.N.; Kazanskiy, N.L.; Butt, M.A. Grayscale Lithography and a Brief Introduction to Other Widely Used Lithographic Methods: A State-of-the-Art Review. Micromachines 2024, 15, 1321. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z. Optical Lithography. In Nanofabrication; Springer International Publishing: Cham, Switzerland, 2024; pp. 9–81. [Google Scholar]
- Sarkar, T.; Nguyen, T.; Moinuddin, S.M.; Stenmark, K.R.; Nozik, E.S.; Saha, D.; Ahsan, F. A Protocol for Fabrication and On-Chip Cell Culture to Recreate PAH-Afflicted Pulmonary Artery on a Microfluidic Device. Micromachines 2022, 13, 1483. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M.; Carvalho, V.; Ribeiro, J.; Lima, R.A.; Teixeira, S.; Pinho, D. Advances in Microfluidic Systems and Numerical Modeling in Biomedical Applications: A Review. Micromachines 2024, 15, 873. [Google Scholar] [CrossRef]
- Cao, X.; Jiang, C.; Tan, D.; Li, Q.; Bi, S.; Song, J. Recent Mechanical Processing Techniques of Two-Dimensional Layered Materials: A Review. J. Sci. Adv. Mater. Devices 2021, 6, 135–152. [Google Scholar] [CrossRef]
- Stokes, K.; Clark, K.; Odetade, D.; Hardy, M.; Goldberg Oppenheimer, P. Advances in Lithographic Techniques for Precision Nanostructure Fabrication in Biomedical Applications. Discov. Nano 2023, 18, 153. [Google Scholar] [CrossRef]
- Ma, R.; Zhang, X.; Sutherland, D.; Bochenkov, V.; Deng, S. Nanofabrication of Nanostructure Lattices: From High-Quality Large Patterns to Precise Hybrid Units. Int. J. Extrem. Manuf. 2024, 6, 062004. [Google Scholar] [CrossRef]
- Yang, W.; Zhou, J.; Tsai, D.P.; Xiao, S. Advanced Manufacturing of Dielectric Meta-Devices. Photonics Insights 2024, 3, R04. [Google Scholar] [CrossRef]
- Zhang, Y.; Remy, M.; Leste-Lasserre, T.; Durrieu, M.-C. Manipulating Stem Cell Fate with Disordered Bioactive Cues on Surfaces: The Role of Bioactive Ligand Selection. ACS Appl. Mater. Interfaces 2024, 16, 18474–18489. [Google Scholar] [CrossRef] [PubMed]
- Yi, K.; Ao, M.; Ding, T.; Zheng, D.; Li, L. Bowtie Nanoantenna LSPR Biosensor for Early Prediction of Preeclampsia. Biosensors 2024, 14, 317. [Google Scholar] [CrossRef] [PubMed]
- Wen, B.; Yang, J.; Hu, C.; Cai, J.; Zhou, J. Top-Down Fabrication of Ordered Nanophotonic Structures for Biomedical Applications. Adv. Mater. Interfaces 2024, 11, 2300856. [Google Scholar] [CrossRef]
- Zhu, C.; Ekinci, H.; Pan, A.; Cui, B.; Zhu, X. Electron Beam Lithography on Nonplanar and Irregular Surfaces. Microsystems Nanoeng. 2024, 10, 52. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Lyu, Y.; Bosiakov, S.; Zhu, H.; Ren, Y. A Review on the Mechanical Metamaterials and Their Applications in the Field of Biomedical Engineering. Front. Mater. 2023, 10, 1273961. [Google Scholar] [CrossRef]
- Trucillo, P. Biomaterials for Drug Delivery and Human Applications. Materials 2024, 17, 456. [Google Scholar] [CrossRef]
- Lakkala, P.; Munnangi, S.R.; Bandari, S.; Repka, M. Additive Manufacturing Technologies with Emphasis on Stereolithography 3D Printing in Pharmaceutical and Medical Applications: A Review. Int. J. Pharm. X 2023, 5, 100159. [Google Scholar] [CrossRef]
- Sabbioni, A.; Rosa, L.; Bujari, A.; Foschini, L.; Corradi, A. DIFFUSE: A DIstributed and Decentralized PlatForm Enabling Function Composition in Serverless Environments. Comput. Networks 2022, 210, 108993. [Google Scholar] [CrossRef]
- Brooks, A.K.; Yadavalli, V.K. Post-Print Processing to Minimize Cytotoxicity of <scp>3D</Scp> -Printed Photopolymer Resins for Biomedical Applications. J. Appl. Polym. Sci. 2025, 142, e56545. [Google Scholar] [CrossRef]
- Naresh, V.; Lee, N. A Review on Biosensors and Recent Development of Nanostructured Materials-Enabled Biosensors. Sensors 2021, 21, 1109. [Google Scholar] [CrossRef]
- Aazmi, A.; Zhang, D.; Mazzaglia, C.; Yu, M.; Wang, Z.; Yang, H.; Huang, Y.Y.S.; Ma, L. Biofabrication Methods for Reconstructing Extracellular Matrix Mimetics. Bioact. Mater. 2024, 31, 475–496. [Google Scholar] [CrossRef]
- Huang, Z.; Chen, Z.; Cheng, J.; Zhang, J.; Wang, S.; Chen, T.; Zhang, X.; Pang, H. Recent Progress in High-Throughput On-Chip Synthesis, Screening, and Data-Driven Optimization: Toward an Electrocatalyst Chip for Catalysis Universe Exploration. Adv. Funct. Mater. 2024, 35, 2416117. [Google Scholar] [CrossRef]
