Seeking Solutions for Inclusively Economic, Rapid, and Safe Molecular Detection of Respiratory Infectious Diseases: Comprehensive Review from Polymerase Chain Reaction Techniques to Amplification-Free Biosensing
Abstract
1. Introduction
2. Analysis of Molecular Diagnostic Technologies
2.1. Temporal-Domain PCR
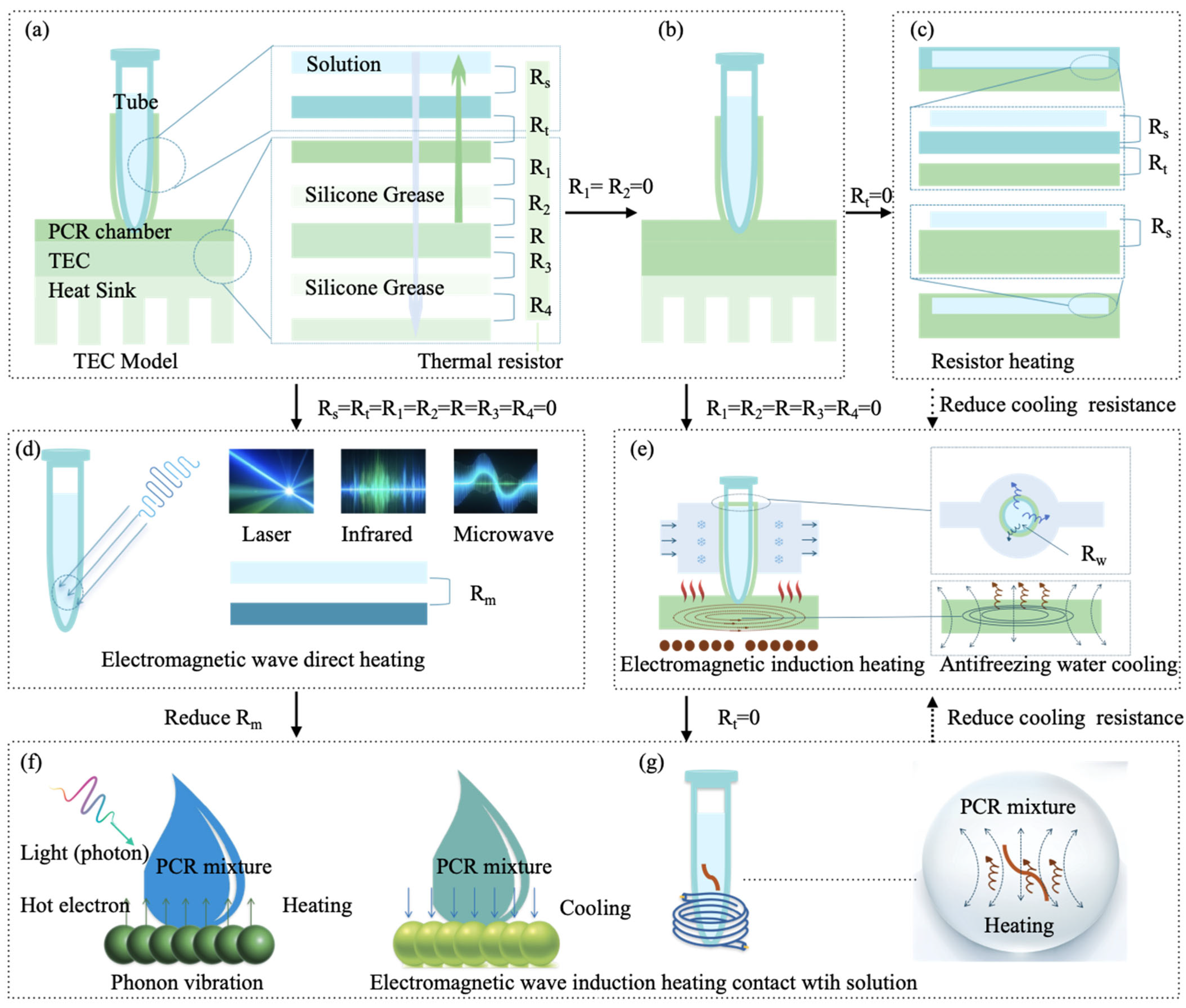
2.1.1. Traditional TEC Thermal Cycling Mode
2.1.2. Impedance Joule Heating Mode
2.1.3. Electromagnetic Wave Direct Heating Mode
Microwave Heating
Infrared Heating
Laser Heating
2.1.4. Electromagnetic Wave-Induced Heating (EWIH)
Magnetic-Induced Heating (MIH)
Plasmonic Photothermal Heating
2.2. Spatial-Domain PCR
2.2.1. Physical Space Exchange
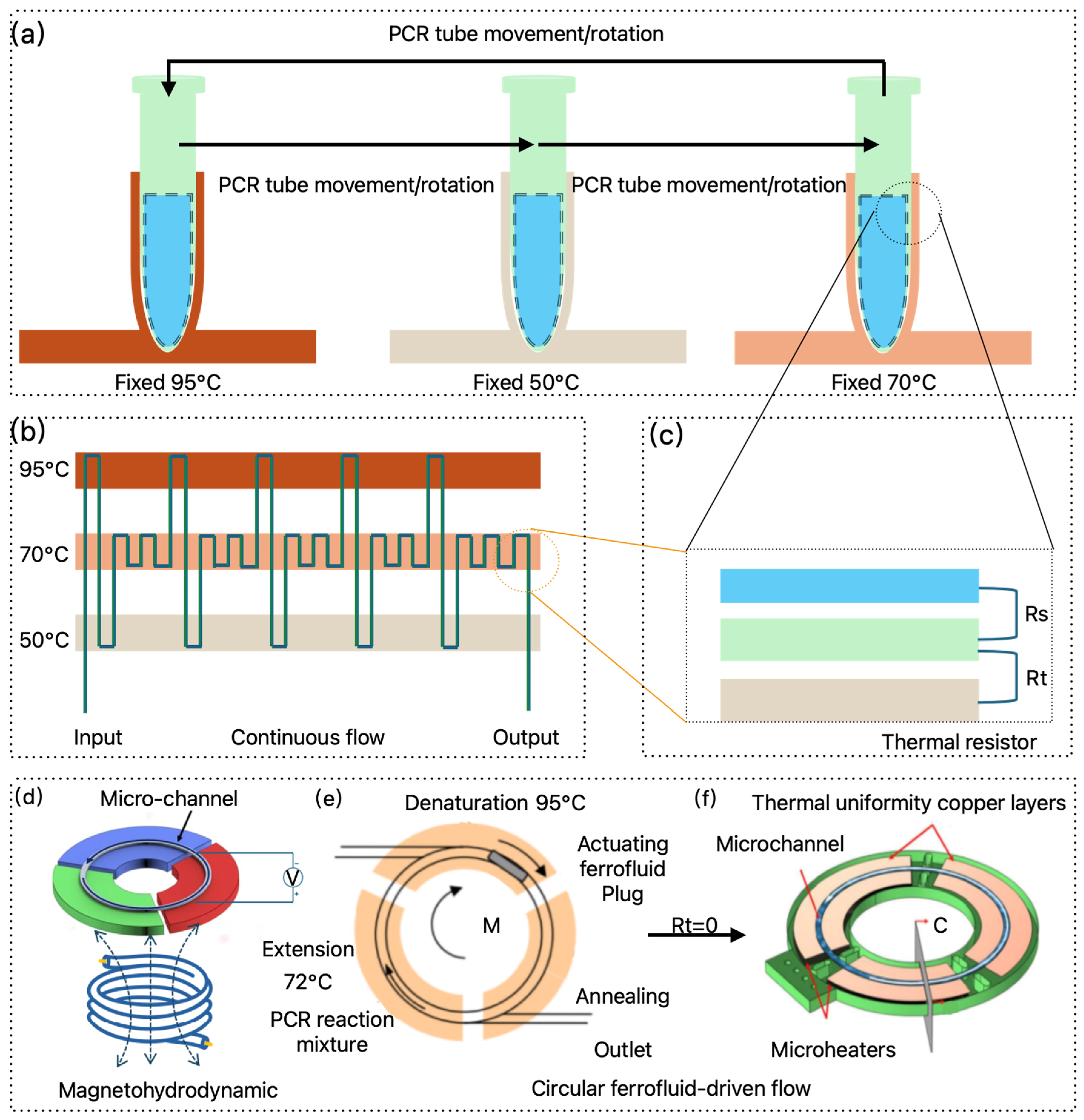
2.2.2. Continuous Flow PCR
2.2.3. Magnet-Driven PCR
2.3. Spatiotemporal Unified PCR, Isothermal Amplification, and Biosensors
2.3.1. Convective PCR
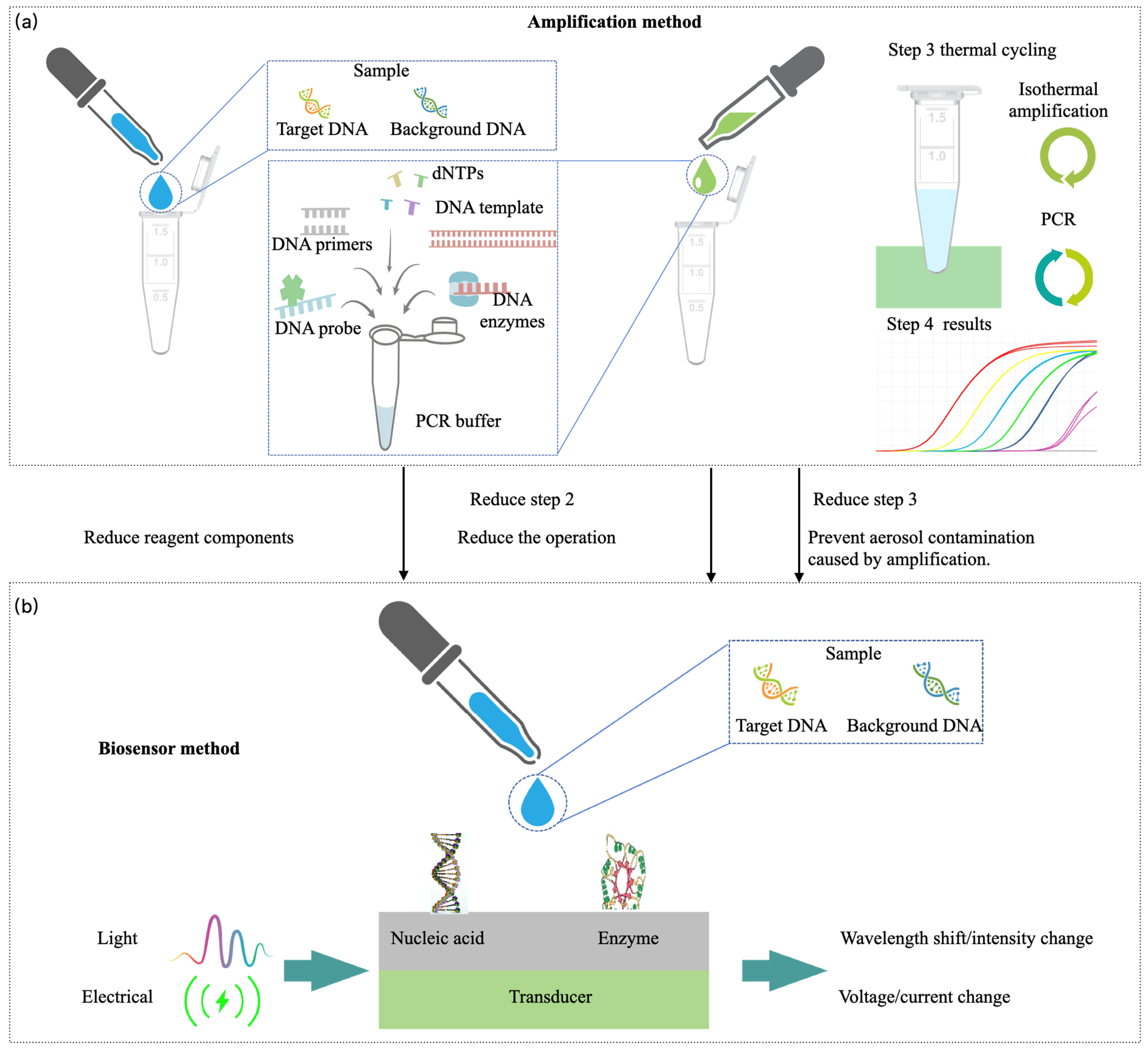
2.3.2. Isothermal Amplification
2.3.3. Biosensor
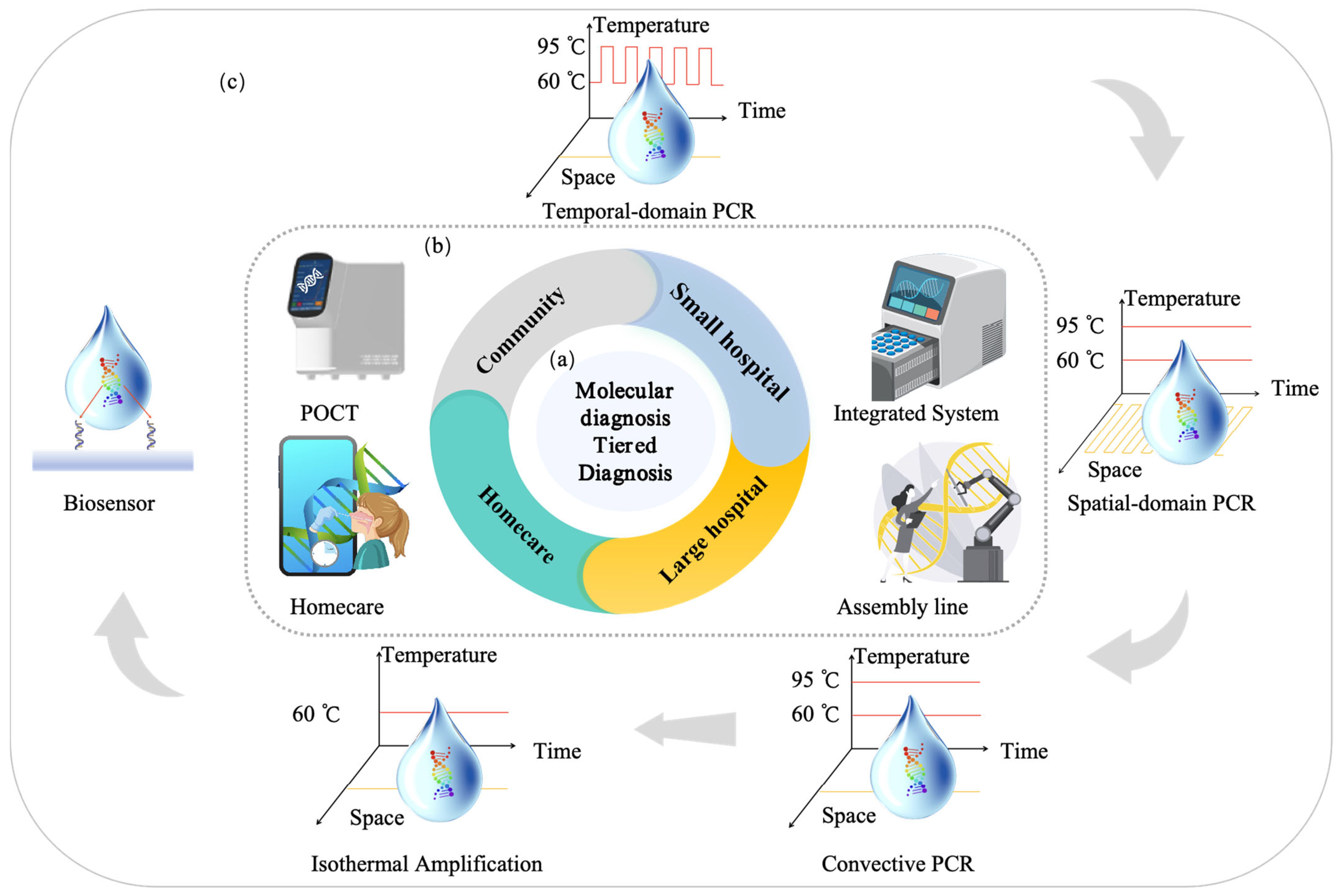
3. Full-Scenario Molecular Infectious Disease Detection Solutions
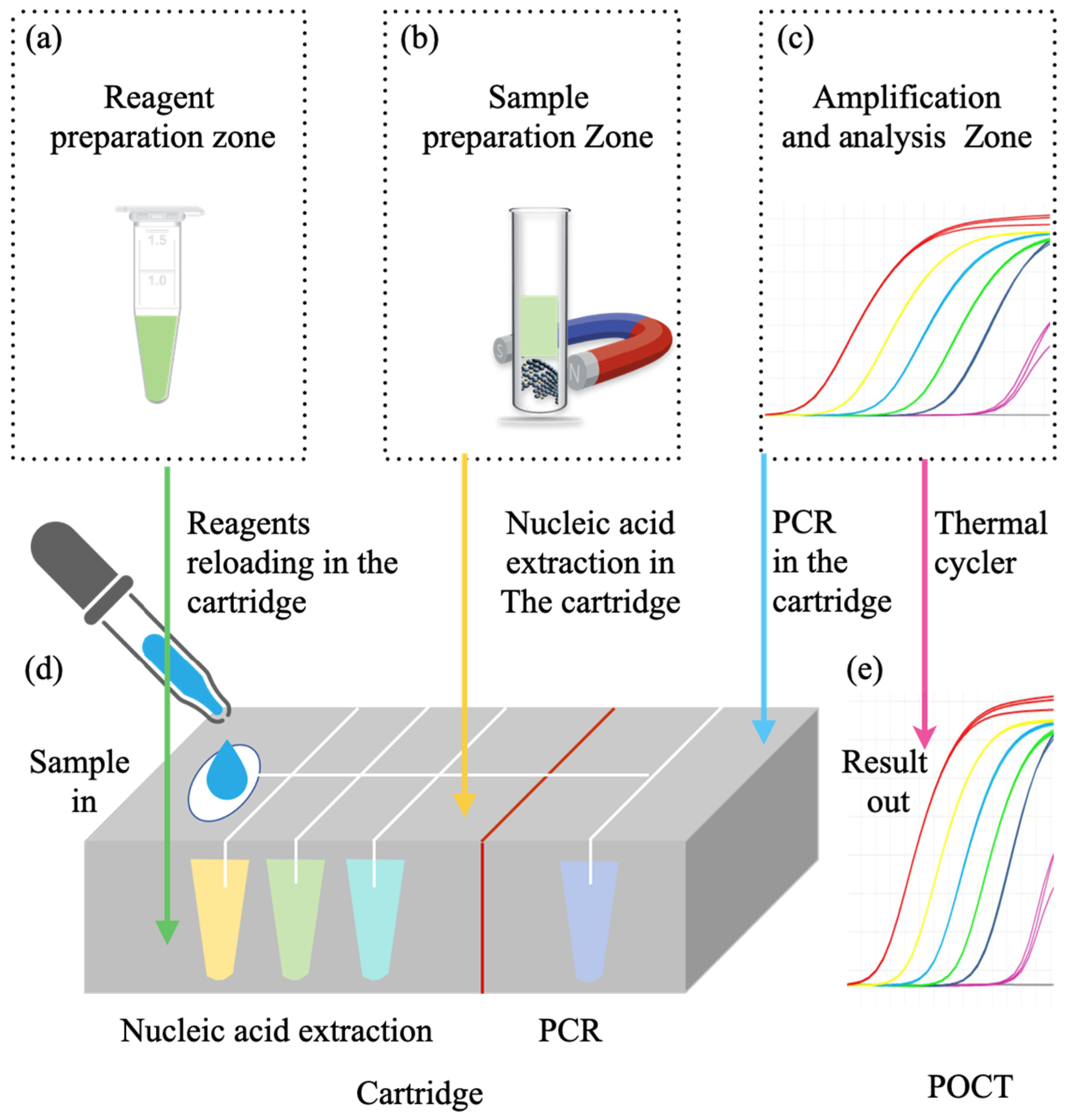
3.1. Hospital-Based Respiratory Infectious Disease Molecular Detection Solutions
3.2. POCT-Based Respiratory Infectious Disease Molecular Detection Solutions
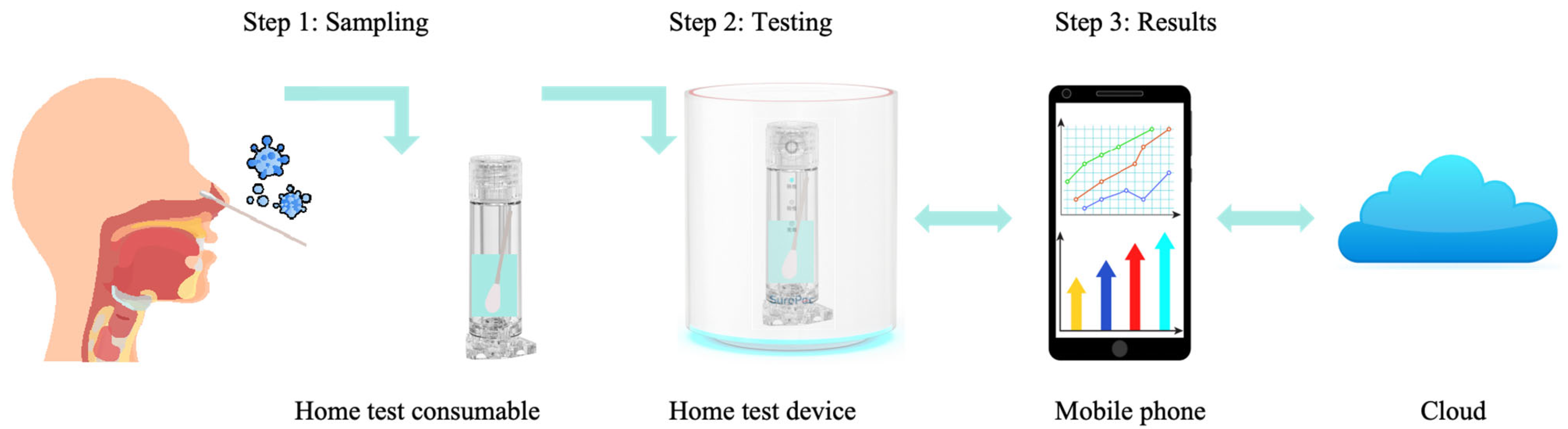
3.3. Home-Based Respiratory Infectious Disease Molecular Detection Solutions
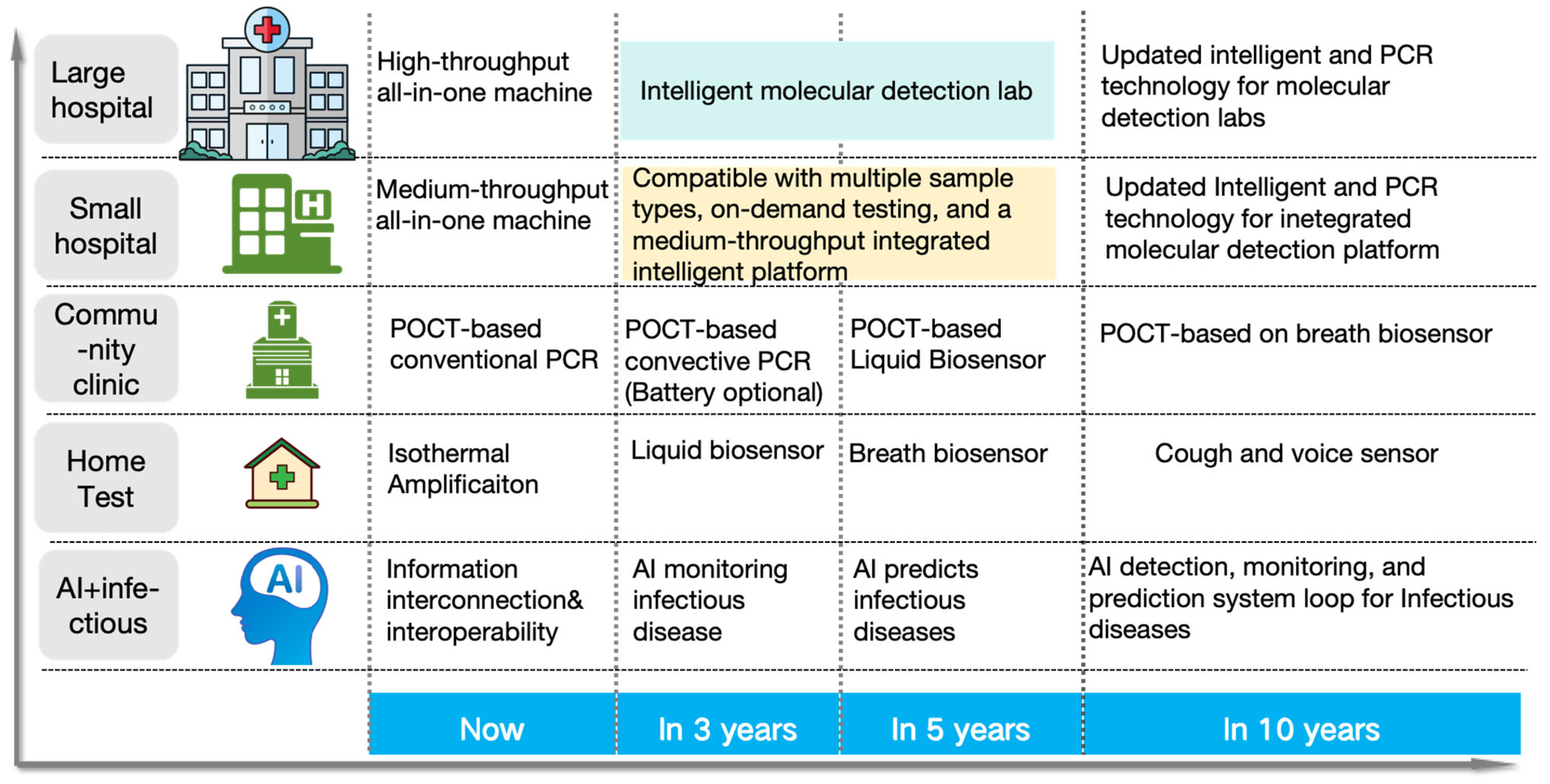
4. Conclusions and Outlook
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cherry, J.D.; Krogstad, P. SARS: The First Pandemic of the 21st Century. Pediatr. Res. 2004, 56, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Al Hajjar, S.; Memish, Z.A.; McIntosh, K. Middle East Respiratory Syndrome Coronavirus (MERS-CoV): A Perpetual Challenge. Ann. Saudi Med. 2013, 33, 427–436. [Google Scholar] [CrossRef]
- Cucinotta, D.; Vanelli, M. WHO Declares COVID-19 a Pandemic. Acta Biomed. 2020, 91, 157–160. [Google Scholar]
- COVID-19 Cases | WHO COVID-19 Dashboard. Available online: https://data.who.int/dashboards/covid19/cases?n=c (accessed on 10 March 2025).
- Hauck, K. The Economics of Infectious Diseases. In Oxford Research Encyclopedia of Economics and Finance; Oxford University Press: Oxford, UK, 2018. [Google Scholar]
