Electrostatic Field Modification Enhances the Electrocatalytic Oxygen Evolution Reaction Stability of CoFe2O4 Catalysts
Abstract
1. Introduction
2. Experimental Section
2.1. Chemicals
2.2. Fabrication of CoFe2O4
2.3. Annealing Treatment
2.4. Materials Characterization
2.5. Electrochemical Measurements
3. Results and Discussion
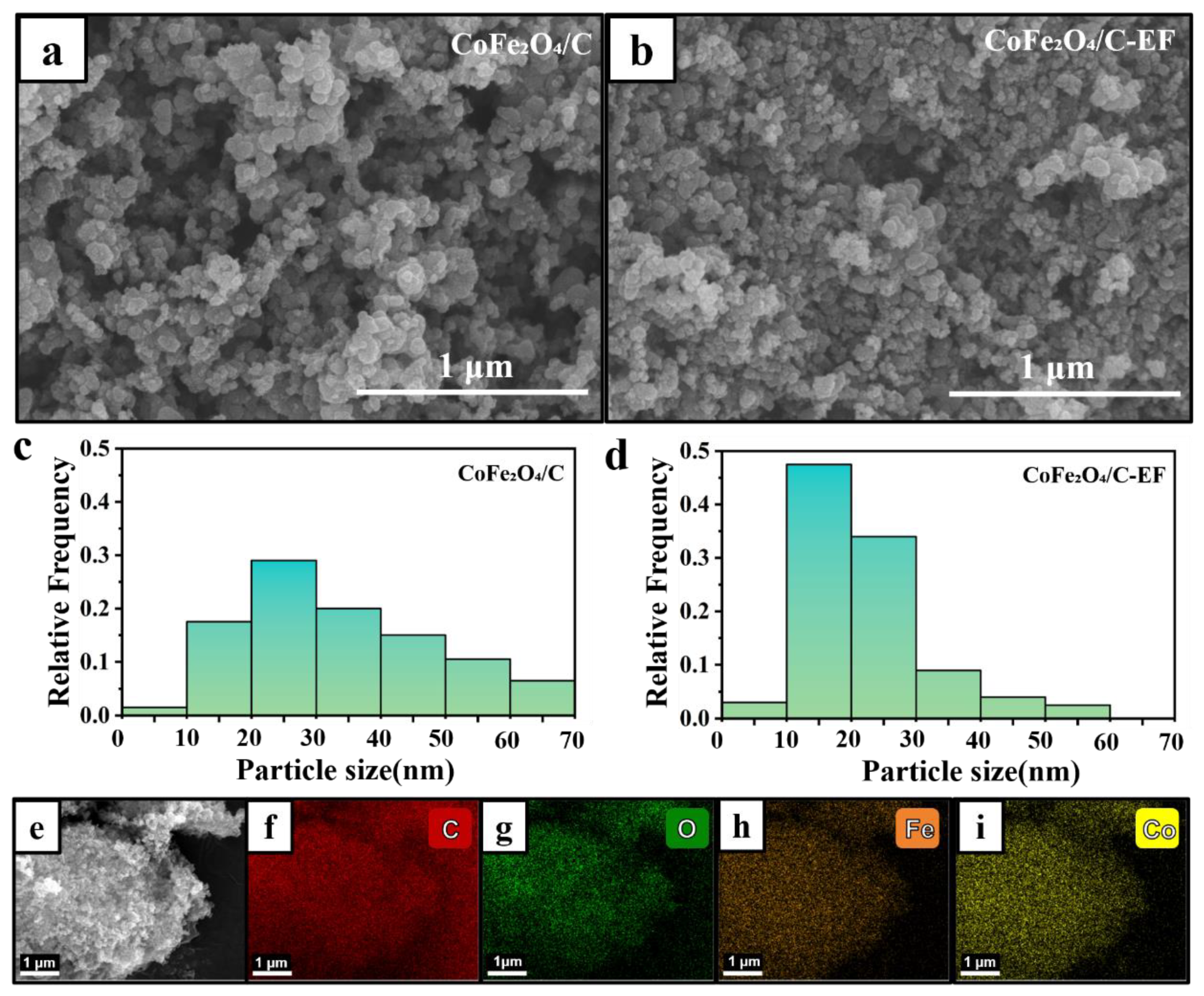
3.1. Morphological Characterization
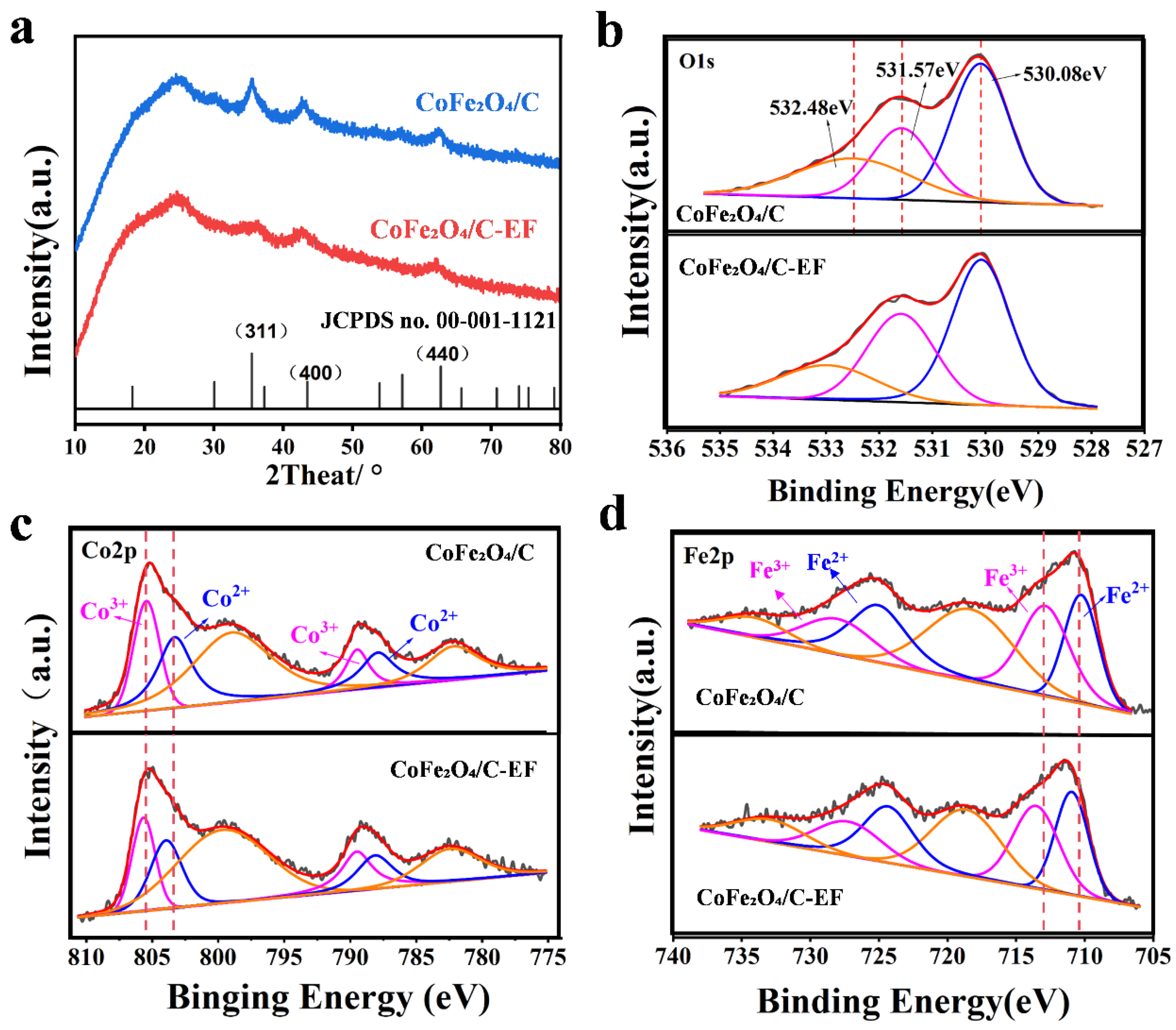
3.2. Structural Characterization
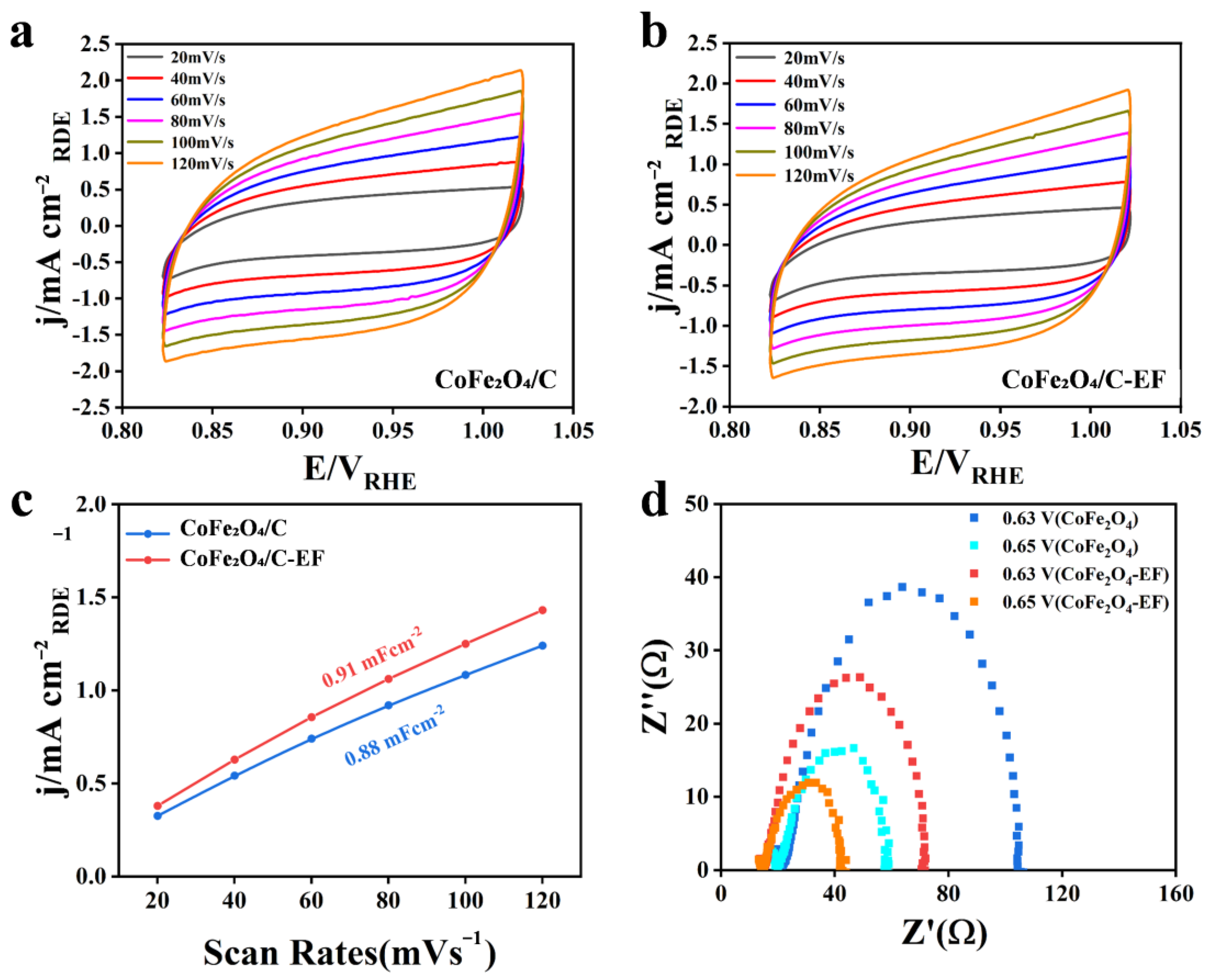
3.3. Electrocatalytic Performance
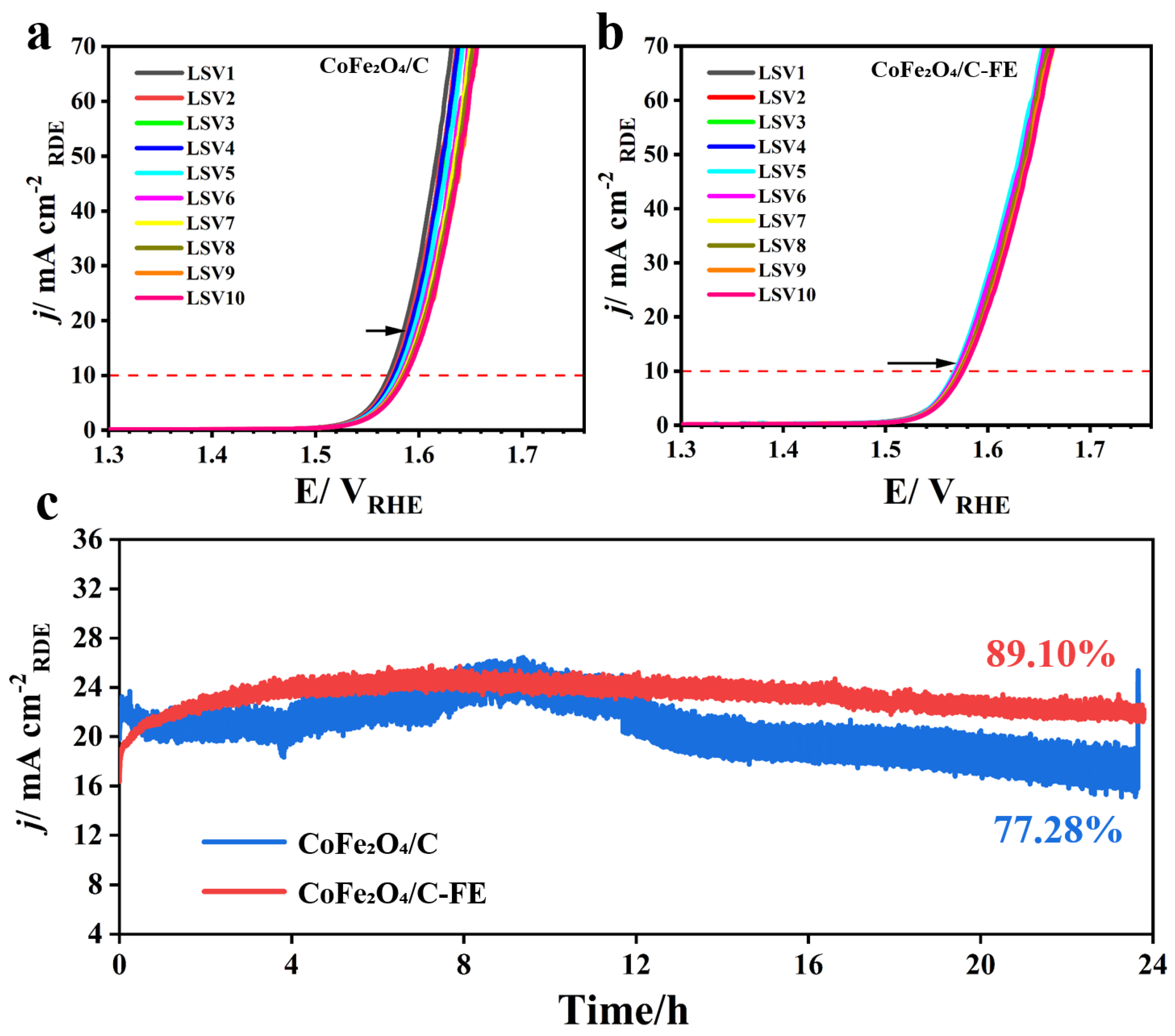
3.4. Stability Assessment
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Oliveira, A.M.; Beswick, R.R.; Yan, Y. A Green Hydrogen Economy for a Renewable Energy Society. Curr. Opin. Chem. Eng. 2021, 33, 100701–100708. [Google Scholar] [CrossRef]
- Amirante, R.; Cassone, E.; Distaso, E.; Tamburrano, P. Overview on Recent Developments in Energy Storage: Mechanical, Electrochemical and Hydrogen Technologies. Energy Convers. Manag. 2017, 132, 372–387. [Google Scholar] [CrossRef]
- Luo, X.; Wang, J.; Dooner, M.; Clarke, J. Overview of Current Development in Electrical Energy Storage Technologies and the Application Potential in Power System Operation. Appl. Energy 2015, 137, 511–536. [Google Scholar] [CrossRef]