- Cai, M.; Yang, J.; Lu, X.; Lu, X. Layer-by-Layer Self-Assembly Strategies of Atomically Thin Two-Dimensional Nanomaterials: Principles, Methods, and Functional Applications. ACS Appl. Nano Mater. 2024, 7, 27940–27959. [Google Scholar] [CrossRef]
- Araste, F.; Aliabadi, A.; Abnous, K.; Taghdisi, S.M.; Ramezani, M.; Alibolandi, M. Self-Assembled Polymeric Vesicles: Focus on Polymersomes in Cancer Treatment. J. Control. Release 2021, 330, 502–528. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Wu, J.; Wang, R.; Zhong, Q. Nanostructures Self-Assembled from Food-Grade Molecules with PH-Cycle as Functional Food Ingredients. Trends Food Sci. Technol. 2022, 120, 36–47. [Google Scholar] [CrossRef]
- Chung, Y.-H.; Oh, J.K. Research Trends in the Development of Block Copolymer-Based Biosensing Platforms. Biosensors 2024, 14, 542. [Google Scholar] [CrossRef]
- Yan, J.; Savenije, T.J.; Mazzarella, L.; Isabella, O. Progress and Challenges on Scaling up of Perovskite Solar Cell Technology. Sustain. Energy Fuels 2022, 6, 243–266. [Google Scholar] [CrossRef]
- Vlachopoulos, A.; Karlioti, G.; Balla, E.; Daniilidis, V.; Kalamas, T.; Stefanidou, M.; Bikiaris, N.D.; Christodoulou, E.; Koumentakou, I.; Karavas, E.; et al. Poly(Lactic Acid)-Based Microparticles for Drug Delivery Applications: An Overview of Recent Advances. Pharmaceutics 2022, 14, 359. [Google Scholar] [CrossRef]
- Blasi, P. Poly(Lactic Acid)/Poly(Lactic-Co-Glycolic Acid)-Based Microparticles: An Overview. J. Pharm. Investig. 2019, 49, 337–346. [Google Scholar] [CrossRef]
- Visan, A.I.; Negut, I. Development and Applications of PLGA Hydrogels for Sustained Delivery of Therapeutic Agents. Gels 2024, 10, 497. [Google Scholar] [CrossRef] [PubMed]
- Madani, F.; Esnaashari, S.S.; Bergonzi, M.C.; Webster, T.J.; Younes, H.M.; Khosravani, M.; Adabi, M. Paclitaxel/Methotrexate Co-Loaded PLGA Nanoparticles in Glioblastoma Treatment: Formulation Development and in Vitro Antitumor Activity Evaluation. Life Sci. 2020, 256, 117943. [Google Scholar] [CrossRef]
- Yarahmadi, A.; Dousti, B.; Karami-Khorramabadi, M.; Afkhami, H. Materials Based on Biodegradable Polymers Chitosan/Gelatin: A Review of Potential Applications. Front. Bioeng. Biotechnol. 2024, 12, 1397668. [Google Scholar] [CrossRef]
- Nguyen, H.T.T.; Do, N.H.N.; Lac, H.D.; Nguyen, P.L.N.; Le, P.K. Synthesis, Properties, and Applications of Chitosan Hydrogels as Anti-Inflammatory Drug Delivery System. J. Porous Mater. 2023, 30, 655–670. [Google Scholar] [CrossRef]
- Zhao, Y.; Tian, C.; Wu, P.; Chen, F.; Xiao, A.; Ye, Q.; Shi, X.; Wang, Z.; Han, X.; Chen, Y. Hydroxypropyl Chitosan/Soy Protein Isolate Conduits Promote Peripheral Nerve Regeneration. Tissue Eng. Part A 2022, 28, 225–238. [Google Scholar] [CrossRef] [PubMed]
- Halder, A.; Sun, Y. Biocompatible Propulsion for Biomedical Micro/Nano Robotics. Biosens. Bioelectron. 2019, 139, 111334. [Google Scholar] [CrossRef] [PubMed]
- Shariatinia, Z. Big Family of Nano- and Microscale Drug Delivery Systems Ranging from Inorganic Materials to Polymeric and Stimuli-Responsive Carriers as Well as Drug-Conjugates. J. Drug Deliv. Sci. Technol. 2021, 66, 102790. [Google Scholar] [CrossRef]
- Kalyane, D.; Raval, N.; Maheshwari, R.; Tambe, V.; Kalia, K.; Tekade, R.K. Employment of Enhanced Permeability and Retention Effect (EPR): Nanoparticle-Based Precision Tools for Targeting of Therapeutic and Diagnostic Agent in Cancer. Mater. Sci. Eng. C 2019, 98, 1252–1276. [Google Scholar] [CrossRef]
- Odeh, F.; Nsairat, H.; Alshaer, W.; Ismail, M.A.; Esawi, E.; Qaqish, B.; Bawab, A.A.; Ismail, S.I. Aptamers Chemistry: Chemical Modifications and Conjugation Strategies. Molecules 2019, 25, 3. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Li, Z.; Si, J. Nanocarriers in Gene Therapy: A Review. J. Biomed. Nanotechnol. 2014, 10, 3483–3507. [Google Scholar] [CrossRef]
- Yao, T.; Baker, M.B.; Moroni, L. Strategies to Improve Nanofibrous Scaffolds for Vascular Tissue Engineering. Nanomaterials 2020, 10, 887. [Google Scholar] [CrossRef]
