Fabrication of a Spiral Microfluidic Chip for the Mass Production of Lipid Nanoparticles Using Laser Engraving
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
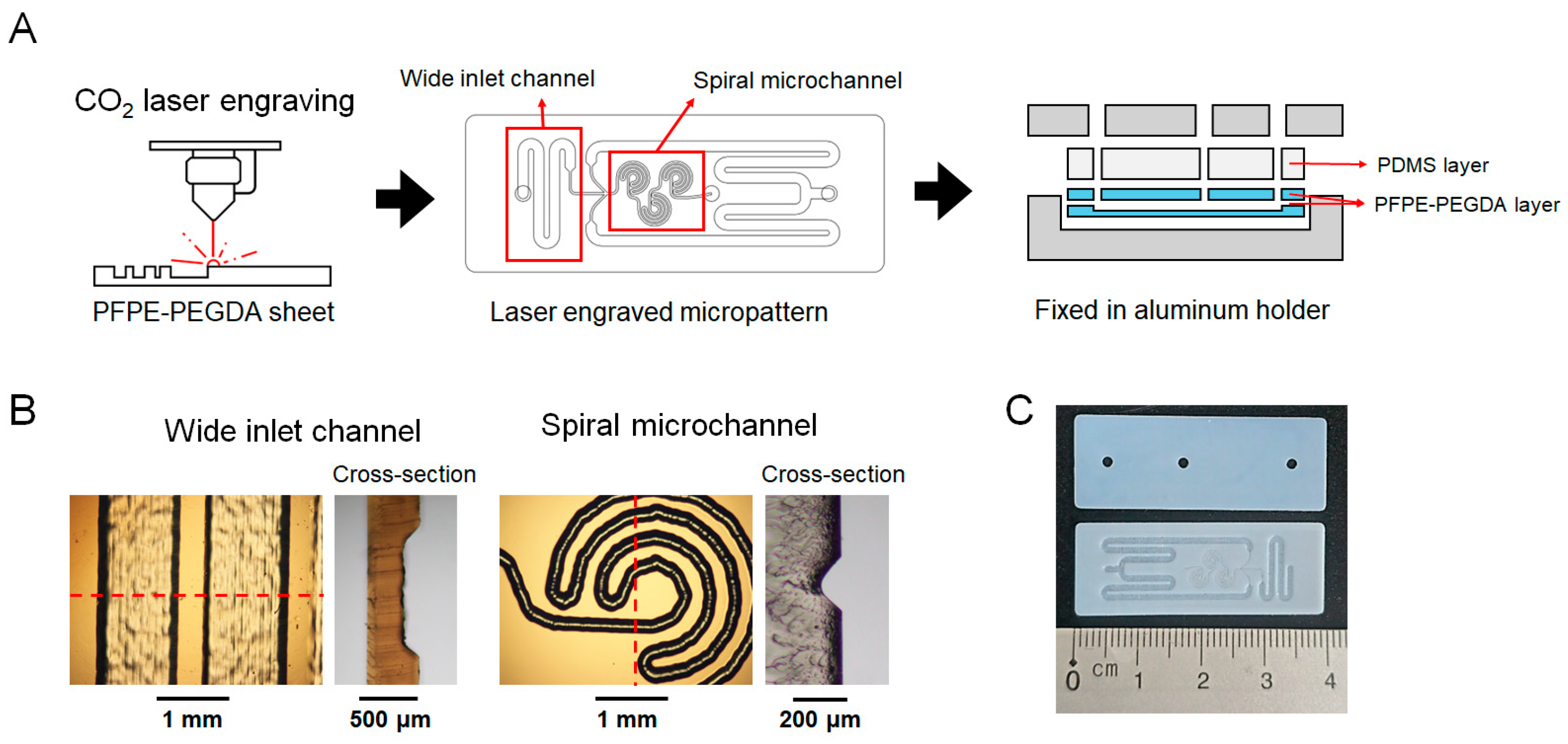
2.2. Fabrication of Microfluidic Chip
2.3. Fabrication of LNP with Microfluidic Chips
3. Results and Discussion
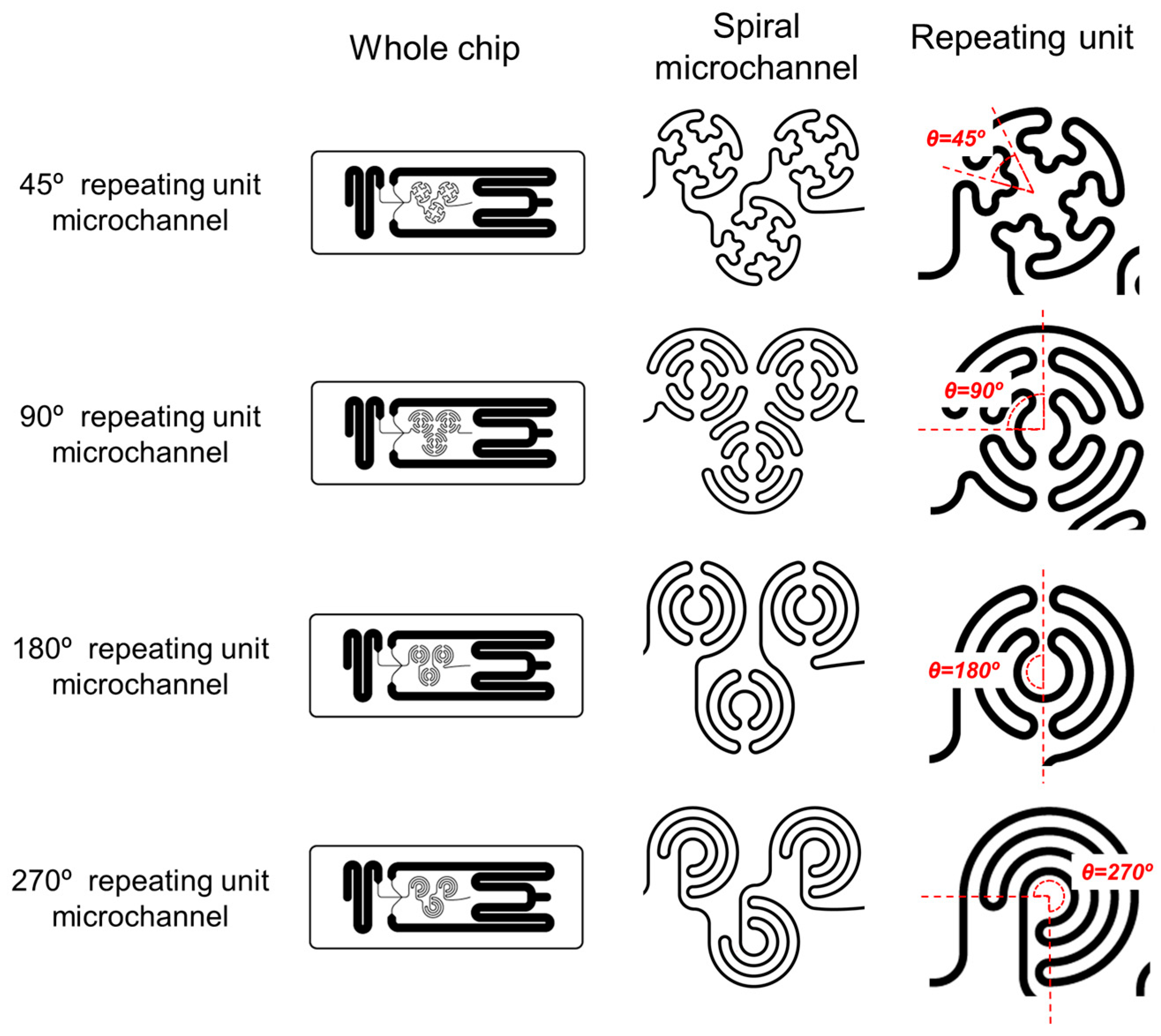
3.1. Fabrication and Characterization of the Spiral Microfluidic Chip
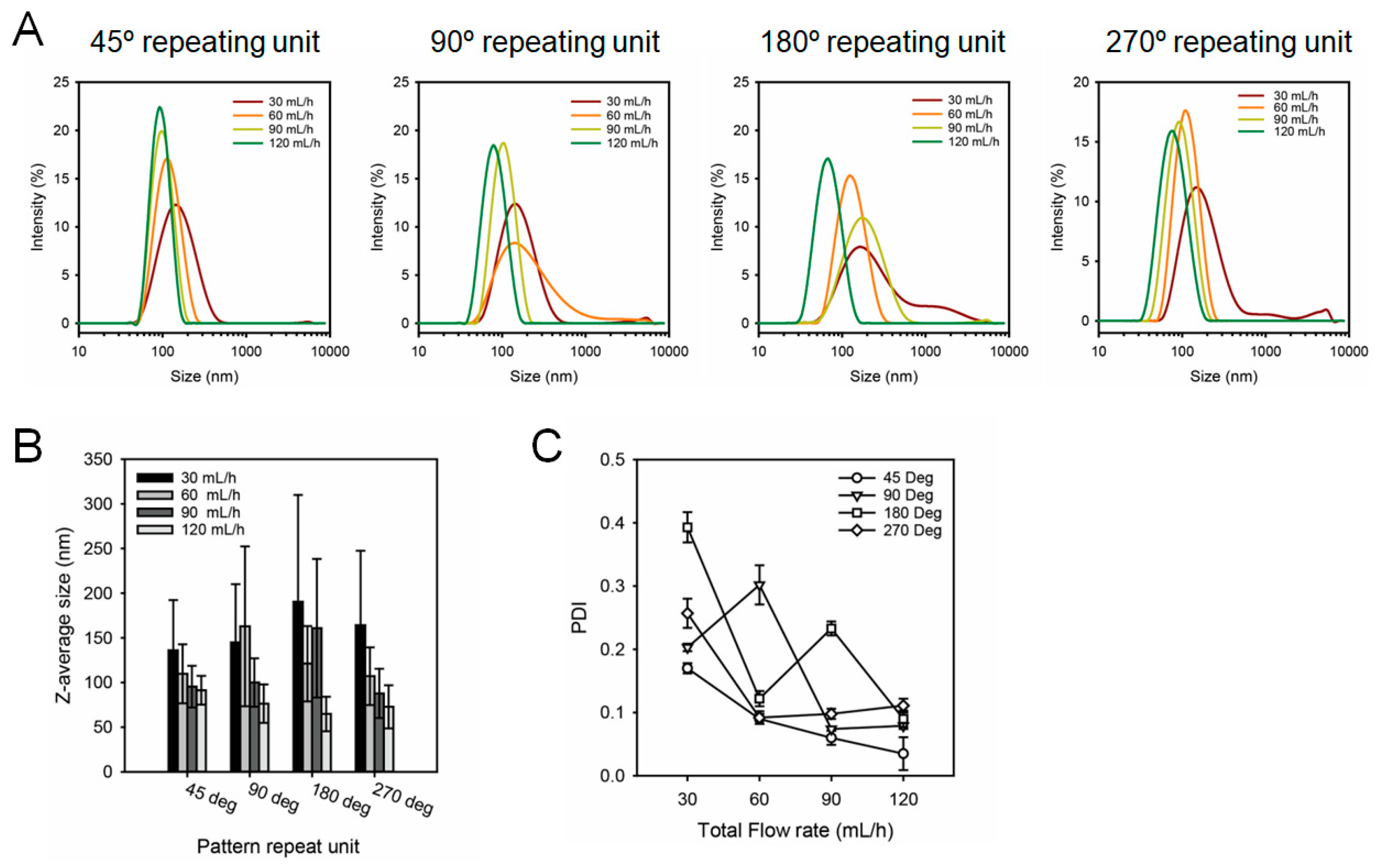
3.2. Production and Characterization of Lipid Nanoparticles Using SMC
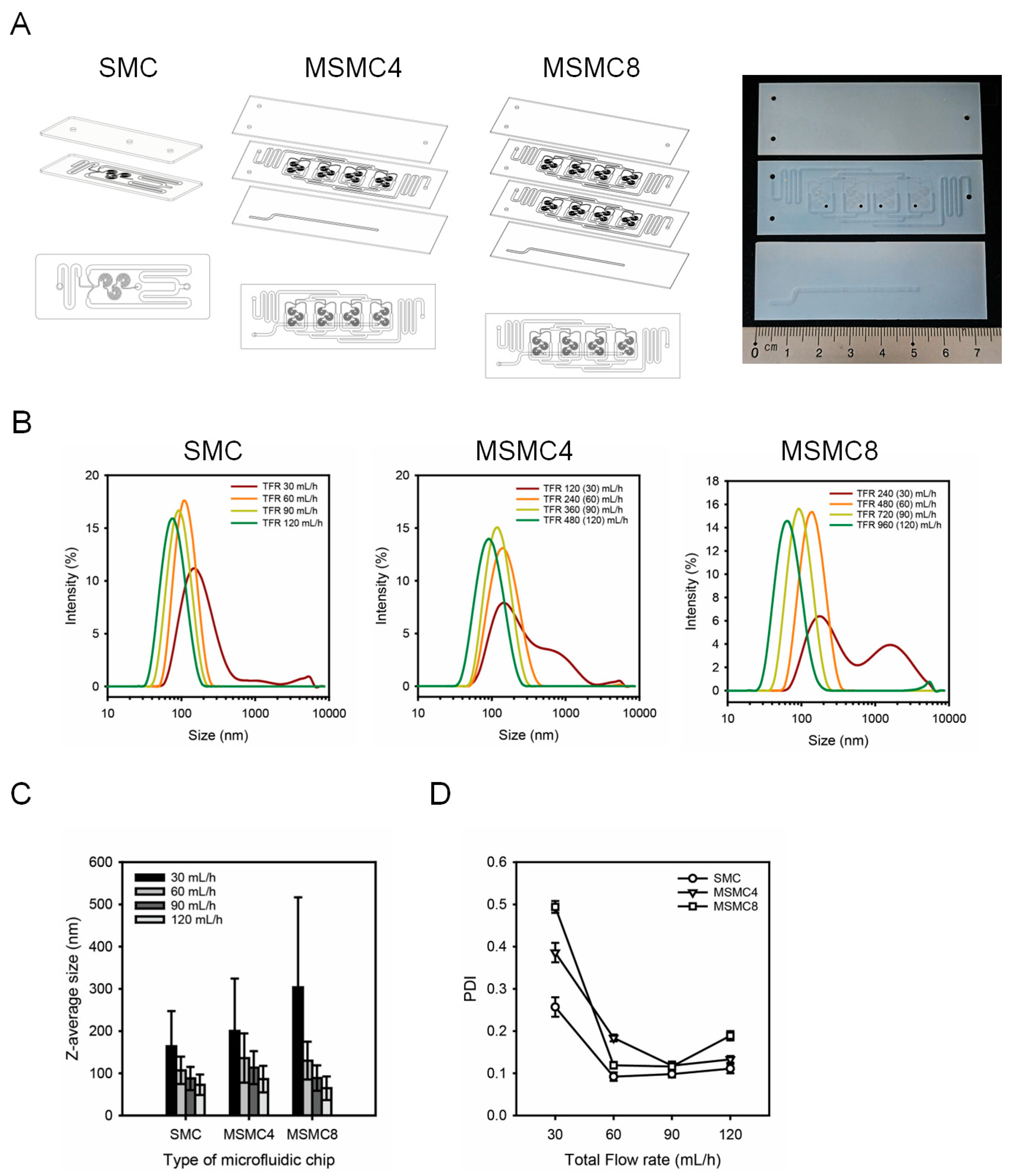
3.3. Fabrication of MSMC for the Mass Production of LNPs
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hou, X.; Zaks, T.; Langer, R.; Dong, Y. Lipid nanoparticles for mRNA delivery. Nat. Rev. Mater. 2021, 6, 1078–1094. [Google Scholar] [CrossRef] [PubMed]
- Tenchov, R.; Bird, R.; Curtze, A.E.; Zhou, Q. Lipid Nanoparticles—From Liposomes to mRNA Vaccine Delivery, a Landscape of Research Diversity and Advancement. ACS Nano 2021, 15, 16982–17015. [Google Scholar] [CrossRef]
- Aldosari, B.N.; Alfagih, I.M.; Almurshedi, A.S. Lipid Nanoparticles as Delivery Systems for RNA-Based Vaccines. Pharmaceutics 2021, 13, 206. [Google Scholar] [CrossRef]
- Floudas, C.S.; Sarkizova, S.; Ceccarelli, M.; Zheng, W. Leveraging mRNA technology for antigen based immuno-oncology therapies. J. Immunother. Cancer 2025, 13, e010569. [Google Scholar] [CrossRef]