- Novella, E.J. COVID-19 and the Emotional Culture of Pandemics: A Retrospective and Prospective View. Paedagog. Hist. 2022, 58, 660–675. [Google Scholar] [CrossRef]
- Proença-Módena, J.L.; Acrani, G.O.; Snider, C.B.; Arruda, E. Respiratory Viral Infections. Trop. Infect. Dis. Princ. Pathog. Pract. 2011, 58, 378–391. [Google Scholar]
- Dong, X.; Wang, Y. The Geography of Healthcare: Mapping Patient Flow and Medical Resource Allocation in China. Econ. Hum. Biol. 2024, 55, 101431. [Google Scholar] [CrossRef]
- Rajakaruna, S.J.; Liu, W.B.; Ding, Y.B.; Cao, G.W. Strategy and Technology to Prevent Hospital-Acquired Infections: Lessons from SARS, Ebola, and MERS in Asia and West Africa. Mil. Med. Res. 2017, 4, 32. [Google Scholar] [CrossRef] [PubMed]
- Mardian, Y.; Kosasih, H.; Karyana, M.; Neal, A.; Lau, C.-Y. Review of Current COVID-19 Diagnostics and Opportunities for Further Development. Front. Med. 2021, 8, 615099. [Google Scholar] [CrossRef]
- Seyedin, H.; Bagherzadeh, R.; Dowlati, M. Hospital Management in Infectious Disease Outbreak: Lessons Learned From COVID-19 Epidemic. Health Emergencies Disasters Q. 2022, 7, 161–166. [Google Scholar] [CrossRef]
- Light, D.W. Universal Health Care: Lessons From the British Experience. Am. J. Public. Health 2003, 93, 25–30. [Google Scholar] [CrossRef]
- World Health Organization Guidance for Managing Ethical Issues in Infectious Disease Outbreaks; World Health Organization: Geneva, Switzerland, 2016; ISBN 978-92-4-154983-7.
- Adalja, A.A. At-Home Infectious Disease Testing: An Idea Whose Time Has Come. Antimicrob. Steward. Healthc. Epidemiol. 2022, 2, e170. [Google Scholar] [CrossRef]
- Kozel, T.R.; Burnham-Marusich, A.R. Point-of-Care Testing for Infectious Diseases: Past, Present, and Future. J. Clin. Microbiol. 2017, 55, 2313–2320. [Google Scholar] [CrossRef] [PubMed]
- Jonguitud-Borrego, N.; Malcı, K.; Anand, M.; Baluku, E.; Webb, C.; Liang, L.; Barba-Ostria, C.; Guaman, L.P.; Hui, L.; Rios-Solis, L. High—Throughput and Automated Screening for COVID-19. Front. Med. Technol. 2022, 4, 969203. [Google Scholar] [CrossRef]
