Rapid Synthesis of Fast-Charging TiNb2O7 for Lithium-Ion Storage via Ultrafast Carbothermal Shock
Abstract
1. Introduction
2. Experiment
2.1. Ultrafast Carbothermal Shock Rapid Synthesis of TiNb2O7
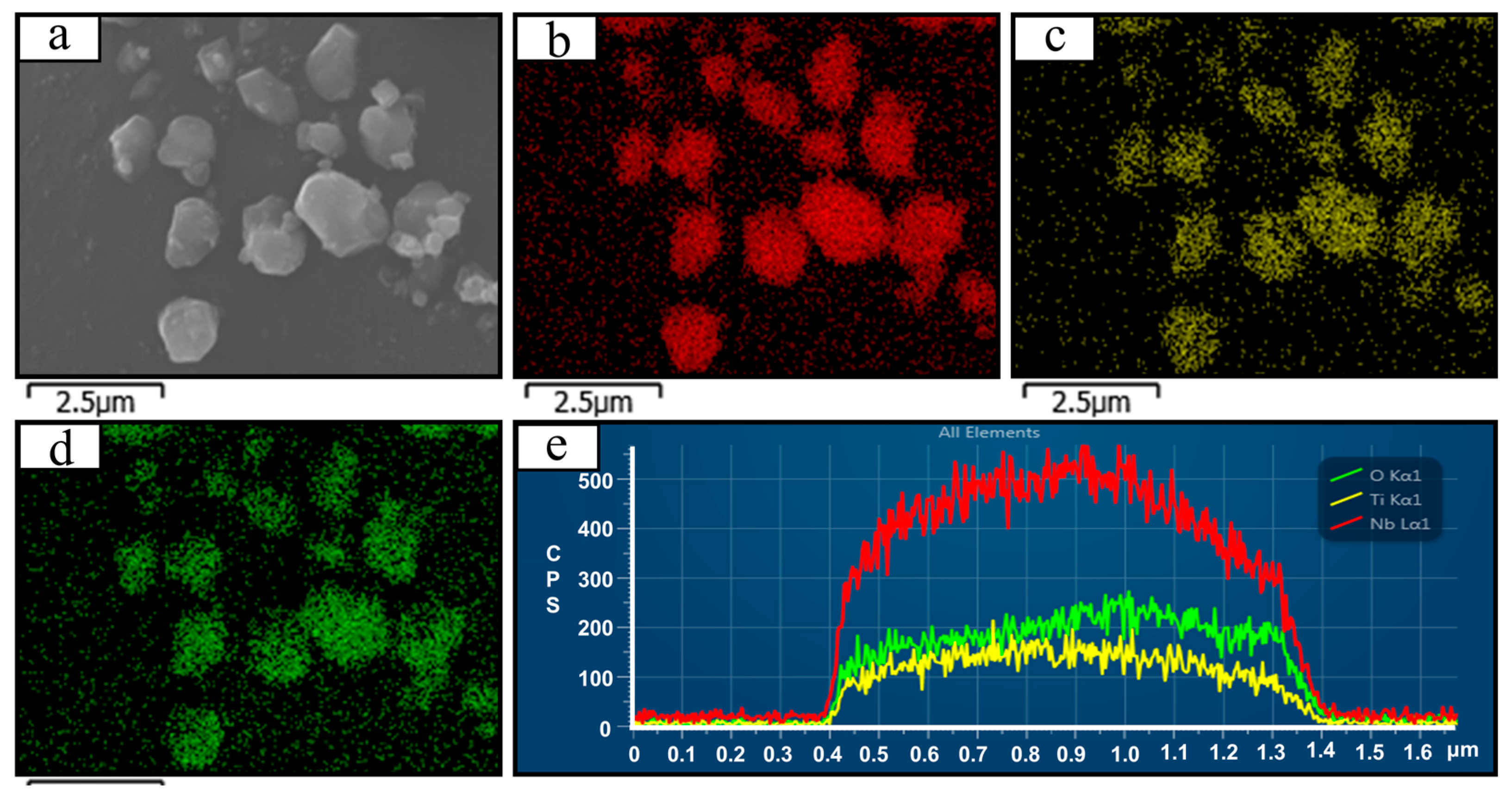
2.2. Material Characterization
2.3. Electrochemical Test
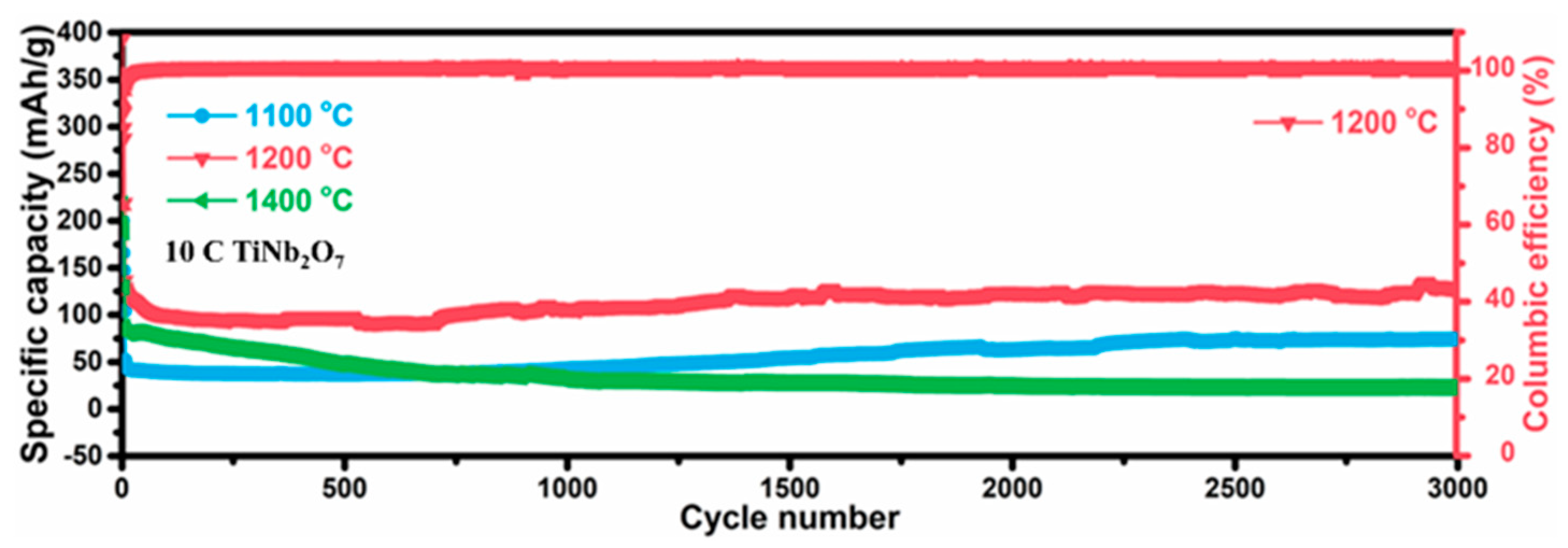
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fellner, J.P.; Loeber, G.J.; Sandhu, S.S. Testing of lithium-ion 18650 cells and characterizing/predicting cell performance. J. Power Sources 1999, 81, 867–871. [Google Scholar] [CrossRef]
- Kida, Y.; Kinoshita, A.; Yanagida, K.; Funahashi, A.; Nohma, T.; Yonezu, I. A study on the cycle performance of lithium secondary batteries using lithium nickel–cobalt composite oxide and graphite/coke hybrid carbon. Electrochim. Acta 2002, 47, 1691–1696. [Google Scholar] [CrossRef]
- Xiao, C.; Wang, H.; Usiskin, R.; van Aken, P.A.; Maier, J.J.S. Unification of insertion and supercapacitive storage concepts: Storage profiles in titania. Science 2024, 386, 407–413. [Google Scholar] [CrossRef]
- Shin, J.Y.; Samuelis, D.; Maier, J. Sustained lithium-storage performance of hierarchical, nanoporous anatase TiO2 at high rates: Emphasis on interfacial storage phenomena. Adv. Funct. Mater. 2011, 21, 3464–3472. [Google Scholar] [CrossRef]
- Li, M.; An, H.; Song, Y.; Liu, Q.; Wang, J.; Huo, H.; Lou, S.; Wang, J. Ion-Dipole-Interaction-Induced Encapsulation of Free Residual Solvent for Long-Cycle Solid-State Lithium Metal Batteries. J. Am. Chem. Soc. 2023, 145, 25632–25642. [Google Scholar] [CrossRef]
- Lou, S.; Liu, Q.; Zhang, F.; Liu, Q.; Yu, Z.; Mu, T.; Zhao, Y.; Borovilas, J.; Chen, Y.; Ge, M.; et al. Insights into interfacial effect and local lithium-ion transport in polycrystalline cathodes of solid-state batteries. Nat. Commun. 2020, 11, 5700. [Google Scholar] [CrossRef]
- Lou, S.; Yu, Z.; Liu, Q.; Wang, H.; Chen, M.; Wang, J. Multi-scale Imaging of Solid-State Battery Interfaces: From Atomic Scale to Macroscopic Scale. Chem 2020, 6, 2199–2218. [Google Scholar] [CrossRef]