- Brandi, R.; Paganelli, A.; D’Amelio, R.; Giuliani, P.; Lista, F.; Salemi, S.; Paganelli, R. mRNA Vaccines Against COVID-19 as Trailblazers for Other Human Infectious Diseases. Vaccines 2024, 12, 1418. [Google Scholar] [CrossRef]
- de Mey, W.; Autaers, D.; Bertazzon, G.; Esprit, A.; Marco Aragon, M.; Franceschini, L.; Breckpot, K. Cancer Vaccines Based on mRNA: Hype or Hope? In Trends in mRNA Vaccine Research, 1st ed.; Szabo, G.T., Pardi, N., Eds.; WILEY-VCH GmbH: Weinheim, Berlin, 2025; pp. 337–400. [Google Scholar]
- Ahamad, S.K.; Laxmi, R.V.; Kumar, A.R.; Kumar, M.N.; Chakala, V.; Tadikonda, R.R. mRNA Vaccines: Transforming Disease Prevention for the Modern Era. Int. J. Health Sci. Res. 2024, 14, 505–515. [Google Scholar] [CrossRef]
- Shi, R.; Liu, X.; Wang, Y.; Pan, M.; Wang, S.; Shi, L.; Ni, B. Long-term stability and immunogenicity of lipid nanoparticle COVID-19 mRNA vaccine is affected by particle size. Hum. Vaccines Immunother. 2024, 20, 2342592. [Google Scholar] [CrossRef] [PubMed]
- Manolova, V.; Flace, A.; Bauer, M.; Schwarz, K.; Saudan, P.; Bachmann, M. Nanoparticles target distinct dendritic cell populations according to their size. Eur. J. Immunol. 2008, 38, 1404–1413. [Google Scholar] [CrossRef]
- Di, J.; Wu, K.; Hou, P.; Corpstein, C.D.; Xu, Y.; Li, T. Multiphysics-Informed Pharmacokinetic Modeling of Systemic Exposure of Intramuscularly Injected LNPs. Mol. Pharm. 2023, 20, 6162–6168. [Google Scholar] [CrossRef]
- Hardianto, A.; Muscifa, Z.S.; Widayat, W.; Yusuf, M.; Subroto, T. The Effect of Ethanol on Lipid Nanoparticle Stabilization from a Molecular Dynamics Simulation Perspective. Molecules 2023, 28, 4836. [Google Scholar] [CrossRef]
