Progress in CO2 Gas Sensing Technologies: Insights into Metal Oxide Nanostructures and Resistance-Based Methods
Abstract
:1. Introduction
2. Fundamental Aspects of Metal Oxide-Based Sensors: Key Considerations
- (1)
- Static environment method
- (2)
- Dynamic environment method
Superiority over Other Technologies
- (1)
- Cost-effectiveness [24]: Chemiresistive CO2 sensors use low-cost materials and processes for manufacturing, making them more affordable compared to other sensors.
- (2)
- Miniaturization [41]: These sensors can be designed in compact and miniaturized forms to be suitable for integration into portable devices and IoT (Internet of Things) applications.
- (3)
- Low power consumption: These sensors are designed to operate with low power consumption, extending the operational life of the sensor.
- (4)
- Fast response time [42]: These sensors exhibit a fast response time for real-time monitoring and control applications. This feature is crucial for applications where rapid changes in CO2 levels need to be detected and acted upon quickly.
- (5)
- Selective sensitivity [43]: The sensing materials in the sensor can be designed to show selectivity for the target gas rather than other gases. Due to selective sensitivity, the sensor’s performance increases as the interference from other gases is reduced, providing actual readings.
- (6)
- Operational stability: Chemiresistive sensors show long-term stability and consistent, reliable performance.
- (7)
- Ease of integration [44]: The integration of chemiresistive sensors into various electronic devices is relatively easier than others, facilitating their adoption in various applications, from industrial processes to electronic devices.
- (8)
- Room-temperature operation [22]: Chemiresistive sensors operate very effectively at room temperature, reducing the need for high-temperature conditions.
3. Carbon Dioxide (CO2)
| MO Sensor | Concentration (ppm) | T (°C) | Response (min)/ Recovery (s) | Reference |
|---|---|---|---|---|
| SnO2 film | 1000 | 350 | 1.16/- | [48] |
| 2000 | 240 | 1.24/4 | [49] | |
| 4000 | 240 | 1.71/59 | ||
| 8000 | 240 | 5.86/- | ||
| ZnO film | 1000 | 300 | 1.01/20 | [50] |
| ZnO nanowires | 15 lit/min | 200 | 1.04/40 | [51] |
| ZnO film | 400 | 350 | 2.86/108 | [52] |
| ZnO nanopowder | 5000 | 400 | 1.11/38 | [53] |
| CdO nanowires | 5000 | 250 | 1.03/- | [54] |
| CdO nanoparticles | 4000 | 250 | 1.02/- | [55] |
| CuO film | 100 | RT | 1.04/6 | [56] |
| CeO2 nanopellets | 80 | 400 | 1.32/- | [57] |
| La2O3 film | 350 | 250 | 1.92/73 | [58] |
| TiO2 film | 1500 | 450 | 0.45/55 | [59] |
| Ni-SnO2 nanoparticles | 100 | 27 | 0.04/- | [60] |
| BaTiO3 film | 10,000 | 550 | 1.04/- | [61] |
| Ca-ZnO nanoparticles | 5000 | 300 | 2.0/- | [62] |
| CoAl2O4 mesoporous | 100 | 400 | 0.76/45 | [63] |
| SnO2 | 5000 | 279 | 3.0/- | [58] |
| La2O3 film | 350 | 321 | 1.75/73 | [64] |
| MoO3 | 1000 | 200 | 0.83/20 | [45] |
| ZnO | 500 | RT | 0.24/15.38 | [65] |
| 1000 | RT | 0.38/23.73 | ||
| 1500 | RT | 0.52/32.98 | ||
| 2000 | RT | 0.68/43.21 | ||
| NiO | 500 | RT | 0.30/21.6 | [65] |
| 1000 | RT | 0.37/25.47 | ||
| 1500 | RT | 0.44/30.84 | ||
| 2000 | RT | 0.53/35.28 | ||
| Ni-ZnO | 500 | RT | 0.24/14.67 | [65] |
| 1000 | RT | 0.33/22.4 | ||
| 1500 | RT | 0.39/28.32 | ||
| 2000 | RT | 0.45/33.25 | ||
| 50% La-loaded ZnO | 5000 | 400 | 1.5/38 | [53] |
| Ni-SnO2 nanoparticles | 100 | 275 | 0.067/- | [60] |
| ZnO:Ca nanopowders | 10,000 | 450 | 0.17/10 | [66] |
| LaOCl | 2000 | 260 | 3.40/- | [67] |
| Nd2O2CO3 | 1000 | 350 | 4.00/- | [68] |
| La2O2CO3 nanorods | 3000 | 325 | 7.08/180 | [69] |
| 2500 | 320 | 2.25/120 | ||
| LaFeO3 nanocrystalline | 2000 | 300 | 2.19/- | [70] |
| In2Te3 film | 1000 | RT | 1.12/- | [71] |
| ZnO nanostructures | 100 | 350 | 4.00/5 | [72] |
| 12 | 350 | Poor/- | ||
| BaTiO3-CuO film | 5000 | 300 | 0.3/- | [73] |
| ZnO (unloaded) | 8.5 mbar | 100 | 0.036/- | [74] |
| La-coated SnO2 film | 2500 | 400 | 0.029/- | [75] |
| TiO2 | 1500 | 450 | 0.70/50 | [76] |
| rGO/TiO2 | 1500 | 450 | 0.5/25 | [76] |
| LaOCl-SnO2 nanofibers | 1000 | 300 | 3.7/- | [77] |
| La2O3-SnO2 | 1000 | 350 | 1.6/- | [48] |
| La2O3-SnO2 film | 500 | 250 | 1.42/- | [78] |
| LaOCl-SnO2 | 2000 | 425 | 1.38/- | [79] |
| LaOCl-SnO2 nanowires | 2000 | 400 | 5.6/- | [80] |
| CuO-BaTiO3 | 100 | 456 | 0.42/- | [81] |
| CuO nanoparticles | 400–4000 (r.h-45%) | 25 | 10.0/- | [82] |
| 400–4000 (r.h-45%) | 50 | 7.5/- | ||
| 400–4000 (r.h-45%) | 65 | 6.7/- | ||
| 400–4000 (r.h-45%) | 80 | 5.83/- | ||
| 400–4000 (r.h-45%) | 95 | 2.5/- | ||
| 400–4000 (r.h-45%) | 150 | 1.83/- | ||
