A Slanted-Finger Interdigitated Transducer Microfluidic Device for Particles Sorting
Abstract
:1. Introduction
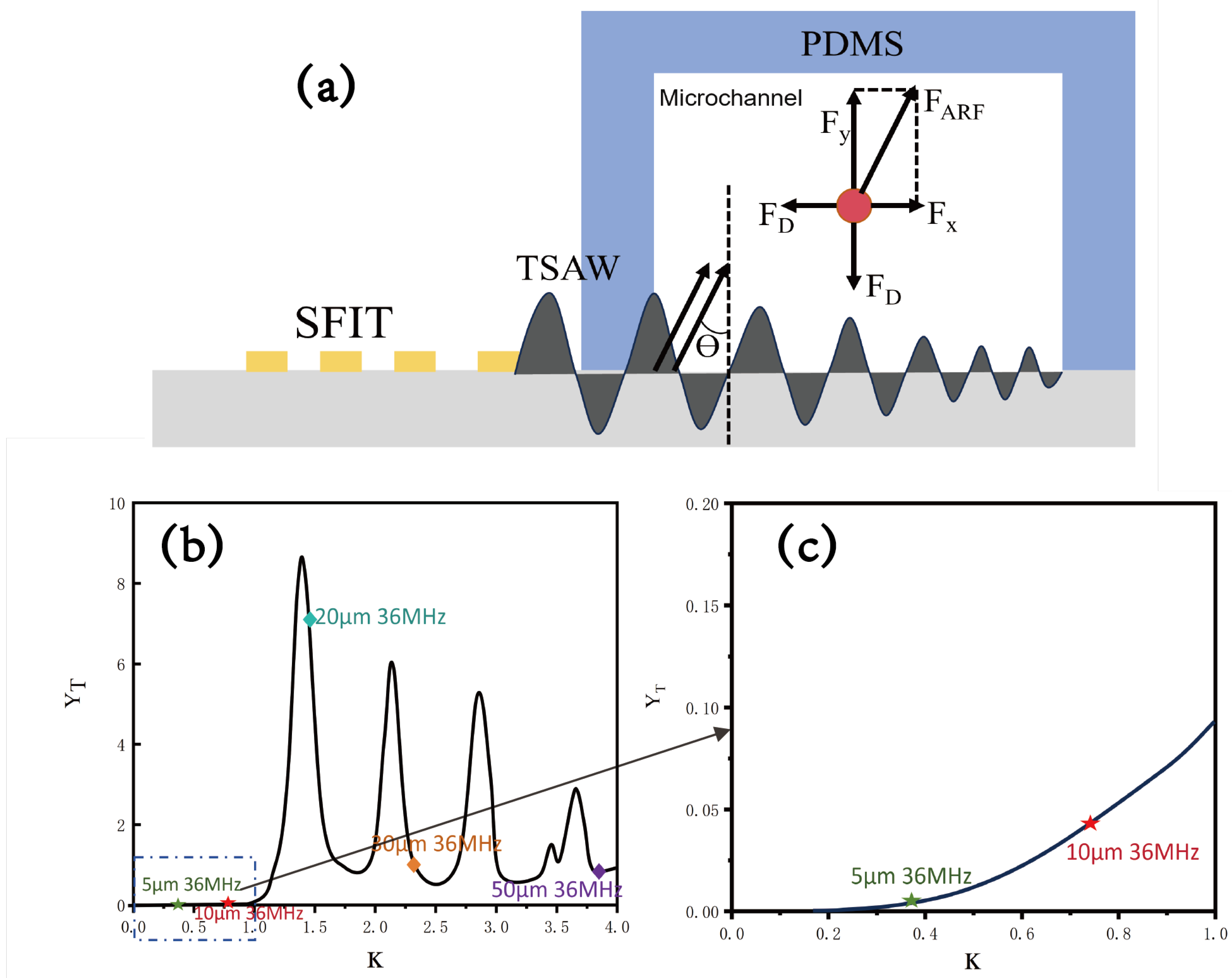
2. Theoretical Analysis
3. Materials and Methods
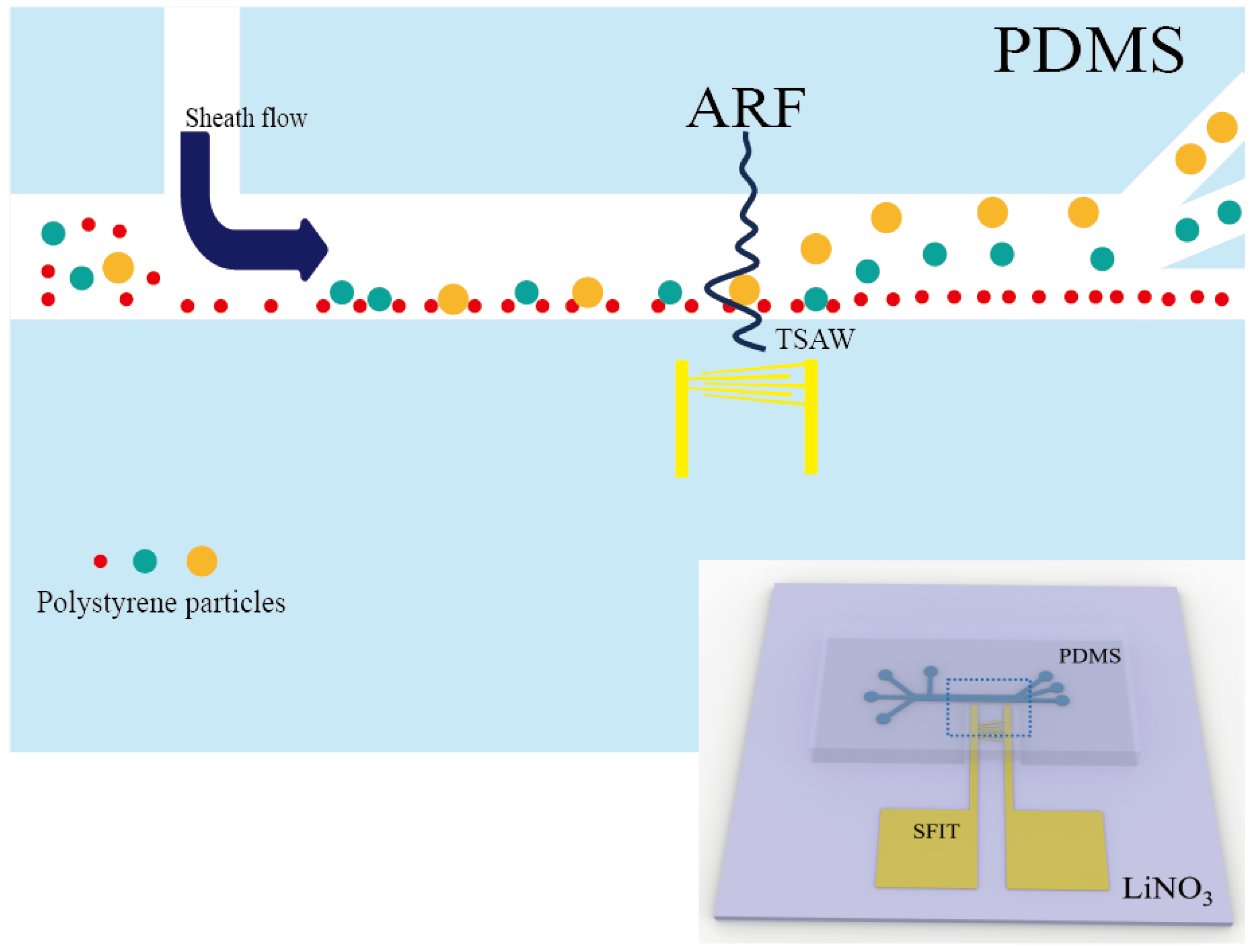
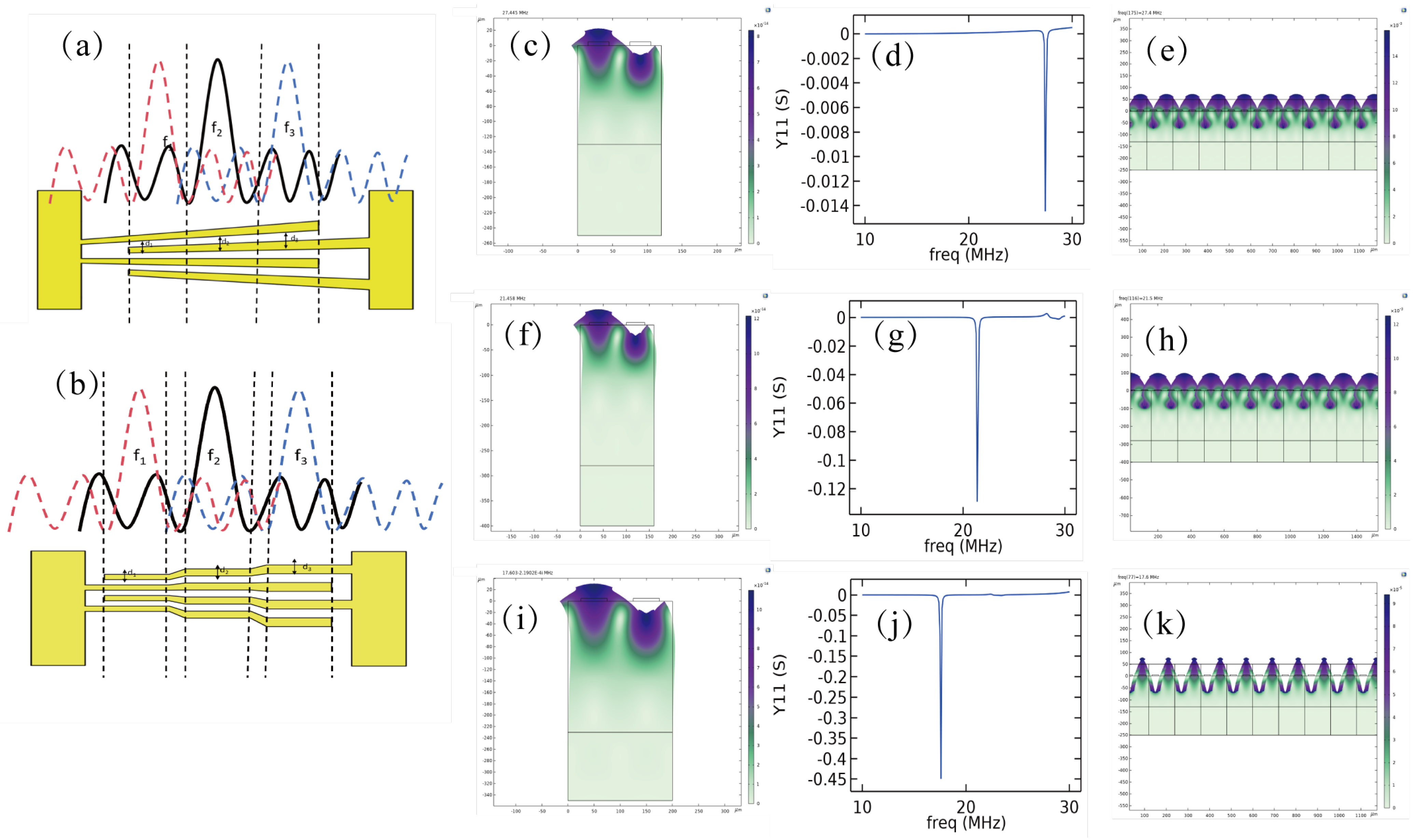
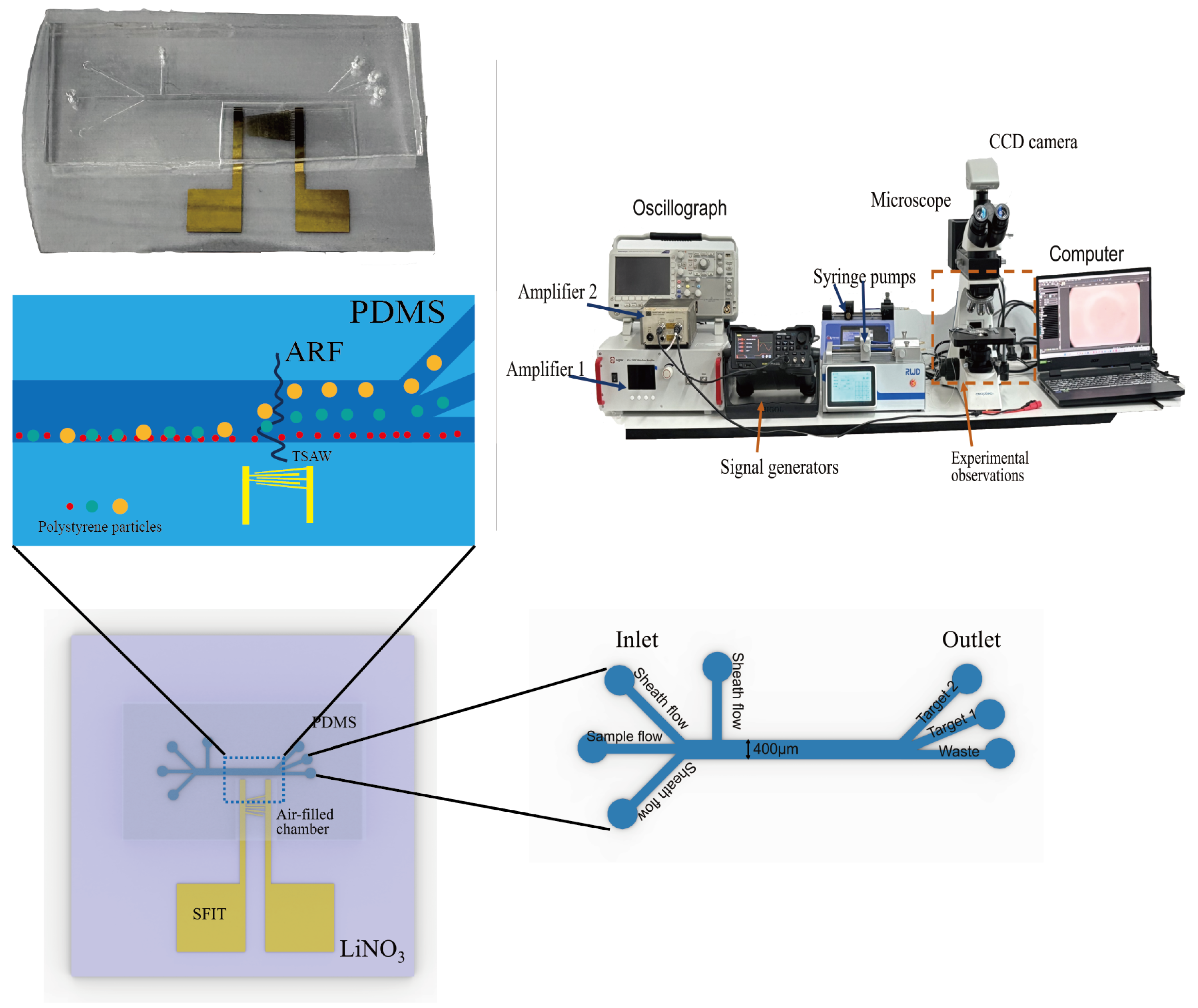
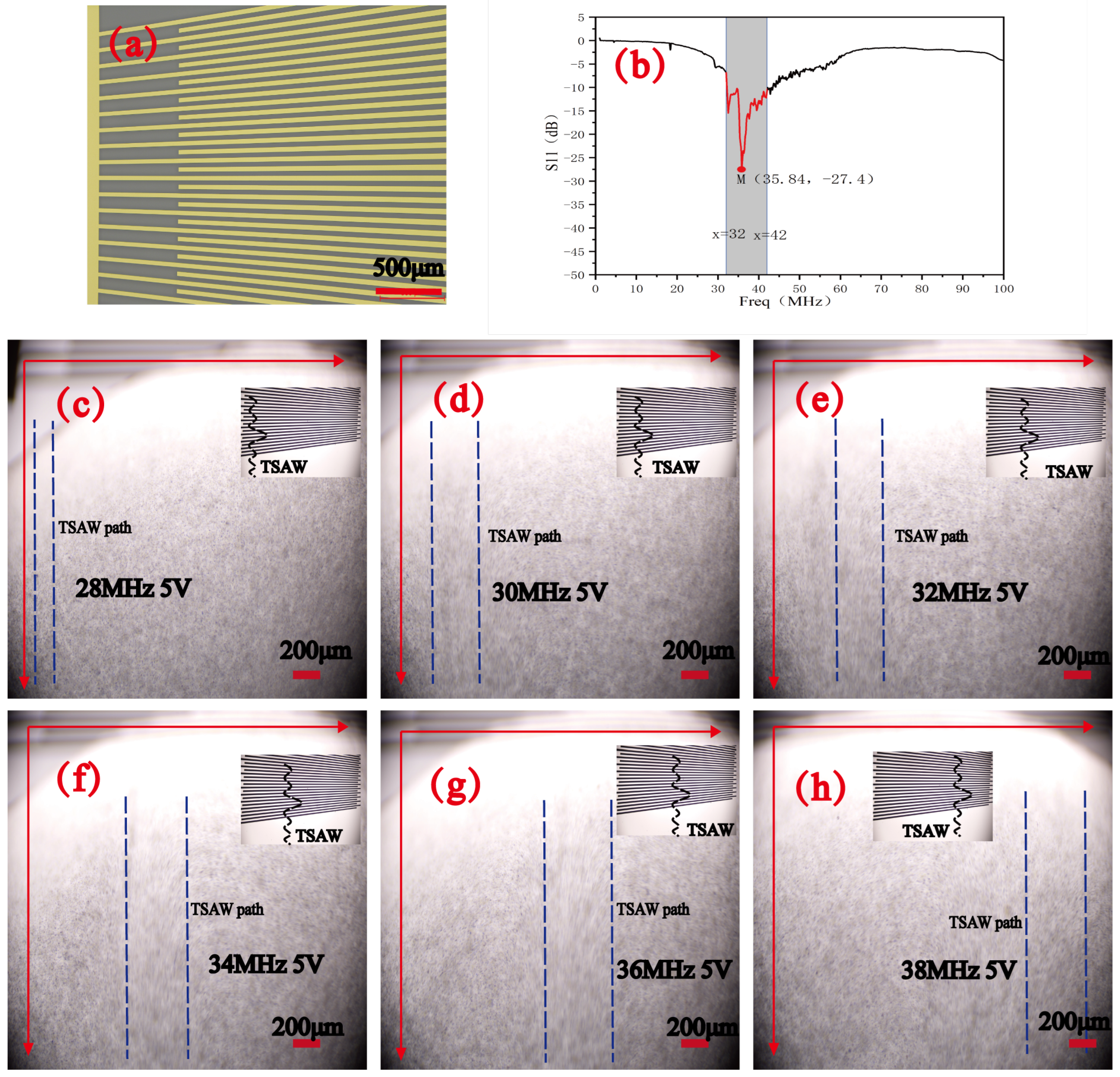
3.1. Device Design and Fabrication
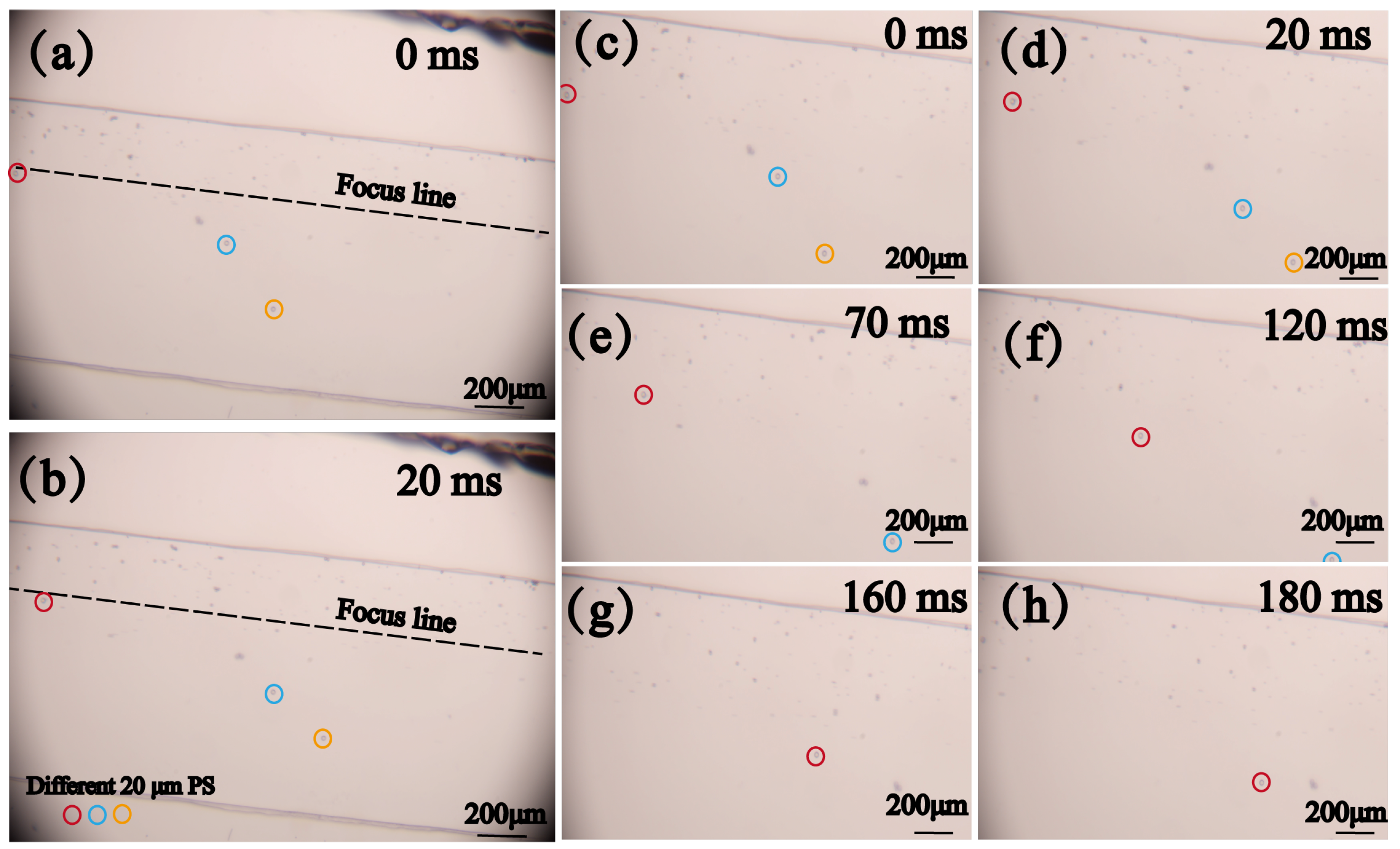
3.2. Experimental Procedures
4. Results and Discussion
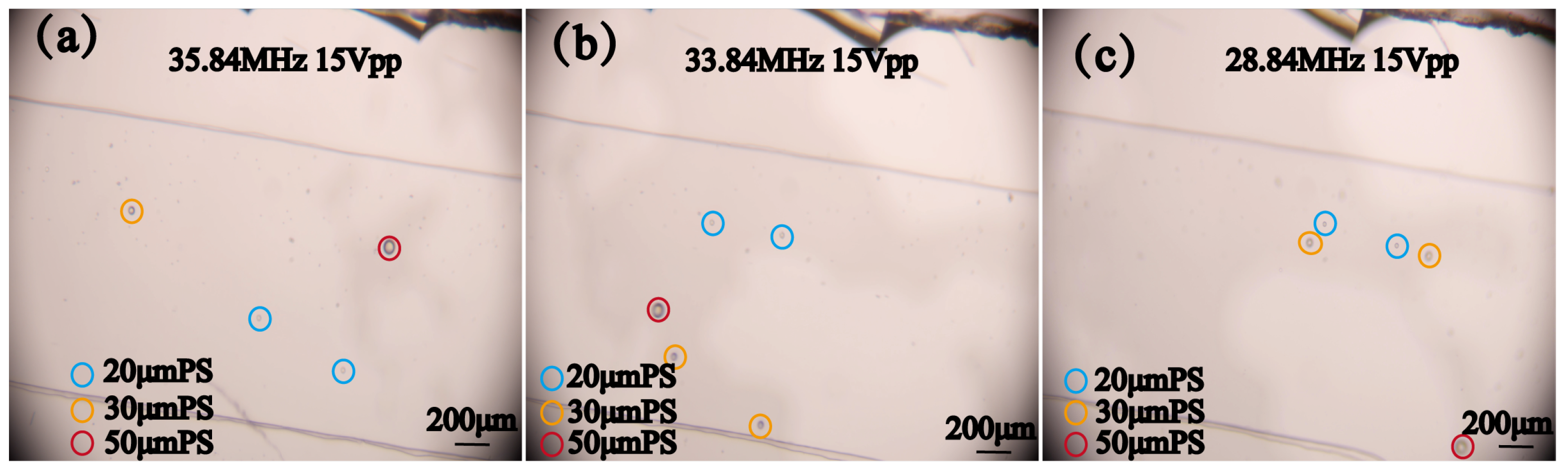
4.1. Operating Mode of SFIT
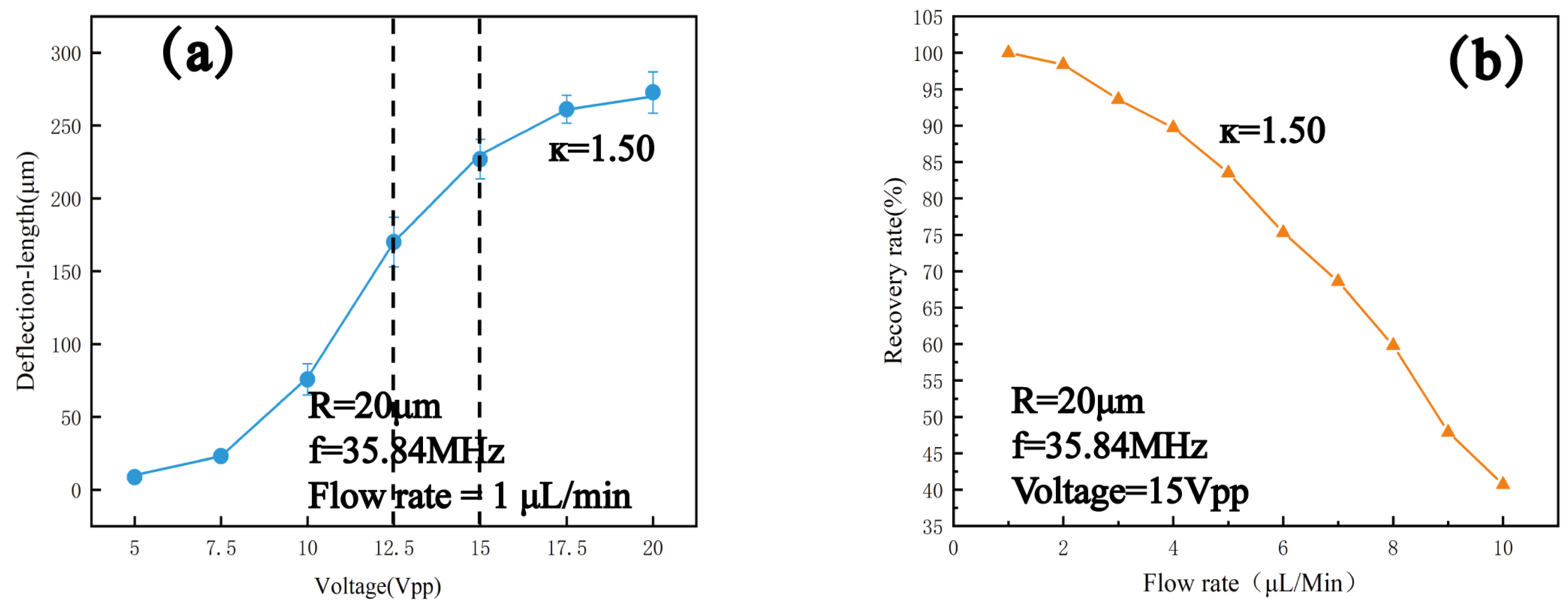
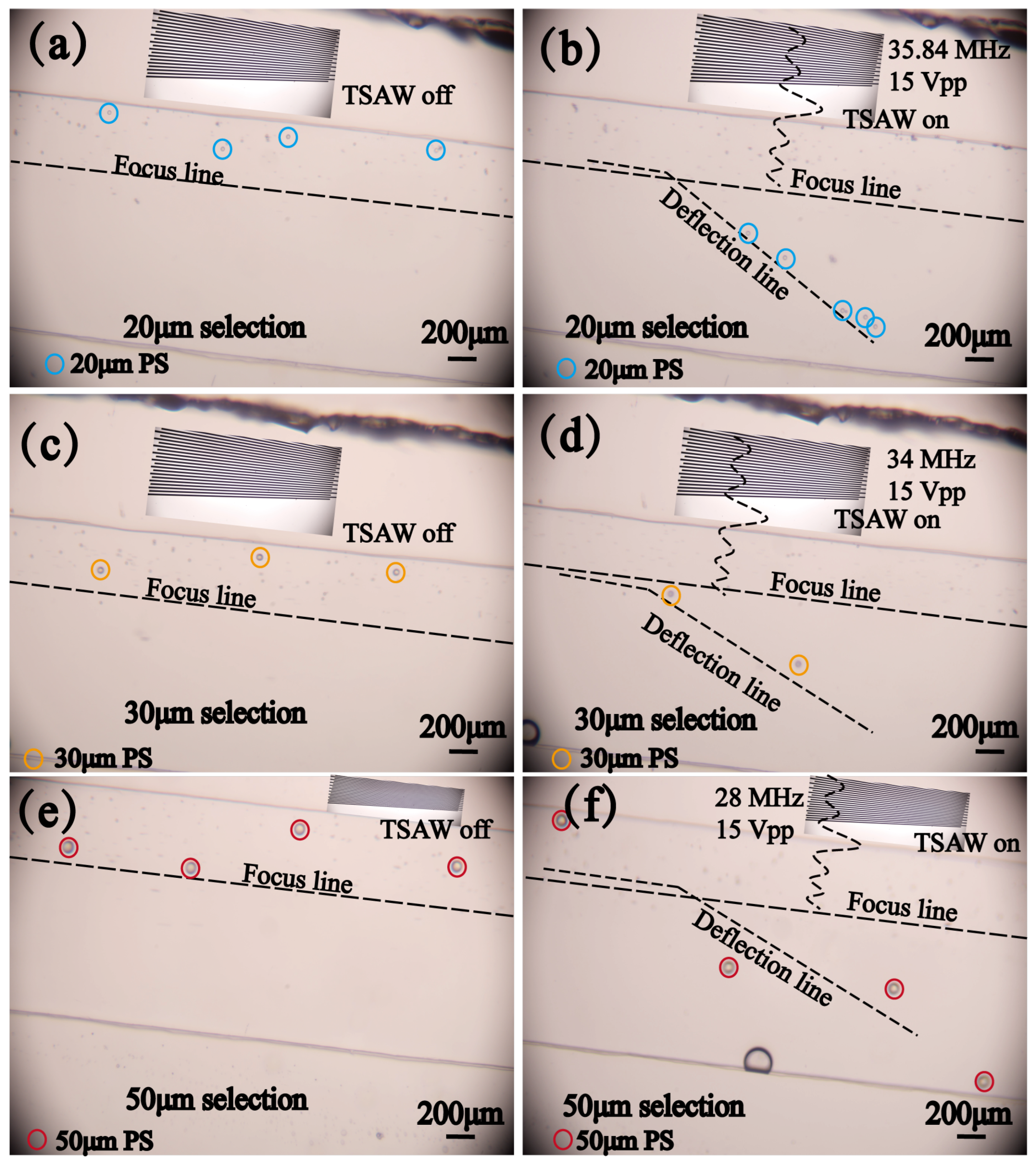
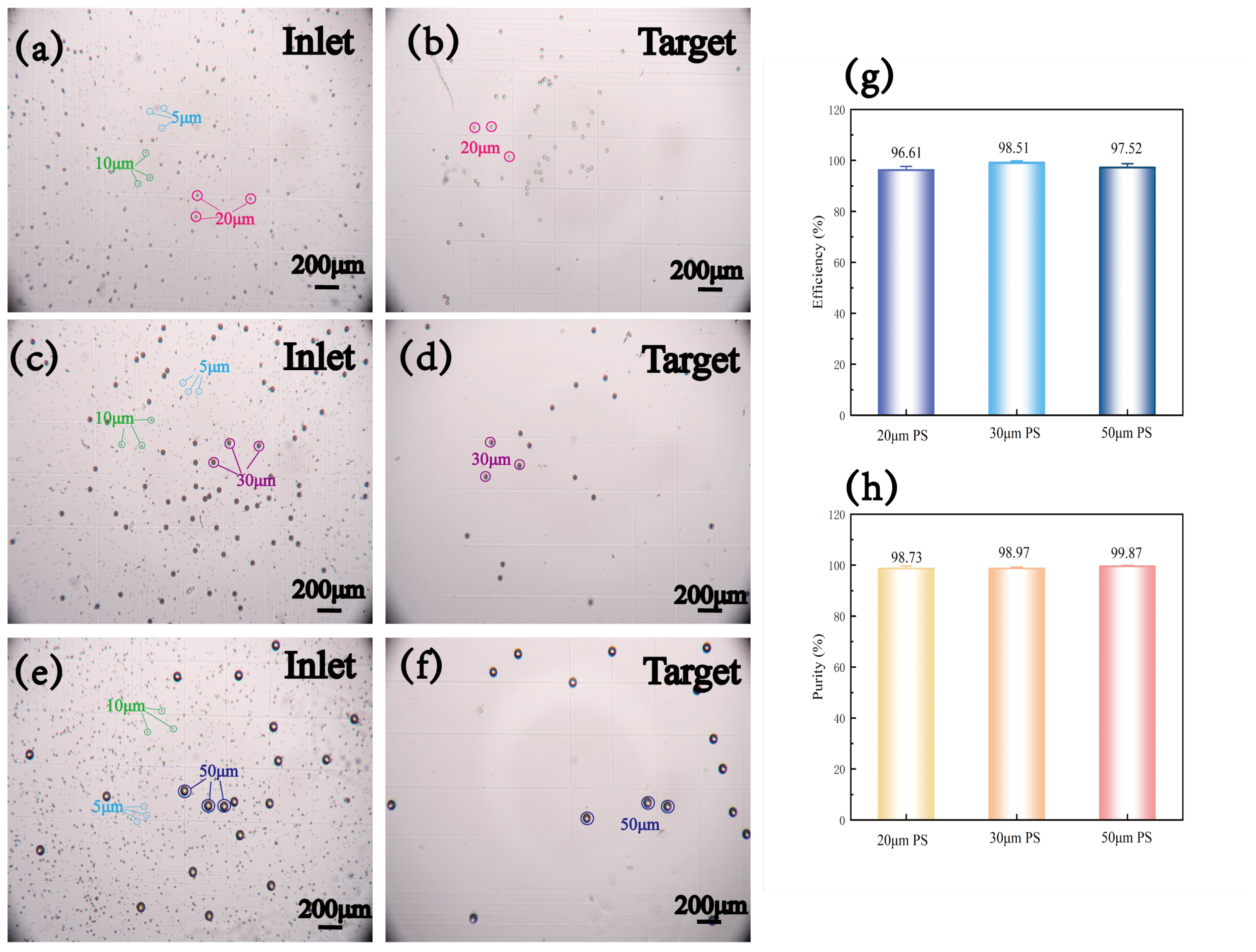
4.2. Sorting of Polystyrene Particles with Different Sizes
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tomlinson, M.J.; Tomlinson, S.; Yang, X.B.; Kirkham, J. Cell separation: Terminology and practical considerations. J. Tissue Eng. 2013, 4, 2041731412472690. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, A.J.; Marengo, M.S.; Oltean, S.; Kemeny, G.; Bitting, R.L.; Turnbull, J.D.; Herold, C.I.; Marcom, P.K.; George, D.J.; Garcia-Blanco, M.A. Circulating tumor cells from patients with advanced prostate and breast cancer display both epithelial and mesenchymal markers. Mol. Cancer Res. 2011, 9, 997–1007. [Google Scholar] [CrossRef]
- Wu, M.; Ouyang, Y.; Wang, Z.; Zhang, R.; Huang, P.H.; Chen, C.; Li, H.; Li, P.; Quinn, D.; Dao, M.; et al. Isolation of exosomes from whole blood by integrating acoustics and microfluidics. Proc. Natl. Acad. Sci. USA 2017, 114, 10584–10589. [Google Scholar] [CrossRef]
