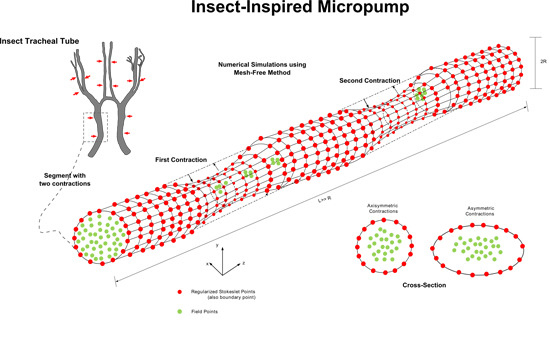
Insect-Inspired Micropump: Flow in a Tube with Local Contractions
Abstract
:1. Introduction
2. Methods
3. Results and Discussion

3.1. Wall Profile, R(z,t)
3.2. Algorithm for Finding Accurate Stokeslets Strengths
| Algorithm 1 Algorithm for finding the strengths of each Stokeslet-source point. |
| Input: , b, h, |
| 1. = 0, =b - =b |
| 2. Do while |
| 3. |
| 4. = + |
| 5. |
| end Do |
| Output: Stokeslets strengths: , |
3.3. Tube with Two Local Rhythmic Wall Contractions



3.4. Unidirectional Net Flow

3.5. Channel vs. Tube Simulations
4. Conclusions
Conflicts of Interest
References
- Aboelkassem, Y.; Staples, A.E. Flow transport in a microchannel induced by moving wall contractions: A novel micropumping mechanism. Acta Mech. 2012, 223, 463–480. [Google Scholar] [CrossRef]
- Aboelkassem, Y.; Staples, A.E. Stokeslets-mesh-free computations and theory for flow in a collapsible microchannel. Theor. Comput. Fluid Dyn. 2012, 27, 681–700. [Google Scholar] [CrossRef]
- Aboelkassem, Y.; Staples, A.E.; Socha, J. Microscale flow pumping inspired by rhythmic tracheal compressions in insects. In Proceedings of the ASME Pressure Vessels and Piping Conference, Baltimore, Maryland, USA, 17–21 July 2011; Volume 4, pp. 471–479.
- Aboelkassem, Y. Novel Bioinspired Pumping Models for Microscale Flow Transport. PhD. Thesis, Virginia Tech, Blacksburg, VA, USA, 2012. [Google Scholar]
- Aboelkassem, Y.; Staples, A.E. Selective pumping in a network: Insect-style microscale flow transport. Bioinspir. Biomim. 2013, 8, 026004. [Google Scholar] [CrossRef] [PubMed]
- Aboelkassem, Y.; Staples, A.E. A bioinspired pumping model for flow in a microtube with rhythmic wall contractions. J. Fluids Struct. 2013, 42, 187–204. [Google Scholar] [CrossRef]
- Aboelkassem, Y.; Staples, A.E. A three-dimensional model for flow pumping in a microchannel inspired by insect respiration. Acta Mech. 2014, 225, 493–507. [Google Scholar] [CrossRef]
- Aboelkassem, Y. A Ghost-Valve micropumping paradigm for biomedical applications. Biomed. Eng. Lett. 2015, 5, 45–50. [Google Scholar] [CrossRef]
- Chu, A.K.H. Transport Control within a Microtube. Phys. Rev. E 2004, 70, 061902. [Google Scholar] [CrossRef]
- Uchida, S.; Aoki, H. Unsteady Flows in a Semi-infinite Contracting or Expanding Pipe. J. Fluid Mech. 1977, 82, 371–387. [Google Scholar] [CrossRef]
- Secomb, T. Flow in a channel with Pulsating Walls. J. Fluid Mech. 1978, 88, 273–288. [Google Scholar] [CrossRef]
- Skalak, F.; Wang, C.Y. On the Unsteady Squeezing of a Viscous Fluid from a Tube. J. Austral. Math. Soc. 1978, 21, 65–74. [Google Scholar] [CrossRef]
- Wang, C.Y. Arbitrary Squeezing of Fluid from a Tube at Low Squeeze Numbers. J. Appl. Math. Phys. (ZAMP) 1980, 31, 620–627. [Google Scholar] [CrossRef]
- Singh, P.; Radhakrishnan, V.; Narayan, K.A. Squeezing Flow Between Parallel Plates. Ingenieur-Archiv 1990, 60, 274–281. [Google Scholar] [CrossRef]
