Micro-Machined Flow Sensors Mimicking Lateral Line Canal Neuromasts
Abstract
:1. Introduction
1.1. The Fish Lateral Line: A Biological Blueprint for Sensor Development

1.2. Biomimetic Flow Sensors: State of the Art
2. Experimental Section
2.1. Biomimetic Transfer: Technical Sensors Based on Canal Neuromasts


2.2. Cross-Correlation Flow Metering: Estimated Bulk Flow Rates from Flow Unsteadiness
2.3. Separation of Lateral Line Sensors by Means of Membranes
3. Results and Discussion
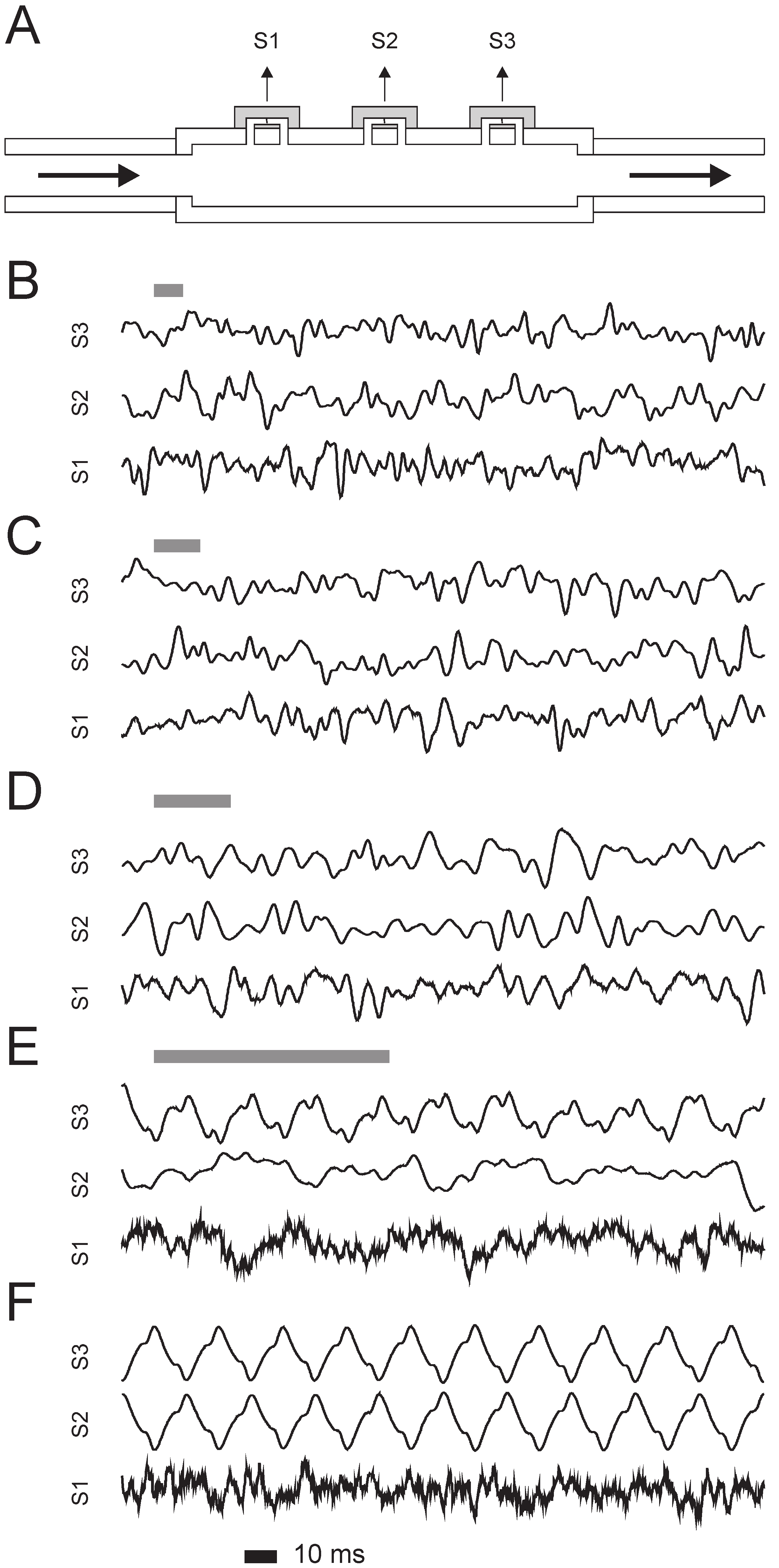
3.1. Proof of Principle: Flow Detection by Cross-Correlation Using an Eight-Fold Sensor Array



3.2. Industrial Application: Flow Measurements in Tap Water Systems


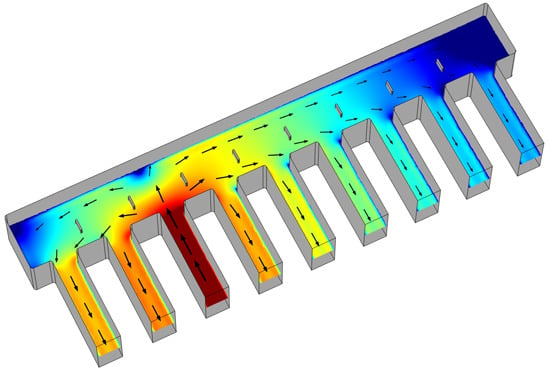
3.3. Influence of Canal Properties on Flow Metering: A FEM Study

3.4. A Segregated Artificial Lateral Line: Medical and Pharmaceutical Applications

4. Conclusions
Acknowledgments
Author Contributions
Supplementary Materials
Conflicts of Interest
References
- Dijkgraaf, S. The functioning and significance of the lateral-line organs. Biol. Rev. 1962, 38, 51–105. [Google Scholar] [CrossRef]
- Coombs, S. Smart Skins: Information Processing by Lateral Line Flow Sensors. Autono. Robot. 2001, 11, 255–261. [Google Scholar] [CrossRef]
- Coombs, S.; New, J.G.; Nelson, M. Information-processing demands in electrosensory and mechanosensory lateral line systems. J. Physiol. Paris 2002, 96, 341–354. [Google Scholar] [CrossRef]
- Bleckmann, H. Peripheral and central processing of lateral line information. J. Comp. Physiol. A 2008, 194, 145–158. [Google Scholar] [CrossRef] [PubMed]
- Bleckmann, H.; Klein, A.; Meyer, G. Nature as a model for technical sensors. In Frontiers in Sensing; Springer: Wien, Austria, 2012; pp. 3–18. [Google Scholar]
- Montgomery, J.; Coombs, S.; Halstead, M. Biology of the mechanosensory lateral line in fishes. Rev. Fish Biol. Fish. 1995, 5, 399–416. [Google Scholar] [CrossRef]
- Montgomery, J.C.; McDonald, F.; Baker, C.F.; Carton, A.G.; Ling, N. Sensory integration in the hydrodynamic world of rainbow trout. Proc. R. Soc. Lond. Ser. B 2003, 270, 195–197. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, A.; Bleckmann, H.; Mogdans, J. Organization of the Superficial Neuromast System in Goldfish, Carassius auratus. J. Morphol. 2008, 269, 751–761. [Google Scholar] [CrossRef] [PubMed]
- Dijkgraaf, S. Untersuchungen über die Funktion der Seitenlinienorgane an Fischen. Z. Physiol. 1933, 20, 162–214. (In German) [Google Scholar]
- Flock, Å. Electronmicroscopic and electrophysiological studies on the lateral line canal organ. Acta Otolaryngol. 1965, 199, 1–90. [Google Scholar]