- Antognoli, M.; Stoecklein, D.; Galletti, C.; Brunazzi, E.; Di Carlo, D. Optimized design of obstacle sequences for micfrofluidic mixing in an inertial regime. Lab Chip 2021, 21, 3910–3923. [Google Scholar] [CrossRef] [PubMed]
- Maeki, M.; Saito, T.; Sato, Y.; Yasui, T.; Kaji, N.; Ishida, A.; Tani, H.; Baba, Y.; Harashima, H.; Tokeshi, M. A strategy for synthesis of lipid nanoparticles using microfluidic devices with a mixer structure. Lap Chip 2015, 5, 46181–46185. [Google Scholar] [CrossRef]
- Lee, B.; Kim, M.; Oh, S.; Lee, D.B.; Lee, S.-G.; Kim, H.M.; Kim, K.H.; Song, J.; Lee, C.-S. Characterization of passive microfluidic mixer with a three-dimensional zig-zag channel for cryo-EM sampling. Chem. Eng. Sci. 2023, 281, 119161. [Google Scholar] [CrossRef]
- Zhang, J.; Yan, S.; Sluyter, R.; Li, W.; Alici, G.; Nguyen, N.-T. Inertial particle separation by differential equilibrium positions in a symmetrical serpentine micro-channel. Sci. Rep. 2014, 4, 4527. [Google Scholar] [CrossRef]
- Ahn, G.-Y.; Choi, I.; Song, M.; Han, S.K.; Choi, K.; Ryu, Y.-H.; Oh, D.-H.; Kang, H.-W.; Choi, S.-W. Fabrication of Microfiber-Templated Microfluidic Chips with Microfibrous Channels for High Throughput and Continuous Production of Nanoscale Droplets. ACS Macro Lett. 2022, 11, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Juraeva, M.; Kang, D.-J. Mixing Performance of a Passive Micromixer Based on Split-to-Circulate (STC) Flow Characteristics. Micromachines 2024, 15, 773. