CD-Based Microfluidics for Primary Care in Extreme Point-of-Care Settings
Abstract
1. The Need for Extreme Point-of-Care
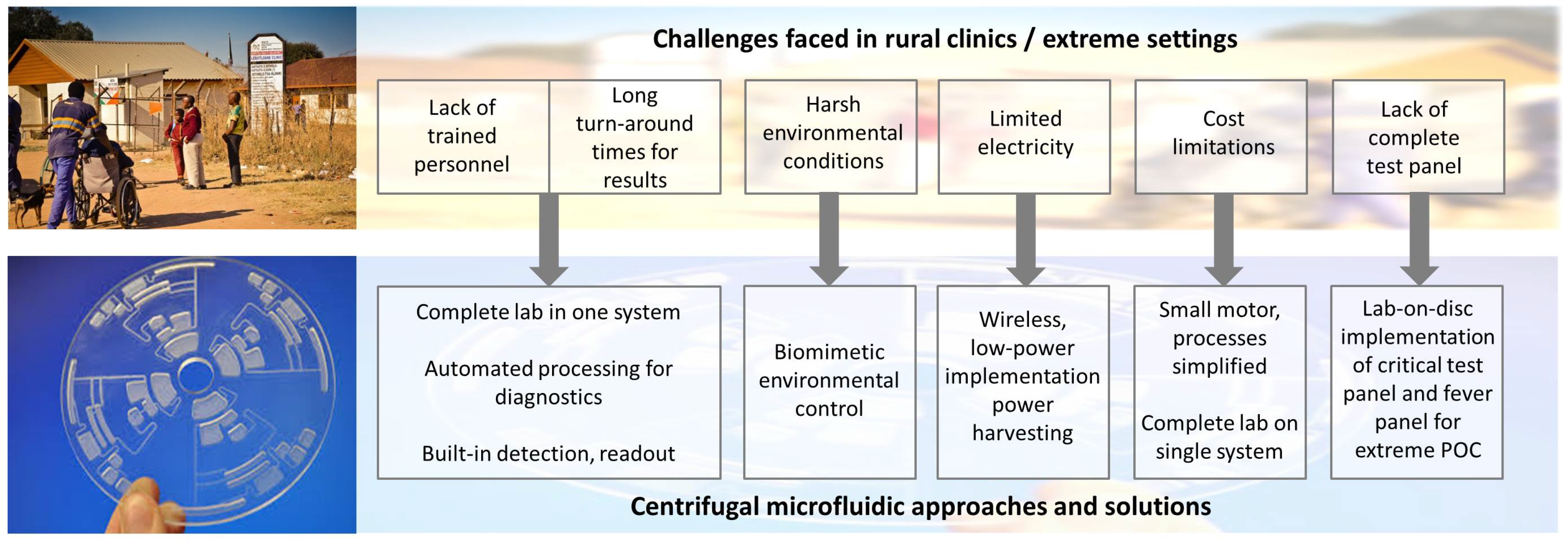
2. Fluidic Approaches
3. Introduction to CD-Based Microfluidics
3.1. Theory of Operation

3.2. Recent Advances in CD Fluidics
3.2.1. Mixing

3.2.2. Valving and Timing Control



3.2.3. Cell Handling
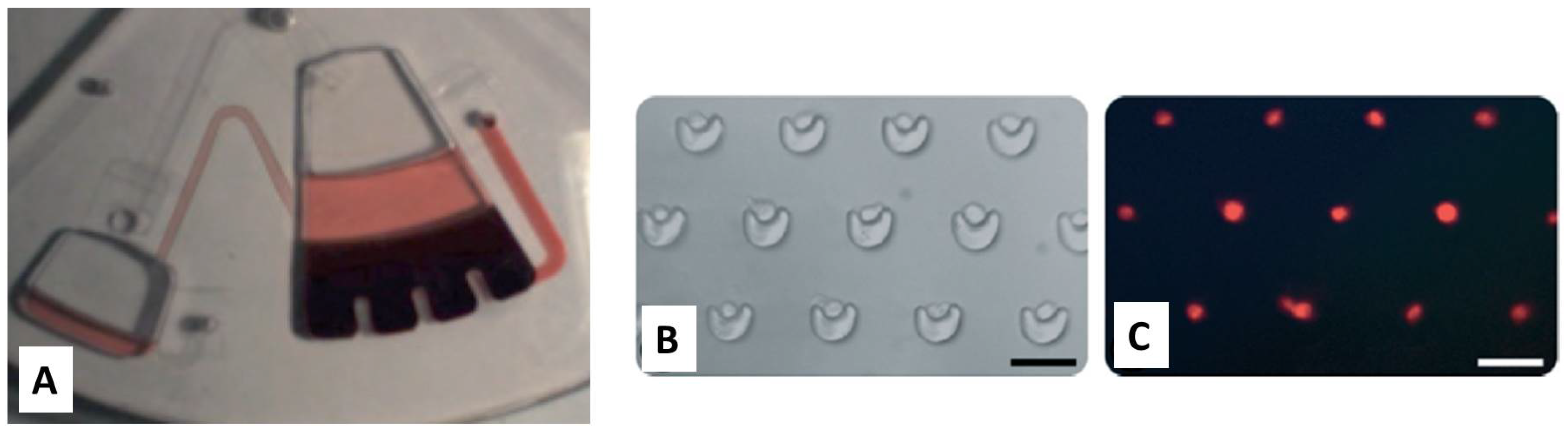
4. Centrifugal-Based Systems for Extreme Point-of-Care
4.1. Lab on a CD
| Laboratory EquipmentFunction | Implementation Method | Example Applications |
|---|---|---|
| Centrifuge | Motor for spinning disc. High speed for centrifuge action. Motor speed can be varied accurately. | Blood plasmaseparation [85]. |
| Vortex | Motor for spinning disc clockwise or counter-clockwise. Turbulence created causing vortex effect. Rotation direction changes vary acceleration, causing turbulence. | RotaPrep, Inc. [103], cell culture [104], vortexing [47]. |
| Mixer | Ceramic beads incorporated into disc. Mixing of fluids using beads when turbulence created. Changes in spin direction cause turbulence and mixing. | Mixing of different reagents [47]. |
| Lysis | Magnets and glass beads incorporated into disc. Lysis of cells as a result of mechanical impaction and shear forces. Rotational dual magnetic field moves magnet and beads inside chambers. | Lysis of bacteria [105,106], RotaPrep, Inc. [103]. |
| Microscope | CD or DVD player. System components used as laser scanning microscope. Photodetector module detects absorbance of objects, images reconstructed. | Detection of cells [107,108]. |
| x-y table/spotter | CD or DVD player. Rotational and linear motors for positioning. Microarrays applied onto disc using piezoelectric inkjet applicator and positioning system. | Immunoassay microarrays [109]. |
| Sample concentration | Pneumatic pressure chambers on a disc. Reciprocation pump implemented. Fluid flushed back and forth, concentration/capture of analyte. | Immunoassays [49]. |
| Cell counter | CD or DVD player. Locating and counting of cells, microparticles, biomolecules. Laser in drive detects errors on disc where particles are located. | Counting of cells, microparticles [107,110]. |
| Thermal cycler | Peltier elements. Thermal treatment of small chambers. Current direction changes mode from heating to cooling. | DNA amplification via PCR [111]. |
| Hypergravity simulation | Spinning microfluidic disc. Chambers containnutrients for cultivation. Centrifugal force simulateshigh g-forces on live samples trapped in fluidic chambers. | C. elegans stress response cultivation platform [112]. |

4.2. Innovative Centrifugal Approaches for Extreme Point-of-Care
4.2.1. Sensing and Detection
| Parameter | Optical Sensors | Electrochemical Sensors |
|---|---|---|
| Instrument cost and size | Often expensive and bulky | Inexpensive and compact |
| Sensor cost | Fair | Low |
| Optically transparent substrate | Required | Not required |
| Selectivity | Good | Fair |
| Limit of detection (LOD) | Very good | Good and very good (using redox amplification |
| Response time | Long (up to tens of seconds) | Less than a second |
| Simplicity of the method | Often simple | Simple |
| Analysis of turbid solutions | Sometimes problematic | Not problematic |
| Electromagnetic interface | No | Yes |
| Resistance to radiation and corrosion | Yes | No |
| Cross-talk | No | Yes |
| Ambient light | Problematic | Not problematic |
| Response curve | Sigmoidal | Nernstian (potentiometric or linear) |
| Sensitivity enhancement | Complicated | Nernstian (potentiometric or linear) |


4.2.2. Energy for Operation
| Module | Power (mW) |
|---|---|
| Qi transmitter: Transmitted power | 5000 |
| On-disc power: Received power (80% efficiency) | 4000 |
| Arduino microcontroller consumption | 190 |
| Bluetooth and SD Card consumption | 200 |
| Energy available for application | 3610 |

4.2.3. Biomimetic Approaches for Environmental Control
4.3. Evolution of the CD Platform
4.3.1. Four Generations of Spin Stands

| Parameter | Slip Ring | Wireless Power Transfer with Qi | Energy Harvesting |