- Zhang, K.; Zou, R. Advanced Transition Metal-based OER Electrocatalysts: Current Status, Opportunities, and Challenges. Small 2021, 17, 2100129–2100169. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Guan, Z.; Guo, D.; Yang, L.; Chen, X.; Wang, S. High-efficiency Oxygen Evolution Reaction: Controllable Reconstruction of Surface Interface. Small 2023, 19, 2304007–2304022. [Google Scholar] [CrossRef]
- Liang, Q.; Brocks, G.; Bieberle-Hütter, A. Oxygen Evolution Reaction (OER) Mechanism under Alkaline and Acidic Conditions. J. Phys. Energy 2021, 3, 26001–26009. [Google Scholar] [CrossRef]
- Ahmed, M.G.; Tay, Y.F.; Chi, X.; Razeen, A.S.; Fang, Y.; Zhang, M.; Sng, A.; Chiam, S.Y.; Rusydi, A.; Wong, L.H. Cation Migration-induced Lattice Oxygen Oxidation in Spinel Oxide for Superior Oxygen Evolution Reaction. Angew. Chem. Int. Ed. 2025, 64, e202416757. [Google Scholar] [CrossRef]
- Hooch Antink, W.; Lee, S.; Lee, H.S.; Shin, H.; Yoo, T.Y.; Ko, W.; Shim, J.; Na, G.; Sung, Y.; Hyeon, T. High-valence Metal-driven Electronic Modulation for Boosting Oxygen Evolution Reaction in High-entropy Spinel Oxide. Adv. Funct. Mater. 2024, 34, 2309438. [Google Scholar] [CrossRef]
- Wang, X.; Hu, J.; Lu, T.; Wang, H.; Sun, D.; Tang, Y.; Li, H.; Fu, G. Importing Atomic Rare-earth Sites to Activate Lattice Oxygen of Spinel Oxides for Electrocatalytic Oxygen Evolution. Angew. Chem. Int. Ed. 2025, 64, e202415306. [Google Scholar] [CrossRef]
- Sun, S.; Sun, Y.; Zhou, Y.; Xi, S.; Ren, X.; Huang, B.; Liao, H.; Wang, L.P.; Du, Y.; Xu, Z.J. Shifting Oxygen Charge towards Octahedral Metal: A Way to Promote Water Oxidation on Cobalt Spinel Oxides. Angew. Chem. Int. Ed. 2019, 58, 6042–6047. [Google Scholar] [CrossRef]