- Yuan, X.; Sui, G.; Zhang, D.; Chen, M.; Zhao, W. Recent Developments and Trends of Automatic Nucleic Acid Detection Systems. J. Biosaf. Biosecurity 2022, 4, 54–58. [Google Scholar] [CrossRef]
- Krishna, N.K.; Cunnion, K.M. Role of Molecular Diagnostics in the Management of Infectious Disease Emergencies. Med. Clin. N. Am. 2012, 96, 1067–1078. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Zhang, H.; Xu, Y.; Laššáková, S.; Korabečná, M.; Neužil, P. PCR Past, Present and Future. BioTechniques 2020, 69, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Pai, N.P.; Vadnais, C.; Denkinger, C.; Engel, N.; Pai, M. Point-of-Care Testing for Infectious Diseases: Diversity, Complexity, and Barriers in Low- And Middle-Income Countries. PLoS Med. 2012, 9, e1001306. [Google Scholar] [CrossRef]
- Mo, X.; Wang, X.; Zhu, Z.; Yu, Y.; Chang, D.; Zhang, X.; Li, D.; Sun, F.; Zhou, L.; Xu, J.; et al. Quality Management for Point-Of-Care Testing of Pathogen Nucleic Acids: Chinese Expert Consensus. Front. Cell. Infect. Microbiol. 2021, 11, 755508. [Google Scholar] [CrossRef]
- Pusterla, N.; Madigan, J.E.; Leutenegger, C.M. Real-Time Polymerase Chain Reaction: A Novel Molecular Diagnostic Tool for Equine Infectious Diseases. J. Veter- Intern. Med. 2006, 20, 3–12. [Google Scholar]
- Mori, Y.; Notomi, T. Loop-Mediated Isothermal Amplification (LAMP): A Rapid, Accurate, and Cost-Effective Diagnostic Method for Infectious Diseases. J. Infect. Chemother. 2009, 15, 62–69. [Google Scholar] [CrossRef]
- Zheng, Y.; Song, X.; Fredj, Z.; Bian, S.; Sawan, M. Challenges and Perspectives of Multi-Virus Biosensing Techniques: A Review. Anal. Chim. Acta 2023, 1244, 34680. [Google Scholar] [CrossRef]
- Son, J.H.; Cho, B.; Hong, S.; Lee, S.H.; Hoxha, O.; Haack, A.J.; Lee, L.P. Ultrafast Photonic PCR. Light Sci. Appl. 2015, 4, e280. [Google Scholar] [CrossRef]
- Li, X.; Wang, J.; Meng, Q.; Yu, D. Theoretical and Experimental Investigation about the Influence of Peltier Effect on the Temperature Loss and Performance Loss of Thermoelectric Generator. Energy Technol. 2022, 10, 202100895. [Google Scholar] [CrossRef]
- CFX96 Touch Deep Well Real-Time PCR Detection System|Bio-Rad. Available online: https://www.bio-rad.com/en-tw/product/cfx96-touch-deep-well-real-time-pcr-detection-system?ID=LZJTUJ15 (accessed on 10 March 2025).