|---|---|---|---|
| Wear | − contacts wear | + no wear | + no wear |
| Direct analog output | + signals can be coupled in and out directly | − only digital signals can be sent to and from the disc | − only digital signals can be sent to and from the disc |
| Energy level | + high voltages and currents can be sent to disc | +/− 5–20 W of power can be transferred (limited power) | +/− 100–500 mW of power can begenerated (relatively low power) |
| Constant energy | + available energy does not depend on spinning frequency | + available energy does not depend on spinning frequency | − induced power depends on the spinning frequency and is zero when stationary |
| Energy storage needed | + no storage is needed since power is always available | + no storage is needed since power is always available | − storage is needed, otherwise there is no power before spinning |
| Weight | + the rotational part can be made to be light weight | + the rotating disk is light weight | − the coils are heavy; hence, a high−torque motor is needed |
| Maintenance | +/− moderate maintenance | + low maintenance | + low maintenance |
| Price | +/− electrical brushes and contacts are mechanically complex | + low cost, below EUR 50 | + low cost, below EUR 50 |
| Interaction with surrounding | + no effect on the surrounding | +/− RF might disturb some applications, but frequency can be adapted | − magnets on the lower disk inhibit magnetic sorting applications |
| Applicable to standard spin stands | − can only be integrated with a special spin stand head | +/− has only a few geometrical demands to enable integration | +/− has only a few geometrical demands to enable integration |
| Power source | − additional power source required | − additional power source required | + no need for additional power source |
4.3.2. Scale-Up of CD-Based Microfluidic Systems
4.3.3. Examples of Existing Centrifugal Systems for Point-of-Care

5. Ideal Panel of Tests for Extreme Point-of-Care
| Critical Test for Under-Resourced POC | Lab-on-Disc Implementation? | Methods and References |
|---|---|---|
| Complete blood count | Yes | Cell capture and counting [94]. Blood fractionation, density gradient tests [87,88,89]. Hemoglobin [163] and Hematocrit [164]. From these tests, all remaining complete blood count values can be calculated. |
| Blood group (ABO and rhesus) | Yes | Agglutination of cells [165]. |
| Blood sugar (F and PP ), urea, creatinine, uric acid | Yes | Colorimetric glucose assay [116]. Uric acid, glucose and lactate tests using whole blood and electrochemistry [166]. Urine analysis [167]. |
| Serum sodium, potassium | Yes | Plasma separation and automated assay [155]. Commercially available: Abaxis Picollo Xpress. |
| Liver function test: bilirubin, liver enzymes (SGOT, SGPT, SAKP) | Yes | Liver function on a disc [117]. |
| HbA1C (diabetes) | Yes | Commercially available: Roche Cobas b 101. |
| HbsAG (hepatitis B) | Yes | Integrated ELISA for detecting antigens and antibodies of hepatitis B virus, HBsAg and anti-HBs in parallel using whole blood [168]. |
| HIV | Yes | CD4+ cell counts [154,169]. |
| TB | In progress, some existing components. | Bacterial pathogen detection [170]. |
| Urine-routine and microscopic, culture | In progress, some existing components. | Individual cell capture: counting can be performed using microscopy [94]. ELISA from cell culture [121]. |
6. Summary and Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Confederation of Indian Industry and PricewaterhouseCoopers. Addressing the Unfinished Agenda: Universal Healthcare; Confederation of Indian Industry and PricewaterhouseCoopers: New Delhi, India, 2011. [Google Scholar]
- Peeling, R.; Mabey, D. Point-of-Care Tests for Diagnosing Infections in the Developing World. Clin. Microbiol. Infect. 2010, 16, 1062–1069. [Google Scholar] [CrossRef] [PubMed]
- 2014-2015 Annual Report of Ministry of Women and Child Development, Government of India. Ministry of Women and Child Development, Government of India, 2015. Available online: http://wcd.nic.in/sites/default/files/AR2014-15.pdf (accessed on 27 October 2015).