- Deng, Q.; Li, H.; Pei, K.; Wong, L.W.; Zheng, X.; Tsang, C.S.; Chen, H.; Shen, W.; Ly, T.H.; Zhao, J.; et al. Strategic Design for High-Efficiency Oxygen Evolution Reaction (OER) Catalysts by Triggering Lattice Oxygen Oxidation in Cobalt Spinel Oxides. ACS Nano 2024, 18, 33718–33728. [Google Scholar] [CrossRef] [PubMed]
- Wygant, B.R.; Kawashima, K.; Mullins, C.B. Catalyst or Precatalyst? The Effect of Oxidation on Transition Metal Carbide, Pnictide, and Chalcogenide Oxygen Evolution Catalysts. ACS Energy Lett. 2018, 3, 2956–2966. [Google Scholar] [CrossRef]
- Vij, V.; Sultan, S.; Harzandi, A.M.; Meena, A.; Tiwari, J.N.; Lee, W.-G.; Yoon, T.; Kim, K.S. Nickel-Based Electrocatalysts for Energy-Related Applications: Oxygen Reduction, Oxygen Evolution, and Hydrogen Evolution Reactions. ACS Catal. 2017, 7, 7196–7225. [Google Scholar] [CrossRef]
- Sher, F.; Curnick, O.; Azizan, M.T. Sustainable Conversion of Renewable Energy Sources. Sustainability 2021, 13, 2940. [Google Scholar] [CrossRef]
- Han, N.; Guo, X.; Cheng, J.; Liu, P.; Zhang, S.; Huang, S.; Rowles, M.R.; Fransaer, J.; Liu, S. Inhibiting in Situ Phase Transition in Ruddlesden-Popper Perovskite via Tailoring Bond Hybridization and Its Application in Oxygen Permeation. Matter 2021, 4, 1720–1734. [Google Scholar] [CrossRef]