- Yamaguchi, S.; Suzuki, T.; Inoue, K.; Azumi, Y. DC-Driven Thermoelectric Peltier Device for Precise DNA Amplification. Jpn. J. Appl. Phys. 2015, 54, 057001. [Google Scholar] [CrossRef]
- Yang, J.; Liu, Y.; Rauch, C.B.; Stevens, R.L.; Liu, R.H.; Lenigk, R.; Grodzinski, P. High Sensitivity PCR Assay in Plastic Micro Reactors. Lab A Chip 2002, 2, 179–187. [Google Scholar] [CrossRef]
- Khandurina, J.; McKnight, T.E.; Jacobson, S.C.; Waters, L.C.; Foote, R.S.; Ramsey, J.M. Integrated System for Rapid PCR-Based DNA Analysis in Microfluidic Devices. Anal. Chem. 2000, 72, 2995–3000. [Google Scholar] [CrossRef] [PubMed]
- Wilding, P.; Shoffner, M.A.; Kricka, L.J. PCR in a Silicon Microstructure. Clin. Chem. 1994, 40, 1815–1818. [Google Scholar] [CrossRef]
- Shoffner, M.A.; Cheng, J.; Hvichia, G.E.; Kricka, L.J.; Wilding, P. Chip PCR. I. Surface Passivation of Microfabricated Silicon-Glass Chips for PCR. Nucleic Acids Res. 1996, 24, 375–379. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, Z.; Liu, L.; Zhang, T.; Hu, L.; Hu, C.; Chen, H.; Ding, R.; Liu, B.; Chen, C. Ultrafast Nucleic Acid Detection Equipment with Silicon-Based Microfluidic Chip. Biosensors 2023, 13, 234. [Google Scholar] [CrossRef]
- Jia, G.; Siegrist, J.; Deng, C.; Zoval, J.V.; Stewart, G.; Peytavi, R.; Huletsky, A.; Bergeron, M.G.; Madou, M.J. A Low-Cost, Disposable Card for Rapid Polymerase Chain Reaction. Colloids Surf. B Biointerfaces 2007, 58, 52–60. [Google Scholar] [CrossRef]
- Sreejith, K.R.; Gorgannezhad, L.; Jin, J.; Ooi, C.H.; Takei, T.; Hayase, G.; Stratton, H.; Lamb, K.; Shiddiky, M.; Dao, D.V.; et al. Core-Shell Beads Made by Composite Liquid Marble Technology as a Versatile Microreactor for Polymerase Chain Reaction. Micromachines 2020, 11, 242. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.; Jeong, S.; Kim, M.; Lee, J.H. Battery-Operated Portable PCR System with Enhanced Stability of Pt RTD. PLoS ONE 2018, 14, e0218571. [Google Scholar] [CrossRef]
- Koo, C.; Malapi-Wight, M.; Kim, H.S.; Cifci, O.S.; Vaughn-Diaz, V.L.; Ma, B.; Kim, S.; Abdel-Raziq, H.; Ong, K.; Jo, Y.K.; et al. Development of a Real-Time Microchip PCR System for Portable Plant Disease Diagnosis. PLoS ONE 2013, 8, e82704. [Google Scholar] [CrossRef] [PubMed]
- Scorzoni, A.; Tavernelli, M.; Placidi, P.; Zampolli, S. Thermal Modeling and Characterization of a Thin-Film Heater on Glass Substrate for Lab-on-Chip Applications. IEEE Trans. Instrum. Meas. 2015, 64, 1215–1222. [Google Scholar] [CrossRef]
- Jeon, H.G.; Choi, J.W.; Lee, H.U.; Chung, B.G. Conductive Silver/Carbon Fiber Films for Rapid Detection of Human Coronavirus. Polymers 2022, 14, 1983. [Google Scholar] [CrossRef]
- Kim, K.; Lee, B.; Park, J.H.; Park, J.H.; Lee, K.J.; Kwak, T.J.; Son, T.; Shin, Y.B.; Im, H.; Kim, M.G. Rapid PCR Kit: Lateral Flow Paper Strip with Joule Heater for SARS-CoV-2 Detection. Mater. Horiz. 2023, 10, 1697–1704. [Google Scholar] [CrossRef]
- Chung, K.H.; Choi, Y.H.; Choi, H.K.; Kim, J.T.; Yu, Y.-J.; Choi, J.S.; Youn, D.-H.; Choi, C.-G. Convection-Based Realtime Polymerase Chain Reaction (PCR) Utilizing Transparent Graphene Heaters. In Proceedings of the IEEE SENSORS 2014 Proceedings, Valencia, Spain, 2–5 November 2014; IEEE: Piscataway, NJ, USA, 2014; pp. 1006–1009. [Google Scholar]
- Nguyen, K.H.T. A Rapid and Power-Efficient Laser-Induced Graphene Micro-Thermocycler for DNA Polymerase Chain Reaction. Ph.D. Dissertation, The University of Minnesota, Minneapolis, MN, USA, 2023. [Google Scholar]
- Kaprou, G.D.; Papadopoulos, V.; Loukas, C.M.; Kokkoris, G.; Tserepi, A. Towards PCB-Based Miniaturized Thermocyclers for DNA Amplification. Micromachines 2020, 11, 258. [Google Scholar] [CrossRef]
- Yeom, D.; Kim, J.; Kim, S.; Ahn, S.; Choi, J.; Kim, Y.; Koo, C. A Thermocycler Using a Chip Resistor Heater and a Glass Microchip for a Portable and Rapid Microchip-Based PCR Device. Micromachines 2022, 13, 339. [Google Scholar] [CrossRef]
- Belgrader, P.; Young, S.; Yuan, B.; Primeau, M.; Christel, L.A.; Pourahmadi, F.; Northrup, M.A. A Battery-Powered Notebook Thermal Cycler for Rapid Multiplex Real-Time PCR Analysis. Anal. Chem. 2001, 73, 286–289. [Google Scholar] [CrossRef]
- Heap, D.M.; Herrmann, M.G.; Witter, C.T. PCR Amplification Using Electrolytic Resistance for Heating and Temperature Monitoring. BioTechniques 2000, 29, 1006–1012. [Google Scholar] [CrossRef]
- Shah, J.J.; Sundaresan, S.G.; Geist, J.; Reyes, D.R.; Booth, J.C.; Rao, M.V.; Gaitan, M. Microwave Dielectric Heating of Fluids in an Integrated Microfluidic Device. J. Micromech. Microeng. 2007, 17, 2224–2230. [Google Scholar] [CrossRef]
- Fermér, C.; Nilsson, P.; Larhed, M. Microwave-Assisted High-Speed PCR. Eur. J. Pharm. Sci. 2003, 18, 129–132. [Google Scholar] [CrossRef]
- Orrling, K.; Nilsson, P.; Gullberg, M.; Larhed, M. An Efficient Method to Perform Milliliter-Scale PCR Utilizing Highly Controlled Microwave Thermocycling. Chem. Commun. 2004, 4, 790–791. [Google Scholar] [CrossRef]
- Shaw, K.J.; Docker, P.T.; Yelland, J.V.; Dyer, C.E.; Greenman, J.; Greenway, G.M.; Haswell, S.J. Rapid PCR Amplification Using a Microfluidic Device with Integrated Microwave Heating and Air Impingement Cooling. Lab A Chip 2010, 10, 1725–1728. [Google Scholar] [CrossRef]
- Kempitiya, A.; Borca-Tasciuc, D.A.; Mohamed, H.S.; Hella, M.M. Localized Microwave Heating in Microwells for Parallel DNA Amplification Applications. Appl. Phys. Lett. 2009, 94, 064106. [Google Scholar] [CrossRef]
- Marchiarullo, D.J.; Sklavounos, A.H.; Oh, K.; Poe, B.L.; Barker, N.S.; Landers, J.P. Low-Power Microwave-Mediated Heating for Microchip-Based PCR. Lab A Chip 2013, 13, 3417–3425. [Google Scholar] [CrossRef]
- Boybay, M.S.; Jiao, A.; Glawdel, T.; Ren, C.L. Microwave Sensing and Heating of Individual Droplets in Microfluidic Devices. Lab A Chip 2013, 13, 3840–3846. [Google Scholar] [CrossRef] [PubMed]
- Martinic, M.; Schreurs, D.; Markovic, T.; Nauwelaers, B. CSRR-Based Low Power Microwave Heater for PCR Applications. In Proceedings of the 2023 IEEE MTT-S International Microwave Biomedical Conference, IMBioC 2023, Leuven, Belgium, 11–13 September 2023; Institute of Electrical and Electronics Engineers Inc.: Minneapolis, MN, USA, 2023; pp. 106–108. [Google Scholar]
- Martinic, M.; Schreurs, D.; Markovic, T.; Nauwelaers, B. Model-Based Frequency Adaptive Microwave Heating for PCR Applications. IEEE J. Electromagn. RF Microw. Med. Biol. 2024, 8, 238–244. [Google Scholar] [CrossRef]
- Pal, D.; Dey, N. PCR Compatible Miniprep DNA Isolation in Rice Using Microwave and Dry Bath Based Heating Devices. Rev. Bras. Bot. 2024, 47, 1001–1005. [Google Scholar] [CrossRef]
- Oda, R.P.; Strausbauch, M.A.; Huhmer, A.F.R.; Borson, N.; Jurrens, S.R.; Craighead, J.; Wettstein, P.J.; Eckloff, B.; Kline, B.; Landers, J.P. Infrared-Mediated Thermocycling for Ultrafast Polymerase Chain Reaction Amplification of DNA. Anal. Chem. 1998, 70, 4361–4368. [Google Scholar] [CrossRef]
- Huhmer, A.F.R.; Landers, J.P. Noncontact Infrared-Mediated Thermocycling for Effective Polymerase Chain Reaction Amplification of DNA in Nanoliter Volumes. Anal. Chem. 2000, 72, 5507–5512. [Google Scholar] [CrossRef] [PubMed]
- Roper, M.G.; Easley, C.J.; Legendre, L.A.; Humphrey, J.A.C.; Landers, J.P. Infrared Temperature Control System for a Completely Noncontact Polymerase Chain Reaction in Microfluidic Chips. Anal. Chem. 2007, 79, 1294–1300. [Google Scholar] [CrossRef] [PubMed]
- Im, K.; Eun, D.; Kong, S.; Shin, J.; Lee, J. Fabrication of a Polymerase Chain Reaction Micro-Reactor Using Infrared Heating. J. Korean Sens. Soc. 2005, 14, 337–342. [Google Scholar] [CrossRef]
- Yu, Y.; Li, B.; Baker, C.A.; Zhang, X.; Roper, M.G. Quantitative Polymerase Chain Reaction Using Infrared Heating on a Microfluidic Chip. Anal. Chem. 2012, 84, 2825–2829. [Google Scholar] [CrossRef]
- Warden, A.R.; Liu, W.; Chen, H.; Ding, X. Portable Infrared Isothermal PCR Platform for Multiple Sexually Transmitted Diseases Strand Detection. Anal. Chem. 2018, 90, 11760–11763. [Google Scholar] [CrossRef]