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, S.J.; Warzecha, C.C.; Yadavali, S.; El-Mayta, R.; Alameh, M.-G.; Wang, L.; Weissman, D.; Wilson, J.M.; Issadore, D.; Mitchell, M.J. Scalable mRNA and siRNA Lipid Nanoparticle Production Using a Parallelized Microfluidic Device. Nano Lett. 2021, 21, 5671–5680. [Google Scholar] [CrossRef]
- Maeki, M.; Okada, Y.; Uno, S.; Sugiura, K.; Suzuki, Y.; Okuda, K.; Sato, Y.; Ando, M.; Yamazaki, H.; Takeuchi, M.; et al. Mass production system for RNA-loaded lipid nanoparticles using piling up microfluidic device. Appl. Mater. Today 2023, 31, 101754. [Google Scholar] [CrossRef]
- Dudala, S.; Rao, L.T.; Dubey, S.K.; Javed, A.; Goel, S. Experimental characterization to fabricate CO2 laser ablated PMMA microchannel with homogeneous surface. Mater. Today Proc. 2020, 28, 804–807. [Google Scholar] [CrossRef]
- Mohammed, M.I.; Quayle, K.; Alexander, R.; Doeven, E.; Nai, R.; Haswell, S.J.; Kouzani, A.Z.; Gibson, I. Improved manufacturing quality and bonding of laser machined microfluidic systems. Procedia Technol. 2015, 20, 219–224. [Google Scholar] [CrossRef]
- Vargas, M.J.; Nieuwoudt, M.; Yong, R.M.; Vanholsbeeck, F.; Williams, D.E.; Simpson, M.C. Excellent quality microchannels for rapid microdevice prototyping: Direct CO2 laser writing with efficient chemical postprocessing. Microfluid. Nanofluidics 2019, 23, 1–13. [Google Scholar] [CrossRef]
- Thomas, T.; Agrawalm, A. Designandfabrication of microfluidic devices: Acost-effective approach for high throughput production. J. Micromech. Microeng. 2023, 34, 015008. [Google Scholar] [CrossRef]