- Stoltzfus, R.J.; Mullany, L.; Black, R.E. Iron Deficiency Anaemia. In Comparative Quantification of Health Risks: Global and Regional Burden of Disease Attributable to Selected Major Risk Factors; World Health Organization: Geneva, Switzerland, 2004; pp. 163–209. [Google Scholar]
- Millenium Development Goals. World Health Organization, Regional Office for South-East Asia, 2008. Available online: http://repository.searo.who.int/handle/123456789/15248 (accessed on 27 October 2015).
- Millenium Development Goals 4 and 5. Partnership for Maternal, Newborn and Child Health (PMNCH), 2015. Available online: http://www.who.int/pmnch/about/about_mdgs/en/ (accessed on 27 October 2015).
- National Development Plan 2030: Our Future—Make it Work. National Planning Commission of the Republic of South Africa, 2012. Available online: http://www.gov.za/issues/national-development-plan- 2030 (accessed on 27 October 2015).
- Gubala, V.; Harris, L.F.; Ricco, A.J.; Tan, M.X.; Williams, D.E. Point of Care Diagnostics: Status and Future. Anal. Chem. 2012, 84, 487–515. [Google Scholar] [CrossRef] [PubMed]
- Drain, P.K.; Hyle, E.P.; Noubary, F.; Freedberg, K.A.; Wilson, D.; Bishai, W.R.; Rodriguez, W.; Bassett, I.V. Diagnostic Point-of-Care Tests in Resource-Limited Settings. Lancet Infect. Dis. 2014, 14, 239–249. [Google Scholar] [CrossRef]
- UNITAID; World Health Organization. HIV/AIDS Diagnostics Technology Landscape, 4th ed.; UNITAID, World Health Organization: Geneva, Switzerland, 2014. [Google Scholar]
- UNITAID; World Health Organization. Malaria Diagnostics Landscape Update; UNITAID, World Health Organization: Geneva, Switzerland, 2015. [Google Scholar]
- UNITAID; World Health Organization. Hepatitis C Diagnostics Technology Landscape, 1st ed.; UNITAID, World Health Organization: Geneva, Switzerland, 2015. [Google Scholar]
- FIND; McGill International TB Centre and UNITAID; World Health Organization. TB Diagnostics Market in Select High-Burden Countries: Current Market and Future Opportunities for Novel Diagnostics; FIND, McGill International TB Centre and UNITAID, World Health Organization: Geneva, Switzerland, 2015. [Google Scholar]
- Kettler, H.; White, K.; Hawkes, S. Mapping the Landscape of Diagnostics for Sexually Transmitted Infections: Key Findings and Recommandations; Technical Report; World Health Organization: Geneva, Switzerland, 2004. [Google Scholar]
- Glynn, M.T.; Kinahan, D.J.; Ducrée, J. CD4 Counting Technologies for HIV Therapy Monitoring in Resource-Poor Settings—State-of-the-Art and Emerging Microtechnologies. Lab Chip 2013, 13, 2731–2748. [Google Scholar] [CrossRef] [PubMed]
- Richards-Kortum, R.; Gray, L.V.; Oden, M. Engaging Undergraduates in Global Health Technology Innovation. Science 2012, 336, 430–431. [Google Scholar] [CrossRef] [PubMed]
- Moleko, W.; Msibi, E.B.; Marshall, C. Developments in Ensuring Quality of Care in Health Establishments in South Africa. In South African Health Review 2013/14; Padarath, A., English, R., Eds.; Health Systems Trust: Durban, South Africa, 2014. [Google Scholar]
- Begg, K.; Tucker, T.; Manyike, P.; Ramabulane, F.; Smith, Y.; Miller, R.; Young, T.; Eksteen, D.; Galloway, M.; Tichapondwa, S.; et al. Analysis of POCT/VCT Performed at South African Primary Health Care Clinics. Technical Report, SEAD—Strategic Evaluation; Advisory and Development Consulting (Pty) Ltd. Available online: http://www.sead.co.za/downloads/POCT-clinics-2011.pdf (accessed on 20 September 2014).
- Blattner, K.; Nixon, G.; Jaye, C.; Dovey, S. Introducing Point-of-Care Testing into a Rural Hospital Setting: Thematic Analysis of Interviews with Providers. J. Prim. Health Care 2010, 2, 54–60. [Google Scholar] [PubMed]
- Lawn, S.D.; Mwaba, P.; Bates, M.; Piatek, A.; Alexander, H.; Marais, B.J.; Cuevas, L.E.; McHugh, T.D.; Zijenah, L.; Kapata, N.; et al. Advances in Tuberculosis Diagnostics: The Xpert MTB/RIF Assay and Future Prospects for a Point-of-Care Test. Lancet Infect. Dis. 2013, 13, 349–361. [Google Scholar] [CrossRef]
- Mark, D.; Haeberle, S.; Roth, G.; von Stetten, F.; Zengerle, R. Microfluidic Lab-on-a-Chip Platforms: Requirements, Characteristics and Applications. Chem. Soc. Rev. 2010, 39, 1153–1182. [Google Scholar] [CrossRef] [PubMed]
- Nge, P.N.; Rogers, C.I.; Woolley, A.T. Advances in Microfluidic Materials, Functions, Integration, and Applications. Chem. Rev. 2013, 113, 2550–2583. [Google Scholar] [CrossRef] [PubMed]
- Haeberle, S.; Zengerle, R. Microfluidic Platforms for Lab-on-a-Chip Applications. Lab Chip 2007, 7, 1094–1110. [Google Scholar] [CrossRef] [PubMed]
- Jung, W.; Han, J.; Choi, J.W.; Ahn, C.H. Point-of-Care Testing (POCT) Diagnostic Systems Using Microfluidic Lab-on-a-Chip Technologies. Microelectron. Eng. 2015, 132, 46–57. [Google Scholar] [CrossRef]
- Weaver, W.; Kittur, H.; Dhar, M.; di Carlo, D. Research Highlights: Microfluidic Point-of-Care Diagnostics. Lab Chip 2014, 14, 1962–1965. [Google Scholar] [CrossRef]
- Gomez, F.A. The Future of Microfluidic Point-of-Care Diagnostic Devices. Bioanalysis 2013, 5, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Sia, S.K.; Kricka, L.J. Microfluidics and Point-of-Care Testing. Lab Chip 2008, 8, 1982–1983. [Google Scholar] [CrossRef] [PubMed]