| 400–4000 (r.h-60%) | 25 | 11.6/- | ||
| 400–4000 (r.h-60%) | 50 | 8.33/- | ||
| 400–4000 (r.h-60%) | 65 | 7.17/- | ||
| 400–4000 (r.h-60%) | 80 | 5.33/- | ||
| 400–4000 (r.h-60%) | 95 | 2.6/- | ||
| 400–4000 (r.h-60%) | 150 | 1.84/- |
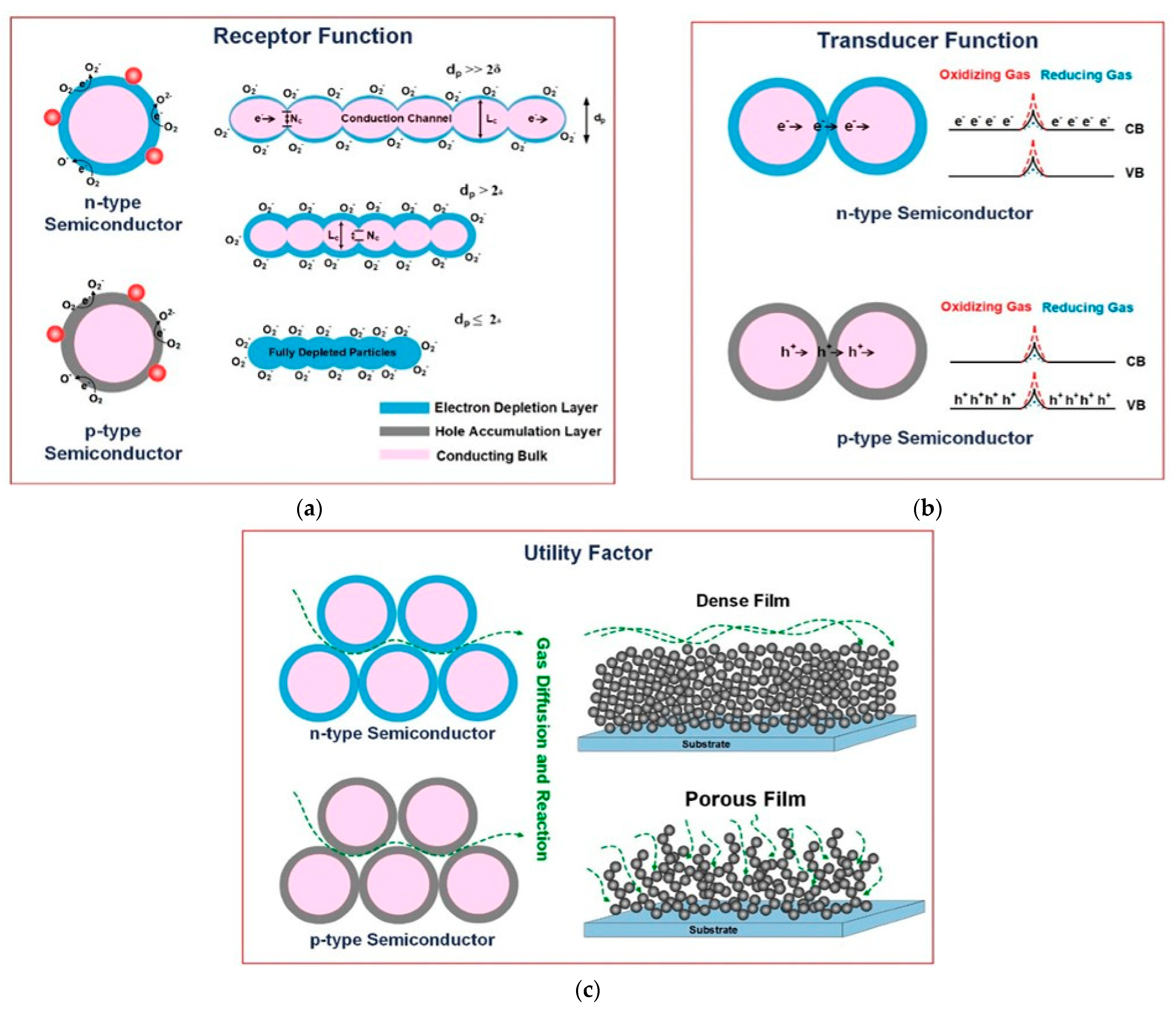
3.1. Detection of CO2 by MO Gas Sensors: Working Principle
3.2. CO2 Specific Sensing Mechanism
3.3. Pristine Metal Oxide
4. Types of MOS Sensing Materials
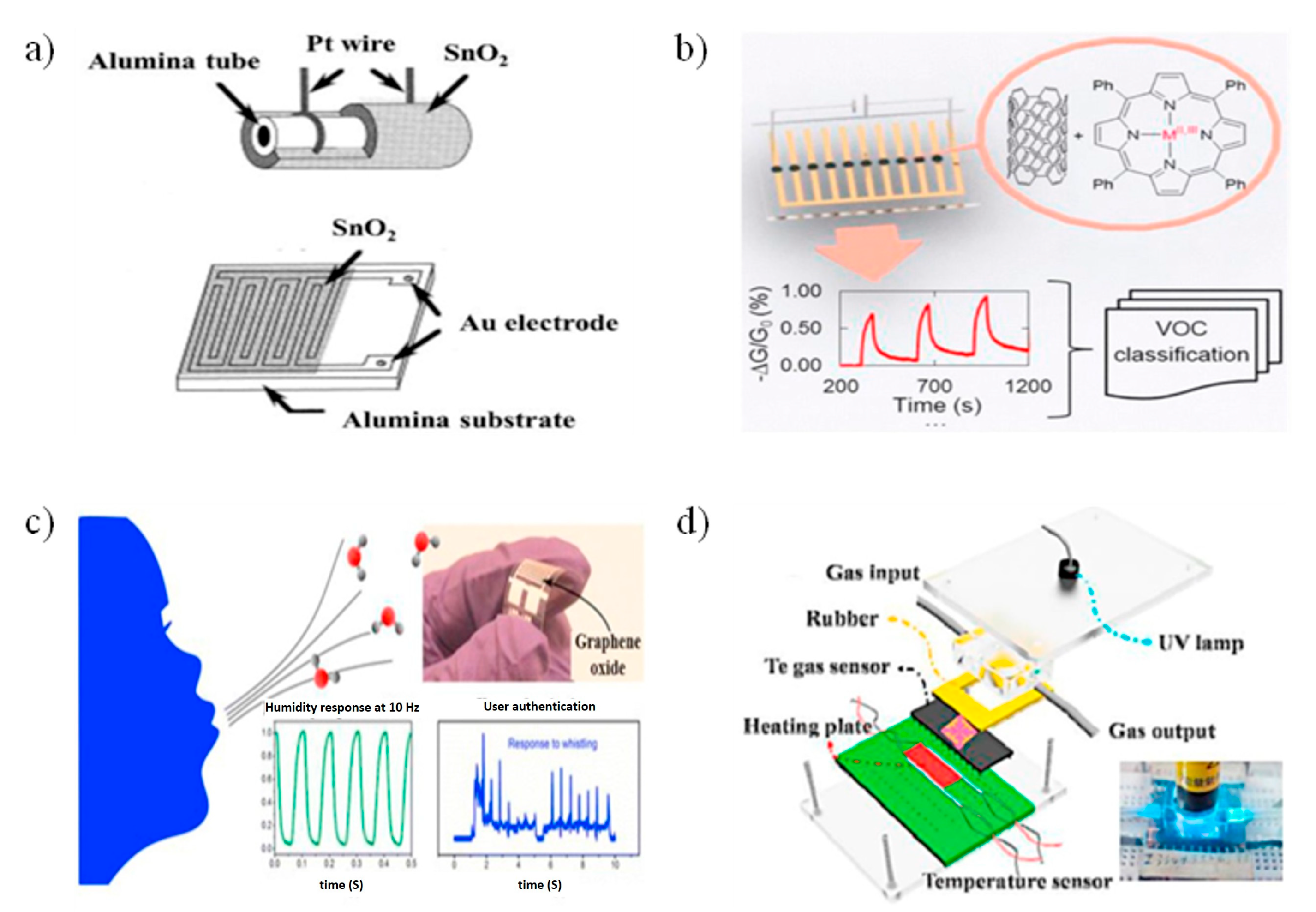
4.1. Tin Oxide (SnO2)
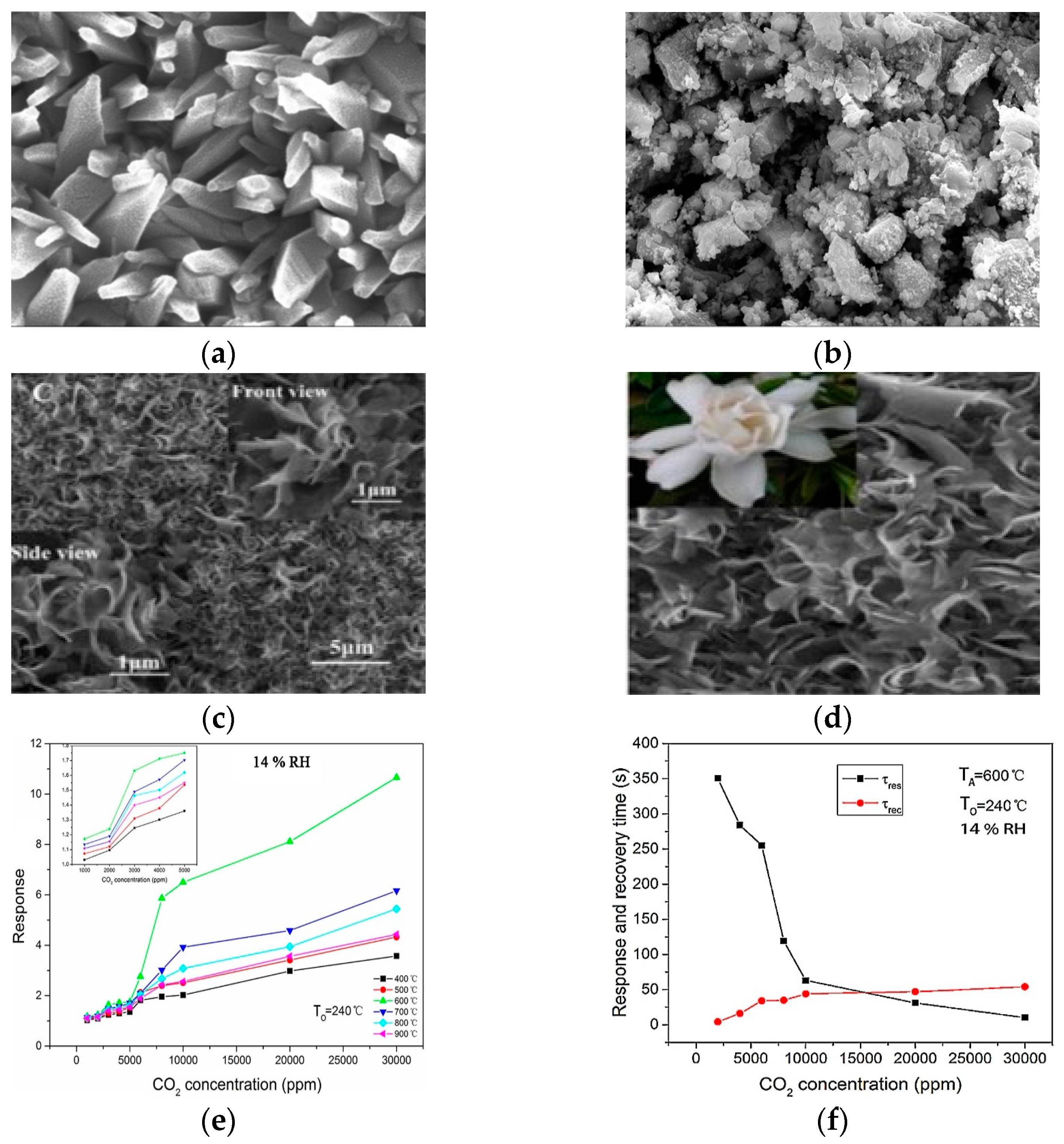
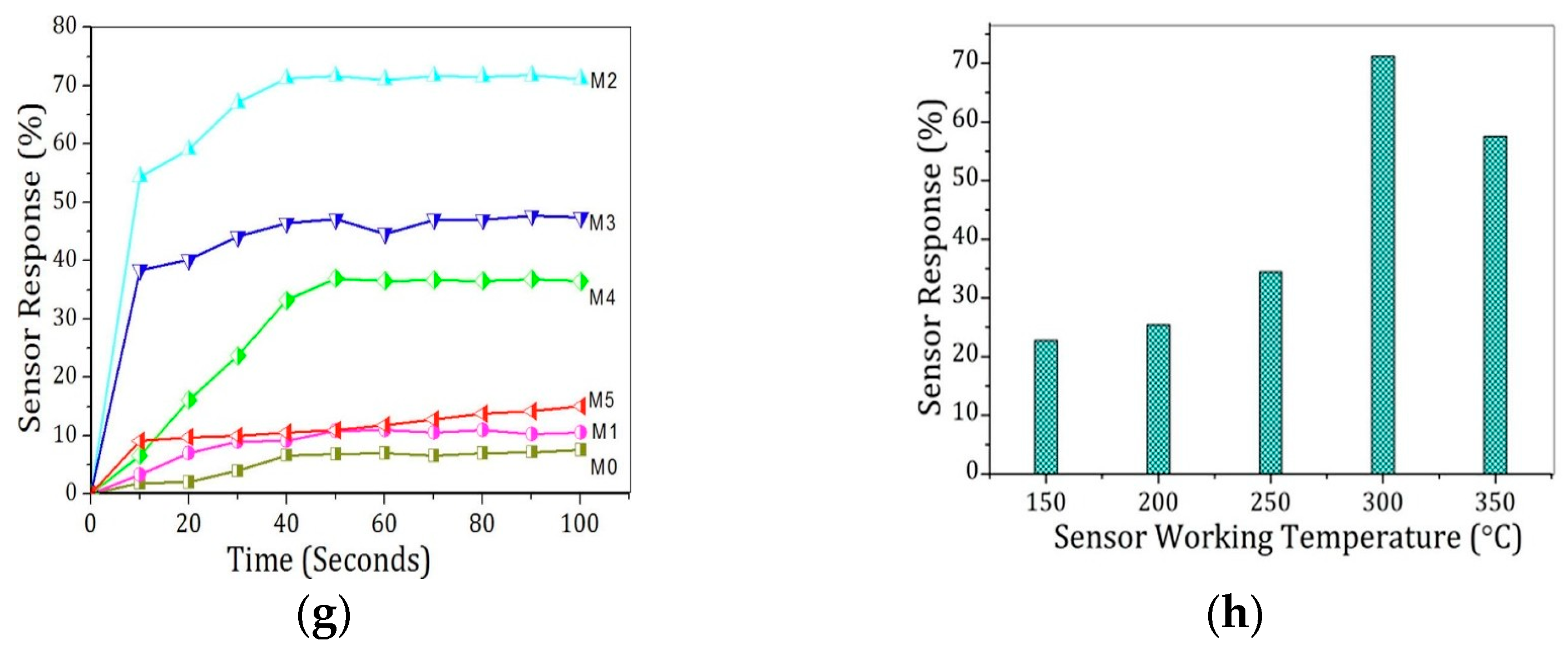
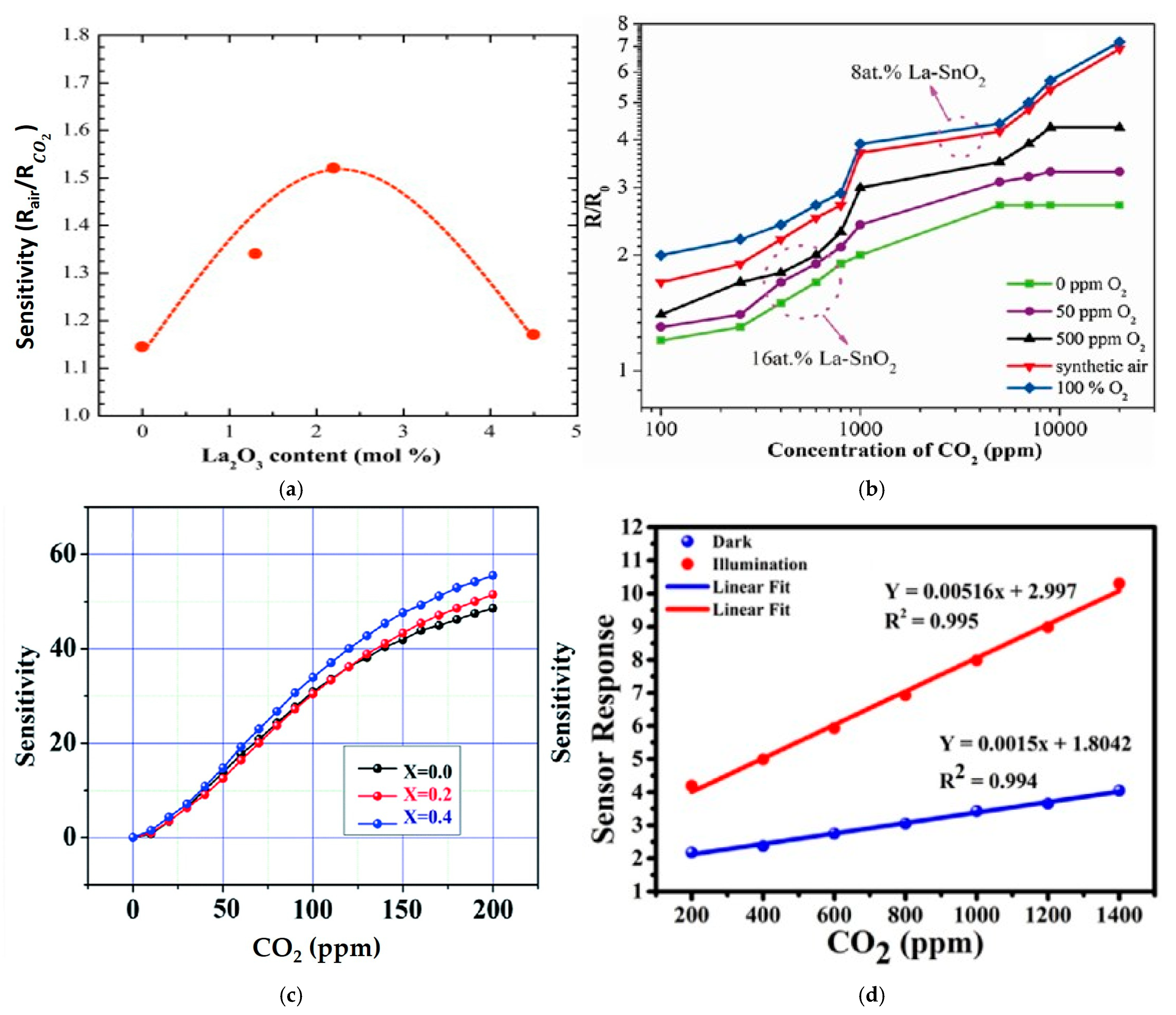
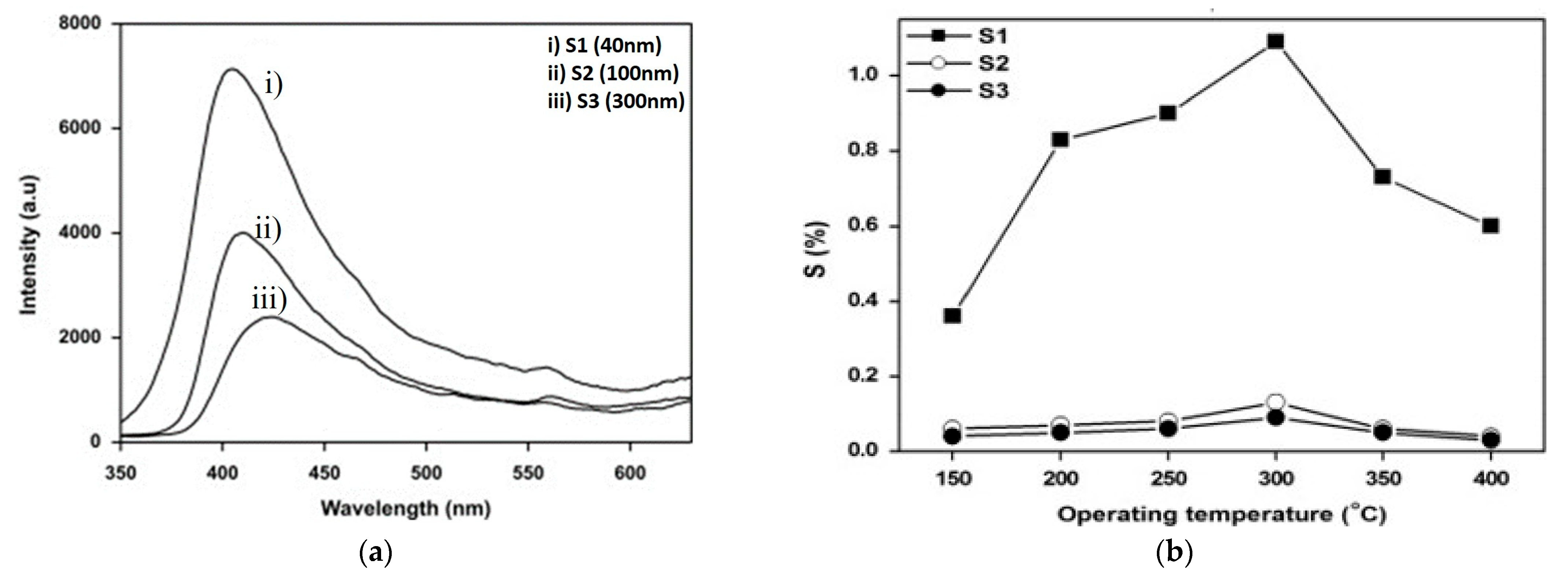
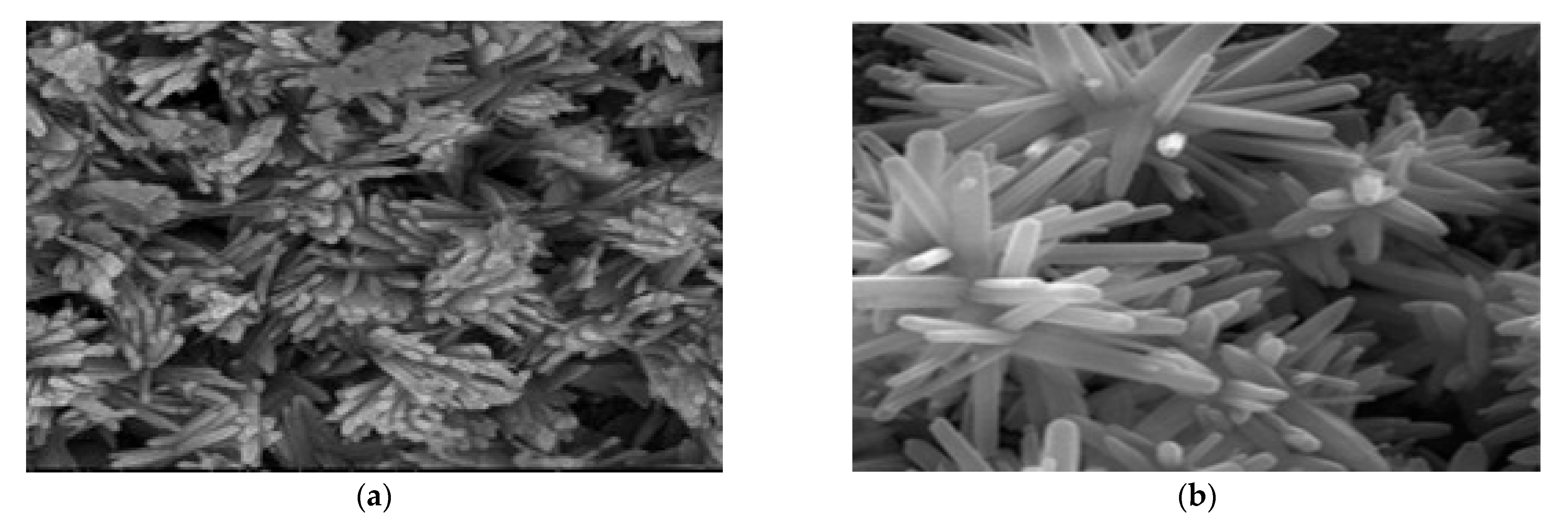
4.2. Zinc Oxide (ZnO)
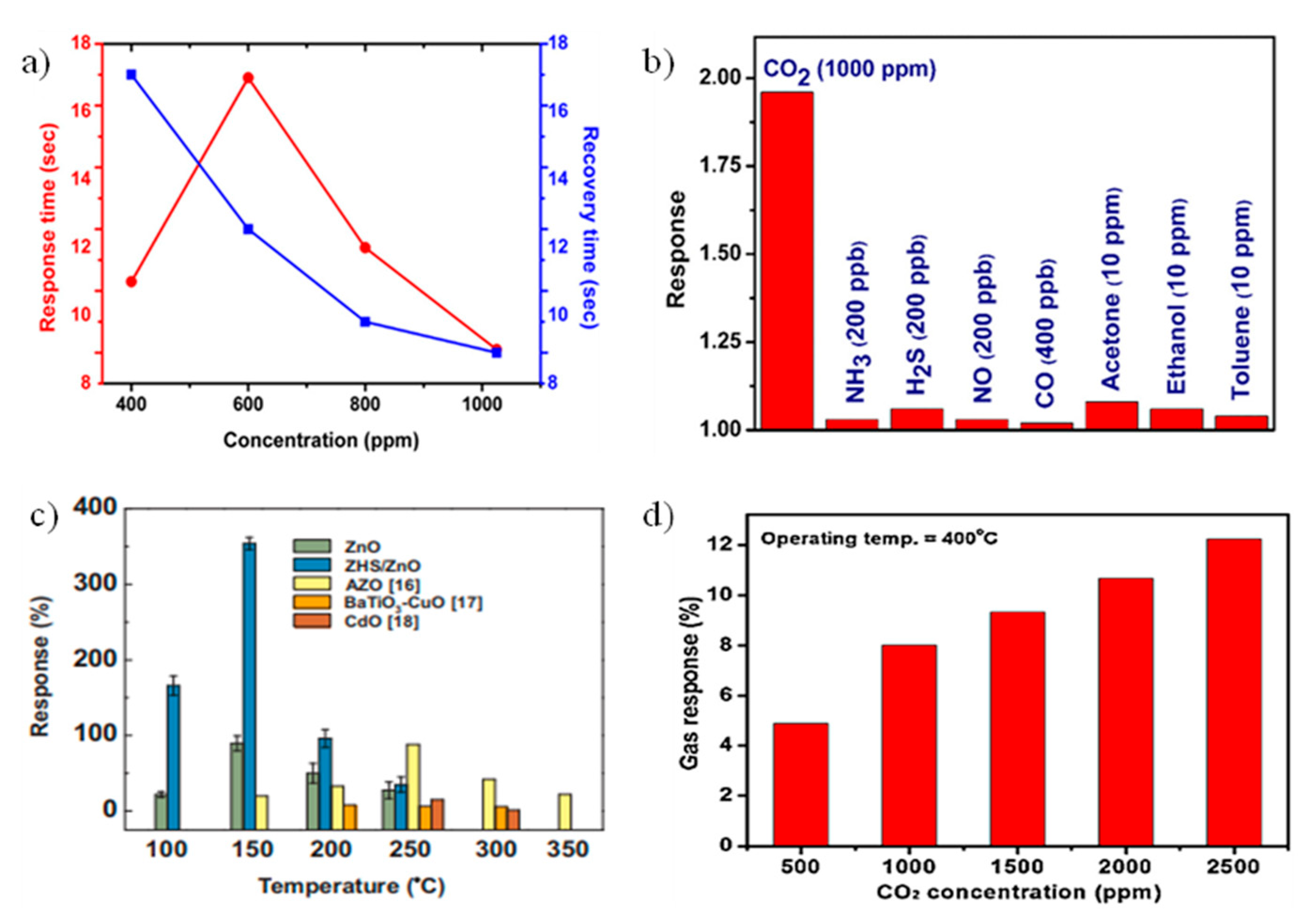
4.3. Other Metal Oxide (In2Te3, CuO, NiO, WO3, and TiO2)

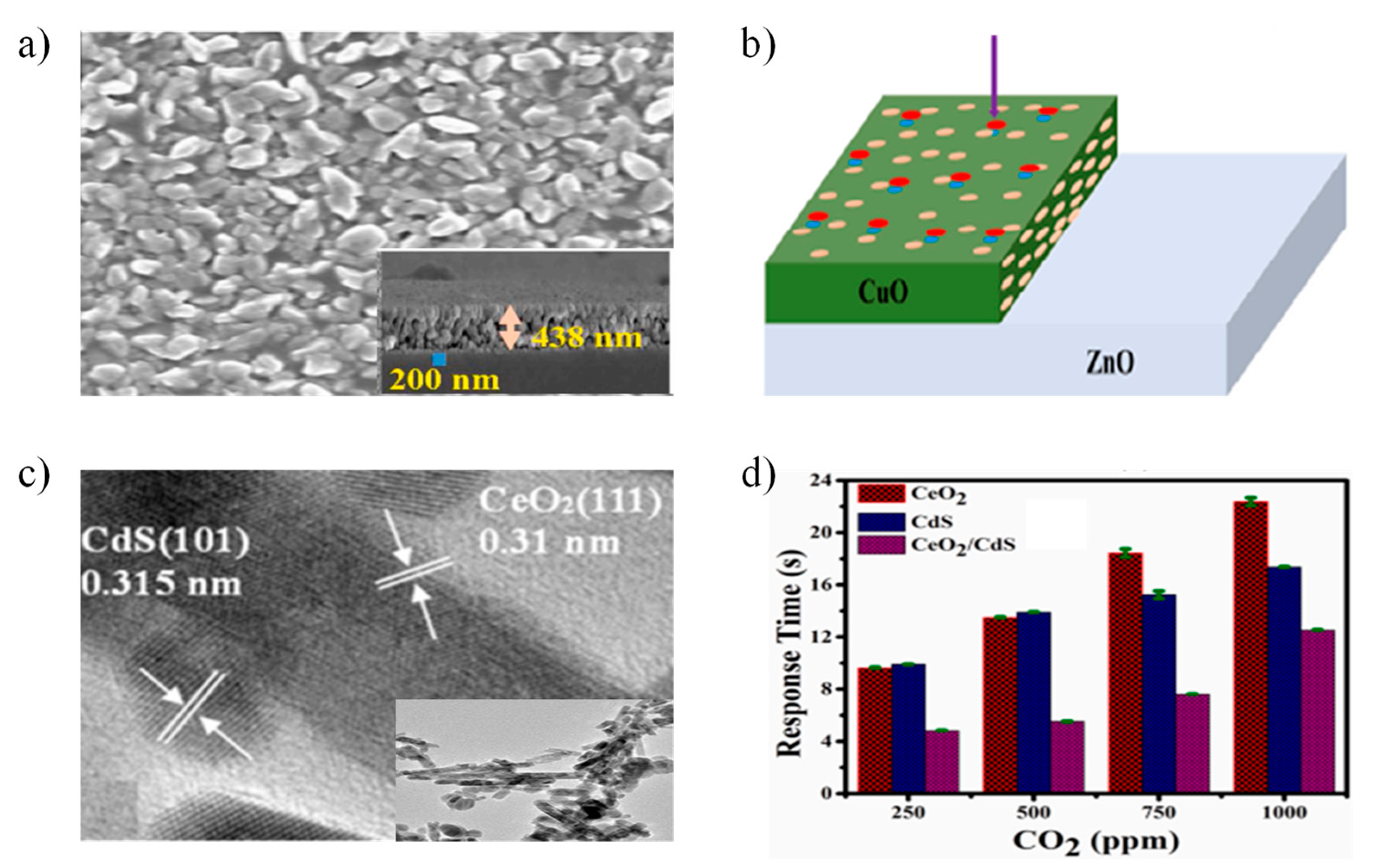
5. Recent Progress on Functionalization and Heterostructures
5.1. Noble Metal-Decorated Metal Oxide Semiconductor Sensors

5.2. Single Noble Metal-Decorated Metal Oxide Semiconductor Sensors
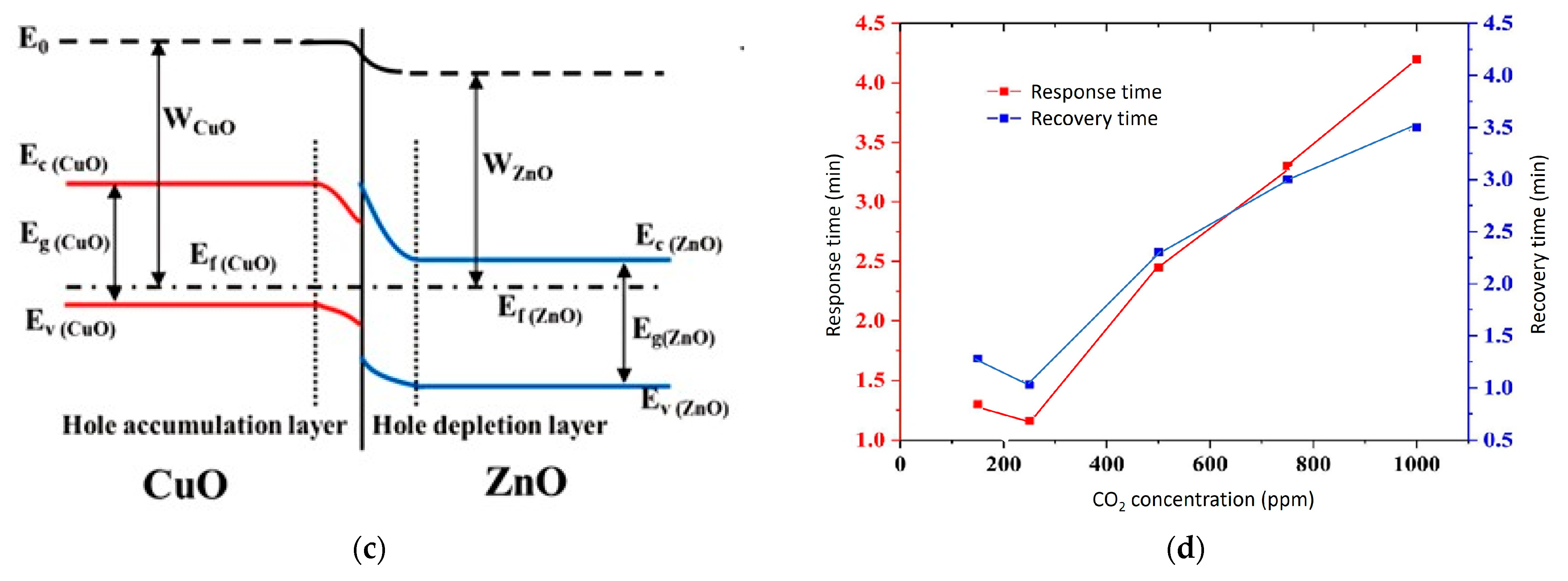
5.3. P–N Heterojunction MOS
6. Sensing Mechanism in Chemiresistive CO2 Sensors
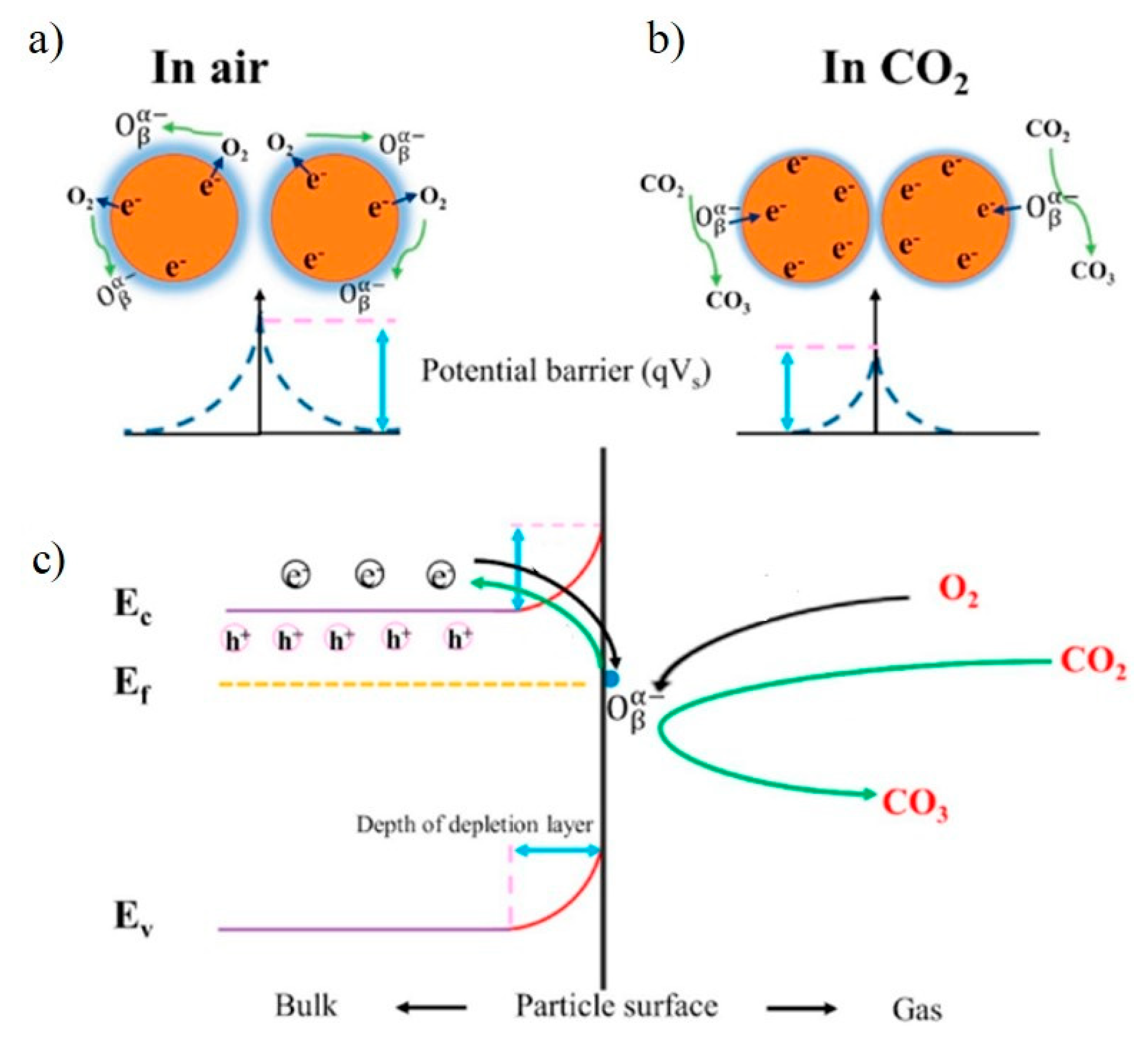
6.1. Ionosorption Model
6.2. Oxygen Vacancy Model
7. In-Depth Examination
7.1. Challenges
7.2. Future Prospective
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yoro, K.O.; Daramola, M.O. CO2 emission sources, greenhouse gases, and the global warming effect. In Advances in Carbon Capture; Elsevier: Amsterdam, The Netherlands, 2020; pp. 3–28. [Google Scholar]
- Fine, G.F.; Cavanagh, L.M.; Afonja, A.; Binions, R. Metal Oxide Semi-Conductor Gas Sensors in Environmental Monitoring. Sensors 2010, 10, 5469–5502. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.R. Oxidative stress and the cardiovascular effects of air pollution. Free Radic. Biol. Med. 2020, 151, 69–87. [Google Scholar] [CrossRef] [PubMed]
- Bhaliya, J.; Shah, V.; Katariya, H.; Suthar, V.; Patel, G. Fluoropolymer nanocomposites for volatile organic compounds and gas-sensing application. In Advanced Fluoropolymer Nanocomposites; Elsevier: Amsterdam, The Netherlands, 2023; pp. 445–483. [Google Scholar]
- Lin, Y.; Fan, Z. Compositing strategies to enhance the performance of chemiresistive CO2 gas sensors. Mater. Sci. Semicond. Process. 2020, 107, 104820. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, Y.; Hakkarainen, J.; Ju, W.; Liu, Y.; Jiang, F.; He, W. Distinguishing Anthropogenic CO2 Emissions from Different Energy Intensive Industrial Sources Using OCO-2 Observations: A Case Study in Northern China. J. Geophys. Res. Atmos. 2018, 123, 9462–9473. [Google Scholar] [CrossRef]
- Dixon, T.; Romanak, K.D. Improving monitoring protocols for CO2 geological storage with technical advances in CO2 attribution monitoring. Int. J. Greenh. Gas Control 2015, 41, 29–40. [Google Scholar] [CrossRef]
- Moumen, S.; Raible, I.; Krauß, A.; Wöllenstein, J. Infrared investigation of CO2 sorption by amine based materials for the development of a NDIR CO2 sensor. Sens. Actuators B Chem. 2016, 236, 1083–1090. [Google Scholar] [CrossRef]
- Zhang, C.; Xu, K.; Liu, K.; Xu, J.; Zheng, Z. Metal oxide resistive sensors for carbon dioxide detection. Coord. Chem. Rev. 2022, 472, 214758. [Google Scholar] [CrossRef]
- Huber, J.; Weber, C.; Eberhardt, A.; Wöllenstein, J. Photoacoustic CO2-Sensor for Automotive Applications. Procedia Eng. 2016, 168, 3–6. [Google Scholar] [CrossRef]
- Malcolm, A.; Wright, S.; Syms, R.R.A.; Dash, N.; Schwab, M.-A.; Finlay, A. Miniature Mass Spectrometer Systems Based on a Microengineered Quadrupole Filter. Anal. Chem. 2010, 82, 1751–1758. [Google Scholar] [CrossRef]
- SAKTHIVEL, M. A portable limiting current solid-state electrochemical diffusion hole type hydrogen sensor device for biomass fuel reactors: Engineering aspect. Int. J. Hydrogen Energy 2008, 33, 905–911. [Google Scholar] [CrossRef]
- Miller, D.R.; Akbar, S.A.; Morris, P.A. Nanoscale metal oxide-based heterojunctions for gas sensing: A review. Sens. Actuators B Chem. 2014, 204, 250–272. [Google Scholar] [CrossRef]
- Srinivasan, P.; Ezhilan, M.; Kulandaisamy, A.J.; Babu, K.J.; Rayappan, J.B.B. Room temperature chemiresistive gas sensors: Challenges and strategies—A mini review. J. Mater. Sci. Mater. Electron. 2019, 30, 15825–15847. [Google Scholar] [CrossRef]
- Mehta, S.; Shah, V.; Patel, G.; Conte-Junior, C.A.; Joshi, N. A holistic review of recent advances in nano-based drug delivery systems for the treatment of triple-negative breast cancer (TNBC). J. Nanopart. Res. 2024, 26, 87. [Google Scholar] [CrossRef]