- Zhang, Y.; Kang, C.; Zhao, W.; Song, Y.; Zhu, J.; Huo, H.; Ma, Y.; Du, C.; Zuo, P.; Lou, S.; et al. d-p Hybridization-Induced “Trapping-Coupling-Conversion” Enables High-Efficiency Nb Single-Atom Catalysis for Li-S Batteries. J. Am. Chem. Soc. 2023, 145, 1728–1739. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Zhao, W.; Zuo, P.; Tong, Y.; Yin, G.; Zhu, T.; Lou, S. Delocalized electronic engineering of TiNb2O7 enables low temperature capability for high-areal-capacity lithium-ion batteries. Nat. Commun. 2024, 15, 6299. [Google Scholar] [CrossRef]
- Aravindan, V.; Sundaramurthy, J.; Jain, A.; Kumar, P.S.; Ling, W.C.; Ramakrishna, S.; Srinivasan, M.P.; Madhavi, S. Unveiling TiNb2O7 as an insertion anode for lithium ion capacitors with high energy and power density. ChemSusChem 2014, 7, 1858–1863. [Google Scholar] [CrossRef]
- Griffith, K.J.; Seymour, I.D.; Hope, M.A.; Butala, M.M.; Lamontagne, L.K.; Preefer, M.B.; Koçer, C.P.; Henkelman, G.; Morris, A.J.; Cliffe, M.J. Ionic and electronic conduction in TiNb2O7. J. Am. Chem. Soc. 2019, 141, 16706–16725. [Google Scholar] [CrossRef]
- Zhao, Z.; Xue, Z.; Xiong, Q.; Zhang, Y.; Hu, X.; Chi, H.; Qin, H.; Yuan, Y.; Ni, H. Titanium niobium oxides (TiNb2O7): Design, fabrication and application in energy storage devices. Sustain. Mater. Technol. 2021, 30, e00357. [Google Scholar] [CrossRef]
- He, Y.-B.; Li, B.; Liu, M.; Zhang, C.; Lv, W.; Yang, C.; Li, J.; Du, H.; Zhang, B.; Yang, Q.-H. Gassing in Li4Ti5O12-based batteries and its remedy. Sci. Rep. 2012, 2, 913. [Google Scholar] [CrossRef]
- Chen, L.; Yu, H.; Zhu, D.; Liu, S.; Zhang, L.; Sun, J.; Zhao, Z.; Li, Q.; Chen, G.; Li, Q. Designing electron/ion dual-phase conductor Ni@ TiO2 for high-performance lithium-ion storage: Combining insertion and space charge mechanism. Appl. Phys. Lett. 2024, 124, 133901. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, J.; He, A.; Li, J.; Ma, W.; Record, M.-C.; Boulet, P.; Wang, J.; Albina, J.-M. Core–Double-Shell TiO2@ Fe3O4@ C Microspheres with Enhanced Cycling Performance as Anode Materials for Lithium-Ion Batteries. Materials 2024, 17, 2543. [Google Scholar] [CrossRef]