- Chin, C.D.; Linder, V.; Sia, S.K. Lab-on-a-Chip Devices for Global Health: Past Studies and Future Opportunities. Lab Chip 2007, 7, 41–57. [Google Scholar] [CrossRef] [PubMed]
- Yager, P.; Edwards, T.; Fu, E.; Helton, K.; Nelson, K.; Tam, M.; Weigl, B. Microfluidic Diagnostic Technologies for Global Public Health. Nature 2006, 442, 412–418. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Akay, A.; Wei, H.; Wang, S.; Pingguan-Murphy, B.; Erlandsson, B.E.; Li, X.; Lee, W.; Hu, J.; Wang, L.; et al. Advances in Smartphone-Based Point-of-Care Diagnostics. IEEE Proc. 2015, 103, 236–247. [Google Scholar] [CrossRef]
- Hu, J.; Wang, S.; Wang, L.; Li, F.; Pingguan-Murphy, B.; Lu, T.J.; Xu, F. Advances in paper-based point-of-care diagnostics. Biosens. Bioelectron. 2014, 54, 585–597. [Google Scholar] [CrossRef] [PubMed]
- Christodouleas, D.C.; Nemiroski, A.; Kumar, A.A.; Whitesides, G.M. Broadly Available Imaging Devices Enable High-Quality Low-Cost Photometry. Anal. Chem. 2015, 87, 9170–9178. [Google Scholar] [CrossRef] [PubMed]
- Nemiroski, A.; Christodouleas, D.C.; Hennek, J.W.; Kumar, A.A.; Maxwell, E.J.; Fernández-Abedul, M.T.; Whitesides, G.M. Universal mobile electrochemical detector designed for use in resource-limited applications. Proc. Natl. Acad. Sci. USA 2014, 111, 11984–11989. [Google Scholar] [CrossRef] [PubMed]
- Dell, N.; Borriello, G. Mobile Tools for Point-of-care Diagnostics in the Developing World. In Proceedings of the 3rd ACM Symposium on Computing for Development, New York, NY, USA, 11–12 January 2013; pp. 1–10.
- Gorkin, R.; Park, J.; Siegrist, J.; Amasia, M.; Lee, B.; Park, J.M.; Kim, J.; Kim, H.; Madou, M.; Cho, Y.K. Centrifugal Microfluidics for Biomedical Applications. Lab Chip 2010, 10, 1758–1773. [Google Scholar] [CrossRef] [PubMed]
- Hugo, S.; Land, K.; Madou, M.; Kido, H. A Centrifugal Microfluidic Platform for Point-of-Care Diagnostic Applications. South Afr. J. Sci. 2014, 110, 1–7. [Google Scholar] [CrossRef]
- Strohmeier, O.; Keller, M.; Schwemmer, F.; Zehnle, S.; Mark, D.; von Stetten, F.; Zengerle, R.; Paust, N. Centrifugal Microfluidic Platforms: Advanced Unit Operations and Applications. Chem. Soc. Rev. 2015, 44, 6187–6229. [Google Scholar] [CrossRef] [PubMed]
- Madou, M.; Zoval, J.; Jia, G.; Kido, H.; Kim, J.; Kim, N. Lab on a CD. Annu. Rev. Biomed. Eng. 2006, 8, 601–628. [Google Scholar] [CrossRef] [PubMed]
- Sin, M.; Gao, J.; Liao, J.; Wong, P. System Integration—A Major Step Toward Lab on a Chip. J. Biol. Eng. 2011, 5, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Burger, R.; Kirby, D.; Glynn, M.; Nwankire, C.; O’Sullivan, M.; Siegrist, J.; Kinahan, D.; Aguirre, G.; Kijanka, G.; Gorkin, R.A.; et al. Centrifugal Microfluidics for Cell Analysis. Curr. Opin Chem. Biol. 2012, 16, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Price, C.P.; Spencer, K. Centrifugal Analysers in Clinical Chemistry, 1st ed.; Praeger: Westport, CT, USA, 1980. [Google Scholar]
- Brenner, T.; Glatzel, T.; Zengerle, R.; Ducrée, J. Frequency-dependent transversal flow control in centrifugal microfluidics. Lab Chip 2005, 5, 146–150. [Google Scholar] [CrossRef] [PubMed]
- Burger, R.; Ducrée, J. Handling and Analysis of Cells and Bioparticles on Centrifugal Microfluidic Platforms. Exp. Rev. Mol. Diagn. 2012, 12, 407–421. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kido, H.; Rangel, R.H.; Madou, M.J. Passive Flow Switching Valves on a Centrifugal Microfluidic Platform. Sens. Actuators B Chem. 2008, 128, 613–621. [Google Scholar] [CrossRef]
- Soroori, S.; Kulinsky, L.; Madou, M. Centrifugal Mircofluidics: Characteristics and Possibilities. In Microfluidics and Microscale Transport Processes; Chakraborty, S., Ed.; CRC Press: Boca Raton, FL, USA, 2013; pp. 149–186. [Google Scholar]
- Ducrée, J.; Haeberle, S.; Lutz, S.; Pausch, S.; von Stetten, F.; Zengerle, R. The Centrifugal Microfluidic Bio-Disk Platform. J. Micromech. Microeng. 2007, 17, S103. [Google Scholar] [CrossRef]
- Grumann, M.; Geipel, A.; Riegger, L.; Zengerle, R.; Ducrée, J. Batch-Mode Mixing on Centrifugal Microfluidic Platforms. Lab Chip 2005, 5, 560–565. [Google Scholar] [CrossRef] [PubMed]
- Ducrée, J.; Haeberle, S.; Brenner, T.; Glatzel, T.; Zengerle, R. Patterning of Flow and Mixing in Rotating Radial Microchannels. Microfluid. Nanofluid. 2006, 2, 97–105. [Google Scholar] [CrossRef]
- Noroozi, Z.; Kido, H.; Micic, M.; Pan, H.; Bartolome, C.; Princevac, M.; Zoval, J.; Madou, M. Reciprocating Flow-Based Centrifugal Microfluidics Mixer. Rev. Sci. Instrum. 2009, 80, 075102. [Google Scholar] [CrossRef] [PubMed]
- Clime, L.; Brassard, D.; Geissler, M.; Veres, T. Active Pneumatic Control of Centrifugal Microfluidic Flows for Lab-on-a-Chip Applications. Lab Chip 2015, 15, 2400–2411. [Google Scholar] [CrossRef] [PubMed]
- Kong, M.C.R.; Bouchard, A.P.; Salin, E.D. Displacement Pumping of Liquids Radially Inward on Centrifugal Microfluidic Platforms in Motion. Micromachines 2012, 3, 1–9. [Google Scholar] [CrossRef]