- Tan, M.K.; Yeo, L.; Friend, J. Rapid fluid flow and mixing induced in microchannels using surface acoustic waves. Europhys. Lett. 2009, 87, 47003. [Google Scholar] [CrossRef]
- Zhao, K.; Wei, Y.; Dong, J.; Zhao, P.; Wang, Y.; Pan, X.; Wang, J. Separation and characterization of microplastic and nanoplastic particles in marine environment. Environ. Pollut. 2022, 297, 118773. [Google Scholar] [CrossRef] [PubMed]
- Regnault, C.; Dheeman, D.S.; Hochstetter, A. Microfluidic devices for drug assays. High Throughput 2018, 7, 18. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.M.; Lv, S.; Zhang, W.; Cui, Y. Microfluidic point-of-care (POC) devices in early diagnosis: A review of opportunities and challenges. Sensors 2022, 22, 1620. [Google Scholar] [CrossRef]
- Wheeler, M.A.; Clark, I.C.; Lee, H.G.; Li, Z.; Linnerbauer, M.; Rone, J.M.; Blain, M.; Akl, C.F.; Piester, G.; Giovannoni, F.; et al. Droplet-based forward genetic screening of astrocyte–microglia cross-talk. Science 2023, 379, 1023–1030. [Google Scholar] [CrossRef]
- Kulkarni, M.B.; Goel, S. Microfluidic devices for synthesizing nanomaterials—A review. Nano Express 2020, 1, 032004. [Google Scholar] [CrossRef]
- Karabacak, N.M.; Spuhler, P.S.; Fachin, F.; Lim, E.J.; Pai, V.; Ozkumur, E.; Martel, J.M.; Kojic, N.; Smith, K.; Chen, P.i.; et al. Microfluidic, marker-free isolation of circulating tumor cells from blood samples. Nat. Protoc. 2014, 9, 694–710. [Google Scholar] [CrossRef]