- Kim, H.; Dixit, S.; Green, C.J.; Faris, G.W.; Liu, J.G.L.; Kim, Y.; Lu, L.P. Nanodroplet Real-Time PCR System with Laser Assisted Heating. Opt. Express 2009, 17, 218–227. [Google Scholar] [CrossRef]
- Kim, H.; Vishniakou, S.; Faris, G.W. Petri Dish PCR: Laser-Heated Reactions in Nanoliter Droplet Arrays. Lab A Chip 2009, 9, 1230–1235. [Google Scholar] [CrossRef]
- Hagan, K.A.; Norris, J.V.; Root, B.E.; Scott, O.N.; Lovaglio, R.; Egan, M.; Trost, P.; Bievenue, J.M.; Landers, J.P. PCR amplification of str loci using an infrared laser source. In Proceedings of the 15th International Conference onMiniaturized Systems for Chemistry and Life Sciences, Seattle, WA, USA, 2–6 October 2011; pp. 365–367. [Google Scholar]
- Terazono, H.; Hattori, A.; Takei, H.; Takeda, K.; Yasuda, K. Development of 1480 Nm Photothermal High-Speed Real-Time Polymerase Chain Reaction System for Rapid Nucleotide Recognition. Jpn. J. Appl. Phys. 2008, 47, 5212–5216. [Google Scholar] [CrossRef]
- Pak, N.; Saunders, D.C.; Phaneuf, C.R.; Forest, C.R. Plug-and-Play, Infrared, Laser-Mediated PCR in a Microfluidic Chip. Biomed. Microdevices 2012, 14, 427–433. [Google Scholar] [CrossRef]
- Vincent, C.; Voronin, A.A.; Sower, K.; Belousov, V.V.; Belousov, V.V.; Belousov, V.V.; Sokolov, A.V.; Sokolov, A.V.; Scully, M.O.; Scully, M.O.; et al. Photonic Toolbox for Fast Real-Time Polymerase Chain Reaction. Laser Phys. Lett. 2020, 17, 076202. [Google Scholar] [CrossRef]
- Hettiarachchi, K.; Kim, H.; Faris, G.W. Optical Manipulation and Control of Real-Time PCR in Cell Encapsulating Microdroplets by IR Laser. Microfluid. Nanofluidics 2012, 13, 967–975. [Google Scholar] [CrossRef]
- Wang, Z.; Liang, X.; Su, H.; Li, S.; Chen, Y. Temperature Control of a Droplet Heated by an Infrared Laser for PCR Applications. Ind. Eng. Chem. Res. 2021, 60, 14341–14353. [Google Scholar] [CrossRef]
- Le Roux, D.; Root, B.E.; Reedy, C.R.; Hickey, J.A.; Scott, O.N.; Bienvenue, J.M.; Landers, J.P.; Chassagne, L.; De Mazancourt, P. DNA Analysis Using an Integrated Microchip for Multiplex PCR Amplification and Electrophoresis for Reference Samples. Anal. Chem. 2014, 86, 8192–8199. [Google Scholar] [CrossRef]
- Pal, D.; Venkataraman, V. A Portable Battery-Operated Chip Thermocycler Based on Induction Heating. Sens. Actuators A 2002, 102, 151–156. [Google Scholar] [CrossRef]
- Pal, D.; Venkataraman, V.; Mohan, K.N.; Chandra, H.S.; Natarajan, V. A Power-Efficient Thermocycler Based on Induction Heating for DNA Amplification by Polymerase Chain Reaction. Rev. Sci. Instrum. 2004, 75, 2880–2883. [Google Scholar] [CrossRef]
- Kim, J.A.; Lee, S.H.; Park, H.; Kim, J.H.; Park, T.H. Microheater Based on Magnetic Nanoparticle Embedded PDMS. Nanotechnology 2010, 21, 165102. [Google Scholar] [CrossRef]
- Xie, Y.; Jiang, Q.; Chang, C.; Zhao, X.; Yong, H.; Ke, X.; Wu, Z. A Thermal Cycler Based on Magnetic Induction Heating and Anti-Freezing Water Cooling for Rapid PCR. Micromachines 2024, 15, 1462. [Google Scholar] [CrossRef] [PubMed]
- Baek, S.K.; Min, J.; Park, J.H. Wireless Induction Heating in a Microfluidic Device for Cell Lysis. Lab A Chip 2010, 10, 909–917. [Google Scholar] [CrossRef]
- Ahn, M.H.; Baek, S.K.; Min, J.H.; Park, J.H. A Portable Electromagnetic Induction Heating Device for Point-of-Care Diagnostics. Biochip J. 2016, 10, 208–214. [Google Scholar] [CrossRef]
- Chen, X.; Song, L.; Babak, A.; Fang, J.; Mohamed, S.M.A.; Kenichi, T. Wirelessly Addressable Heater Array for Centrifugal Microfluidics and Escherichia Coli Sterilization. In Proceedings of the 2013 35th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Osaka, Japan, 3–7 July 2013; pp. 5505–5508. [Google Scholar]
- Roche, P.J.R.; Beitel, L.K.; Khan, R.; Lumbroso, R.; Najih, M.; Cheung, M.C.K.; Thiemann, J.; Veerasubramanian, V.; Trifiro, M.; Chodavarapu, V.P.; et al. Demonstration of a Plasmonic Thermocycler for the Amplification of Human Androgen Receptor DNA. Analyst 2012, 137, 4475–4481. [Google Scholar] [CrossRef]
- Roche, P.J.R.; Najih, M.; Lee, S.S.; Beitel, L.K.; Carnevale, M.L.; Paliouras, M.; Kirk, A.G.; Trifiro, M.A. Real Time Plasmonic qPCR: How Fast Is Ultra-Fast? 30 Cycles in 54 Seconds. Analyst 2017, 142, 1746–1755. [Google Scholar] [CrossRef] [PubMed]
- Restaino, S.M.; White, I.M. Real-Time Multiplexed PCR Using Surface Enhanced Raman Spectroscopy in a Thermoplastic Chip. Lab A Chip 2018, 18, 832–839. [Google Scholar] [CrossRef]
- Amadeh, A.; Ghazimirsaeed, E.; Shamloo, A.; Dizani, M. Improving the Performance of a Photonic PCR System Using TiO2 Nanoparticles. J. Ind. Eng. Chem. 2021, 94, 195–204. [Google Scholar] [CrossRef]
- Kim, B.K.; Lee, S.A.; Park, M.; Jeon, E.J.; Kim, M.J.; Kim, J.M.; Kim, H.; Jung, S.; Kim, S.K. Ultrafast Real-Time PCR in Photothermal Microparticles. ACS Nano 2022, 16, 20533–20544. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Jiang, K.; Wu, J.; Mi, H.; Peng, Y.K.; Go, Y.Y.; Park, H.J.; Lee, J.H. Fast and Sensitive Immuno-PCR Assisted by Plasmonic Magnetic Nanoparticles. Appl. Mater. Today 2021, 23, 101054. [Google Scholar] [CrossRef]
- Kim, J.; Kim, H.; Park, J.H.; Jon, S. Gold Nanorod-Based Photo-Pcr System for One-Step, Rapid Detection of Bacteria. Nanotheranostics 2017, 1, 178–185. [Google Scholar] [CrossRef]
- Cheong, J.; Yu, H.; Lee, C.Y.; Lee, J.; Choi, H.J.; Lee, J.H.; Lee, H.; Cheon, J. Fast Detection of SARS-CoV-2 RNA via the Integration of Plasmonic Thermocycling and Fluorescence Detection in a Portable Device. Nat. Biomed. Eng. 2020, 4, 1159–1167. [Google Scholar] [CrossRef]
- Shrestha, K.; Kim, S.; Han, J.; Florez, G.M.; Truong, H.; Hoang, T.; Parajuli, S.; Tiara, A.M.; Kim, B.; Jung, Y.; et al. Mobile Efficient Diagnostics of Infectious Diseases via On-Chip RT-qPCR: MEDIC-PCR. Adv. Sci. 2023, 10, 2302072. [Google Scholar] [CrossRef] [PubMed]
- Jalili, A.; Bagheri, M.; Shamloo, A.; Kazemipour Ashkezari, A.H. A Plasmonic Gold Nanofilm-Based Microfluidic Chip for Rapid and Inexpensive Droplet-Based Photonic PCR. Sci. Rep. 2021, 11, 23338. [Google Scholar] [CrossRef]
- Monshat, H.; Wu, Z.; Pang, J.; Zhang, Q.; Lu, M. Integration of Plasmonic Heating and On-Chip Temperature Sensor for Nucleic Acid Amplification Assays. J. Biophotonics 2020, 13, e202000060. [Google Scholar] [CrossRef]
- Han, S.; An, H.J.; Kwak, T.; Kim, M.; Kim, D.; Lee, L.P.; Choi, I. Plasmonic Optical Wells-Based Enhanced Rate PCR. Nano Lett. 2024, 24, 1738–1745. [Google Scholar] [CrossRef] [PubMed]
- Bartsch, M.S.; Edwards, H.S.; Lee, D.; Moseley, C.E.; Tew, K.E.; Renzi, R.F.; Van De Vreugde, J.L.; Kim, H.; Knight, D.L.; Sinha, A.; et al. The Rotary Zone Thermal Cycler: A Low-Power System Enabling Automated Rapid PCR. PLoS ONE 2015, 10, e0118182. [Google Scholar] [CrossRef]
- Wong, G.; Wong, I.; Chan, K.; Hsieh, Y.; Wong, S. A Rapid and Low-Cost PCR Thermal Cycler for Low Resource Settings. PLoS ONE 2015, 10, e0131701. [Google Scholar] [CrossRef]
- Chan, K.; Wong, P.Y.; Yu, P.; Hardick, J.; Wong, K.Y.; Wilson, S.A.; Wu, T.; Hui, Z.; Gaydos, C.; Wong, S.S. A Rapid and Low-Cost PCR Thermal Cycler for Infectious Disease Diagnostics. PLoS ONE 2016, 11, e0149150. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Kim, D.; Han, J.; Yun, J.; Lee, K.H.; Kim, G.M.; Kwon, O.; Lee, J. Integrated, Automated, Fast Pcr System for Point-of-Care Molecular Diagnosis of Bacterial Infection. Sensors 2021, 21, 377. [Google Scholar] [CrossRef] [PubMed]
- Millington, A.L.; Houskeeper, J.A.; Quackenbush, J.F.; Trauba, J.M.; Wittwer, C.T. The Kinetic Requirements of Extreme qPCR. Biomol. Detect. Quantif. 2019, 17, 100081. [Google Scholar] [CrossRef]
- Song, W.; Zhang, C.; Lin, H.; Zhang, T.; Liu, H.; Huang, X. Portable Rotary PCR System for Real-Time Detection of Pseudomonas Aeruginosa in Milk. Lab A Chip 2023, 23, 4592–4599. [Google Scholar] [CrossRef]
- An, Y.Q.; Huang, S.L.; Xi, B.C.; Gong, X.L.; Ji, J.H.; Hu, Y.; Ding, Y.J.; Zhang, D.X.; Ge, S.X.; Zhang, J.; et al. Ultrafast Microfluidic PCR Thermocycler for Nucleic Acid Amplification. Micromachines 2023, 14, 658. [Google Scholar] [CrossRef]
- Xi, B.; Huang, S.; An, Y.; Gong, X.; Yang, J.; Zeng, J.; Ge, S.; Zhang, D. Sophisticated and Precise: Design and Implementation of a Real-Time Optical Detection System for Ultra-Fast PCR. RSC Adv. 2023, 13, 19770–19781. [Google Scholar] [CrossRef]