- Zhang, T.; Wu, J.; Wang, Z.; Wei, Z.; Liu, J.; Gong, X. Transfer of Molecular Oxygen and Electrons Improved by the Regulation of C-N/C = O for Highly Efficient 2e-ORR. Chem. Eng. J. 2022, 433, 133591–133602. [Google Scholar] [CrossRef]
- Vijay, A.; Ramanujachary, K.V.; Lofland, S.E.; Vaidya, S. Role of Crystal Structure and Electrical Polarization of an Electrocatalyst in Enhancing Oxygen Evolution Performance: Bi-Fe-O System as a Case Study. Electrochim. Acta 2022, 407, 139887–139897. [Google Scholar] [CrossRef]
- Cui, T.; Zhai, X.; Guo, L.; Chi, J.-Q.; Zhang, Y.; Zhu, J.; Sun, X.; Wang, L. Controllable Synthesis of a Self-Assembled Ultralow Ru, Ni-Doped Fe2O3 Lily as a Bifunctional Electrocatalyst for Large-Current-Density Alkaline Seawater Electrolysis. Chin. J. Catal. 2022, 43, 2202–2211. [Google Scholar] [CrossRef]
- Shao, L.; Han, X.; Shi, L.; Wang, T.; Zhang, Y.; Jiang, Z.; Yin, Z.; Zheng, X.; Li, J.; Han, X.; et al. In Situ Generation of Molybdate-modulated Nickel-iron Oxide Electrodes with High Corrosion Resistance for Efficient Seawater Electrolysis. Adv. Energy Mater. 2024, 14, 2303261–2303271. [Google Scholar] [CrossRef]
- Wang, S.; Yang, P.; Sun, X.; Xing, H.; Hu, J.; Chen, P.; Cui, Z.; Zhu, W.; Ma, Z. Synthesis of 3D Heterostructure Co-Doped Fe2P Electrocatalyst for Overall Seawater Electrolysis. Appl. Catal., B 2021, 297, 120386–120397. [Google Scholar] [CrossRef]