- Abdulla, A.; Zhang, T.; Ahmad, K.Z.; Li, S.; Lou, J.; Ding, X. Label-free separation of circulating tumor cells using a self-amplified inertial focusing (SAIF) microfluidic chip. Anal. Chem. 2020, 92, 16170–16179. [Google Scholar] [CrossRef] [PubMed]
- Pei, H.; Li, L.; Wang, Y.; Sheng, R.; Wang, Y.; Xie, S.; Shui, L.; Si, H.; Tang, B. Single-cell phenotypic profiling of CTCs in whole blood using an integrated microfluidic device. Anal. Chem. 2019, 91, 11078–11084. [Google Scholar] [CrossRef]
- Ding, X.; Peng, Z.; Lin, S.C.S.; Geri, M.; Li, S.; Li, P.; Chen, Y.; Dao, M.; Suresh, S.; Huang, T.J. Cell separation using tilted-angle standing surface acoustic waves. Proc. Natl. Acad. Sci. USA 2014, 111, 12992–12997. [Google Scholar] [CrossRef] [PubMed]
- Bu, Y.; Wang, J.; Ni, S.; Guo, Y.; Yobas, L. Continuous-flow label-free size fractionation of extracellular vesicles through electrothermal fluid rolls and dielectrophoresis synergistically integrated in a microfluidic device. Lab A Chip 2023, 23, 2421–2433. [Google Scholar] [CrossRef] [PubMed]
- Dowling, R.; Kostylev, M. Controlled Capture of Magnetic Nanoparticles from Microfluidic Flows by Ferromagnetic Antidot and Dot Nanostructures. Nanomaterials 2025, 15, 132. [Google Scholar] [CrossRef]
- Sajeesh, P.; Sen, A.K. Particle separation and sorting in microfluidic devices: A review. Microfluid. Nanofluidics 2014, 17, 1–52. [Google Scholar] [CrossRef]