- Kanaparthi, S.; Singh, S.G. Chemiresistive Sensor Based on Zinc Oxide Nanoflakes for CO2 Detection. ACS Appl. Nano Mater. 2019, 2, 700–706. [Google Scholar] [CrossRef]
- Shah, V.; Bhaliya, J.; Patel, G.M.; Popaliya, M.; Mishra, A.; Patel, P.R. Biodegradable polymer nanocomposites as electrode materials for electrochemical double-layer capacitors and hybrid supercapacitor applications. In Biodegradable and Biocompatible Polymer Nanocomposites; Elsevier: Amsterdam, The Netherlands, 2023; pp. 311–352. [Google Scholar]
- Patel, G.M.; Shah, V.; Bhaliya, J.; Mehta, K. Nanomaterials for construction building products designed to withstand natural disasters. In Nanotechnology-Based Smart Remote Sensing Networks for Disaster Prevention; Elsevier: Amsterdam, The Netherlands, 2022; pp. 19–42. [Google Scholar]
- Koralkar, N.; Mehta, S.; Upadhyay, A.; Patel, G.; Deshmukh, K. MOF-Based Nanoarchitectonics for Lithium-Ion Batteries: A Comprehensive Review. J. Inorg. Organomet. Polym. Mater. 2024, 34, 903–929. [Google Scholar] [CrossRef]
- Rezk, M.Y.; Sharma, J.; Gartia, M.R. Nanomaterial-Based CO2 Sensors. Nanomaterials 2020, 10, 2251. [Google Scholar] [CrossRef]
- Shah, V.; Bhaliya, J.; Patel, G.M.; Deshmukh, K. Advances in polymeric nanocomposites for automotive applications: A review. Polym. Adv. Technol. 2022, 33, 3023–3048. [Google Scholar] [CrossRef]
- Shah, V.; Bhaliya, J.; Patel, G.M.; Joshi, P. Room-Temperature Chemiresistive Gas Sensing of SnO2 Nanowires: A Review. J. Inorg. Organomet. Polym. Mater. 2022, 32, 741–772. [Google Scholar] [CrossRef]
- Nikita, K.; Patel, D.; Patel, G. Additive Manufacturing of Multifunctional Polymer Nanocomposites: From 3D to 4D. In Nanotechnology-Based Additive Manufacturing; Wiley: Hoboken, NJ, USA, 2023; pp. 277–313. [Google Scholar]
- Srinives, S.; Sarkar, T.; Hernandez, R.; Mulchandani, A. A miniature chemiresistor sensor for carbon dioxide. Anal. Chim. Acta 2015, 874, 54–58. [Google Scholar] [CrossRef]
- Molina, A.; Escobar-Barrios, V.; Oliva, J. A review on hybrid and flexible CO2 gas sensors. Synth. Met. 2020, 270, 116602. [Google Scholar] [CrossRef]
- Chai, H.; Zheng, Z.; Liu, K.; Xu, J.; Wu, K.; Luo, Y.; Liao, H.; Debliquy, M.; Zhang, C. Stability of Metal Oxide Semiconductor Gas Sensors: A Review. IEEE Sens. J. 2022, 22, 5470–5481. [Google Scholar] [CrossRef]
- Alam, M.; Pooja, P.; Aamir, M.; Souayeh, B.; Mushtaq, S.; Khan, M.; Amin, M.; Khan, K.; Shajahan, S. The Recent Development in Chemoresistive-Based Heterostructure Gas Sensor Technology, Their Future Opportunities and Challenges: A Review. Membranes 2022, 12, 555. [Google Scholar] [CrossRef] [PubMed]
- Fritea, L.; Banica, F.; Costea, T.; Moldovan, L.; Dobjanschi, L.; Muresan, M.; Cavalu, S. Metal Nanoparticles and Carbon-Based Nanomaterials for Improved Performances of Electrochemical (Bio)Sensors with Biomedical Applications. Materials 2021, 14, 6319. [Google Scholar] [CrossRef] [PubMed]
- Gurlo, A. Insights into the Mechanism of Gas Sensor Operation. In Metal Oxide Nanomaterials for Chemical Sensors; Springer: New York, NY, USA, 2013; pp. 3–34. [Google Scholar]
- Nikolic, M.V.; Milovanovic, V.; Vasiljevic, Z.Z.; Stamenkovic, Z. Semiconductor Gas Sensors: Materials, Technology, Design, and Application. Sensors 2020, 20, 6694. [Google Scholar] [CrossRef]
- Shearer, C.J.; Cherevan, A.; Eder, D. Application and Future Challenges of Functional Nanocarbon Hybrids. Adv. Mater. 2014, 26, 2295–2318. [Google Scholar] [CrossRef]
- Mirzaei, A.; Hashemi, B.; Janghorban, K. α-Fe2O3 based nanomaterials as gas sensors. J. Mater. Sci. Mater. Electron. 2016, 27, 3109–3144. [Google Scholar] [CrossRef]
- Patel, G.; Pillai, V.; Vora, M. Liquid Phase Exfoliation of Two-Dimensional Materials for Sensors and Photocatalysis—A Review. J. Nanosci. Nanotechnol. 2019, 19, 5054–5073. [Google Scholar] [CrossRef]
- Bhaliya, J.D.; Shah, V.R.; Patel, G.; Deshmukh, K. Recent Advances of MOF-Based Nanoarchitectonics for Chemiresistive Gas Sensors. J. Inorg. Organomet. Polym. Mater. 2023, 33, 1453–1494. [Google Scholar] [CrossRef]
- Nasiri, N.; Clarke, C. Nanostructured Chemiresistive Gas Sensors for Medical Applications. Sensors 2019, 19, 462. [Google Scholar] [CrossRef]
- Jafari, M.; Jung, J. Direct Measurement of Static and Dynamic Contact Angles Using a Random Micromodel Considering Geological CO2 Sequestration. Sustainability 2017, 9, 2352. [Google Scholar] [CrossRef]
- Ramgir, N.; Pathak, A.; Sinju, K.R.; Bhangare, B.; Debnath, A.K.; Muthe, K.P. Chemiresistive Sensors for H2S Gas: State of the Art. In Recent Advances in Thin Films; Springer: Berlin/Heidelberg, Germany, 2020; pp. 625–663. [Google Scholar]