- Feng, F.; Wang, L.; Hu, N.; Wang, Q.; Hu, Q.; Guo, S.; Jin, W.; Li, C. The novel α-TiO2@ g-C3N4 heterostructure for ultra rapid ionic pumping and effective capacitive deionization. Sep. Purif. Technol. 2025, 355, 129632. [Google Scholar] [CrossRef]
- Pillai, A.M.; Gopinadh, S.V.; Phanendra, P.V.; Salini, P.S.; John, B.; SarojiniAmma, S.; Devassy, M.T. Bio-synthesized TiO2 nanoparticles and the aqueous binder-based anode derived thereof for lithium-ion cells. Discov. Nano 2024, 19, 69. [Google Scholar] [CrossRef]
- Wu, C.; Wang, X.; Zhu, Y.; Dong, L.; Xu, J. SN-doping TiO2@ MXene heterostructure in-situ derived from MXene frameworks as high-rate anodes for Lithium/Sodium-ion batteries. J. Electroanal. Chem. 2025, 977, 118833. [Google Scholar] [CrossRef]
- Wu, W.; Lin, Y.; Hu, Y.; He, Z.; Yang, Y. Phase-field modelling for degradation/failure research in lithium battery: Progress and prospects. J. Energy Chem. 2024, 102, 628–650. [Google Scholar] [CrossRef]
- Dahlman, C.J.; Heo, S.; Zhang, Y.; Reimnitz, L.C.; He, D.; Tang, M.; Milliron, D.J. Dynamics of lithium insertion in electrochromic titanium dioxide nanocrystal ensembles. J. Am. Chem. Soc. 2021, 143, 8278–8294. [Google Scholar] [CrossRef]
- Fan, M.; Yang, Z.; Lin, Z.; Xiong, X. Facile synthesis of uniform N-doped carbon-coated TiO2 hollow spheres with enhanced lithium storage performance. Nanoscale 2021, 13, 2368–2372. [Google Scholar] [CrossRef]
- Kim, M.-S.; Lee, B.-H.; Park, J.-H.; Lee, H.S.; Hooch Antink, W.; Jung, E.; Kim, J.; Yoo, T.Y.; Lee, C.W.; Ahn, C.-Y. Operando identification of the chemical and structural origin of Li-ion battery aging at near-ambient temperature. J. Am. Chem. Soc. 2020, 142, 13406–13414. [Google Scholar] [CrossRef]