- Al-Faqheri, W.; Thio, T.H.G.; Qasaimeh, M.A.; Dietzel, A.; Madou, M.; Al-Halhouli, A. Particle/cell separation on microfluidic platforms based on centrifugation effect: A review. Microfluid. Nanofluidics 2017, 21, 102. [Google Scholar] [CrossRef]
- Chen, R.; He, S.; He, X.; Xie, J.; Zhu, X. Picosecond Laser Etching of Glass Spiral Microfluidic Channel for Microparticles Dispersion and Sorting. Micromachines 2025, 16, 66. [Google Scholar] [CrossRef]
- Chen, X.; Feng, Q.; Zhang, Y. Insights into a deterministic lateral displacement sorting chip with new cross-section micropillars. Chaos Solitons Fractals 2022, 156, 111884. [Google Scholar] [CrossRef]
- Li, G.; Ji, Y.; Wu, Y.; Liu, Y.; Li, H.; Wang, Y.; Chi, M.; Sun, H.; Zhu, H. Multistage microfluidic cell sorting method and chip based on size and stiffness. Biosens. Bioelectron. 2023, 237, 115451. [Google Scholar] [CrossRef]
- Yao, Y.; Lin, Y.; Wu, Z.; Li, Z.; He, X.; Wu, Y.; Sun, Z.; Ding, W.; He, L. Solute-particle separation in microfluidics enhanced by symmetrical convection. RSC Adv. 2024, 14, 1729–1740. [Google Scholar] [CrossRef] [PubMed]
- Clark, I.C.; Thakur, R.; Abate, A.R. Concentric electrodes improve microfluidic droplet sorting. Lab A Chip 2018, 18, 710–713. [Google Scholar] [CrossRef] [PubMed]
- Amorim, D.; Sousa, P.C.; Abreu, C.; Catarino, S.O. A Review of SAW-Based Micro-and Nanoparticle Manipulation in Microfluidics. Sensors 2025, 25, 1577. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Ma, X.; Li, K.; Yue, Y.; Zhang, Z.; Meng, Y.; Wang, S. Bipolar electrode-based sheath-less focusing and continuous acoustic sorting of particles and cells in an integrated microfluidic device. Anal. Chem. 2024, 96, 3627–3635. [Google Scholar] [CrossRef]