- Stramarkou, M.; Bardakas, A.; Krokida, M.; Tsamis, C. ZnO-based chemi-resistive sensors for CO2 detection: A review. Sens. Rev. 2022, 42, 682–706. [Google Scholar] [CrossRef]
- Majhi, S.M.; Mirzaei, A.; Kim, H.W.; Kim, S.S.; Kim, T.W. Recent advances in energy-saving chemiresistive gas sensors: A review. Nano Energy 2021, 79, 105369. [Google Scholar] [CrossRef] [PubMed]
- Shah, V.; Bhaliya, J.; Patel, G.M.; Joshi, P. Recent Advancement in Pd-Decorated Nanostructures for Its Catalytic and Chemiresistive Gas Sensing Applications: A Review. Top. Catal. 2022. [Google Scholar] [CrossRef]
- Doshi, M.; Fahrenthold, E.P. Functionalized metallic carbon nanotube arrays for gas phase explosives detection. Comput. Theor. Chem. 2021, 1205, 113460. [Google Scholar] [CrossRef]
- Basyooni, M.A.; Zaki, S.E.; Ertugrul, S.; Yilmaz, M.; Eker, Y.R. Fast response of CO2 room temperature gas sensor based on Mixed-Valence Phases in Molybdenum and Tungsten Oxide nanostructured thin films. Ceram. Int. 2020, 46, 9839–9853. [Google Scholar] [CrossRef]
- Pandey, S. Highly sensitive and selective chemiresistor gas/vapor sensors based on polyaniline nanocomposite: A comprehensive review. J. Sci. Adv. Mater. Devices 2016, 1, 431–453. [Google Scholar] [CrossRef]
- Chiang, C.-J.; Tsai, K.-T.; Lee, Y.-H.; Lin, H.-W.; Yang, Y.-L.; Shih, C.-C.; Lin, C.-Y.; Jeng, H.-A.; Weng, Y.-H.; Cheng, Y.-Y.; et al. In situ fabrication of conducting polymer composite film as a chemical resistive CO2 gas sensor. Microelectron. Eng. 2013, 111, 409–415. [Google Scholar] [CrossRef]
- Manasa, M.V.; Sarala Devi, G.; Prasada Reddy, P.S.; Sreedhar, B. High performance CO2 gas sensor based on noble metal functionalized semiconductor nanomaterials for health and environmental safety. Mater. Res. Express 2019, 6, 125041. [Google Scholar] [CrossRef]
- Manzoli, A.; Steffens, C.; Paschoalin, R.T.; Correa, A.A.; Alves, W.F.; Leite, F.L.; Herrmann, P.S.P. Low-Cost Gas Sensors Produced by the Graphite Line-Patterning Technique Applied to Monitoring Banana Ripeness. Sensors 2011, 11, 6425–6434. [Google Scholar] [CrossRef]
- Idso, S.B.; Idso, K.E. Effects of atmospheric CO2 enrichment on plant constituents related to animal and human health. Environ. Exp. Bot. 2001, 45, 179–199. [Google Scholar] [CrossRef]
- Kim, M.Y.; Choi, Y.N.; Bae, J.M.; Oh, T.S. Carbon dioxide sensitivity of La-doped thick film tin oxide gas sensor. Ceram. Int. 2012, 38, S657–S660. [Google Scholar] [CrossRef]
- Wang, D.; Chen, Y.; Liu, Z.; Li, L.; Shi, C.; Qin, H.; Hu, J. CO2-sensing properties and mechanism of nano-SnO2 thick-film sensor. Sens. Actuators B Chem. 2016, 227, 73–84. [Google Scholar] [CrossRef]
- Kannan, P.K.; Saraswathi, R.; Rayappan, J.B.B. CO2 gas sensing properties of DC reactive magnetron sputtered ZnO thin film. Ceram. Int. 2014, 40, 13115–13122. [Google Scholar] [CrossRef]
- Habib, M.; Hussain, S.S.; Riaz, S.; Naseem, S. Preparation and Characterization of ZnO Nanowires and their Applications in CO2 Gas Sensors. Mater. Today Proc. 2015, 2, 5714–5719. [Google Scholar] [CrossRef]
- Hunge, Y.M.; Yadav, A.A.; Kulkarni, S.B.; Mathe, V.L. A multifunctional ZnO thin film based devices for photoelectrocatalytic degradation of terephthalic acid and CO2 gas sensing applications. Sens. Actuators B Chem. 2018, 274, 1–9. [Google Scholar] [CrossRef]
- Jeong, Y.-J.; Balamurugan, C.; Lee, D.-W. Enhanced CO2 gas-sensing performance of ZnO nanopowder by La loaded during simple hydrothermal method. Sens. Actuators B Chem. 2016, 229, 288–296. [Google Scholar] [CrossRef]
- Krishnakumar, T.; Jayaprakash, R.; Prakash, T.; Sathyaraj, D.; Donato, N.; Licoccia, S.; Latino, M.; Stassi, A.; Neri, G. CdO-based nanostructures as novel CO2 gas sensors. Nanotechnology 2011, 22, 325501. [Google Scholar] [CrossRef]
- Rajesh, N.; Kannan, J.C.; Krishnakumar, T.; Bonavita, A.; Leonardi, S.G.; Neri, G. Microwave irradiated Sn-substituted CdO nanostructures for enhanced CO2 sensing. Ceram. Int. 2015, 41, 14766–14772. [Google Scholar] [CrossRef]
- Abdelmounaïm, C.; Amara, Z.; Maha, A.; Mustapha, D. Effects of molarity on structural, optical, morphological and CO2 gas sensing properties of nanostructured copper oxide films deposited by spray pyrolysis. Mater. Sci. Semicond. Process. 2016, 43, 214–221. [Google Scholar] [CrossRef]
- Aboud, A.A.; Al-Kelesh, H.; El Rouby, W.M.A.; Farghali, A.A.; Hamdedein, A.; Khedr, M.H. CO2 responses based on pure and doped CeO2 nano-pellets. J. Mater. Res. Technol. 2018, 7, 14–20. [Google Scholar] [CrossRef]