- Bružauskaitė, I.; Bironaitė, D.; Bagdonas, E.; Bernotienė, E. Scaffolds and Cells for Tissue Regeneration: Different Scaffold Pore Sizes—Different Cell Effects. Cytotechnology 2016, 68, 355–369. [Google Scholar] [CrossRef] [PubMed]
- Marin, D.; Marchesan, S. Self-Assembled Peptide Nanostructures for ECM Biomimicry. Nanomaterials 2022, 12, 2147. [Google Scholar] [CrossRef] [PubMed]
- Novosel, E.C.; Kleinhans, C.; Kluger, P.J. Vascularization Is the Key Challenge in Tissue Engineering. Adv. Drug Deliv. Rev. 2011, 63, 300–311. [Google Scholar] [CrossRef] [PubMed]
- Chu, S.; Shi, X.; Tian, Y.; Gao, F. PH-Responsive Polymer Nanomaterials for Tumor Therapy. Front. Oncol. 2022, 12, 855019. [Google Scholar] [CrossRef]
- Joseph, T.M.; Thomas, M.G.; Mahapatra, D.K.; Unni, A.B.; Kianfar, E.; Haponiuk, J.T.; Thomas, S. Adaptive and Intelligent Polyurethane Shape-Memory Polymers Enabling next-Generation Biomedical Platforms. Case Stud. Chem. Environ. Eng. 2025, 11, 101165. [Google Scholar] [CrossRef]
- Parak, A.; Pradeep, P.; du Toit, L.C.; Kumar, P.; Choonara, Y.E.; Pillay, V. Functionalizing Bioinks for 3D Bioprinting Applications. Drug Discov. Today 2019, 24, 198–205. [Google Scholar] [CrossRef]
- Tian, M.; Keshavarz, M.; Demircali, A.A.; Han, B.; Yang, G. Localized Microrobotic Delivery of Enzyme-Responsive Hydrogel-Immobilized Therapeutics to Suppress Triple-Negative Breast Cancer. Small 2024. ahead of print. [Google Scholar] [CrossRef]
- Gomte, S.S.; Agnihotri, T.G.; Khopade, S.; Jain, A. Exploring the Potential of PH-Sensitive Polymers in Targeted Drug Delivery. J. Biomater. Sci. Polym. Ed. 2024, 35, 228–268. [Google Scholar] [CrossRef]
- Lacroce, E.; Rossi, F. Polymer-Based Thermoresponsive Hydrogels for Controlled Drug Delivery. Expert Opin. Drug Deliv. 2022, 19, 1203–1215. [Google Scholar] [CrossRef]
- Zhao, W.; Zhao, Y.; Wang, Q.; Liu, T.; Sun, J.; Zhang, R. Remote Light-Responsive Nanocarriers for Controlled Drug Delivery: Advances and Perspectives. Small 2019, 15, e1903060. [Google Scholar] [CrossRef]
- Sun, W.; Chai, X.; Zhang, Y.; Yu, T.; Wang, Y.; Zhao, W.; Liu, Y.; Yin, D.; Zhang, C. Combination Using Magnetic Iron Oxide Nanoparticles and Magnetic Field for Cancer Therapy. Chem. Rec. 2024, 24, e202400179. [Google Scholar] [CrossRef] [PubMed]
- Sadraei, A.; Naghib, S.M. 4D Printing of Physical Stimuli-Responsive Hydrogels for Localized Drug Delivery and Tissue Engineering. Polym. Rev. 2025, 65, 104–168. [Google Scholar] [CrossRef]
- Shabani, M.; Bodaghi, M. Mechanical-Responsive Materials: Properties, Design, and Applications. In Stimuli-Responsive Materials for Biomedical Applications; ACS Publications: Washington, DC, USA, 2023; pp. 129–144. [Google Scholar]
- Li, Y.; Yang, G.; Gerstweiler, L.; Thang, S.H.; Zhao, C. Design of Stimuli-Responsive Peptides and Proteins. Adv. Funct. Mater. 2023, 33, 2210387. [Google Scholar] [CrossRef]
- Cao, Z.-Q.; Wang, G.-J. Multi-Stimuli-Responsive Polymer Materials: Particles, Films, and Bulk Gels. Chem. Rec. 2016, 16, 1398–1435. [Google Scholar] [CrossRef]
- Dotta, T.C.; D’Ercole, S.; Iezzi, G.; Pedrazzi, V.; Galo, R.; Petrini, M. The Interaction between Oral Bacteria and 3D Titanium Porous Surfaces Produced by Selective Laser Melting—A Narrative Review. Biomimetics 2024, 9, 461. [Google Scholar] [CrossRef] [PubMed]
- Udriște, A.S.; Burdușel, A.C.; Niculescu, A.-G.; Rădulescu, M.; Grumezescu, A.M. Coatings for Cardiovascular Stents—An Up-to-Date Review. Int. J. Mol. Sci. 2024, 25, 1078. [Google Scholar] [CrossRef]
- Safarkhani, M.; Aldhaher, A.; Heidari, G.; Zare, E.N.; Warkiani, M.E.; Akhavan, O.; Huh, Y.; Rabiee, N. Nanomaterial-Assisted Wearable Glucose Biosensors for Noninvasive Real-Time Monitoring: Pioneering Point-of-Care and Beyond. Nano Mater. Sci. 2024, 6, 263–283. [Google Scholar] [CrossRef]
- Homa, K.; Zakrzewski, W.; Dobrzyński, W.; Piszko, P.J.; Piszko, A.; Matys, J.; Wiglusz, R.J.; Dobrzyński, M. Surface Functionalization of Titanium-Based Implants with a Nanohydroxyapatite Layer and Its Impact on Osteoblasts: A Systematic Review. J. Funct. Biomater. 2024, 15, 45. [Google Scholar] [CrossRef]
- Wasilewski, T.; Kamysz, W.; Gębicki, J. AI-Assisted Detection of Biomarkers by Sensors and Biosensors for Early Diagnosis and Monitoring. Biosensors 2024, 14, 356. [Google Scholar] [CrossRef]