- Peng, T.; Qiang, J.; Yuan, S. Investigation on a cascaded inertial and acoustic microfluidic device for sheathless and label-free separation of circulating tumor cells. Phys. Fluids 2023, 35, 082009. [Google Scholar] [CrossRef]
- Hossein, F.; Angeli, P. A review of acoustofluidic separation of bioparticles. Biophys. Rev. 2023, 15, 2005–2025. [Google Scholar] [CrossRef]
- Zhao, Z.; Yang, S.; Feng, L.; Zhang, L.; Wang, J.; Chang, K.; Chen, M. Acoustofluidics: A versatile tool for micro/nano separation at the cellular, subcellular, and biomolecular levels. Adv. Mater. Technol. 2023, 8, 2202201. [Google Scholar] [CrossRef]
- Shi, J.; Huang, H.; Stratton, Z.; Huang, Y.; Huang, T.J. Continuous particle separation in a microfluidic channel via standing surface acoustic waves (SSAW). Lab A Chip 2009, 9, 3354–3359. [Google Scholar] [CrossRef]
- Ding, X.; Lin, S.C.S.; Lapsley, M.I.; Li, S.; Guo, X.; Chan, C.Y.; Chiang, I.K.; Wang, L.; McCoy, J.P.; Huang, T.J. Standing surface acoustic wave (SSAW) based multichannel cell sorting. Lab A Chip 2012, 12, 4228–4231. [Google Scholar] [CrossRef]
- Li, S.; Ding, X.; Guo, F.; Chen, Y.; Lapsley, M.I.; Lin, S.C.S.; Wang, L.; McCoy, J.P.; Cameron, C.E.; Huang, T.J. An on-chip, multichannel droplet sorter using standing surface acoustic waves. Anal. Chem. 2013, 85, 5468–5474. [Google Scholar] [CrossRef]