- Yadav, A.A.; Lokhande, A.C.; Kim, J.H.; Lokhande, C.D. Highly sensitive CO2 sensor based on microrods-like La2O3 thin film electrode. RSC Adv. 2016, 6, 106074–106080. [Google Scholar] [CrossRef]
- Karthik, P.; Gowthaman, P.; Venkatachalam, M.; Saroja, M. Design and fabrication of g-C3N4 nanosheets decorated TiO2 hybrid sensor films for improved performance towards CO2 gas. Inorg. Chem. Commun. 2020, 119, 108060. [Google Scholar] [CrossRef]
- Manikandan, V.; Petrila, I.; Vigneselvan, S.; Mane, R.S.; Vasile, B.; Dharmavarapu, R.; Lundgaard, S.; Juodkazis, S.; Chandrasekaran, J. A reliable chemiresistive sensor of nickel-doped tin oxide (Ni-SnO2) for sensing carbon dioxide gas and humidity. RSC Adv. 2020, 10, 3796–3804. [Google Scholar] [CrossRef]
- Keller, P.; Ferkel, H.; Zweiacker, K.; Naser, J.; Meyer, J.-U.; Riehemann, W. The application of nanocrystalline BaTiO3-composite films as CO2-sensing layers. Sens. Actuators B Chem. 1999, 57, 39–46. [Google Scholar] [CrossRef]
- Dhahri, R.; Leonardi, S.G.; Hjiri, M.; El Mir, L.; Bonavita, A.; Donato, N.; Iannazzo, D.; Neri, G. Enhanced performance of novel calcium/aluminum co-doped zinc oxide for CO2 sensors. Sens. Actuators B Chem. 2017, 239, 36–44. [Google Scholar] [CrossRef]
- Michel, C.R. CO and CO2 gas sensing properties of mesoporous CoAl2O4. Sens. Actuators B Chem. 2010, 147, 635–641. [Google Scholar] [CrossRef]
- Yadav, A.A.; Lokhande, A.C.; Kim, J.H.; Lokhande, C.D. Improvement in CO2 sensing characteristics using Pd nanoparticles decorated La2O3 thin films. J. Ind. Eng. Chem. 2017, 49, 76–81. [Google Scholar] [CrossRef]
- Kumar, V.; Singh, A.; Yadav, B.C.; Singh, H.K.; Singh, D.P.; Singh, S.K.; Chaurasiya, N. Environment-sensitive and fast room temperature CO2 gas sensor based on ZnO, NiO and Ni-ZnO nanocomposite materials. Environ. Funct. Mater. 2023, 2, 167–177. [Google Scholar] [CrossRef]
- Dhahri, R.; Hjiri, M.; El Mir, L.; Fazio, E.; Neri, F.; Barreca, F.; Donato, N.; Bonavita, A.; Leonardi, S.G.; Neri, G. ZnO:Ca nanopowders with enhanced CO2 sensing properties. J. Phys. D Appl. Phys. 2015, 48, 255503. [Google Scholar] [CrossRef]
- Marsal, A.; Dezanneau, G.; Cornet, A.; Morante, J.R. A new CO2 gas sensing material. Sens. Actuators B Chem. 2003, 95, 266–270. [Google Scholar] [CrossRef]
- Djerdj, I.; Haensch, A.; Koziej, D.; Pokhrel, S.; Barsan, N.; Weimar, U.; Niederberger, M. Neodymium Dioxide Carbonate as a Sensing Layer for Chemoresistive CO2 Sensing. Chem. Mater. 2009, 21, 5375–5381. [Google Scholar] [CrossRef]
- Chen, G.; Han, B.; Deng, S.; Wang, Y.; Wang, Y. Lanthanum Dioxide Carbonate La2O2CO3 Nanorods as a Sensing Material for Chemoresistive CO2 Gas Sensor. Electrochim. Acta 2014, 127, 355–361. [Google Scholar] [CrossRef]
- Wang, X.; Qin, H.; Sun, L.; Hu, J. CO2 sensing properties and mechanism of nanocrystalline LaFeO3 sensor. Sens. Actuators B Chem. 2013, 188, 965–971. [Google Scholar] [CrossRef]
- Desai, R.R.; Lakshminarayana, D.; Patel, P.B.; Panchal, C.J. Indium sesquitelluride (In2Te3) thin film gas sensor for detection of carbon dioxide. Sens. Actuators B Chem. 2005, 107, 523–527. [Google Scholar] [CrossRef]
- Radhakrishnan, J.K.; Kumara, M. Geetika Effect of temperature modulation, on the gas sensing characteristics of ZnO nanostructures, for gases O2, CO and CO2. Sensors Int. 2021, 2, 100059. [Google Scholar] [CrossRef]
- Herrán, J.; Ga Mandayo, G.; Castaño, E. Semiconducting BaTiO3-CuO mixed oxide thin films for CO2 detection. Thin Solid Films 2009, 517, 6192–6197. [Google Scholar] [CrossRef]
- Samarasekara, P.; Yapa, N.U.S.; Kumara, N.T.R.N.; Perera, M.V.K. CO2 gas sensitivity of sputtered zinc oxide thin films. Bull. Mater. Sci. 2007, 30, 113–116. [Google Scholar] [CrossRef]
- Kim, D.H.; Yoon, J.Y.; Park, H.C.; Kim, K.H. CO2-sensing characteristics of SnO2 thick film by coating lanthanum oxide. Sens. Actuators B Chem. 2000, 62, 61–66. [Google Scholar] [CrossRef]
- Karthik, P.; Gowthaman, P.; Venkatachalam, M.; Rajamanickam, A.T. Propose of high performance resistive type H2S and CO2 gas sensing response of reduced graphene oxide/titanium oxide (rGO/TiO2) hybrid sensors. J. Mater. Sci. Mater. Electron. 2020, 31, 3695–3705. [Google Scholar] [CrossRef]
- Xiong, Y.; Xue, Q.; Ling, C.; Lu, W.; Ding, D.; Zhu, L.; Li, X. Effective CO2 detection based on LaOCl-doped SnO2 nanofibers: Insight into the role of oxygen in carrier gas. Sens. Actuators B Chem. 2017, 241, 725–734. [Google Scholar] [CrossRef]
- Xiong, Y.; Zhang, G.; Zhang, S.; Zeng, D.; Xie, C. Tin oxide thick film by doping rare earth for detecting traces of CO2: Operating in oxygen-free atmosphere. Mater. Res. Bull. 2014, 52, 56–64. [Google Scholar] [CrossRef]