- Hudspeth, A.J.; Corey, D.P. Sensitivity, polarity, and conductance change in the response of vertebrate hair cells to controlled mechanical stimuli. Proc. Natl. Acad. Sci. USA 1977, 74, 2407–2411. [Google Scholar] [CrossRef] [PubMed]
- Hudspeth, A.J. Mechanoelectrical transduction by hair cells in the acousticolateralis sensory system. Ann. Rev. Neurosci. 1983, 6, 187–215. [Google Scholar] [CrossRef] [PubMed]
- Kroese, A.; van Netten, S. Sensory Transduction in lateral line hair cells. In The Mechanosensory Lateral Line: Neurobiology and Evolution; Coombs, S., Görner, P., Münz, H., Eds.; Springer: New York, NY, USA, 1989; pp. 265–284. [Google Scholar]
- Yamada, Y.; Hama, K. Fine Structure of the Lateral-Line Organ of the Common Eel, Anguilla japonica. Z. Zellforsch. 1972, 124, 454–464. [Google Scholar] [CrossRef] [PubMed]
- Hama, K.; Yamada, Y. Fine Structure of the Ordinary Lateral Line Organ II. The Lateral Line Canal Organ of Spotted Shark, Mustelus manazo. Cell Tissue Res. 1977, 176, 23–36. [Google Scholar] [PubMed]
- Hama, K.M. Some observations on the fine structure of the lateral line organ of the Japanese sea eel Lyncozymba nystromi. J. Cell Biol. 1965, 24, 193–210. [Google Scholar] [CrossRef] [PubMed]
- Görner, P. Untersuchungen zur Morphologie und Elektrophysiologie des Seitenlinienogans vom Krallenfrosch (Xenopus Laevis Daudin). Z. Vergleichende Physiol. 1963, 47, 316–338. (In German) [Google Scholar] [CrossRef]
- Bauknight, R.; Strelioff, D.; Honrubia, V. Effective stimulus for the Xenopus laevis lateral-line hair-cell system. Laryngoscope 1976, 86, 1836–1844. [Google Scholar] [CrossRef] [PubMed]
- Faucherre, A.; Pujol-Martí, J.; Kawakami, K.; López-Schier, H. Afferent Neurons of the Zebrafish Lateral Line Are Strict Selectors of Hair-Cell Orientation. PLoS ONE 2009, 4, e4477. [Google Scholar] [CrossRef] [PubMed]
- Coombs, S.; Janssen, J.; Webb, J. Diversity of lateral line systems: Evolutionary and functional considerations. In Sensory Biology of Aquatic Animals.; Atema, J., Fay, R.R., Popper, A.N., Tavolga, W.N., Eds.; Springer: New York, NY, USA, 1988; pp. 553–593. [Google Scholar]
- Kroese, A.B.A.; Schellart, N.A.M. Velocity- and Acceleration-Sensitive Units in the Trunk Lateral Line of the Trout. J. Neurophysiol. 1992, 68, 2212–2221. [Google Scholar] [PubMed]
- Kalmijn, A. Hydrodynamic and acoustic field detection. In Sensory Biology of Aquatic Animals; Springer: New York, NY, USA, 1988; pp. 151–186. [Google Scholar]