- Le-Petross, H.T.; Scoggins, M.E.; Clemens, M.W. Assessment, Complications, and Surveillance of Breast Implants: Making Sense of 2022 FDA Breast Implant Guidance. J. Breast Imaging 2023, 5, 360–372. [Google Scholar] [CrossRef]
- Glicksman, C.; Wolfe, A.; McGuire, P. The Study of the Safety and Effectiveness of Motiva SmoothSilk Silicone Gel-Filled Breast Implants in Patients Undergoing Primary and Revisional Breast Augmentation: Three-Year Clinical Data. Aesthetic Surg. J. 2024, 44, 1273–1285. [Google Scholar] [CrossRef]
- Bender, C.; Vestergaard, P.; Cichosz, S.L. The History, Evolution and Future of Continuous Glucose Monitoring (CGM). Diabetology 2025, 6, 17. [Google Scholar] [CrossRef]
- Hayano, J.; Yamamoto, H.; Nonaka, I.; Komazawa, M.; Itao, K.; Ueda, N.; Tanaka, H.; Yuda, E. Quantitative Detection of Sleep Apnea with Wearable Watch Device. PLoS ONE 2020, 15, e0237279. [Google Scholar] [CrossRef]
- Hemdan, M.; Ali, M.A.; Doghish, A.S.; Mageed, S.S.A.; Elazab, I.M.; Khalil, M.M.; Mabrouk, M.; Das, D.B.; Amin, A.S. Innovations in Biosensor Technologies for Healthcare Diagnostics and Therapeutic Drug Monitoring: Applications, Recent Progress, and Future Research Challenges. Sensors 2024, 24, 5143. [Google Scholar] [CrossRef]
- Huang, X.; O’Connor, R.; Kwizera, E.A. Gold Nanoparticle Based Platforms for Circulating Cancer Marker Detection. Nanotheranostics 2017, 1, 80–102. [Google Scholar] [CrossRef]
- Ince, B.; Sezgintürk, M.K. Lateral Flow Assays for Viruses Diagnosis: Up-to-Date Technology and Future Prospects. TrAC Trends Anal. Chem. 2022, 157, 116725. [Google Scholar] [CrossRef]
- Peng, Y.; Wang, X.; Wang, Y.; Gao, Y.; Guo, R.; Shi, X.; Cao, X. Macrophage-Laden Gold Nanoflowers Embedded with Ultrasmall Iron Oxide Nanoparticles for Enhanced Dual-Mode CT/MR Imaging of Tumors. Pharmaceutics 2021, 13, 995. [Google Scholar] [CrossRef]
- Mitchell, K.R.; Esene, J.E.; Woolley, A.T. Advances in Multiplex Electrical and Optical Detection of Biomarkers Using Microfluidic Devices. Anal. Bioanal. Chem. 2022, 414, 167–180. [Google Scholar] [CrossRef]
- Shand, H.; Dutta, S.; Rajakumar, S.; James Paulraj, S.; Mandal, A.K.; KT, R.D.; Ghorai, S. New Age Detection of Viruses: The Nano-Biosensors. Front. Nanotechnol. 2022, 3, 814550. [Google Scholar] [CrossRef]
- Kumar, S.; Singh, H.; Feder-Kubis, J.; Nguyen, D.D. Recent Advances in Nanobiosensors for Sustainable Healthcare Applications: A Systematic Literature Review. Environ. Res. 2023, 238, 117177. [Google Scholar] [CrossRef]
- Naseri, N.; Ajorlou, E.; Asghari, F.; Pilehvar-Soltanahmadi, Y. An Update on Nanoparticle-Based Contrast Agents in Medical Imaging. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1111–1121. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Hou, H.; Li, J. Frontiers in Fluorescence Imaging: Tools for the In Situ Sensing of Disease Biomarkers. J. Mater. Chem. B 2025, 13, 1133–1158. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B.; Sharma, A. Microfluidics: Recent Advances Toward Lab-on-Chip Applications in Bioanalysis. Adv. Eng. Mater. 2022, 24, 2100738. [Google Scholar] [CrossRef]
- Kuru, C.İ.; Ulucan-Karnak, F.; Akgöl, S. Lab-on-a-Chip Sensors. In Fundamentals of Sensor Technology; Elsevier: Amsterdam, The Netherlands, 2023; pp. 65–98. [Google Scholar]
- Ji, Y.; Wang, Y.; Wang, X.; Lv, C.; Zhou, Q.; Jiang, G.; Yan, B.; Chen, L. Beyond the Promise: Exploring the Complex Interactions of Nanoparticles within Biological Systems. J. Hazard. Mater. 2024, 468, 133800. [Google Scholar] [CrossRef] [PubMed]
- Pei, B.; Wang, W.; Fan, Y.; Wang, X.; Watari, F.; Li, X. Fiber-Reinforced Scaffolds in Soft Tissue Engineering. Regen. Biomater. 2017, 4, 257–268. [Google Scholar] [CrossRef]
- Kangarshahi, B.M.; Naghib, S.M.; Kangarshahi, G.M.; Mozafari, M.R. Bioprinting of Self-Healing Materials and Nanostructures for Biomedical Applications: Recent Advances and Progresses on Fabrication and Characterization Techniques. Bioprinting 2024, 38, e00335. [Google Scholar] [CrossRef]
- Karimi, K.; Fardoost, A.; Mhatre, N.; Rajan, J.; Boisvert, D.; Javanmard, M. A Thorough Review of Emerging Technologies in Micro- and Nanochannel Fabrication: Limitations, Applications, and Comparison. Micromachines 2024, 15, 1274. [Google Scholar] [CrossRef]