- Pan, Y.; Wang, X.; Zhang, W.; Tang, L.; Mu, Z.; Liu, C.; Tian, B.; Fei, M.; Sun, Y.; Su, H.; et al. Boosting the Performance of Single-Atom Catalysts via External Electric Field Polarization. Nat. Commun. 2022, 13, 3063. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; He, Q.; Li, X.; Su, X.; Zhang, Y.; Chen, S.; Zhang, S.; Zhang, G.; Jiang, J.; Luo, Y.; et al. Tracking Structural Self-reconstruction and Identifying True Active Sites toward Cobalt Oxychloride Precatalyst of Oxygen Evolution Reaction. Adv. Mater. 2019, 31, 1805127–1805135. [Google Scholar] [CrossRef]
- Sun, J.; Xue, H.; Guo, N.; Song, T.; Hao, Y.; Sun, J.; Zhang, J.; Wang, Q. Synergetic Metal Defect and Surface Chemical Reconstruction into NiCo2 S4/ZnS Heterojunction to Achieve Outstanding Oxygen Evolution Performance. Angew. Chem. Int. Ed. 2021, 60, 19435–19441. [Google Scholar] [CrossRef] [PubMed]
- Nie, J.; Hu, C.; Yan, Q.; Luo, J. Discovery of Electrochemically Induced Grain Boundary Transitions. Nat. Commun. 2021, 12, 2374. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Shen, Y.; Luo, K.; An, Q.; Gao, P.; Xiao, P.; Zou, Y. Harnessing Dislocation Motion Using an Electric Field. Nat. Mater. 2023, 22, 958–963. [Google Scholar] [CrossRef]
- Ashfaq, K.; Saleem, M.I.; Ibrahim, A.; Dahshan, A.; Aslam, M.; Henaish, A.M.A.; Khan, M.J.; Ahmad, K. CoFe2O4 Supported on G-CN Nanosheet for Oxygen Evolution Reaction in Basic Media. Int. J. Hydrog. Energy 2024, 80, 554–563. [Google Scholar] [CrossRef]