- Coombs, S.; Montgomery, J.C. The enigmatic lateral line system. In Comparative Hearing: Fish and Amphibians; Springer: New York, NY, USA, 1999; pp. 319–362. [Google Scholar]
- Chagnaud, B.P.; Bleckmann, H.; Hofmann, M.H. Lateral line nerve fibers do not code bulk water flow direction in turbulent flow. Zoology 2008, 111, 204–217. [Google Scholar] [CrossRef] [PubMed]
- Chagnaud, B.P.; Brücker, C.; Hofmann, M.H.; Bleckmann, H. Measuring Flow Velocity and Flow Direction by Spatial and Temporal Analysis of Flow Fluctuations. J. Neurosci. 2008, 28, 4479–4487. [Google Scholar] [CrossRef] [PubMed]
- Voth, G.A.; La Porta, A.; Crawford, A.M.; Alexander, J.I.M.; Bodenschatz, E. Measurement of particle accelerations in fully developed turbulence. J. Fluid Mech. 2002, 469, 121–160. [Google Scholar] [CrossRef]
- Barth, S.; Koch, H.; Kittel, A.; Peinke, J.; Burgold, J.; Wurmus, H. Laser-cantilever anemometer: A new high-resolution sensor for air and liquid flows. Rev. Sci. Instrum. 2005, 76, 075110. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, J.; Engel, J.; Pandya, S.; Chen, N.; Tucker, C.; Coombs, S.; Jones, D.L.; Liu, C. Distant touch hydrodynamic imaging with an artificial lateral line. Proc. Nal. Acad. Sci. USA 2006, 103, 18891–18895. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Shen, Y.; Xi, N.; Tan, X. Integrated sensing for ionic polymer-metal composite actuators using PVDF thin films. Smart Mater. Struct. 2007, 16, 262–271. [Google Scholar] [CrossRef]
- Toschi, F.; Bodenschatz, E. Lagrangian Properties of Particles in Turbulence. Annual Rev. Fluid Mech. 2009, 41, 375–404. [Google Scholar] [CrossRef]
- Zhang, Y.; Tadigadapa, S.; Najafi, N. A micromachined Coriolis-force-based mass flowmeter for direct mass flow and fluid density measurements. Transducers 2001, 1, 1460. [Google Scholar]
- Mogdans, J.; Engelmann, J.; Hanke, W.; Kröther, S. The Fish Lateral Line: How to Detect Hydrodynamic Stimuli. In Sensors and Sensing in Biology and Engineering; Springer: New York, NY, USA, 2003; pp. 173–185. [Google Scholar]
- Colgate, J.E.; Lynch, K.M. Mechanics and Control of Swimming: A Review. IEEE J. Ocean. Eng. 2004, 29, 660–673. [Google Scholar] [CrossRef]
- Yang, Y.; Nguyen, N.; Chen, N.; Lockwood, M.; Tucker, C.; Hu, H.; Bleckmann, H.; Liu, C.; Jones, D.L. Artificial lateral line with biomimetic neuromasts to emulate fish sensing. Bioinspir. Biomim. 2010, 5, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Bogue, R. Inspired by nature: Developments in biomimetic sensors. Sens. Rev. 2009, 29, 107–111. [Google Scholar]