- Lee, M.D.; Lee, G.J.; Nam, I.; Abbas, M.A.; Bang, J.H. Exploring the effect of cation vacancies in TiO2: Lithiation behavior of n-type and p-type TiO2. ACS Appl. Mater. Interfaces 2022, 14, 6560–6569. [Google Scholar] [CrossRef]
- Liu, Y.; Pan, X.; Chen, W.; Zhao, X. Titanate-derived Nb-doped TiO2 nanoparticles displaying improved lithium storage performance. Dalton Trans. 2022, 51, 2506–2511. [Google Scholar] [CrossRef]
- Babu, B.; Simon, P.; Balducci, A. Fast charging materials for high power applications. Adv. Energy Mater. 2020, 10, 2001128. [Google Scholar] [CrossRef]
- Hao, Z.; Chen, Q.; Dai, W.; Ren, Y.; Zhou, Y.; Yang, J.; Xie, S.; Shen, Y.; Wu, J.; Chen, W. Oxygen-deficient blue TiO2 for ultrastable and fast lithium storage. Adv. Energy Mater. 2020, 10, 1903107. [Google Scholar] [CrossRef]
- Liang, Y.; Xiong, X.; Xu, Z.; Xia, Q.; Wan, L.; Liu, R.; Chen, G.; Chou, S.L. Ultrathin 2D Mesoporous TiO2/rGO Heterostructure for High-Performance Lithium Storage. Small 2020, 16, 2000030. [Google Scholar] [CrossRef]
- Niu, J.; Zhang, Z.; Aurbach, D. Alloy anode materials for rechargeable Mg ion batteries. Adv. Energy Mater. 2020, 10, 2000697. [Google Scholar] [CrossRef]
- Liu, J.; Xu, Z.; Wu, M.; Wang, Y.; Karim, Z. Capacity contribution induced by pseudo-capacitance adsorption mechanism of anode carbonaceous materials applied in potassium-ion battery. Front. Chem. 2019, 7, 640. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, Y.; Yu, L.; Wu, N.-L.; Huang, H.; Wei, M. TiO2-B nanowires via topological conversion with enhanced lithium-ion intercalation properties. J. Mater. Chem. A 2019, 7, 3842–3847. [Google Scholar] [CrossRef]
- Ha, J.U.; Lee, J.; Abbas, M.A.; Lee, M.D.; Lee, J.; Bang, J.H. Designing hierarchical assembly of carbon-coated TiO2 nanocrystals and unraveling the role of TiO2/carbon interface in lithium-ion storage in TiO2. ACS Appl. Mater. Interfaces 2019, 11, 11391–11402. [Google Scholar] [CrossRef]