- Abi-Samra, K.; Hanson, R.; Madou, M.; Gorkin, R.A. Infrared Controlled Waxes for Liquid Handling and Storage on a CD-Microfluidic Platform. Lab Chip 2011, 11, 723–726. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Cordero, J.L.; Kurzbuch, D.; Benito-Lopez, F.; Diamond, D.; Lee, L.P.; Ricco, A.J. Optically Addressable Single-Use Microfluidic Valves by Laser Printer Lithography. Lab Chip 2010, 10, 2680–2687. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.S.; Lee, Y.U.; Kim, H.S.; Kim, T.H.; Park, J.; Lee, J.G.; Kim, J.; Kim, H.; Lee, W.G.; Cho, Y.K. Fully Integrated Lab-on-a-Disc for Simultaneous Analysis of Biochemistry and Immunoassay from Whole Blood. Lab Chip 2011, 11, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Al-Faqheri, W.; Ibrahim, F.; Thio, T.H.G.; Moebius, J.; Joseph, K.; Arof, H.; Madou, M. Vacuum/Compression Valving (VCV) Using Parrafin-Wax on a Centrifugal Microfluidic CD Platform. PLoS ONE 2013, 8, e58523. [Google Scholar]
- Kawai, T.; Naruishi, N.; Nagai, H.; Tanaka, Y.; Hagihara, Y.; Yoshida, Y. Rotatable Reagent Cartridge for High-Performance Microvalve System on a Centrifugal Microfluidic Device. Anal. Chem. 2013, 85, 6587–6592. [Google Scholar] [CrossRef] [PubMed]
- Geissler, M.; Clime, L.; Hoa, X.D.; Morton, K.J.; Hébert, H.; Poncelet, L.; Mounier, M.; Deschênes, M.; Gauthier, M.E.; Huszczynski, G.; et al. Microfluidic Integration of a Cloth-Based Hybridization Array System (CHAS) for Rapid, Colorimetric Detection of Enterohemorrhagic Escherichia coli (EHEC) Using an Articulated, Centrifugal Platform. Anal. Chem. 2015, 87, 10565–10572. [Google Scholar] [CrossRef] [PubMed]
- Höfflin, J.; Torres Delgado, S.; Suárez Sandoval, F.; Korvink, J.; Mager, D. Electrifying the Disk: A Modular Rotating Platform for Wireless Power and Data Transmission for Lab on a Disk Application. Lab Chip 2015, 15, 2584–2587. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Huang, P.C.; Lin, M.G. Analysis and Experiment of Capillary Valves for Microfluidics on a Rotating Disk. Microfluid. Nanofluid. 2008, 4, 427–437. [Google Scholar] [CrossRef]
- Moore, J.; McCuiston, A.; Mittendorf, I.; Ottway, R.; Johnson, R. Behavior of Capillary Valves in Centrifugal Microfluidic Devices Prepared by Three-Dimensional Printing. Microfluid. Nanofluid. 2011, 10, 877–888. [Google Scholar] [CrossRef]
- Mark, D.; Weber, P.; Lutz, S.; Focke, M.; Zengerle, R.; von Stetten, F. Aliquoting on the Centrifugal Microfluidic Platform Based on Centrifugo-Pneumatic Valves. Microfluid. Nanofluid. 2011, 10, 1279–1288. [Google Scholar] [CrossRef]
- Gorkin, R.; Nwankire, C.E.; Gaughran, J.; Zhang, X.; Donohoe, G.G.; Rook, M.; O’Kennedy, R.; Ducrée, J. Centrifugo-Pneumatic Valving Utilizing Dissolvable Films. Lab Chip 2012, 12, 2894–2902. [Google Scholar] [CrossRef] [PubMed]
- Van Oordt, T.; Barb, Y.; Smetana, J.; Zengerle, R.; von Stetten, F. Miniature Stick-Packaging—An Industrial Technology for Pre-Storage and Release of Reagents in Lab-on-a-Chip Systems. Lab Chip 2013, 13, 2888–2892. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.; Kim, H.H.; Cho, Y.K. Elastomeric Membrane Valves in a Disc. Lab Chip 2011, 11, 1434–1436. [Google Scholar] [CrossRef] [PubMed]
- Siegrist, J.; Gorkin, R.; Clime, L.; Roy, E.; Peytavi, R.; Kido, H.; Bergeron, M.; Veres, T.; Madou, M. Serial Siphon Valving for Centrifugal Microfluidic Platforms. Microfluid. Nanofluid. 2010, 9, 55–63. [Google Scholar] [CrossRef]
- Gorkin, R.; Clime, L.; Madou, M.; Kido, H. Pneumatic Pumping in Centrifugal Microfluidic Platforms. Microfluid. Nanofluid. 2010, 9, 541–549. [Google Scholar] [CrossRef]
- Godino, N.; Gorkin, R.; Linares, A.V.; Burger, R.; Ducrée, J. Comprehensive Integration of Homogeneous Bioassays via Centrifugo-Pneumatic Cascading. Lab Chip 2013, 13, 685–694. [Google Scholar] [CrossRef] [PubMed]
- Aeinehvand, M.M.; Ibrahim, F.; Harun, S.W.; Al-Faqheri, W.; Thio, T.H.G.; Kazemzadeh, A.; Madou, M. Latex Micro-Balloon Pumping in Centrifugal Microfluidic Platforms. Lab Chip 2014, 14, 988–997. [Google Scholar] [CrossRef] [PubMed]
- Kinahan, D.J.; Kearney, S.M.; Dimov, N.; Glynn, M.T.; Ducrée, J. Event-Triggered Logical Flow Control for Comprehensive Process Integration of Multi-Step Assays on Centrifugal Microfluidic Platforms. Lab Chip 2014, 14, 2249–2258. [Google Scholar] [CrossRef] [PubMed]
- Schwemmer, F.; Zehnle, S.; Mark, D.; von Stetten, F.; Zengerle, R.; Paust, N. A Microfluidic Timer for Timed Valving and Pumping in Centrifugal Microfluidics. Lab Chip 2015, 15, 1545–1553. [Google Scholar] [CrossRef] [PubMed]
- Ukita, Y.; Takamura, Y.; Utsumi, Y. Water-Clock-Based Autonomous Flow Sequencing in Steadily Rotating Centrifugal Microfluidic Device. Sens. Actuators B Chem. 2015, 220, 180–183. [Google Scholar] [CrossRef]
- Kinahan, D.J.; Kearney, S.M.; Faneuil, O.P.; Glynn, M.T.; Dimov, N.; Ducrée, J. Paper Imbibition for Timing of Multi-Step Liquid Handling Protocols on Event-Triggered Centrifugal Microfluidic Lab-on-a-Disc Platforms. RSC Adv. 2015, 5, 1818–1826. [Google Scholar] [CrossRef]
- Mishra, R.; Alam, R.; Kinahan, D.; Anderson, K.; Ducrée, J. Lipophilic-Membrane Based Routing for Centrifugal Automation of Heterogeneous Immunoassays. In Proceedings of the 28th IEEE 28th International Conference Micro Electro Mechanical Systems (MEMS), Estoril, Portugal, 18–22 January 2015; pp. 523–526.