- Diagne, E.H.A.; Lumbreras, M. Elaboration and characterization of tin oxide–lanthanum oxide mixed layers prepared by the electrostatic spray pyrolysis technique. Sens. Actuators B Chem. 2001, 78, 98–105. [Google Scholar] [CrossRef]
- Trung, D.D.; Toan, L.D.; Hong, H.S.; Lam, T.D.; Trung, T.; Van Hieu, N. Selective detection of carbon dioxide using LaOCl-functionalized SnO2 nanowires for air-quality monitoring. Talanta 2012, 88, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, T.; Kometani, K.; Nishi, Y.; Takita, Y. Improved sensitivity of CuO-BaTiO3 capacitive-type CO2 sensor by additives. Sens. Actuators B Chem. 1995, 28, 49–54. [Google Scholar] [CrossRef]
- Tanvir, N.B.; Yurchenko, O.; Urban, G. Optimization Study for Work Function Based CO2 Sensing Using CuO-nanoparticles in Respect to Humidity and Temperature. Procedia Eng. 2015, 120, 667–670. [Google Scholar] [CrossRef]
- McCreary, J.L.; Gray, P.R. All-MOS charge redistribution analog-to-digital conversion techniques. I. IEEE J. Solid-State Circuits 1975, 10, 371–379. [Google Scholar] [CrossRef]
- Kashyout, A.B.; Soliman, H.M.A.; Shokry Hassan, H.; Abousehly, A.M. Fabrication of ZnO and ZnO:Sb Nanoparticles for Gas Sensor Applications. J. Nanomater. 2010, 2010, 341841. [Google Scholar] [CrossRef]
- Herberger, S.; Herold, M.; Ulmer, H.; Burdack-Freitag, A.; Mayer, F. Detection of human effluents by a MOS gas sensor in correlation to VOC quantification by GC/MS. Build. Environ. 2010, 45, 2430–2439. [Google Scholar] [CrossRef]
- Samanta, P.K.; Saha, A. Wet chemical synthesis of ZnO nanoflakes and photoluminescence. Optik 2015, 126, 3786–3788. [Google Scholar] [CrossRef]
- Shen, B.; Zhang, F.; Jiang, L.; Liu, X.; Song, X.; Qin, X.; Li, X. Improved Sensing Properties of Thermal Conductivity-Type CO2 Gas Sensors by Loading Multi-Walled Carbon Nanotubes Into Nano-Al2O3 Powders. Front. Energy Res. 2021, 9, 634321. [Google Scholar] [CrossRef]
- Xu, F.; HO, H.-P. Light-Activated Metal Oxide Gas Sensors: A Review. Micromachines 2017, 8, 333. [Google Scholar] [CrossRef] [PubMed]
- Helwig, A.; Müller, G.; Sberveglieri, G.; Eickhoff, M. On the Low-Temperature Response of Semiconductor Gas Sensors. J. Sens. 2009, 2009, 620720. [Google Scholar] [CrossRef]
- Star, A.; Han, T.-R.; Joshi, V.; Gabriel, J.-C.P.; Grüner, G. Nanoelectronic Carbon Dioxide Sensors. Adv. Mater. 2004, 16, 2049–2052. [Google Scholar] [CrossRef]
- Lin, T.; Lv, X.; Li, S.; Wang, Q. The Morphologies of the Semiconductor Oxides and Their Gas-Sensing Properties. Sensors 2017, 17, 2779. [Google Scholar] [CrossRef]
- Yoon, J.-W.; Kim, H.-J.; Jeong, H.-M.; Lee, J.-H. Gas sensing characteristics of p-type Cr2O3 and Co3O4 nanofibers depending on inter-particle connectivity. Sens. Actuators B Chem. 2014, 202, 263–271. [Google Scholar] [CrossRef]
- Matheswaran, P.; Sathyamoorthy, R.; Asokan, K. Effect of 130 MeV Au ion irradiation on CO2 gas sensing properties of In2Te3 thin films. Sens. Actuators B Chem. 2013, 177, 8–13. [Google Scholar] [CrossRef]
- Giberti, A.; Carotta, M.C.; Malagù, C.; Aldao, C.M.; Castro, M.S.; Ponce, M.A.; Parra, R. Permittivity measurements in nanostructured TiO2 gas sensors. Phys. Status Solidi 2011, 208, 118–122. [Google Scholar] [CrossRef]
- Pokhrel, S.; Simion, C.E.; Quemener, V.; Bârsan, N.; Weimar, U. Investigations of conduction mechanism in Cr2O3 gas sensing thick films by ac impedance spectroscopy and work function changes measurements. Sens. Actuators B Chem. 2008, 133, 78–83. [Google Scholar] [CrossRef]
- Yu, Y.; Xia, Y.; Zeng, W.; Liu, R. Synthesis of multiple networked NiO nanostructures for enhanced gas sensing performance. Mater. Lett. 2017, 206, 80–83. [Google Scholar] [CrossRef]
- Volanti, D.P.; Felix, A.A.; Orlandi, M.O.; Whitfield, G.; Yang, D.; Longo, E.; Tuller, H.L.; Varela, J.A. The Role of Hierarchical Morphologies in the Superior Gas Sensing Performance of CuO-Based Chemiresistors. Adv. Funct. Mater. 2013, 23, 1759–1766. [Google Scholar] [CrossRef]
- Anithaa, A.C.; Lavanya, N.; Asokan, K.; Sekar, C. WO3 nanoparticles based direct electrochemical dopamine sensor in the presence of ascorbic acid. Electrochim. Acta 2015, 167, 294–302. [Google Scholar] [CrossRef]
- Onkar, S.G.; Raghuwanshi, F.C.; Patil, D.R.; Krishnakumar, T. Synthesis, Characterization and Gas Sensing Study of SnO2 Thick Film Sensor towards H2S, NH3, LPG and CO2. Mater. Today Proc. 2020, 23, 190–201. [Google Scholar] [CrossRef]