- Goulet, J.; Engelmann, J.; Chagnaud, B.P.; Franosch, J.M.P.; Suttner, M.D.; van Hemmen, J.L. Object localization through the lateral line system of fish: theory and experiment. J. Comp. Physiol. A 2008, 194, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Yan, G.H.; Chen, Z.F.; Sun, J.C. Using a linear array to estimate the velocity of underwater moving targets. J. Mar. Sci. Appl. 2009, 8, 343–347. [Google Scholar] [CrossRef]
- Asadnia, M.; Kottapalli, A.G.P.; Shen, Z.; Miao, J.; Triantafyllou, M. Flexible and surface-mountable piezoelectric sensor arrays for underwater sensing in marine vehicles. IEEE Sens. J. 2013, 13, 3918–3925. [Google Scholar] [CrossRef]
- Peleshanko, S.; Julian, M.D.; Ornatska, M.; McConney, M.E.; LeMieux, M.C.; Chen, N.; Tucker, C.; Yang, Y.; Liu, C.; Humphrey, J.A.C.; et al. Hydrogel-Encapsulated Microfabricated Haircells Mimicking Fish Cupula Neuromast. Adv. Mater. 2007, 19, 2903–2909. [Google Scholar] [CrossRef]
- Liu, C. Micromachined biomimetic artificial haircell sensors. Bioinspir. Biomim. 2007, 2, S162–S169. [Google Scholar] [CrossRef] [PubMed]
- McConney, M.E.; Chen, N.; Lu, D.; Hu, H.A.; Coombs, S.; Liu, C.; Tsukruk, V.V. Biologically inspired design of hydrogel-capped hair sensors for enhanced underwater flow detection. Soft Matter 2009, 5, 292–295. [Google Scholar] [CrossRef]
- Su, Y.; Evans, A.G.R.; Brunnschweiler, A.; Ensell, G. Characterization of a highly sensitive ultra-thin piezoresistive silicon cantilever probe and its application in gas flow velocity sensing. J. Micromech. Microeng. 2002, 12, 780–785. [Google Scholar] [CrossRef]
- Chen, N.; Tucker, C.; Engel, J.M.; Yang, Y.; Pandya, S.; Liu, C. Design and Characterization of Artificial Haircell Sensor for Flow Sensing with Ultrahigh Velocity and Angular Sensitivity. J. Microelectromech. Syst. 2007, 16, 999–1014. [Google Scholar] [CrossRef]
- Dijkstra, M.; van Baar, J.J.; Wiegerink, R.J.; Lammerink, T.S.J.; de Boer, J.H.; Krijnen, G.J.M. Artificial sensory hairs based on the flow sensitive receptor hairs of crickets. J. Micromech. Microeng. 2005, 15, 132–138. [Google Scholar] [CrossRef]
- Krijnen, G.J.M.; Dijkstra, M.; van Baar, J.J.; Shankar, S.S.; Kuipers, W.J.; de Boer, R.J.H.; Altpeter, D.; Lammerink, T.S.J.; Wiegerink, R. MEMS based hair flow-sensors as model systems for acoustic perception studies. Nanotechnology 2006, 17, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Izadi, N.; de Boer, M.; Berenschot, J.; Wiegerink, R.; Lammerink, T.; Jansen, H.; Mogdans, J.; Krijnen, G. Fabrication of dense flow sensor arrays on flexible membranes. In Proceedings of the International Solid-State Sensors, Actuators and Microsystems Conference, Denver, CO, USA, 21–25 June 2009; pp. 1075–1078.