- Destgeer, G.; Lee, K.H.; Jung, J.H.; Alazzam, A.; Sung, H.J. Continuous separation of particles in a PDMS microfluidic channel via travelling surface acoustic waves (TSAW). Lab A Chip 2013, 13, 4210–4216. [Google Scholar] [CrossRef]
- Destgeer, G.; Ha, B.H.; Jung, J.H.; Sung, H.J. Submicron separation of microspheres via travelling surface acoustic waves. Lab A Chip 2014, 14, 4665–4672. [Google Scholar] [CrossRef] [PubMed]
- Mezzanzanica, G.; Agazzi, L.; Siena, M.; Français, O.; Mariani, S. A microfluidic device based on standing surface acoustic waves for sorting and trapping microparticles. Eng. Proc. 2022, 27, 28. [Google Scholar] [CrossRef]
- Liu, Y.; Ji, M.; Yu, N.; Zhao, C.; Xue, G.; Fu, W.; Qiao, X.; Zhang, Y.; Chou, X.; Geng, W. Enhanced detection in droplet microfluidics by acoustic vortex modulation of particle rings and particle clusters via asymmetric propagation of surface acoustic waves. Biosensors 2022, 12, 399. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Park, J. Ultrasonic surface acoustic wave-assisted separation of microscale droplets with varying acoustic impedance. Ultrason. Sonochem. 2023, 93, 106305. [Google Scholar] [CrossRef]
- Geng, W.; Liu, Y.; Yu, N.; Qiao, X.; Ji, M.; Niu, Y.; Niu, L.; Fu, W.; Zhang, H.; Bi, K.; et al. An ultra-compact acoustofluidic device based on the narrow-path travelling surface acoustic wave (np-TSAW) for label-free isolation of living circulating tumor cells. Anal. Chim. Acta 2023, 1255, 341138. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Lin, S.C.S.; Kiraly, B.; Yue, H.; Li, S.; Chiang, I.K.; Shi, J.; Benkovic, S.J.; Huang, T.J. On-chip manipulation of single microparticles, cells, and organisms using surface acoustic waves. Proc. Natl. Acad. Sci. USA 2012, 109, 11105–11109. [Google Scholar] [CrossRef]