- Gurunathan, S.; Kim, J.-H. Synthesis, Toxicity, Biocompatibility, and Biomedical Applications of Graphene and Graphene-Related Materials. Int. J. Nanomed. 2016, 11, 1927–1945. [Google Scholar] [CrossRef]
- Manivasagam, V.K.; Sabino, R.M.; Kantam, P.; Popat, K.C. Surface Modification Strategies to Improve Titanium Hemocompatibility: A Comprehensive Review. Mater. Adv. 2021, 2, 5824–5842. [Google Scholar] [CrossRef]
- Fuchs, S.; Shariati, K.; Ma, M. Specialty Tough Hydrogels and Their Biomedical Applications. Adv. Healthc. Mater. 2020, 9, e1901396. [Google Scholar] [CrossRef]
- Srivastav, R.S.; More, A.P. A Comprehensive Review of Self-Healing Polymers: Mechanisms, Types, and Industry Implications. Polym. Adv. Technol. 2025, 36, e70092. [Google Scholar] [CrossRef]
- Chen, Z.; Lin, Y.-T.; Salehi, H.; Che, Z.; Zhu, Y.; Ding, J.; Sheng, B.; Zhu, R.; Jiao, P. Advanced Fabrication of Mechanical Metamaterials Based on Micro/Nanoscale Technology. Adv. Eng. Mater. 2023, 25, 2300750. [Google Scholar] [CrossRef]
- Ayanda, O.S.; Mmuoegbulam, A.O.; Okezie, O.; Durumin Iya, N.I.; Mohammed, S.E.; James, P.H.; Muhammad, A.B.; Unimke, A.A.; Alim, S.A.; Yahaya, S.M.; et al. Recent Progress in Carbon-Based Nanomaterials: Critical Review. J. Nanoparticle Res. 2024, 26, 106. [Google Scholar] [CrossRef]
- Melchels, F.P.W.; Domingos, M.A.N.; Klein, T.J.; Malda, J.; Bartolo, P.J.; Hutmacher, D.W. Additive Manufacturing of Tissues and Organs. Prog. Polym. Sci. 2012, 37, 1079–1104. [Google Scholar] [CrossRef]
- Kanamala, M.; Wilson, W.R.; Yang, M.; Palmer, B.D.; Wu, Z. Mechanisms and Biomaterials in PH-Responsive Tumour Targeted Drug Delivery: A Review. Biomaterials 2016, 85, 152–167. [Google Scholar] [CrossRef]
- de Jesús Plasencia-Salgueiro, A. Deep Reinforcement Learning for Autonomous Mobile Robot Navigation. In Artificial Intelligence for Robotics and Autonomous Systems Applications; Springer: Cham, Switzerland, 2023; pp. 195–237. [Google Scholar]
- Alghamdi, M.A.; Fallica, A.N.; Virzì, N.; Kesharwani, P.; Pittalà, V.; Greish, K. The Promise of Nanotechnology in Personalized Medicine. J. Pers. Med. 2022, 12, 673. [Google Scholar] [CrossRef]
- Mazumdar, H.; Khondakar, K.R.; Das, S.; Halder, A.; Kaushik, A. Artificial Intelligence for Personalized Nanomedicine; from Material Selection to Patient Outcomes. Expert Opin. Drug Deliv. 2025, 22, 85–108. [Google Scholar] [CrossRef]
- Betz, U.A.K.; Arora, L.; Assal, R.A.; Azevedo, H.; Baldwin, J.; Becker, M.S.; Bostock, S.; Cheng, V.; Egle, T.; Ferrari, N.; et al. Game Changers in Science and Technology—Now and Beyond. Technol. Forecast. Soc. Change 2023, 193, 122588. [Google Scholar] [CrossRef]
- Molla, G.; Bitew, M. Revolutionizing Personalized Medicine: Synergy with Multi-Omics Data Generation, Main Hurdles, and Future Perspectives. Biomedicines 2024, 12, 2750. [Google Scholar] [CrossRef]
- Sabet, M. From Self-Assembly to Sustainability: Advanced Polymerization Techniques for Energy, Healthcare, and Robotics. Polym. Technol. Mater. 2024, 64, 763–793. [Google Scholar] [CrossRef]
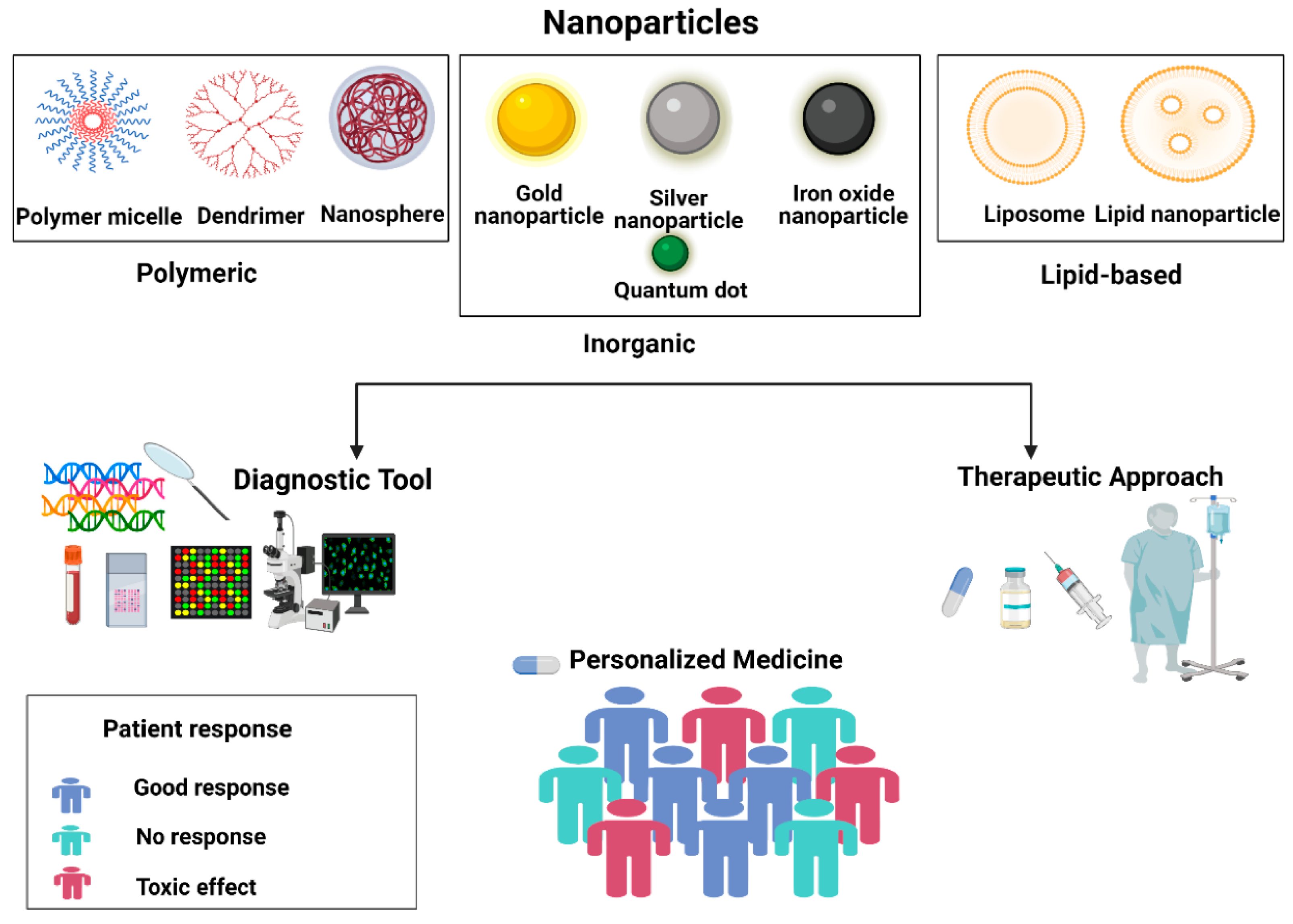

| Material Type | Examples | Key Properties | Applications in Biomedical Engineering | Ref. |
|---|---|---|---|---|
| Polymeric Materials | PLA, PCL, PEG, Chitosan, Gelatin | Biocompatibility, biodegradability, tunable mechanical properties | Drug delivery, tissue scaffolding, wound healing, controlled release systems | [7] |
| Inorganic Materials | Gold nanoparticles, Iron oxide, Silica | Stability, optical and magnetic properties | Imaging agents, contrast-enhancement, drug delivery, diagnostics | [8] |
| Natural Biomaterials | Collagen, Alginate, Silk fibroin | Biodegradable, bioactive, cell-interactive | Tissue engineering, wound healing, cell culture scaffolds | [9] |
| Robotic/Bioinspired | Self-assembled structures, 3D-printed scaffolds | High-precision, complex geometries | Fabrication of complex tissue architectures, personalized implants | [10] |
| Nanostructures | Carbon nanotubes, Quantum dots | High surface area; electrical, optical, and magnetic properties | Biosensing, diagnostics, targeted therapy, drug delivery | [11] |