- Abdulsadda, A.T.; Tan, X. An artificial lateral line system using IPMC sensor arrays. Int. J. Smart Nano Mater. 2012, 3, 226–242. [Google Scholar] [CrossRef]
- Große, S.; Schröder, W.; Brücker, C. Nano-newton drag sensor based on flexible micro-pillars. Meas. Sci. Technol. 2006, 17, 2689–2697. [Google Scholar] [CrossRef]
- Brücker, C.; Bauer, D.; Chaves, H. Dynamic response of micro-pillar sensors measuring fluctuating wall-shear-stress. Exp. Fluids 2007, 42, 737–749. [Google Scholar] [CrossRef]
- Lien, V.; Vollmer, F. Microfluidic flow rate detection based on integrated optical fiber cantilever. Lab Chip 2007, 7, 1352–1356. [Google Scholar] [CrossRef] [PubMed]
- Klein, A.T.; Bleckmann, H. Determination of object position, vortex shedding frequency and flow velocity using artificial lateral line canals. Beilstein J. Nanotechnol. 2011, 2, 276–283. [Google Scholar] [CrossRef] [PubMed]
- McConney, M.E.; Anderson, K.D.; Brott, L.L.; Naik, R.R.; Tsukruk, V.V. Bioinspired Material Approaches to Sensing. Adv. Funct. Mater. 2009, 19, 2527–2544. [Google Scholar] [CrossRef]
- Fan, Z.; Chen, J.; Zou, J.; Bullen, D.; Liu, C.; Delcomyn, F. Design and fabrication of artificial lateral line flow sensors. J. Micromech. Microeng. 2002, 12, 655–661. [Google Scholar] [CrossRef]
- Qualtieri, A.; Rizzi, F.; Todaro, M.; Passaseo, A.; Cingolani, R.; Vittorio, M.D. Stress-driven AlN cantilever-based flow sensor for fish lateral line system. Microelectron. Eng. 2011, 88, 2376–2378. [Google Scholar] [CrossRef]
- Qualtieri, A.; Rizzi, F.; Epifani, G.; Ernits, A.; Kruusmaa, M.; Vittorio, M.D. Parylene-coated bioinspired artificial hair cell for liquid flow sensing. Microelectron. Eng. 2012, 98, 516–519. [Google Scholar] [CrossRef]
- Tang, L.; Zhang, K.; Chen, S.; Zhang, G.; Liu, G. MEMS inclinometer based on a novel piezoresistor structure. Microelectron. J. 2009, 40, 78–82. [Google Scholar] [CrossRef]
- Xue, C.; Chen, S.; Zhang, W.; Zhang, B.; Zhang, G.; Qiao, H. Design, fabrication, and preliminary characterization of a novel MEMS bionic vector hydrophone. Microelectron. J. 2007, 38, 1021–1026. [Google Scholar] [CrossRef]
- Zhang, G.; Wang, P.; Guan, L.; Xiong, J.; Zhang, W. Improvement of the MEMS bionic vector hydrophone. Microelectron. J. 2011, 42, 815–819. [Google Scholar] [CrossRef]
- Fernandez, V.I.; Hou, S.M.; Hover, F.S.; Lang, J.H.; Triantafyllou, M.S. Lateral-Line-Inspired MEMS-Array Pressure Sensing for Passive Underwater Navigation; Sea Grant College Program; Massachusetts Institiute of Technology: Cambridge, MA, USA, 2007. [Google Scholar]
- Ozaki, Y.; Yasuda, T.; Shimoyama, I. An Air flow Sensor Modeled on Wind Receptor Hairs of Insects. In Proceedings of the 13th Annual International Conference on Micro Electro Mechanical Systems (MEMS 2000), Miyazaki, Japan, 23–27 January 2000; pp. 531–536.