- Kodzius, R.; Xiao, K.; Wu, J.; Yi, X.; Gong, X.; Foulds, I.G.; Wen, W. Inhibitory Effect of Common Microfluidic Materials on PCR Outcome. Sens. Actuators B Chem. 2012, 161, 349–358. [Google Scholar] [CrossRef]
- Abi-Samra, K.; Clime, L.; Kong, L.; Gorkin, R.; Kim, T.H.; Cho, Y.K.; Madou, M. Thermo-Pneumatic Pumping in Centrifugal Microfluidic Platforms. Microfluid. Nanofluid. 2011, 11, 643–652. [Google Scholar] [CrossRef]
- Noroozi, Z.; Kido, H.; Madou, M.J. Electrolysis-Induced Pneumatic Pressure for Control of Liquids in a Centrifugal System. J. Electrochem. Soc. 2011, 158, P130–P135. [Google Scholar] [CrossRef]
- Kinahan, D.; Burger, R.; Vembadi, A.; Kilcawley, N.; Lawlor, D.; Glynn, M.; Ducrée, J. Baking-Powder Driven Centripetal Pumping Controlled by Event-Triggering of Functional Liquids. In Proceedings of the IEEE 28th International Conference Micro Electro Mechanical Systems (MEMS), Estoril, Portugal, 18–22 January 2015; pp. 504–507.
- Zehnle, S.; Schwemmer, F.; Roth, G.; von Stetten, F.; Zengerle, R.; Paust, N. Centrifugo-Dynamic Inward Pumping of Liquids on a Centrifugal Microfluidic Platform. Lab Chip 2012, 12, 5142–5145. [Google Scholar] [CrossRef] [PubMed]
- Soroori, S.; Kulinsky, L.; Kido, H.; Madou, M. Design and Implementation of Fluidic Micro-Pulleys for Flow Control on Centrifugal Microfluidic Platforms. Microfluid. Nanofluid. 2014, 16, 1117–1129. [Google Scholar] [CrossRef] [PubMed]
- Kilcawley, N.; Kinahan, D.; Nwankire, C.; Glynn, M.; Ducrée, J. Buoyancy-Driven Centripetal Pumping for Nested Sample Preparation in Bioassays. In Proceedings of the 18th International Conference Miniaturized Systems for Chemistry and Life Sciences (MicroTAS 2014), San Antonio, TX, USA, 26–30 October 2014.
- Godino, N.; Vereshchagina, E.; Gorkin, R.; Ducrée, J. Hybrid Paper-Polymer Lab-on-a-Disc for Bioassay Integration. In Proceedings of the 16th International Conference Miniaturized Systems for Chemistry and Life Sciences, Okinawa, Japan, 28 October–1 November 2012; pp. 1369–1371.
- Vereshchagina, E.; Bourke, K.; Meehan, L.; Dixit, C.; Glade, D.; Ducrée, J. Multi-Material Paper-Disc Devices for Low Cost Biomedical Diagnostics. In Proceedings of the IEEE 26th International Conference Micro Electro Mechanical Systems (MEMS), Taipei, Taiwan, 20–24 January 2013; pp. 1049–1052.