- Pedley, T.; Stephanoff, K.D. Flow along Channel with a Time-Dependent Indentation in One Wall: The Generation of Vorticity Waves. J. Fluid Mech. 1985, 160, 337–367. [Google Scholar] [CrossRef]
- Ralph, M.; Pedley, T.J. Flow in a Channel with Moving Indentation. J. Fluid Mech. 1988, 190, 87–112. [Google Scholar] [CrossRef]
- Mahmood, T.; Merkin, J. The flow in a narrow duct with an indentation or hump on one wall. Warme-und Stoffubertrangung 1990, 22, 69–76. [Google Scholar] [CrossRef]
- Aranda, V.; Cortez, R.; Fauci, L. Stokesian peristaltic pumping in a three-dimensional tube with a phase-shift asymmetry. Phys. Fluids 2011, 23, 081901. [Google Scholar] [CrossRef]
- Macagno, E.; Christensen, J. Fluid mechanics of the duodenum. Ann. Rev. Fluid Mech. 1980, 12, 139–158. [Google Scholar] [CrossRef]
- Macagno, E.; Christensen, J.; Lee, L. Modeling the effect of wall movement on absorption in the intestine. Am. J. Physiol. 1982, 243, G541–G550. [Google Scholar] [PubMed]
- Cortez, R. The method of regularized Stokeslets. SIAM J. Sci. Comput. 2001, 23, 1204–1225. [Google Scholar] [CrossRef]
- Cortez, R.; Fauci, L.; Medovikov, A. The method of regularized Stokeslets in three dimensions: analysis, validation, and applications to helical swimming. Phys. Fluids 2005, 17, 031504. [Google Scholar] [CrossRef]
- Ainley, J.; Durkin, S.; Embid, R.; Boindala, P.; Cortez, R. The method of images for regularized Stokeslets. J. Comput. Phys. 2008, 227, 4600–4616. [Google Scholar] [CrossRef]
- Young, D.L.; Jane, S.J.; Fan, C.M.; Murugesan, K.; Tsai, C.C. The method of fundamental solutions for 2D and 3D Stokes problems. J. Comput. Phys. 2006, 211, 1–8. [Google Scholar] [CrossRef]
- Alves, C.; Silvestre, A. Density results using Stokeslets and a method of fundamental solutions for the Stokes equations. Eng. Anal. Bound. Elem. 2004, 28, 1245–1252. [Google Scholar] [CrossRef]
- Young, D.L.; Chen, C.; Fan, C.M.; Murugesan, K.; Tsai, C.C. The method of fundamental solutions for Stokes flow in a rectangular cavity with cylinders. J. Mech. B/Fluids 2005, 24, 703–716. [Google Scholar] [CrossRef]
- Neumaier, A. Solving Ill-Conditioned and Singular Linear Systems : A Tutorial on Regularization. SIAM Rev. 1998, 40, 636–666. [Google Scholar] [CrossRef]
© 2015 by the author; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aboelkassem, Y. Insect-Inspired Micropump: Flow in a Tube with Local Contractions. Micromachines 2015, 6, 1143-1156. https://doi.org/10.3390/mi6081143
Aboelkassem Y. Insect-Inspired Micropump: Flow in a Tube with Local Contractions. Micromachines. 2015; 6(8):1143-1156. https://doi.org/10.3390/mi6081143
Chicago/Turabian StyleAboelkassem, Yasser. 2015. "Insect-Inspired Micropump: Flow in a Tube with Local Contractions" Micromachines 6, no. 8: 1143-1156. https://doi.org/10.3390/mi6081143