- Li, Z.; Ma, X.; Zhang, Z.; Wang, X.; Yang, B.; Yang, J.; Zeng, Y.; Yuan, X.; Zhang, D.; Yamaguchi, Y. A Rapid and Low-Cost Platform for Detection of Bacterial Based on Microchamber PCR Microfluidic Chip. Biomed. Microdevices 2024, 26, 20. [Google Scholar] [CrossRef]
- Sun, Y.; Kwok, Y.C.; Nguyen, N.T. A Circular Ferrofluid Driven Microchip for Rapid Polymerase Chain Reaction. Lab A Chip 2007, 7, 1012–1017. [Google Scholar] [CrossRef] [PubMed]
- Skaltsounis, P.; Kokkoris, G.; Papaioannou, T.G.; Tserepi, A. Closed-Loop Microreactor on PCB for Ultra-Fast DNA Amplification: Design and Thermal Validation. Micromachines 2023, 14, 172. [Google Scholar] [CrossRef]
- Kopp, M.U.; De Mello, A.J.; Manz, A. Chemical Amplification: Continuous-Flow PCR on a Chip. Science 1998, 280, 1046–1048. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Yamaguchi, A.; Ishida, Y.; Matsuo, S.; Misawa, H. A Heater-Integrated Transparent Microchannel Chip for Continuous-Flow PCR. Sens. Actuators B 2002, 84, 283–289. [Google Scholar] [CrossRef]
- Xue, N.; Yan, W. Glass-Based Continuous-Flow PCR Chip with a Portable Control System for DNA Amplification. IEEE Sens. J. 2012, 12, 1914–1918. [Google Scholar] [CrossRef]
- Hashimoto, M.; Chen, P.C.; Mitchell, M.W.; Nikitopoulos, D.E.; Soper, S.A.; Murphy, M.C. Rapid PCR in a Continuous Flow Device. Lab A Chip 2004, 4, 638–645. [Google Scholar] [CrossRef]
- Hatch, A.C.; Ray, T.; Lintecum, K.; Youngbull, C. Continuous Flow Real-Time PCR Device Using Multi-Channel Fluorescence Excitation and Detection. Lab A Chip 2014, 14, 562–568. [Google Scholar] [CrossRef]
- Crews, N.; Wittwer, C.; Gale, B. Continuous-Flow Thermal Gradient PCR. Biomed. Microdevices 2008, 10, 187–195. [Google Scholar] [CrossRef]
- Thomas, S.; Orozco, R.L.; Ameel, T. Thermal Gradient Continuous-Flow PCR: A Guide to Design. Microfluid. Nanofluidics 2014, 17, 1039–1051. [Google Scholar] [CrossRef]
- Fernández-Carballo, B.L.; McGuiness, I.; McBeth, C.; Kalashnikov, M.; Borrós, S.; Sharon, A.; Sauer-Budge, A.F. Low-Cost, Real-Time, Continuous Flow PCR System for Pathogen Detection. Biomed. Microdevices 2016, 18, 34. [Google Scholar] [CrossRef]
- Fernández-Carballo, B.L.; McBeth, C.; McGuiness, I.; Kalashnikov, M.; Baum, C.; Borrós, S.; Sharon, A.; Sauer-Budge, A.F. Continuous-Flow, Microfluidic, qRT-PCR System for RNA Virus Detection. Anal. Bioanal. Chem. 2018, 410, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, M.B.; Goel, S. Miniaturized DNA Amplification Platform with Soft-Lithographically Fabricated Continuous-Flow PCR Microfluidic Device on a Portable Temperature Controller. Microfluid. Nanofluidics 2021, 25, 69. [Google Scholar] [CrossRef]
- Li, Z.; Li, Y.; Sekine, S.; Xi, H.; Amano, A.; Zhang, D.; Yamaguchi, Y. Design and Fabrication of Portable Continuous Flow PCR Microfluidic Chip for DNA Replication. Biomed. Microdevices 2020, 22, 5. [Google Scholar] [CrossRef]
- Li, Z.; Wang, Y.; Gao, Z.; Sekine, S.; You, Q.; Zhuang, S.; Zhang, D.; Feng, S.; Yamaguchi, Y. Lower Fluidic Resistance of Double-Layer Droplet Continuous Flow PCR Microfluidic Chip for Rapid Detection of Bacteria. Anal. Chim. Acta 2023, 1251, 340995. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.J.; Qiu, X.C. The Effect of the Surface Passivation on Polymerase Chain Reaction inside a Continuous Flow Microfluidic Chip. Microsyst. Technol. 2025, 31, 25–43. [Google Scholar] [CrossRef]
- Wang, K.; Wu, D.; Wu, W. A New Self-Activated Micropumping Mechanism Capable of Continuous-Flow and Real-Time PCR Amplification inside 3D Spiral Microreactor. Micromachines 2019, 10, 685. [Google Scholar] [CrossRef]
- West, J.; Karamata, B.; Lillis, B.; Gleeson, J.P.; Alderman, J.; Collins, J.K.; Lane, W.; Mathewson, A.; Berney, H. Application of Magnetohydrodynamic Actuation to Continuous Flow Chemistry. Lab A Chip 2002, 2, 224–230. [Google Scholar] [CrossRef]
- Sun, Y.; Kwok, Y.C.; Foo-Peng Lee, P.; Nguyen, N.T. Rapid Amplification of Genetically Modified Organisms Using a Circular Ferrofluid-Driven PCR Microchip. Proc. Anal. Bioanal. Chem. 2009, 394, 1505–1508. [Google Scholar] [CrossRef]
- Sun, Y.; Nguyen, N.T.; Yien, C.K. High-Throughput Polymerase Chain Reaction in Parallel Circular Loops Using Magnetic Actuation. Anal. Chem. 2008, 80, 6127–6130. [Google Scholar] [CrossRef]
- Krishnan, M.; Ugaz, V.M.; Burns, M.A. PCR in a Rayleigh-Bénard Convection Cell. Science 2002, 298, 25. [Google Scholar] [CrossRef]
- Agrawal, N.; Ugaz, V.M. A Buoyancy-Driven Compact Thermocycler for Rapid PCR. J. Assoc. Lab. Autom. 2006, 11, 217–221. [Google Scholar] [CrossRef]
- Agrawal, N.; Hassan, Y.A.; Ugaz, V.M. A Pocket-Sized Convective PCR Thermocycler. Angew. Chem.—Int. Ed. 2007, 46, 4316–4319. [Google Scholar] [CrossRef] [PubMed]
- Shu, B.; Zhang, C.; Xing, D. A Sample-to-Answer, Real-Time Convective Polymerase Chain Reaction System for Point-of-Care Diagnostics. Biosens. Bioelectron. 2017, 97, 360–368. [Google Scholar] [CrossRef]
- Wheeler, E.K.; Benett, W.; Stratton, P.; Richards, J.; Chen, A.; Christian, A.; Ness, K.D.; Ortega, J.; Li, L.G.; Weisgraber, T.H.; et al. Convectively Driven Polymerase Chain Reaction Thermal Cycler. Anal. Chem. 2004, 76, 4011–4016. [Google Scholar] [CrossRef] [PubMed]
- Espulgar, W.V.; Saito, M.; Takahashi, K.; Ushiro, S.; Yamamoto, N.; Akeda, Y.; Hamaguchi, S.; Tomono, K.; Tamiya, E. Deskilled and Rapid Drug-Resistant Gene Detection by Centrifugal Force-Assisted Thermal Convection PCR Device. Sensors 2021, 21, 1225. [Google Scholar] [CrossRef]
- Qiu, X.; Ge, S.; Gao, P.; Li, K.; Yang, Y.; Zhang, S.; Ye, X.; Xia, N.; Qian, S. A Low-Cost and Fast Real-Time PCR System Based on Capillary Convection. SLAS Technol. 2017, 22, 13–17. [Google Scholar] [CrossRef]
- Qiu, X.; Zhang, S.; Mei, L.; Wu, D.; Guo, Q.; Li, K.; Ge, S.; Ye, X.; Xia, N.; Mauk, M.G. Characterization and Analysis of Real-Time Capillary Convective Pcr toward Commercialization. Biomicrofluidics 2017, 11, 024103. [Google Scholar] [CrossRef]
- Zhuo, Z.; Wang, J.; Chen, W.; Su, X.; Chen, M.; Fang, M.; He, S.; Zhang, S.; Ge, S.; Zhang, J.; et al. A Rapid On-Site Assay for the Detection of Influenza A by Capillary Convective PCR. Mol. Diagn. Ther. 2018, 22, 225–234. [Google Scholar] [CrossRef]
- Chang, H.F.G.; Tsai, Y.L.; Tsai, C.F.; Lin, C.K.; Lee, P.Y.; Teng, P.H.; Su, C.; Jeng, C.C. A Thermally Baffled Device for Highly Stabilized Convective PCR. Biotechnol. J. 2012, 7, 662–666. [Google Scholar] [CrossRef]
- Hsieh, Y.F.; Lee, D.S.; Chen, P.H.; Liao, S.K.; Yeh, S.H.; Chen, P.J.; Yang, A.S. A Real-Time Convective PCR Machine in a Capillary Tube Instrumented with a CCD-Based Fluorometer. Sens. Actuators B Chem. 2013, 183, 434–440. [Google Scholar] [CrossRef]
- Rajendran, V.K.; Bakthavathsalam, P.; Bergquist, P.L.; Sunna, A. A Portable Nucleic Acid Detection System Using Natural Convection Combined with a Smartphone. Biosens. Bioelectron. 2019, 134, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Jiang, X.; Zou, T.; Miao, G.; Fu, Q.; Xiang, F.; Feng, L.; Ye, X.; Zhang, L.; Qiu, X. A Microfluidic System for Rapid Nucleic Acid Analysis Based on Real-Time Convective PCR at Point-of-Care Testing. Microfluid. Nanofluidics 2022, 26, 69. [Google Scholar] [CrossRef] [PubMed]
- Miao, G.; Jiang, X.; Yang, D.; Fu, Q.; Zhang, L.; Ge, S.; Ye, X.; Xia, N.; Qian, S.; Qiu, X. A Hand-Held, Real-Time, AI-Assisted Capillary Convection PCR System for Point-of-Care Diagnosis of African Swine Fever Virus. Sens. Actuators B Chem. 2022, 358, 131476. [Google Scholar] [CrossRef]
- Khodakov, D.; Li, J.; Zhang, J.X.; Zhang, D.Y. Highly Multiplexed Rapid DNA Detection with Single-Nucleotide Specificity via Convective PCR in a Portable Device. Nat. Biomed. Eng. 2021, 5, 702–712. [Google Scholar] [CrossRef]
- Sullivan, A.T.; Rao, V.; Rockwood, T.; Gandhi, J.; Gruzka, S.; O’Connor, L.; Wang, B.; Ragan, K.B.; Zhang, D.Y.; Khodakov, D. Rapid, Tunable, and Multiplexed Detection of RNA Using Convective Array PCR. Commun. Biol. 2023, 6, 973. [Google Scholar] [CrossRef]