- Hwang, H.; Kim, S.H.; Kim, T.H.; Park, J.K.; Cho, Y.K. Paper on a Disc: Balancing the Capillary-Driven Flow with a Centrifugal Force. Lab Chip 2011, 11, 3404–3406. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Park, J.; Kim, C.J.; Cho, Y.K. Fully Integrated Lab-on-a-Disc for Nucleic Acid Analysis of Food-Borne Pathogens. Anal. Chem. 2014, 86, 3841–3848. [Google Scholar] [CrossRef] [PubMed]
- Amasia, M.; Madou, M. Large-Volume Centrifugal Microfluidic Device for Blood Plasma Separation. Bioanalysis 2010, 2, 1701–1710. [Google Scholar] [CrossRef] [PubMed]
- Schröder, U.C.; Bokeloh, F.; O’Sullivan, M.; Glaser, U.; Wolf, K.; Pfister, W.; Popp, J.; Ducrée, J.; Neugebauer, U. Rapid, Culture-Independent, Optical Diagnostics of Centrifugally Captured Bacteria from Urine Samples. Biomicrofluidics 2015, 9, 044118. [Google Scholar] [CrossRef] [PubMed]
- Kinahan, D.J.; Kearney, S.M.; Glynn, M.T.; Ducrée, J. Spira Mirabilis Enhanced Whole Blood Processing in a Lab-on-a-Disk. Sens. Actuators A Phys. 2014, 215, 71–76. [Google Scholar] [CrossRef]
- Nwankire, C.E.; Venkatanarayanan, A.; Glennon, T.; Keyes, T.E.; Forster, R.J.; Ducrée, J. Label-Free Impedance Detection of Cancer Cells from Whole Blood on an Integrated Centrifugal Microfluidic Platform. Biosens. Bioelectron. 2015, 68, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Schaff, U.Y.; Sommer, G.J. Whole Blood Immunoassay Based on Centrifugal Bead Sedimentation. Clin. Chem. 2011, 57, 753–761. [Google Scholar] [CrossRef] [PubMed]
- Walsh, D.I.; Sommer, G.J.; Schaff, U.Y.; Hahn, P.S.; Jaffe, G.J.; Murthy, S.K. A Centrifugal Fluidic Immunoassay for Ocular Diagnostics with an Enzymatically Hydrolyzed Fluorogenic Substrate. Lab Chip 2014, 14, 2673–2680. [Google Scholar] [CrossRef] [PubMed]
- Koh, C.Y.; Schaff, U.Y.; Piccini, M.E.; Stanker, L.H.; Cheng, L.W.; Ravichandran, E.; Singh, B.R.; Sommer, G.J.; Singh, A.K. Centrifugal Microfluidic Platform for Ultrasensitive Detection of Botulinum Toxin. Anal. Chem. 2015, 87, 922–928. [Google Scholar] [CrossRef] [PubMed]
- Kirby, D.; Glynn, M.; Kijanka, G.; Ducrée, J. Rapid and Cost-Efficient Enumeration of Rare Cancer Cells from Whole Blood by Low-Loss Centrifugo-Magnetophoretic Purification Under Stopped-Flow Conditions. Cytom. A 2015, 87, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Glynn, M.; Nwankire, C.; Lemass, K.; Kinahan, D.; Ducrée, J. Cluster Size Distribution of Cancer Cells in Blood Using Stopped-Flow Centrifugation Along Scale-Matched Gaps of a Radially Inclined Rail. Microsyst. Nanoeng. 2015, 1, 15018. [Google Scholar] [CrossRef]
- Burger, R.; Kurzbuch, D.; Gorkin, R.; Kijanka, G.; Glynn, M.; McDonagh, C.; Ducrée, J. An Integrated Centrifugo-Opto-Microfluidic Platform for Arraying, Analysis, Identification and Manipulation of Individual Cells. Lab Chip 2015, 15, 378–381. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.; Park, J.; Lim, M.; Sunkara, V.; Kim, S.Y.; Kim, G.H.; Kim, M.H.; Cho, Y.K. All-in-One Centrifugal Microfluidic Device for Size-Selective Circulating Tumor Cell Isolation with High Purity. Anal. Chem. 2014, 86, 11349–11356. [Google Scholar] [CrossRef] [PubMed]
- Park, J.M.; Kim, M.S.; Moon, H.S.; Yoo, C.E.; Park, D.; Kim, Y.J.; Han, K.Y.; Lee, J.Y.; Oh, J.H.; Kim, S.S.; et al. Fully Automated Circulating Tumor Cell Isolation Platform with Large-Volume Capacity Based on Lab-on-a-Disc. Anal. Chem. 2014, 86, 3735–3742. [Google Scholar] [CrossRef] [PubMed]
- Aguirre, G.; Efremov, V.; Kitsara, M.; Ducrée, J. Integrated Micromixer for Incubation and Separation of Cancer Cells on a Centrifugal Platform Using Inertial and Dean Forces. Microfluid. Nanofluid. 2015, 18, 513–526. [Google Scholar] [CrossRef]
- Zoval, J.; Madou, M. Centrifuge-Based Fluidic Platforms. Proc. IEEE 2004, 92, 140–153. [Google Scholar] [CrossRef]
- Sharma, S.; Zapatero-Rodríguez, J.; Estrela, P.; O’Kennedy, R. Point-of-Care Diagnostics in Low Resource Settings: Present Status and Future Role of Microfluidics. Biosensors 2015, 5, 577–601. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.G.; Kim, Y.G.; Chung, B.G.; Demirci, U.; Khademhosseini, A. Nano/Microfluidics for Diagnosis of Infectious Diseases in Developing Countries. Adv. Drug Deliv. Rev. 2010, 62, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Huang, T.J. Microfluidic Diagnostics for the Developing World. Lab Chip 2012, 12, 1412–1416. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.X.; Perebikovsky, A.; Moebius, J.; Kulinsky, L.; Madou, M. Lab-on-a-CD: A Fully Integrated Molecular Diagnostic System. J. Lab. Autom. 2015. [Google Scholar] [CrossRef] [PubMed]
- RotaPrep Sample Preparation Systems. Available online: http://www.rotaprep.com/ (accessed on 12 October 2015).