- Lawrence, J.; Li, L. Modification of the wettability characteristics of polymethyl methacrylate (PMMA) by means of CO2, Nd: YAG, excimer and high power diode laser radiation. Mater. Sci. Eng. A 2001, 303, 142–149. [Google Scholar] [CrossRef]
- Jensen, M.F.; Noerholm, M.; Christensen, L.H.; Geschke, O. Microstructure fabrication with a CO2 laser system: Characterization and fabrication of cavities produced by raster scanning of the laser beam. Lab Chip 2003, 3, 302–307. [Google Scholar] [CrossRef]
- Cheng, J.Y.; Wei, C.W.; Hsu, K.H.; Young, T.H. Direct-write laser micromachining and universal surface modification of PMMA for device development. Sens. Actuators B Chem. 2004, 99, 186–196. [Google Scholar] [CrossRef]
- Chuang, Y.F.; Wu, H.C.; Yang, F.; Yang, T.J.; Lee, S. Cracking and healing in poly(methyl methacrylate): Effect of solvent. J. Polym. Res. 2017, 24, 2. [Google Scholar] [CrossRef]
- Islam, M.M.; Loewen, A.; Allen, P.B. Simple, low-cost fabrication of acrylic based droplet microfluidics and its use to generate DNA-coated particles. Sci. Rep. 2018, 8, 8763. [Google Scholar] [CrossRef]
- Kim, E.S.; Cho, M.; Choi, I.; Choi, S.-W. Fabrication of Perfluoropolyether Microfluidic Devices Using Laser Engraving for Uniform Droplet Production. Micromachines 2024, 15, 599. [Google Scholar] [CrossRef]
- Nguyen, N.-T.; Wu, Z. Micromixers—A review. J. Micromechanics Microengineering 2005, 15, R1–R16. [Google Scholar] [CrossRef]