| Fabrication Technique | Principle | Materials Used | Resolution | Advantages | Limitations | Applications |
|---|---|---|---|---|---|---|
| Lithography-Based Methods | Patterning of materials using light or electron beams to create nanoscale structures | Metals, polymers, semiconductors | Sub-micron to nanometer scale | High precision, high throughput | Expensive, limited to flat surfaces, complex setup | Fabrication of neural scaffolds, microfluidic platforms for organ-on-a-chip systems, nanopatterned surfaces for stem cell differentiation |
| Photolithography | Uses light exposure on a photosensitive material to form patterns | Polymers, silicon | ~100 nm | Mature technology, high throughput | Requires expensive equipment, limited resolution | Microfabrication of lab-on-a-chip devices, biosensor arrays, and microelectrode arrays for neural interfaces |
| Electron Beam Lithography (e-beam) | Uses focused electron beam to create patterns directly on a substrate | Polymers, metals, semiconductors | Sub-10 nm | High resolution, direct patterning | Slow, high cost, complex equipment | Nanostructured substrates for biomolecule detection, nanopatterned surfaces for tissue engineering, fabrication of nanostructured drug carriers |
| 3D Printing Technologies | Layer-by-layer additive manufacturing of materials to create complex 3D structures | Polymers, ceramics, hydrogels, metals | Micro- to millimeter scale | Flexible, customizable, cost-effective for prototyping | Limited resolution, material limitations, slow process | Patient-specific implants, 3D-printed vascularized tissue constructs, bioactive scaffolds for tissue regeneration |
| Stereolithography (SLA) | Uses UV light to cure liquid resin into solid layers | Photopolymer resins | ~50–100 µm | High resolution, fast prototyping | Limited material choice, post-processing required | Dental implants, surgical guides, patient-specific bone grafts |
| Two-Photon Polymerization | Uses focused laser to polymerize materials in a highly localized manner | Photopolymer resins | <100 nm | High resolution, 3D printing of complex structures | Slow, limited material options, high cost | Fabrication of microvascular networks, nanostructured scaffolds for neural regeneration |
| Self-Assembly Techniques | Spontaneous organization of molecules or particles into desired structures | Nanoparticles, block copolymers, biomolecules | Nanometer scale | Low cost, minimal energy input, scalable | Requires precise control over conditions, limited scalability | Smart drug carriers, bioinspired membranes for controlled drug release, self-assembled peptide hydrogels for wound healing |
| Molecular Self-Assembly | Molecules spontaneously form ordered structures due to intermolecular interactions | Organic molecules, nanoparticles | Nanometer scale | Simple, energy-efficient, cost-effective | Limited control over large-scale organization, slow process | Nanoparticle synthesis for targeted therapy, biomimetic hydrogels for wound healing |
| Block Copolymer Self-Assembly | Block copolymers self-assemble into nanoscale structures based on phase separation | Block copolymers, polymers | ~10–100 nm | High precision, versatile | Requires specific conditions, material limitations | Nanostructured drug carriers, porous scaffolds for regenerative medicine, biomimetic membranes for biosensing |
| Type of Stimuli-Responsive Material | Stimulus | Mechanism | Applications | Advantages | Limitations | Ref. |
|---|---|---|---|---|---|---|
| pH-Responsive Polymers | pH changes (e.g., acidic or alkaline environments) | Protonation or deprotonation of functional groups (e.g., carboxyl or amine groups) changes material solubility or swelling behavior. | Tumor-targeted drug delivery, gastrointestinal DDS. | High specificity in acidic environments (e.g., tumors) | Limited to environments with significant pH gradients; risk of premature degradation. | [71] |
| Thermo-Responsive Polymers | Temperature variations | Phase transition occurs at critical solution temperature (LCST or UCST), altering solubility. | Injectable hydrogels for tissue regeneration, smart drug carriers. | Minimally invasive; temperature-sensitive control | Potential loss of function in fluctuating body temperature conditions. | [72] |