- Krijnen, G.J.M.; Lammerink, T.; Wiegerink, R.; Casas, J. Cricket Inspired Flow-Sensor Arrays. In Proceedings of 2007 IEEE on Sensors, Atlanta, GA, USA, 28-31 October 2007; pp. 539–546.
- Izadi, N.; de Boer, M.; Berenschot, J.; Krijnen, G. Fabrication of superficial neuromast inspired capacitive flow sensors. J. Micromechan. Microeng. 2010, 20, 085041. [Google Scholar] [CrossRef]
- Abdulsadda, A.T.; Tan, X. Nonlinear estimation-based dipole source localization for artificial lateral line systems. Bioinspir. Biomim. 2013, 8, 2005–2019. [Google Scholar] [CrossRef] [PubMed]
- Abdulsadda, A.T.; Tan, X. Underwater tracking of a moving dipole source using an artificial lateral line: Algorithm and experimental validation with ionic polymer-metal composite flow sensors. Smart Mater. Struct. 2013, 22, 045010. [Google Scholar] [CrossRef]
- Ganley, T.; Hung, D.L.S.; Zhu, G.; Tan, X. Modeling and Inverse Compensation of Temperature-Dependent Ionic Polymer-Metal Composite Sensor Dynamics. IEEE/ASME Trans. Mechatron. 2011, 16, 80–89. [Google Scholar] [CrossRef]
- Lei, H.; Lim, C.; Tan, X. Modeling and inverse compensation of dynamics of base-excited ionic polymer-metal composite sensors. J. Intell. Mater. Syst. Struct. 2013, 24, 1557–1571. [Google Scholar] [CrossRef]
- Klein, A.T.; Herzog, H.; Bleckmann, H. Lateral line canal morphology and signal to noise ratio. In Proceedings of the SPIE Smart Structures and Materials+ Nondestructive Evaluation and Health Monitoring, San Diego, CA, USA, 6 March 2011.
- Klein, A.T.; Münz, H.; Bleckmann, H. The functional significance of lateral line canal morphology on the trunk of the marine teleost Xiphister atropurpureus (Stichaeidae). J. Comp. Physiol. A 2013, 199, 735–749. [Google Scholar] [CrossRef] [PubMed]
- Herzog, H.; Klein, A.T.; Bleckmann, H.; Holik, P.; Schmitz, S.; Siebke, G.; Tätzner, S.; Lacher, M.; Steltenkamp, S. μ-biomimetic flow sensors—Introducing light-guiding PDMS structures into MEMS. Bioinspir. Biomim. 2015, 10, 036001. [Google Scholar] [CrossRef] [PubMed]
- Barbier, C.; Humphrey, J.A. Drag force acting on a neuromast in the fish lateral line trunk canal. I. Numerical modelling of external -internal flow coupling. J. R. Soc. Interface 2009, 6, 627–640. [Google Scholar] [PubMed]
- Humphrey, J.A. Drag force acting on a neuromast in the fish lateral line trunk canal. II. Analytical modelling of parameter dependencies. J. R. Soc. Interface 2009, 6, 641–653. [Google Scholar] [CrossRef] [PubMed]
- Denton, E.J.; Gray, J. Mechanical Factors in the Excitation of Clupeid Lateral Lines. Proc. R. Soc. Lond. Ser. B 1983, 218, 1–26. [Google Scholar] [CrossRef]
- Denton, E.; Gray, J. Some Observations on the Forces Acting on Neuromasts in Fish Lateral Line Canals. In The Mechanosensory Lateral Line: Neurobiology and Evolution; Coombs, S., Görner, P., Münz, H., Eds.; Springer: New York, NY, USA, 1989; pp. 229–246. [Google Scholar]