- Stroock, A.D.; Dertinger, S.K.W.; Ajdari, A.; Mezić, I.; Stone, H.A.; Whitesides, G.M. Chaotic Mixer for Microchannels. Science 2002, 295, 647–651. [Google Scholar] [CrossRef]
- Zhou, T.; Xu, Y.; Liu, Z.; Joo, S.W. An Enhanced One-Layer Passive Microfluidic Mixer with an Optimized Lateral Structure with the Dean Effect. J. Fluids Eng. 2015, 137, 091102. [Google Scholar] [CrossRef]
- Juraeva, M.; Kang, D.-J. Design and Mixing Analysis of a Passive Micromixer with Circulation Promoters. Micromachines 2024, 15, 831. [Google Scholar] [CrossRef] [PubMed]
| Repeating Unit | TFR (mL/h) | 30 | 60 | 90 | 120 | |
|---|---|---|---|---|---|---|
| Parameter | ||||||
| 45° | Z-average size (nm) | 136.1 ± 56.16 | 109.7 ± 32.99 | 95.34 ± 23.35 | 91.35 ± 16.14 | |
| PDI | 0.170 ± 0.008 | 0.090 ± 0.008 | 0.060 ± 0.011 | 0.035 ± 0.026 | ||
| 90° | Z-average size (nm) | 144.8 ± 65.22 | 162.9 ± 89.49 | 100.0 ± 27.18 | 76.34 ± 21.47 | |
| PDI | 0.203 ± 0.006 | 0.302 ± 0.031 | 0.074 ± 0.003 | 0.079 ± 0.005 | ||
| 180° | Z-average size (nm) | 190.5 ± 119.4 | 121.1 ± 42.17 | 160.8 ± 77.61 | 64.72 ± 19.34 | |
| PDI | 0.393 ± 0.024 | 0.122 ± 0.012 | 0.233 ± 0.011 | 0.090 ± 0.013 | ||
| 270° | Z-average size (nm) | 164.2 ± 83.22 | 107.1 ± 32.37 | 87.82 ± 27.52 | 72.82 ± 24.14 | |
| PDI | 0.257 ± 0.023 | 0.092 ± 0.010 | 0.098 ± 0.008 | 0.111 ± 0.011 | ||
| Types of Chips | TFR (mL/h) | 30 | 60 | 90 | 120 | |
|---|---|---|---|---|---|---|
| Parameter | ||||||
| SMC | Z-average size (nm) | 164.2 ± 83.22 | 107.1 ± 32.37 | 87.82 ± 27.52 | 72.82 ± 24.14 | |
| PDI | 0.257 ± 0.023 | 0.092 ± 0.010 | 0.098 ± 0.008 | 0.111 ± 0.011 | ||