- Kharusi, M.; Farnell, G. On diffraction and focusing in anisotropic crystals. Proc. IEEE 1972, 60, 945–956. [Google Scholar] [CrossRef]
- Mazalan, M.B.; Noor, A.M.; Wahab, Y.; Yahud, S.; Zaman, W.S.W.K. Current development in interdigital transducer (IDT) surface acoustic wave devices for live cell in vitro studies: A review. Micromachines 2021, 13, 30. [Google Scholar] [CrossRef]
- Khan, M.S.; Sahin, M.A.; Destgeer, G.; Park, J. Residue-free acoustofluidic manipulation of microparticles via removal of microchannel anechoic corner. Ultrason. Sonochem. 2022, 89, 106161. [Google Scholar] [CrossRef]
- Mutafopulos, K.; Lu, P.J.; Garry, R.; Spink, P.; Weitz, D.A. Selective cell encapsulation, lysis, pico-injection and size-controlled droplet generation using traveling surface acoustic waves in a microfluidic device. Lab A Chip 2020, 20, 3914–3921. [Google Scholar] [CrossRef] [PubMed]
- Settnes, M.; Bruus, H. Forces acting on a small particle in an acoustical field in a viscous fluid. Phys. Rev. E—Stat. Nonlinear Soft Matter Phys. 2012, 85, 016327. [Google Scholar] [CrossRef] [PubMed]
- King, L.V. On the acoustic radiation pressure on spheres. Proc. R. Soc. Lond. Ser. A-Math. Phys. Sci. 1934, 147, 212–240. [Google Scholar]