- George, A.S.; Deepa, S. Influence of defect related oxygen vacancies in tuning the CO2 sensing properties of microwave treated SnO2 thin films. Mater. Today Proc. 2020, 25, 328–332. [Google Scholar] [CrossRef]
- Elango, G.; Kumaran, S.M.; Kumar, S.S.; Muthuraja, S.; Roopan, S.M. Green synthesis of SnO2 nanoparticles and its photocatalytic activity of phenolsulfonphthalein dye. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 145, 176–180. [Google Scholar] [CrossRef]
- Meng, X.; Zhou, M.; Li, X.; Yao, J.; Liu, F.; He, H.; Xiao, P.; Zhang, Y. Synthesis of SnO2 nanoflowers and electrochemical properties of Ni/SnO2 nanoflowers in supercapacitor. Electrochim. Acta 2013, 109, 20–26. [Google Scholar] [CrossRef]
- Bhaliya, J.; Shah, V.; Patel, G. Functionalized nanofibers for gas and volatile organic compound sensing. In Functionalized Nanofibers; Elsevier: Amsterdam, The Netherlands, 2023; pp. 531–577. [Google Scholar]
- Singh, A.; Yadav, B.C. Photo-responsive highly sensitive CO2 gas sensor based on SnO2@CdO heterostructures with DFT calculations. Surf. Interfaces 2022, 34, 102368. [Google Scholar] [CrossRef]
- Musharaf, M.; Karamat, S.; Hassan, M.U.; Khalique, U.; Oral, A.; Behjat, A.B.; Akram, R.; Almohaimeed, Z. Solubility Enhancement of Fe in ZnO Nanoparticles Prepared by Co-Precipitation Method. J. Supercond. Nov. Magn. 2021, 34, 2633–2642. [Google Scholar] [CrossRef]
- Seiyama, T.; Nakahara, T.; Takeuchi, T. Overview of Gas Sensors for Environmental Use. In Studies in Environmental Science; Elsevier: Amsterdam, The Netherlands, 1994; pp. 233–272. [Google Scholar]
- Juang, F.-R.; Chen, B.-Y. Effect of adding ZHS microcubes on ZnO nanorods for CO2 gas sensing applications. Solid. State. Electron. 2020, 164, 107711. [Google Scholar] [CrossRef]
- Li, D.; Haneda, H. Morphologies of zinc oxide particles and their effects on photocatalysis. Chemosphere 2003, 51, 129–137. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, W. Growth process, crystal size and alignment of ZnO nanorods synthesized under neutral and acid conditions. J. Alloys Compd. 2015, 629, 84–91. [Google Scholar] [CrossRef]
- Joshi, S.; Jones, L.A.; Sabri, Y.M.; Bhargava, S.K.; Sunkara, M.V.; Ippolito, S.J. Facile conversion of zinc hydroxide carbonate to CaO-ZnO for selective CO2 gas detection. J. Colloid Interface Sci. 2020, 558, 310–322. [Google Scholar] [CrossRef] [PubMed]
- Keerthana, S.; Rathnakannan, K. Hierarchical ZnO/CuO nanostructures for room temperature detection of carbon dioxide. J. Alloys Compd. 2022, 897, 162988. [Google Scholar] [CrossRef]
- Tai, G.; Miao, C.; Wang, Y.; Bai, Y.; Zhang, H.; Guo, W. Solvothermal synthesis and thermoelectric properties of indium telluride nanostring-cluster hierarchical structures. Nanoscale Res. Lett. 2011, 6, 329. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, K.; Xu, D.; Yang, G.; Huang, H.; Nie, F.; Liu, C.; Yang, S. CuO nanostructures: Synthesis, characterization, growth mechanisms, fundamental properties, and applications. Prog. Mater. Sci. 2014, 60, 208–337. [Google Scholar] [CrossRef]
- Zhu, L.-Y.; Ou, L.-X.; Mao, L.-W.; Wu, X.-Y.; Liu, Y.-P.; Lu, H.-L. Advances in Noble Metal-Decorated Metal Oxide Nanomaterials for Chemiresistive Gas Sensors: Overview. Nano-Micro Lett. 2023, 15, 89. [Google Scholar] [CrossRef]
- Hsu, K.-C.; Fang, T.-H.; Hsiao, Y.-J.; Chan, C.-A. Highly response CO2 gas sensor based on Au-La2O3 doped SnO2 nanofibers. Mater. Lett. 2020, 261, 127144. [Google Scholar] [CrossRef]
- Ehsani, M.; Hamidon, M.N.; Toudeshki, A.; Abadi, M.H.S.; Rezaeian, S. CO2 Gas Sensing Properties of Screen-Printed La2O3/SnO2 Thick Film. IEEE Sens. J. 2016, 16, 6839–6845. [Google Scholar] [CrossRef]
- Hassanpouryouzband, A.; Yang, J.; Tohidi, B.; Chuvilin, E.; Istomin, V.; Bukhanov, B.; Cheremisin, A. Insights into CO2 Capture by Flue Gas Hydrate Formation: Gas Composition Evolution in Systems Containing Gas Hydrates and Gas Mixtures at Stable Pressures. ACS Sustain. Chem. Eng. 2018, 6, 5732–5736. [Google Scholar] [CrossRef]
- Majhi, S.M.; Lee, H.-J.; Choi, H.-N.; Cho, H.-Y.; Kim, J.-S.; Lee, C.-R.; Yu, Y.-T. Construction of novel hybrid PdO–ZnO p–n heterojunction nanostructures as a high-response sensor for acetaldehyde gas. CrystEngComm 2019, 21, 5084–5094. [Google Scholar] [CrossRef]
- Joshi, S.; Satyanarayana, L.; Manjula, P.; Sunkara, M.V.; Ippolito, S.J. Chemo—Resistive CO2 gas sensor based on CuO-SnO2 heterojunction nanocomposite material. In Proceedings of the 2015 2nd International Symposium on Physics and Technology of Sensors (ISPTS), Pune, India, 8–10 March 2015; pp. 43–48. [Google Scholar]