| MSMC4 | Z-average size (nm) | 200.2 ± 124.3 | 136.2 ± 58.28 | 113.5 ± 38.98 | 86.22 ± 31.43 | |
| PDI | 0.386 ± 0.023 | 0.184 ± 0.008 | 0.118 ± 0.010 | 0.133 ± 0.008 | ||
| MSMC8 | Z-average size (nm) | 303.5 ± 213.3 | 130.1 ± 44.85 | 88.77 ± 30.16 | 64.55 ± 28.05 | |
| PDI | 0.494 ± 0.014 | 0.119 ± 0.008 | 0.116 ± 0.008 | 0.189 ± 0.011 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, I.; Cho, M.; Song, M.; Ryu, B.W.; Kang, B.M.; Kim, J.; Ryu, T.-K.; Choi, S.-W. Fabrication of a Spiral Microfluidic Chip for the Mass Production of Lipid Nanoparticles Using Laser Engraving. Micromachines 2025, 16, 501. https://doi.org/10.3390/mi16050501
Choi I, Cho M, Song M, Ryu BW, Kang BM, Kim J, Ryu T-K, Choi S-W. Fabrication of a Spiral Microfluidic Chip for the Mass Production of Lipid Nanoparticles Using Laser Engraving. Micromachines. 2025; 16(5):501. https://doi.org/10.3390/mi16050501
Chicago/Turabian StyleChoi, Inseong, Mincheol Cho, Minseo Song, Byeong Wook Ryu, Bo Mi Kang, Joonyeong Kim, Tae-Kyung Ryu, and Sung-Wook Choi. 2025. "Fabrication of a Spiral Microfluidic Chip for the Mass Production of Lipid Nanoparticles Using Laser Engraving" Micromachines 16, no. 5: 501. https://doi.org/10.3390/mi16050501
APA StyleChoi, I., Cho, M., Song, M., Ryu, B. W., Kang, B. M., Kim, J., Ryu, T.-K., & Choi, S.-W. (2025). Fabrication of a Spiral Microfluidic Chip for the Mass Production of Lipid Nanoparticles Using Laser Engraving. Micromachines, 16(5), 501. https://doi.org/10.3390/mi16050501