- Jiang, H.; Wei, Z.; Cai, X.; Lai, L.; Ma, J.; Huang, W. A cathode for Li-ion batteries made of vanadium oxide on vertically aligned carbon nanotube arrays/graphene foam. Chem. Eng. J. 2019, 359, 1668–1676. [Google Scholar] [CrossRef]
- Lee, D.-H.; Lee, B.-H.; Sinha, A.K.; Park, J.-H.; Kim, M.-S.; Park, J.; Shin, H.; Lee, K.-S.; Sung, Y.-E.; Hyeon, T. Engineering titanium dioxide nanostructures for enhanced lithium-ion storage. J. Am. Chem. Soc. 2018, 140, 16676–16684. [Google Scholar] [CrossRef] [PubMed]
- Lou, S.; Zhao, Y.; Wang, J.; Yin, G.; Du, C.; Sun, X. Ti-based oxide anode materials for advanced electrochemical energy storage: Lithium/sodium ion batteries and hybrid pseudocapacitors. Small 2019, 15, 1904740. [Google Scholar] [CrossRef]
- Cheng, Q.; Liang, J.; Zhu, Y.; Si, L.; Guo, C.; Qian, Y. Bulk Ti2Nb10O29 as long-life and high-power Li-ion battery anodes. J. Mater. Chem. A 2014, 2, 17258–17262. [Google Scholar] [CrossRef]
- Wagemaker, M.; Mulder, F.M. Properties and promises of nanosized insertion materials for Li-ion batteries. Acc. Chem. Res. 2013, 46, 1206–1215. [Google Scholar] [CrossRef] [PubMed]
- Mei, J.; Liao, T.; Kou, L.; Sun, Z. Oxygen vacancies and cation disorder tailoring electrochemical kinetics in TiNb2O7. Adv. Energy Mater. 2021, 11, 2101713. [Google Scholar]
- Kim, J.-H.; Park, K.-J.; Kim, S.-J.; Yoon, C.S. Atomic-scale engineering of cation channels for fast-charging batteries. Nat. Energy 2022, 7, 312–321. [Google Scholar]



| TiNb2O7 (Monoclinic, Space Group: C2/m) | |||
|---|---|---|---|
| T (K) | 300 | ||
| a, b, c (Å) | 17.6869 (2) | 3.8030 (0) | 11.8976 (0) |
| α, β, γ (°) | 90 | 95.32233 (3) | 90 |
| V (Å3) | 796.830 (1) | ||
| Atom | x | y | z |
| Nb1 | 0.0000 (0) | 0.0000 (0) | 0.0000 (0) |
| Ti1 | 0.0000 (0) | 0.0000 (0) | 0.0000 (0) |
| Nb2 | 0.18483 (36) | 0.0000 (0) | 0.18442 (58) |
| Ti2 | 0.18483 (36) | 0.0000 (0) | 0.18442 (58) |
| Nb3 | 0.07844 (33) | 0.0000 (0) | −0.55762 (56) |
| Ti3 | 0.07844 (33) | 0.0000 (0) | −0.55762 (56) |
| Nb4 | 0.89020 (36) | 0.0000 (0) | 0.25427 (59) |