- Singh, A.; Singh, S.; Yadav, B.C. Gigantic enhancement in response of heterostructured CeO2/CdS nanospheres based self-powered CO2 gas sensor: A comparative study. Sens. Actuators B Chem. 2023, 377, 133085. [Google Scholar] [CrossRef]
- Bhowmick, T.; Ghosh, A.; Nag, S.; Majumder, S.B. Sensitive and selective CO2 gas sensor based on CuO/ZnO bilayer thin-film architecture. J. Alloys Compd. 2022, 903, 163871. [Google Scholar] [CrossRef]
- Lu, X.; Liu, Z.; Zhao, X.; Xu, W.; Hou, H.; Qian, J. CdS Nanoparticles Decorated 1D CeO2 Nanorods for Enhanced Photocatalytic Desulfurization Performance. Catalysts 2022, 12, 1478. [Google Scholar] [CrossRef]
- Baik, N.; Sakai, G.; Miura, N.; Yamazoe, N. Hydrothermally treated sol solution of tin oxide for thin-film gas sensor. Sens. Actuators B Chem. 2000, 63, 74–79. [Google Scholar] [CrossRef]
- Zemel, J.N. Theoretical description of gas-film interaction on SnOx. Thin Solid Films 1988, 163, 189–202. [Google Scholar] [CrossRef]
- Moll, N.G.; Clutter, D.R.; Thompson, W.E. Carbon Trioxide: Its Production, Infrared Spectrum, and Structure Studied in a Matrix of Solid CO2. J. Chem. Phys. 1966, 45, 4469–4481. [Google Scholar] [CrossRef]
- Kowalczyk, T.; Krylov, A.I. Electronic Structure of Carbon Trioxide and Vibronic Interactions Involving Jahn−Teller States. J. Phys. Chem. A 2007, 111, 8271–8276. [Google Scholar] [CrossRef]
- Tricoli, A.; Righettoni, M.; Teleki, A. Semiconductor Gas Sensors: Dry Synthesis and Application. Angew. Chemie Int. Ed. 2010, 49, 7632–7659. [Google Scholar] [CrossRef]
- Gurlo, A. Interplay between O2 and SnO2: Oxygen Ionosorption and Spectroscopic Evidence for Adsorbed Oxygen. ChemPhysChem 2006, 7, 2041–2052. [Google Scholar] [CrossRef]
- Gurlo, A.; Riedel, R. In Situ and Operando Spectroscopy for Assessing Mechanisms of Gas Sensing. Angew. Chemie Int. Ed. 2007, 46, 3826–3848. [Google Scholar] [CrossRef]
| Gas Type | Typical Sensor Response | Temperature Sensitivity | Typical Sensing Mechanism | Selectivity | Sensing Efficiency |
|---|---|---|---|---|---|
| CO2 | Weak interaction | Low | Minimal surface adsorption | Poor | Limited |
| Reducing Gases (H2, CO) | Strong response | High | Surface catalytic reactions | Good | High |
| Oxidizing Gases (O2, NO2) | Strong response | High | Surface charge transfer | Good | High |
| Metal Oxide | Type | Key Properties | Applications |
|---|---|---|---|
| SnO2 | n-type | High sensitivity, excellent chemical stability, low cost. | Indoor air quality monitoring, environmental sensing |
| TiO2 | n-type | High-temperature stability, reversible changes in resistance. | Outdoor air quality monitoring, automotive sensors |
| ZnO | n-type | High surface reactivity, good conductivity. | Environmental monitoring, industrial leak detection |
| In2O3 | n-type | Good sensitivity, stability. | Optimized for specific gas interactions. |
| Fe2O3 | n-type | Cost-effective, abundant. | Industrial and agricultural environments |
| CuO | p-type | Independence from relative humidity. | Low-cost portable applications, automotive sensors |
| NiO | p-type | Lower response compared to n-type oxides but potential for improvement through doping. | Automotive exhaust, air quality sensors |
| MO Sensor | T (°C) | Response/Recovery (s) | Concentration (ppm) | Reference |
|---|---|---|---|---|
| Pd-La2O3-nanoparticles | 250 | 0.83/- | 500 | [64] |
| Au/La2O3/SnO2-nanofibers | 300 | 10.1/- | 100 | [115] |
| La2O3-SnO2-film | 225 | 0.32/- | 1000 | [116] |
| La2O3-SnO2-film | 225 | 4.38/- | 1000 | [116] |
| Au La2O3-SnO2-nanofibers | 300 | 10/- | 100 | [115] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ughade, Y.; Mehta, S.; Patel, G.; Gowda, R.; Joshi, N.; Patel, R. Progress in CO2 Gas Sensing Technologies: Insights into Metal Oxide Nanostructures and Resistance-Based Methods. Micromachines 2025, 16, 466. https://doi.org/10.3390/mi16040466
Ughade Y, Mehta S, Patel G, Gowda R, Joshi N, Patel R. Progress in CO2 Gas Sensing Technologies: Insights into Metal Oxide Nanostructures and Resistance-Based Methods. Micromachines. 2025; 16(4):466. https://doi.org/10.3390/mi16040466
Chicago/Turabian StyleUghade, Yash, Shubham Mehta, Gautam Patel, Roopa Gowda, Nirav Joshi, and Rohan Patel. 2025. "Progress in CO2 Gas Sensing Technologies: Insights into Metal Oxide Nanostructures and Resistance-Based Methods" Micromachines 16, no. 4: 466. https://doi.org/10.3390/mi16040466
APA StyleUghade, Y., Mehta, S., Patel, G., Gowda, R., Joshi, N., & Patel, R. (2025). Progress in CO2 Gas Sensing Technologies: Insights into Metal Oxide Nanostructures and Resistance-Based Methods. Micromachines, 16(4), 466. https://doi.org/10.3390/mi16040466