- Ganesan, M.; Ganapathi, B.; Govindasamy, P.; Parasuraman, B.; Shanmugam, P.; Boddula, R.; Pothu, R.; Thangavelu, P. CoFe2O4/rGO Nanocomposite: Synthesis and Enhanced Ammonia Gas Sensing Properties at Room Temperature. Results Chem. 2024, 7, 101342. [Google Scholar] [CrossRef]
- Asadi, A.; Beigrezaee, S.; Daglioglu, N.; Guzel, E.Y.; Heydari, M.; Ravankhah, N. Combination of Hydrodynamic Cavitation with CoFe2O4 Photocatalyst for Activation of Peracetic Acid under UVC Irradiation in Synergistic Degradation of Bisphenol a. J. Water Process Eng. 2024, 64, 105608. [Google Scholar] [CrossRef]
- Huang, Q.; Li, C.; Tu, Y.; Jiang, Y.; Mei, P.; Yan, X. Spinel CoFe2O4/Carbon Nanotube Composites as Efficient Bifunctional Electrocatalysts for Oxygen Reduction and Oxygen Evolution Reaction. Ceram. Int. 2021, 47, 1602–1608. [Google Scholar] [CrossRef]
- Alharthi, A.I.; Alotaibi, M.A.; Abdel-Fattah, E.; Awadallah, A.E. Understanding the Impact of Support Materials on CoFe2O4 Catalyst Performance for Hydrogen Fuel and Nanocarbon Production via Methane Decomposition. Ceram. Int. 2024, 50, 38029–38039. [Google Scholar] [CrossRef]
- Lai, J.; Zeng, C.; Peng, S.; Zhou, Q.; Zeng, J.; Liu, C.; Qi, X. Constructions of Hierarchical Nitrogen Doped Carbon Nanotubes Anchored on CoFe2O4 Nanoflakes for Efficient Hydrogen Evolution, Oxygen Evolution and Oxygen Reduction Reaction. J. Power Sources 2024, 599, 234218. [Google Scholar] [CrossRef]