- Sheen, S.; Raptis, A. Acoustic Cross-Correlation Flowmeter for Solid-Gas Flow. U.S. Patent 4,598,593, 8 July 1986. [Google Scholar]
- Amemiya, S. Correlation Detection Type Ultrasound Blood Flowmeter. U.S. Patent 4,693,319, 15 September 1987. [Google Scholar]
- Inada, Y.; Sugimoto, K. Cross Correlation Flowmeter. U.S. Patent 4,841,780, 27 June 1989. [Google Scholar]
- Margit, B.; Joerg, R.; Frank, S. Ultrasound Flowmeter Which Functions according to the Delay Correlation Method. DE Patent 19,815,199, 14 October 1999. [Google Scholar]
- Veneruso, A.; Huang, S. Cross Correlation Fluid Flow Meter. U.S. Patent 5,948,995, 7 September 1999. [Google Scholar]
- Jakkula, P.; Luostarinen, K.; Tahkola, E. Method of Measuring Flow, and Flow Meter. U.S. Patent 6,009,760, 29 March 2000. [Google Scholar]
- Fukuhara, S. Ultraschall-Strömungsmesser—Ultrasonic Flow Meter. DE Patent 10,206,134, 29 August 2002. [Google Scholar]
- Ramamurthy, V.; Dabak, A. Flow Meter. CN Patent 103542901A, 9 January 2014. [Google Scholar]
- Venturelli, R.; Akanyeti, O.; Visentin, F.; Ježov, J.; Chambers, L.D.; Toming, G.; Brown, J.; Kruusmaa, M.; Megill, W.M.; Fiorini, P. Hydrodynamic pressure sensing with an artificial lateral line in steady and unsteady flows. Bioinspir. Biomim. 2012, 7, 036004–036016. [Google Scholar] [CrossRef] [PubMed]
- Kaldenbach, F.; (University of Bonn, Bonn, Germany). Personal communication, 2015.
- Ren, Z.; Mohseni, K. A model of the lateral line of fish for vortex sensing. Bioinspir. Biomim. 2012, 7, 036016. [Google Scholar] [CrossRef] [PubMed]
- Klein, A.; Bleckmann, H. Function of lateral line canal morphology. Integr. Zool. 2015, 10, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Casas, J.; Stein, T.; Krijnen, G.J.M. Why do insects have such a high density of flow-sensing hairs? Insights from the hydromechanics of biomimetic MEMS sensors. J. R. Soc. Interface 2010, 7, 1487–1495. [Google Scholar] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herzog, H.; Steltenkamp, S.; Klein, A.; Tätzner, S.; Schulze, E.; Bleckmann, H. Micro-Machined Flow Sensors Mimicking Lateral Line Canal Neuromasts. Micromachines 2015, 6, 1189-1212. https://doi.org/10.3390/mi6081189
Herzog H, Steltenkamp S, Klein A, Tätzner S, Schulze E, Bleckmann H. Micro-Machined Flow Sensors Mimicking Lateral Line Canal Neuromasts. Micromachines. 2015; 6(8):1189-1212. https://doi.org/10.3390/mi6081189
Chicago/Turabian StyleHerzog, Hendrik, Siegfried Steltenkamp, Adrian Klein, Simon Tätzner, Elisabeth Schulze, and Horst Bleckmann. 2015. "Micro-Machined Flow Sensors Mimicking Lateral Line Canal Neuromasts" Micromachines 6, no. 8: 1189-1212. https://doi.org/10.3390/mi6081189
APA StyleHerzog, H., Steltenkamp, S., Klein, A., Tätzner, S., Schulze, E., & Bleckmann, H. (2015). Micro-Machined Flow Sensors Mimicking Lateral Line Canal Neuromasts. Micromachines, 6(8), 1189-1212. https://doi.org/10.3390/mi6081189