- Srivastava, P.; Prasad, D. Isothermal Nucleic Acid Amplification and Its Uses in Modern Diagnostic Technologies. 3 Biotech 2023, 13, 200. [Google Scholar] [CrossRef] [PubMed]
- Rajan, A.; Shrivastava, S.; Janhawi; Kumar, A.; Singh, A.K.; Arora, P.K. CRISPR-Cas System: From Diagnostic Tool to Potential Antiviral Treatment. Appl. Microbiol. Biotechnol. 2022, 106, 5863–5877. [Google Scholar] [CrossRef]
- Lu, X.; Lin, H.; Feng, X.; Lui, G.C.; Hsing, I.M. Disposable and Low-Cost Pen-like Sensor Incorporating Nucleic-Acid Amplification Based Lateral-Flow Assay for at-Home Tests of Communicable Pathogens. Biosens. Bioelectron. X 2022, 12, 100248. [Google Scholar] [CrossRef]
- Bravo-González, S.; González-González, E.; Perales-Salinas, V.; Rodríguez-Sánchez, I.P.; Ortiz-Castillo, J.E.; Vargas-Martínez, A.; Perez-Gonzalez, V.H.; Luna-Aguirre, C.M.; Trujillo-de Santiago, G.; Alvarez, M.M. Self-Diagnosis of SARS-CoV-2 from Saliva Samples at Home: Isothermal Amplification Enabled by Do-It-Yourself Portable Incubators and Laminated Poly-Ethyl Sulfonate Membranes. Diagnostics 2024, 14, 221. [Google Scholar] [CrossRef]
- Li, Q.; Li, Y.; Gao, Q.; Jiang, C.; Tian, Q.; Ma, C.; Shi, C. Real-Time Monitoring of Isothermal Nucleic Acid Amplification on a Smartphone by Using a Portable Electrochemical Device for Home-Testing of SARS-CoV-2: A Portable Device for Home-Testing of SARS-CoV-2 via the Electrochemical Method. Anal. Chim. Acta 2022, 1229, 340343. [Google Scholar] [CrossRef]
- Dai, F.; Zhang, T.; Pang, F.; Jiao, T.; Wang, K.; Zhang, Z.; Wang, N.; Xie, Z.; Zhang, Y.; Wang, Z.; et al. A Compact, Palm-Sized Isothermal Fluorescent Diagnostic Intelligent IoT Device for Personal Health Monitoring and beyond via One-Tube/One-Step LAMP-CRISPR Assay. Biosens. Bioelectron. 2025, 270, 116945. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Lu, X.; Lin, H.; Rodriguez Serrano, A.F.; Lui, G.C.Y.; Hsing, I.M. CoLAMP: CRISPR-Based One-Pot Loop-Mediated Isothermal Amplification Enables at-Home Diagnosis of SARS-CoV-2 RNA with Nearly Eliminated Contamination Utilizing Amplicons Depletion Strategy. Biosens. Bioelectron. 2023, 236, 115402. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Lin, B.; Yuan, G.; Geng, Z.; Zhao, Z.; Wang, J.; Shao, J.; Wang, Z.; Xu, Y.; Yang, X.; et al. A Gamepad-like Nucleic Acid Testing Device for Rapid Detection of SARS-CoV-2 via Visible Nested Recombinase Polymerase Amplification. Commun. Eng. 2024, 3, 83. [Google Scholar] [CrossRef]
- Cao, Y.; Lin, H.; Lu, X.; Wu, X.; Zhu, Y.; Zhao, Z.; Li, Y.; Borje, S.; Lui, G.C.Y.; Lee, S.S.; et al. Benchtop to At-Home Test: Amplicon-Depleted CRISPR-Regulated Loop Mediated Amplification at Skin-Temperature for Viral Load Monitoring. Biosens. Bioelectron. 2025, 267, 116866. [Google Scholar] [CrossRef]
- Zhang, D.; Huang, L.; Liu, B.; Ge, Q.; Dong, J.; Zhao, X. Rapid and Ultrasensitive Quantification of Multiplex Respiratory Tract Infection Pathogen via Lateral Flow Microarray Based on SERS Nanotags. Theranostics 2019, 9, 4849–4859. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Kim, H.; Woo, K.; Kim, J.M.; Jo, H.J.; Jeong, Y.; Lee, K.H. SARS-CoV-2 Variant Screening Using a Virus-Receptor-Based Electrical Biosensor. Nano Lett. 2022, 22, 50–57. [Google Scholar] [CrossRef]
- Narita, F.; Wang, Z.; Kurita, H.; Li, Z.; Shi, Y.; Jia, Y.; Soutis, C. A Review of Piezoelectric and Magnetostrictive Biosensor Materials for Detection of COVID-19 and Other Viruses. Adv. Mater. 2021, 33, 2005448. [Google Scholar] [CrossRef]
- Fozouni, P.; Son, S.; Díaz de León Derby, M.; Knott, G.J.; Gray, C.N.; D’Ambrosio, M.V.; Zhao, C.; Switz, N.A.; Kumar, G.R.; Stephens, S.I.; et al. Amplification-Free Detection of SARS-CoV-2 with CRISPR-Cas13a and Mobile Phone Microscopy. Cell 2021, 184, 323–333. [Google Scholar] [CrossRef]
- Song, D.; Xu, W.; Zhuo, Y.; Liu, J.; Zhu, A.; Long, F. A CRISPR/Cas13a-Powered Catalytic Hairpin Assembly Evanescent Wave Fluorescence Biosensor for Target Amplification-Free SARS-CoV-2 Detection. Sens. Actuators B Chem. 2024, 405, 135296. [Google Scholar] [CrossRef]
- Prétet, J.; Baraquin, A.; Chung, P.Y.J.; Puget, L.; Dhillon, S.K.; Tkachenka, Y.; Jacquot, K.; Lepiller, Q.; Broeck, D.V.; Arbyn, M. The Sansure® Human Papillomavirus DNA Diagnostic Kit Offers Excellent Reproducibility Performance for the Detection of High-risk HPV. J. Med. Virol. 2024, 96, e29961. [Google Scholar] [CrossRef]
- Zha, G.; Xiao, X.; Tian, Y.; Zhu, H.; Chen, P.; Zhang, Q.; Yu, C.; Li, H.; Wang, Y.; Cao, C. An Efficient Isoelectric Focusing of Microcolumn Array Chip for Screening of Adult Beta-Thalassemia. Clin. Chim. Acta 2023, 538, 124–130. [Google Scholar] [CrossRef]
- Hossain, M.W.; Hossain, M.; Arafath, K.; Ety, S.S.; Shetu, M.M.H.; Kabir, M.; Noor, F.A.; Mannoor, K. Real-Time Fast PCR Amplification Using Designated and Conventional Real Time Thermal Cycler Systems: COVID-19 Perspective. PLoS ONE 2022, 17, e0276464. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Zheng, R.; Fu, S.; Li, Z. Epidemiology of Pertussis and the Screening Value of WBC and Lymphocyte Percentage. nt. J. Gen. Med. 2024, 17, 5443–5452. [Google Scholar] [CrossRef] [PubMed]
- Panther Fusion® System|Hologic. Available online: https://www.hologic.com/hologic-products/diagnostic-solutions/panther-fusion-system (accessed on 20 March 2025).
- Molecular Centralized Lab Testing. Available online: https://diagnostics.roche.com/global/en/products/product-category/lab-type/molecular-lab/centralized-lab-testing.html (accessed on 20 March 2025).
- Alinity M Product Overview|Abbott Molecular. Available online: https://www.molecular.abbott/int/en/products/infectious-disease/alinity-m-product-overview#assays (accessed on 20 March 2025).
- Burger, B.; Maffettone, P.M.; Gusev, V.V.; Aitchison, C.M.; Bai, Y.; Wang, X.; Li, X.; Alston, B.M.; Li, B.; Clowes, R.; et al. A Mobile Robotic Chemist. Nature 2020, 583, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Herman, D.S.; Rhoads, D.D.; Schulz, W.L.; Durant, T.J.S. Artificial Intelligence and Mapping a New Direction in Laboratory Medicine: A Review. Clin. Chem. 2021, 67, 1466–1482. [Google Scholar] [CrossRef]
- Nelson, P.P.; Rath, B.A.; Fragkou, P.C.; Antalis, E.; Tsiodras, S.; Skevaki, C. Current and Future Point-of-Care Tests for Emerging and New Respiratory Viruses and Future Perspectives. Front. Cell. Infect. Microbiol. 2020, 10, 181. [Google Scholar] [CrossRef]
- Human Diagnostics Product Catalogue—Sansure. Available online: https://en.sansure.com/products-and-solutions/#mPOCT (accessed on 20 March 2025).
- GeneXpert Xpress Systems. Available online: https://www.cepheid.com/en-US/systems/genexpert-family-of-systems/genexpert-xpress.html (accessed on 20 March 2025).
- BIOFIRE® FILMARRAY® TORCH System. Available online: https://www.biomerieux.com/us/en/our-offer/clinical-products/biofire-torch-system.html (accessed on 20 March 2025).
- Q-POC Platform—Platforms & Assays. Available online: https://www.quantumdx.com/products-solutions/platforms-assays/q-poc-platform/ (accessed on 20 March 2025).
- Ahram Biosystems Inc. Available online: http://www.ahrambio.com/products_palmpcr_S1.html (accessed on 20 March 2025).
- GeneReach Biotechnology Corp. Available online: https://www.genereach.com/index.php?func=product&action=view&product_no=2 (accessed on 20 March 2025).
- Liu, W.; Zhang, E.; Li, W.; Lv, R.; Lin, Y.; Xu, Y.; Li, J.; Lai, Y.; Jiang, Y.; Lin, S.; et al. Advances and Challenges of Mpox Detection Technology. Biosaf. Health 2024, 6, 260–269. [Google Scholar] [CrossRef]
- Sansure—Innovating Diagnostics for All. Available online: https://www.sansureglobal.com/the-tsure-nucleic-acid-analysis-device-from-sansure-obtained-ce-mark/ (accessed on 20 March 2025).
- UPDATE: Do Not Use Cue Health’s COVID-19 Tests Due to Risk of False Results: FDA Safety Communication|FDA. Available online: https://www.fda.gov/medical-devices/safety-communications/update-do-not-use-cue-healths-covid-19-tests-due-risk-false-results-fda-safety-communication (accessed on 20 March 2025).
- AS7341L Spectral Sensor|Ams OSRAM. Available online: https://ams-osram.com/news/press-releases/as7341l-spectral-sensor (accessed on 20 March 2025).