- Liu, Y.; Ye, C.; Chen, L.; Fan, J.; Liu, C.; Xue, L.; Sun, J.; Zhang, W.; Wang, X.; Xiong, P.; et al. High Entropy-driven Role of Oxygen Vacancies for Water Oxidation. Adv. Funct. Mater. 2024, 34, 2314820. [Google Scholar] [CrossRef]
- Wang, X.; Ouyang, T.; Wang, L.; Zhong, J.; Ma, T.; Liu, Z. Redox-inert Fe3+ Ions in Octahedral Sites of Co-fe Spinel Oxides with Enhanced Oxygen Catalytic Activity for Rechargeable Zinc–Air Batteries. Angew. Chem. Int. Ed. 2019, 58, 13291–13296. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, L.; Miao, J.; Feng, X.; Zhong, Y.; Wang, W. Electrostatic Field Modification Enhances the Electrocatalytic Oxygen Evolution Reaction Stability of CoFe2O4 Catalysts. Micromachines 2025, 16, 491. https://doi.org/10.3390/mi16050491
Liang L, Miao J, Feng X, Zhong Y, Wang W. Electrostatic Field Modification Enhances the Electrocatalytic Oxygen Evolution Reaction Stability of CoFe2O4 Catalysts. Micromachines. 2025; 16(5):491. https://doi.org/10.3390/mi16050491
Chicago/Turabian StyleLiang, Liwen, Jiatong Miao, Xiyuan Feng, Yunlei Zhong, and Wei Wang. 2025. "Electrostatic Field Modification Enhances the Electrocatalytic Oxygen Evolution Reaction Stability of CoFe2O4 Catalysts" Micromachines 16, no. 5: 491. https://doi.org/10.3390/mi16050491
APA StyleLiang, L., Miao, J., Feng, X., Zhong, Y., & Wang, W. (2025). Electrostatic Field Modification Enhances the Electrocatalytic Oxygen Evolution Reaction Stability of CoFe2O4 Catalysts. Micromachines, 16(5), 491. https://doi.org/10.3390/mi16050491