- Karlsen, J.T.; Bruus, H. Forces acting on a small particle in an acoustical field in a thermoviscous fluid. Phys. Rev. E 2015, 92, 043010. [Google Scholar] [CrossRef]
- Baasch, T.; Pavlic, A.; Dual, J. Acoustic radiation force acting on a heavy particle in a standing wave can be dominated by the acoustic microstreaming. Phys. Rev. E 2019, 100, 061102. [Google Scholar] [CrossRef]
- Hasegawa, T.; Yosioka, K. Acoustic-radiation force on a solid elastic sphere. J. Acoust. Soc. Am. 1969, 46, 1139–1143. [Google Scholar] [CrossRef]
| Freq (MHz) | |||||
|---|---|---|---|---|---|
| 5 m | 10 m | 20 m | 30 m | 50 m | |
| 32 | 0.34 | 0.67 | 1.34 | 2.02 | 3.36 |
| 34 | 0.36 | 0.71 | 1.43 | 2.14 | 3.57 |
| 36 | 0.38 | 0.76 | 1.51 | 2.27 | 3.78 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, B.; Ren, X.; Xue, T.; Zou, Q. A Slanted-Finger Interdigitated Transducer Microfluidic Device for Particles Sorting. Micromachines 2025, 16, 483. https://doi.org/10.3390/mi16040483
Liu B, Ren X, Xue T, Zou Q. A Slanted-Finger Interdigitated Transducer Microfluidic Device for Particles Sorting. Micromachines. 2025; 16(4):483. https://doi.org/10.3390/mi16040483
Chicago/Turabian StyleLiu, Baoguo, Xiang Ren, Tao Xue, and Qiang Zou. 2025. "A Slanted-Finger Interdigitated Transducer Microfluidic Device for Particles Sorting" Micromachines 16, no. 4: 483. https://doi.org/10.3390/mi16040483
APA StyleLiu, B., Ren, X., Xue, T., & Zou, Q. (2025). A Slanted-Finger Interdigitated Transducer Microfluidic Device for Particles Sorting. Micromachines, 16(4), 483. https://doi.org/10.3390/mi16040483