- Scott, C.; Beck, V. Technology Trends: Speed and Accuracy Are Key for the Pandemic and Beyond. BioProcess Int. 2021, 19, e1. [Google Scholar]
- Full-Automatic Workstation_Suzhou Molarray Co., Ltd. Available online: http://www.molarray.com/enproducts_show.aspx?id=845 (accessed on 20 March 2025).
- Molecular Diagnostics Analyzer|AutoMolec 1600. Available online: https://en.autobio.com.cn/Product/productDetail/fid/63/cid/10/id/155.html (accessed on 20 March 2025).



| Time-Domain PCR | Disadvantages | Reaction Volume | PCR Chamber | Average Heating/Cooling Rate | Fragment Length | Ref. |
|---|---|---|---|---|---|---|
| TEC | The heating efficiency of TEC is low, requiring an effective cooling system, and its power output varies with changes in ambient temperature. | Plastic tube | 3.3 °C/s | N/A | [27] | |
| Plastic tube | 3 °C/s | 1000 bp | [28] | |||
| PC chip | 30 cycles/30 min | 221 bp | [29] | |||
| Glass chip | 1 cycle/1.25 min | 199 bp | [30] | |||
| Silicon chip | 40 cycles/5 min | 92 bp | [33] | |||
| Joule heater | The heat dissipation rate is slow, requiring a redesigned cooling system. | Plastic chip | 30 cycles/70 min | 180 bp | [36] | |
| Glass chip | 1 cycle/1.25 min | 260 bp | [37] | |||
| Glass chip | 35 cycles/10 min | 64/121/172 bp | [39] | |||
| NiCr/glass chip | 11.6–33.3/4.1 °C/s | N/A | [40] | |||
| EWDH-microwave | Complex hardware control is required to implement microwaves. | PP tube | 20 cycles/60 min | 220 bp | [47] | |
| Glass chip | 28 cycles/42 min | 112 bp | [49] | |||
| PC | 7/6 °C/s | N/A | [51] | |||
| N/A | 21/7.6 °C/s | N/A | [54] | |||
| EWDH-infrared | A complex optical system is required; optical components are typically bulky; and precise mechanical design is necessary for energy focusing. | Glass chamber | 10/20 °C/s | N/A | [57] | |
| Capillary | 25 cycles/15 min | 500 bp | [58] | |||
| Glass microchip | 30 cycles/18 min | 211 bp | [59] | |||
| EWDH-laser | A complex optical system is required; optical components are typically bulky; the cost of laser is relatively high; and it is difficult to heat large volumes. | Hybrislip | 40 cycles/6.17 min | 187 bp | [63] | |
| Polymeric | 25 cycles/30 min | N/A | [65] | |||
| Polymeric chip | 25 cycles/12 min | 500 bp | [67] | |||
| EWIH-MIH | It requires a high-frequency power supply, which can generate noise and EMI, and demands complex hardware control and precise system design. | Plastic tube | 6.5/4.28 °C/s | N/A | [72] | |
| Plastic tube | 14.92/13.39 °C/s | N/A | [75] | |||
| Plastic tube | 6.5 J/s | N/A | [77] | |||
| EWIH-PPT | A complex optical system is required; optical components are typically bulky; and precise mechanical design is necessary for energy focusing. | Plastic tube | 30 cycles/54 s | 250–300 bp | [80] | |
| PMMA well | 12.79/6.66 °C/s | N/A | [25] | |||
| PDMS cavity | 30 cycles/12 min | 113 bp | [90] |
| Spatial-Domain-PCR | Disadvantages | Reaction Volume | PCR Chamber | Average Heating/Cooling Rate | Fragment Length | Ref. |
|---|---|---|---|---|---|---|
| Space Exchange | It requires a complex motion mechanism, and without a thermal lid, mineral oil needs to be added. It is difficult to miniaturize. | 25 | Plastic tube | 26 cycles/21 min | 362 bp | [91] |
| 20/25 | Glass capillaries | 15/13 °C/s | 100–1500 bp | [92] | ||
| 50 | Plastic tube | 40 cycles/13.8 min | 70 bp | [94] | ||
| 5 | Capillary tube | 1 cycle/15–60 s | 70 bp | [96] | ||
| 25 | PC chip | 13.33 °C/s | 120 bp | [97] | ||
| CF | This system faces several challenges, including fixed cycle numbers, reagent adsorption on the microfluidic chip, the presence of bubbles in the micro-channels, difficulties in real-time detection, and complexities in the design and production of consumables. | 10 | Glass chip | 20 cylces/1.5–18.7 min | 176 bp | [102] |
| 25 | Glass chip | 1 cycle/45–90 s | 450 bp | [103] | ||
| 20 | TOP chip | 40 cycles/10–40 min | 69/85 bp | [109] | ||
| 25 | TOP chip | 40 cycles/20–50 min | 120 bp | [110] | ||
| 20 | PDMS/glass chip | 30 cycles/20.41 min | 594 bp | [111] | ||
| 25 | Silicon chip | 40 cycles/11 min | 197 bp | [113] | ||
| Magnetic Drive | Magnetohydrodynamic (MHD) applications still need improvement, and the magnetic control equipment is complex. | 6 | Silicon/SU8 | 1 cycle/1.5 min | 142 bp | [116] |
| 2 | PMMA channel | 25 cycles/4 min | 500 bp | [100] | ||
| 10 | PCB channel | 30 cycles/3 min | 126 bp | [117] |
| Convective-PCR | Disadvantages | Reaction Volume | PCR Chamber | Equivalent Time | Fragment Length | Ref. |
|---|---|---|---|---|---|---|
| Triangle | The reaction chamber for PCR should be carefully designed, and the cycle count should not be recorded. There is a risk of non-specific amplification. | FEP tubing | 30 min (1 cycle/42 s) | 191 bp | [121] | |
| PTFE capillary | 30 min (N/A cycle/s) | 111 bp | [122] | |||
| Circle/track | PP chip | 20 min (1 cycle/24 s) | 160 bp | [123] | ||
| COP chip | 15 min (1 cycle/5.7 s) | 159 bp | [124] | |||
| Glass/polymer | 20 min (N/A cycle/s) | 100 bp | [133] | |||
| Capillary (two-temperature zone) | Capillary tubes | 25 min (N/A cycle/s) | 105 bp | [125] | ||
| Capillary tubes | 25 min (N/A cycle/s) | 159 bp | [127] | |||
| Capillary (one-temperature zone) | Capillary tubes | 30 min (N/A cycle/s) | 67 bp | [128] | ||
| Glass capillary | 28 min (1 cycle/30 s) | 122 bp | [129] |
| Isothermal Amplification Techniques | Sensitivity (Copies/mL) | Target | Reaction Time | Primers/Probes | Temperature |
|---|---|---|---|---|---|
| LAMP | 107–109 | DNA, (RNA) | 1–2 h | 4–6 | 60–65 °C |
| NASBA | 106–109 | RNA(DNA) | 90 min | 2 | 41 °C |
| SDA | 107–109 | DNA | 1–1.5 h | 2–4 | 37–70 °C |
| RCA | 103–107 | DNA(RNA) | 1 h | 2 | 65 °C |
| CPA | 104–107 | DNA | 1–2 h | 5 | 60–65 °C |
| EXPAR | 106–109 | Short DNA | 1–2 h | N/A | 60 °C |
| WGA | 103–107 | DNA | 1 h | N/A | 37 °C |
| RPA | 103–106 | DNA | 1 h | 2 | 37–42 °C |
| HDA | 107–109 | DNA | 1–2 h | 2 | 37 °C |
| SMART | 104–10⁵ | RNA | 2 h | 2 | 41 °C |
| SPIA | 107–109 | DNA, RNA | 30–90 min | 1 | 47 °C |
| DAMP | 107–109 | DNA | 1–2 h | 6 | 60 °C |
| Scene | Product Name | Throughput | Detection Time | Detection Technology | Refs. |
|---|---|---|---|---|---|
| Large hospital | Roche cobas® 8800 system (Roche Diagnostics, Basel, Switzerland) | 960/8 h | N/A | PCR | [154] |
| Abbot Anility M (Abbott Molecular, Des Plaines, USA) | 300/8 h | N/A | PCR | [155] | |
| Hologic Panther Fusion® system (Hologic, Inc., Marlborough, USA) | 500/8 h | N/A | PCR | [153] | |
| Small hospital | Roche cobas® 5800 system Roche Diagnostics, Basel, Switzerland) | 144/8 h | N/A | PCR | [154] |
| Molarray MW-1600L (Suzhou Molarray, Suzhou, China) | 1–16 | 70 min | PCR | [170] | |
| Autobio AutoMolec 1600 (Autobio Diagnostics, Zhengzhou, China) | 192/8 h | 100 min | PCR | [171] | |
| Community clinic | Sansure iPonatic (Sansure, Changsha, China) | 1–8 | 45 min | PCR/POCT | [159] |
| Cepheid GeneXpert (Cepheid, Sunnyvale, CA, USA) | 1–4 | 60 min | PCR/POCT | [160] | |
| Biofire Filmarray (BioFire Diagnostics, Salt Lake City, USA) | 1–8 | 60 min | PCR/POCT | [161] | |
| Homecare | Sansure Tsure (Sansure, Changsha, China) | 1 | 15–30 min | LAMP | [166] |
| Cuehealth (Cue Health Inc., San Diego, USA) | 1 | 15–30 min | LAMP | [167] | |
| BiologyWorks (BiologyWorks, Inc., Los Angeles, USA) | 1 | <10 min | Biosensor | [168,169] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, Y.; Zong, Z.; Jiang, Q.; Ke, X.; Wu, Z. Seeking Solutions for Inclusively Economic, Rapid, and Safe Molecular Detection of Respiratory Infectious Diseases: Comprehensive Review from Polymerase Chain Reaction Techniques to Amplification-Free Biosensing. Micromachines 2025, 16, 472. https://doi.org/10.3390/mi16040472
Xie Y, Zong Z, Jiang Q, Ke X, Wu Z. Seeking Solutions for Inclusively Economic, Rapid, and Safe Molecular Detection of Respiratory Infectious Diseases: Comprehensive Review from Polymerase Chain Reaction Techniques to Amplification-Free Biosensing. Micromachines. 2025; 16(4):472. https://doi.org/10.3390/mi16040472
Chicago/Turabian StyleXie, Yaping, Zisheng Zong, Qin Jiang, Xingxing Ke, and Zhigang Wu. 2025. "Seeking Solutions for Inclusively Economic, Rapid, and Safe Molecular Detection of Respiratory Infectious Diseases: Comprehensive Review from Polymerase Chain Reaction Techniques to Amplification-Free Biosensing" Micromachines 16, no. 4: 472. https://doi.org/10.3390/mi16040472
APA StyleXie, Y., Zong, Z., Jiang, Q., Ke, X., & Wu, Z. (2025). Seeking Solutions for Inclusively Economic, Rapid, and Safe Molecular Detection of Respiratory Infectious Diseases: Comprehensive Review from Polymerase Chain Reaction Techniques to Amplification-Free Biosensing. Micromachines, 16(4), 472. https://doi.org/10.3390/mi16040472