| Light-Responsive Materials | UV, visible, or NIR light | Photoisomerization or photothermal conversion induces structural changes or triggers release. | Photothermal therapy, on-demand drug release, bio-imaging. | High spatiotemporal control; non-invasive activation | Limited tissue penetration depth for light (especially UV or visible); phototoxicity. | [73] |
| Magneto-Responsive Materials | Magnetic fields | Magnetic nanoparticles (e.g., Fe3O4) heat under an alternating magnetic field or align for targeted movement. | Hyperthermia therapy, guided drug delivery. | Remote activation; deeper penetration possible | Requires external magnetic fields and specialized equipment. | [74] |
| Electro-Responsive Polymers | Electric fields | Change in electrical potential alters molecular alignment or triggers ion transport. | Neural tissue engineering, electroactive drug release. | Precise electrical control; compatibility with bioelectronics | Risk of local heating or cell damage from high-intensity electrical fields. | [75] |
| Mechanical-Responsive Polymers | Pressure, shear, or strain | Changes in structure (e.g., micropores open/close under stress) or release of encapsulated drugs. | Wound healing dressings, wearable sensors. | Responds to external forces; no additional stimuli needed | Difficult to achieve controlled and uniform response under variable mechanical forces. | [76] |
| Enzyme-Responsive Polymers | Specific enzymes | Enzymatic degradation of polymer matrix or release of drugs upon enzyme-triggered cleavage. | Cancer therapy (elevated enzyme levels in tumors), infection-responsive systems. | High specificity to biological microenvironments | Limited by enzyme activity and concentration in target tissue. | [77] |
| Multi-Responsive Materials | Combination of stimuli | Integration of dual or multiple responses (e.g., pH + light, temperature + magnetic fields). | Cancer therapy, smart scaffolds, controlled release. | Synergistic response; greater versatility | Complex design and fabrication; difficulty in balancing stimuli sensitivity. | [78] |
| Application | Key Technology | Mechanism | Advantages | Limitations | Ref. |
|---|---|---|---|---|---|
| Biosensing and Detection Technologies | Nano-Biosensors | Detection of disease biomarkers (e.g., proteins, DNA, RNA) using nanoparticles or nanomaterials (e.g., gold, graphene) to enhance sensitivity. | High sensitivity, rapid detection, ability to detect low concentrations of biomarkers. | Sensitivity can be affected by non-specific binding; need for precise functionalization of nanoparticles to improve selectivity. | [93] |
| Integration with Point-of-Care Devices | Portable devices that integrate nano-biosensors for onsite disease detection (e.g., glucose testing, cancer biomarker detection) in real-time. | Enables rapid diagnostics in resource-limited settings; portable and easy to use. | Limited in terms of detectable diseases due to sensor specificity, sample preparation challenges, and biomarker stability; difficulties in detecting multiplexed biomarkers in complex biological samples. | [94] | |
| Imaging and Contrast-Enhancing Agents | Nanoparticle-Based Contrast Agents | Use of nanoparticles (e.g., gold, silica, iron oxide) to enhance imaging in techniques such as MRI, CT, and ultrasound. | Enhanced imaging quality, improved tissue contrast, targeted imaging for early detection. | Risk of nanoparticle toxicity; challenges in controlling biodistribution and clearance from the body. | [95] |
| Quantum Dots and Fluorescent Nanoparticles | Fluorescent nanoparticles that provide high-resolution imaging with multi-color capability. | Superior resolution, multiplexing capabilities, non-invasive monitoring. | Potential toxicity in vivo, photobleaching over time affecting long-term imaging accuracy. | [96] | |
| Lab-on-a-Chip and Microfluidic Platforms | Microfluidic Devices | Miniaturized systems that use small-scale fluid handling (microchannels) to analyze biological samples with high efficiency. | High throughput, low sample and reagent consumption, integration with other diagnostic tools. | Complexity in device design; limitations in large-scale manufacturing and high initial costs. | [97] |