| Ti4 | 0.89020 (36) | 0.0000 (0) | 0.25427 (59) |
| Nb5 | 0.29626 (43) | 0.0000 (0) | −0.07822 (66) |
| Ti5 | 0.29626 (43) | 0.0000 (0) | −0.07822 (66) |
| O1 | 0.18303 (0) | 0.0000 (0) | −0.41559 (0) |
| O2 | 0.36568 (0) | 0.0000 (0) | −0.25112 (0) |
| O3 | 0.59550 (0) | 0.0000 (0) | −0.01283 (0) |
| O4 | 0.78760 (0) | 0.0000 (0) | 0.16717 (0) |
| O5 | 0.24841 (0) | 0.0000 (0) | 0.05577 (0) |
| O6 | 0.69940 (0) | 0.0000 (0) | 0.69177 (0) |
| O7 | 0.89633 (0) | 0.0000 (0) | −0.08214 (0) |
| O8 | 0.01655 (0) | 0.0000 (0) | −0.38232 (0) |
| O9 | 0.87235 (0) | 0.0000 (0) | 0.68015 (0) |
| O10 | 0.50000 (0) | 0.0000 (0) | 0.50000 (0) |
| O11 | 0.04006 (0) | 0.0000 (0) | −0.13948 (0) |
| Rp, Rwp, Rexp, χ2 | 5.08, 8.30, 3.68, 5.09 | ||
| Material | Current Density | Capacity (mAh/g) | Cycle Number | Retention Rate |
|---|---|---|---|---|
| TNO-1200 (This Work) | 10 C | 125 | 3000 | 98% |
| TiNb2O7 [11] | 1 C | 250 | 100 | - |
| Ti2Nb10O29 [35] | 10 C | 100 | 500 | 95% |
| Oxygen-deficient TNO [26] | 5 C | 180 | 1000 | 85% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, X.; Zhong, Y.; Hu, X.; Feng, X.; Ye, F. Rapid Synthesis of Fast-Charging TiNb2O7 for Lithium-Ion Storage via Ultrafast Carbothermal Shock. Micromachines 2025, 16, 490. https://doi.org/10.3390/mi16050490
Hu X, Zhong Y, Hu X, Feng X, Ye F. Rapid Synthesis of Fast-Charging TiNb2O7 for Lithium-Ion Storage via Ultrafast Carbothermal Shock. Micromachines. 2025; 16(5):490. https://doi.org/10.3390/mi16050490
Chicago/Turabian StyleHu, Xianyu, Yunlei Zhong, Xiaosai Hu, Xiyuan Feng, and Fengying Ye. 2025. "Rapid Synthesis of Fast-Charging TiNb2O7 for Lithium-Ion Storage via Ultrafast Carbothermal Shock" Micromachines 16, no. 5: 490. https://doi.org/10.3390/mi16050490
APA StyleHu, X., Zhong, Y., Hu, X., Feng, X., & Ye, F. (2025). Rapid Synthesis of Fast-Charging TiNb2O7 for Lithium-Ion Storage via Ultrafast Carbothermal Shock. Micromachines, 16(5), 490. https://doi.org/10.3390/mi16050490