| Lab-on-a-Chip (LOC) Technology | Integration of various laboratory functions (e.g., PCR, immunoassays) on a single chip for rapid diagnostics. | Faster diagnostics, portable, and requires minimal sample handling. | High manufacturing cost, technical challenges in integrating multiple functions onto a single chip. | [98] |
| Design Parameter | Key Considerations | Materials/Technologies | Advantages | Challenges/Limitations | Ref. |
|---|---|---|---|---|---|
| Biocompatibility and Safety | Interaction with biological systems without causing toxicity or inflammation. | Gold nanoparticles, graphene, biocompatible polymers (e.g., PEG), hydrogels, carbon nanotubes. | Reduced immune response, safe degradation products, compatibility with tissue. | Risk of cytotoxicity, potential immune activation from carbon nanotubes, long-term stability issues, toxicity of degradation products. | [103] |
| Surface modification to enhance compatibility and reduce immunogenicity. | Surface-functionalized nanoparticles, biodegradable polymers. | Enhances material stability, prevents immune activation, improves circulation. | Requires sophisticated surface engineering, in vivo validation needed, risk of altered bio-distribution. | [104] | |
| Mechanical Properties and Resilience | Strength, elasticity, and durability under physiological conditions. | Elastomers, hydrogels, bioactive ceramics, carbon nanotubes, graphene oxide. | High mechanical performance, mimics biological tissue characteristics. | Difficulty in matching mechanical properties with native tissues; mechanical fatigue in long-term applications. | [105] |
| Self-healing capability for longevity and functionality. | Self-healing polymers, dynamic hydrogels, supramolecular hydrogels. | Enhanced durability, recovery from mechanical damage, increased lifespan. | Complexity in designing self-healing materials, limited scalability, potential changes in mechanical strength over time. | [106] | |
| Scalability and Cost-Effectiveness | Ability to produce materials at scale without sacrificing quality. | 3D printing, roll-to-roll processing, photolithography, self-assembly. | Potential for large-scale, low-cost manufacturing; high throughput. | Limited scalability of some techniques (e.g., photolithography), expensive fabrication steps, batch-to-batch variations. | [107] |
| Cost considerations for large-scale production. | Carbon-based materials, biodegradable polymers, low-cost metals. | Cost-effective materials and manufacturing methods, easily available. | High material costs (e.g., gold nanoparticles), complex processing for certain biodegradable polymers. | [108] | |
| Ensuring reproducibility across large batches. | Mass production techniques, automated systems (e.g., inkjet printing, microcontact printing). | Consistent quality across large-scale production. | Variability in material properties across different batches, challenges in high-precision manufacturing, need for rigorous quality control. | [109] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parvin, N.; Joo, S.W.; Jung, J.H.; Mandal, T.K. Innovative Micro- and Nano-Architectures in Biomedical Engineering for Therapeutic and Diagnostic Applications. Micromachines 2025, 16, 419. https://doi.org/10.3390/mi16040419
Parvin N, Joo SW, Jung JH, Mandal TK. Innovative Micro- and Nano-Architectures in Biomedical Engineering for Therapeutic and Diagnostic Applications. Micromachines. 2025; 16(4):419. https://doi.org/10.3390/mi16040419
Chicago/Turabian StyleParvin, Nargish, Sang Woo Joo, Jae Hak Jung, and Tapas K. Mandal. 2025. "Innovative Micro- and Nano-Architectures in Biomedical Engineering for Therapeutic and Diagnostic Applications" Micromachines 16, no. 4: 419. https://doi.org/10.3390/mi16040419
APA StyleParvin, N., Joo, S. W., Jung, J. H., & Mandal, T. K. (2025). Innovative Micro- and Nano-Architectures in Biomedical Engineering for Therapeutic and Diagnostic Applications. Micromachines, 16(4), 419. https://doi.org/10.3390/mi16040419