- Ren, Y.; Chow, L.C.; Leung, W.F. Cell Culture Using Centrifugal Microfluidic Platform with Demonstration on Pichia Pastoris. Biomed. Microdevices 2013, 15, 321–337. [Google Scholar] [CrossRef] [PubMed]
- Kido, H.; Micic, M.; Smith, D.; Zoval, J.; Norton, J.; Madou, M. A Novel, Compact Disk-Like Centrifugal Microfluidics System for Cell Lysis and Sample Homogenization. Colloids Surf. B 2007, 58, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Hee Jang, S.; Jia, G.; Zoval, J.V.; da Silva, N.A.; Madou, M.J. Cell Lysis on a Microfluidic CD (Compact Disc). Lab Chip 2004, 4, 516–522. [Google Scholar] [CrossRef] [PubMed]
- Ramachandraiah, H.; Amasia, M.; Cole, J.; Sheard, P.; Pickhaver, S.; Walker, C.; Wirta, V.; Lexow, P.; Lione, R.; Russom, A. Lab-on-DVD: Standard DVD Drives as a Novel Laser Scanning Microscope for Image Based Point of Care Diagnostics. Lab Chip 2013, 13, 1578–1585. [Google Scholar] [CrossRef] [PubMed]
- Madou, M.J. Solid-State Physics, Fluidics, and Analytical Techniques in Micro- and Nanotechnology; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]
- Kido, H.; Maquieira, A.; Hammock, B.D. Disc-Based Immunoassay Microarrays. Anal. Chim. Acta 2000, 411, 1–11. [Google Scholar] [CrossRef]
- Imaad, S.M.; Lord, N.; Kulsharova, G.; Liu, G.L. Microparticle and Cell Counting with Digital Microfluidic Compact Disc Using Standard CD Drive. Lab Chip 2011, 11, 1448–1456. [Google Scholar] [CrossRef] [PubMed]
- Amasia, M.; Cozzens, M.; Madou, M.J. Centrifugal Microfluidic Platform for Rapid PCR Amplification Using Integrated Thermoelectric Heating and Ice-Valving. Sens. Actuators B Chem. 2012, 161, 1191–1197. [Google Scholar] [CrossRef]
- Kim, N.; Dempsey, C.M.; Zoval, J.V.; Sze, J.Y.; Madou, M.J. Automated Microfluidic Compact Disc (CD) Cultivation System of Caenorhabditis Elegans. Sens. Actuators B Chem. 2007, 122, 511–518. [Google Scholar] [CrossRef]
- Noroozi, Z.; Kido, H.; Peytavi, R.; Nakajima-Sasaki, R.; Jasinskas, A.; Micic, M.; Felgner, P.; Madou, M. A multiplexed immunoassay system based upon reciprocating centrifugal microfluidics. Rev. Sci. Instrum. 2011, 82, 064303. [Google Scholar] [CrossRef] [PubMed]
- King, D.; O’Sullivan, M.; Ducrée, J. Optical Detection Strategies for Centrifugal Microfluidic Platforms. J. Mod. Opt. 2014, 61, 85–101. [Google Scholar] [CrossRef]
- Burger, R.; Amato, L.; Boisen, A. Detection Methods for Centrifugal Microfluidic Platforms. Biosens. Bioelectron. 2015, 76, 54–67. [Google Scholar] [CrossRef] [PubMed]
- Grumann, M.; Steigert, J.; Riegger, L.; Moser, I.; Enderle, B.; Riebeseel, K.; Urban, G.; Zengerle, R.; Ducrée, J. Sensitivity Enhancement for Colorimetric Glucose Assays on Whole Blood by On-Chip Beam-Guidance. Biomed. Microdevices 2006, 8, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Nwankire, C.E.; Czugala, M.; Burger, R.; Fraser, K.J.; O’Connell, T.M.; Glennon, T.; Onwuliri, B.E.; Nduaguibe, I.E.; Diamond, D.; Ducrée, J. A Portable Centrifugal Analyser for Liver Function Screening. Biosens. Bioelectron. 2014, 56, 352–358. [Google Scholar] [CrossRef] [PubMed]
- Czugala, M.; Maher, D.; Collins, F.; Burger, R.; Hopfgartner, F.; Yang, Y.; Zhaou, J.; Ducrée, J.; Smeaton, A.; Fraser, K.J.; et al. CMAS: Fully Integrated Portable Centrifugal Microfluidic Analysis System for On-Site Colorimetric Analysis. RSC Adv. 2013, 3, 15928–15938. [Google Scholar] [CrossRef]
- Hwang, H.; Kim, Y.; Cho, J.; Lee, J.Y.; Choi, M.S.; Cho, Y.K. Lab-on-a-Disc for Simultaneous Determination of Nutrients in Water. Anal. Chem. 2013, 85, 2954–2960. [Google Scholar] [CrossRef] [PubMed]
- Kong, M.C.R.; Salin, E.D. Spectrophotometric Determination of Aqueous Sulfide on a Pneumatically Enhanced Centrifugal Microfluidic Platform. Anal. Chem. 2012, 84, 10038–10043. [Google Scholar] [CrossRef] [PubMed]
- Lai, S.; Wang, S.; Luo, J.; Lee, L.J.; Yang, S.T.; Madou, M.J. Design of a Compact Disk-like Microfluidic Platform for Enzyme-Linked Immunosorbent Assay. Anal. Chem. 2004, 76, 1832–1837. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Yuan, Y.; Wang, W.; Chiou Nan-Rong, N.R.; Epstein, A.; Lee, L. Design and Testing of a Microfluidic Biochip for Cytokine Enzyme-Linked Immunosorbent Assay. Biomicrofluidics 2009, 3, 022401. [Google Scholar] [CrossRef] [PubMed]
- La Clair, J.J.; Burkart, M.D. Molecular Screening on a Compact Disc. Org. Biomol. Chem. 2003, 1, 3244–3249. [Google Scholar] [CrossRef] [PubMed]
- Grumann, M.; Brenner, T.; Beer, C.; Zengerle, R.; Ducrée, J. Visualization of Flow Patterning in High-Speed Centrifugal Microfluidics. Rev. Sci. Instrum. 2005, 76, 025101. [Google Scholar] [CrossRef]
- Kim, J.; Liu, G.L.; Lee, L.P. Lens-Scanning Raman Microspectroscopy System Using Compact Disc Optical Pickup Technology. Opt. Express 2005, 13, 4780–4785. [Google Scholar] [CrossRef] [PubMed]
- Kamath, R.R.; Madou, M.J. Three-Dimensional Carbon Interdigitated Electrode Arrays for Redox-Amplification. Anal. Chem. 2014, 86, 2963–2971. [Google Scholar] [CrossRef] [PubMed]
- Madou, M.J. Manufacturing Techniques for Microfabrication and Nanotechnology; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]
- Madou, M.J. From MEMS to Bio-MEMS and Bio-NEMS: Manufacturing Techniques and Applications; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]
- Cruz, A.F.D.; Norena, N.; Kaushik, A.; Bhansali, S. A Low-Cost Miniaturized Potentiostat for Point-of-Care Diagnosis. Biosens. Bioelectron. 2014, 62, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Yellow Springs Instrument Corporation. The Dissolved Oxygen Handbook; YSI, Inc.: Yellow Springs, OH, USA.; p. 76.
- Andreasen, S.Z.; Kwasny, D.; Amato, L.; Brogger, A.L.; Bosco, F.G.; Andersen, K.B.; Svendsen, W.E.; Boisen, A. Integrating Electrochemical Detection with Centrifugal Microfluidics for Real-Time and Fully Automated Sample Testing. RSC Adv. 2015, 5, 17187–17193. [Google Scholar] [CrossRef]
- Cho, H.K.; Lee, Y.H.; Couch, R.A.; Jagadeesh, J.M.; Olson, C.L. Development of a Multichannel Electrochemical Centrifugal Analyzer. Clin. Chem. 1982, 28, 1956–1961. [Google Scholar] [PubMed]
- Kim, T.H.; Abi-Samra, K.; Sunkara, V.; Park, D.K.; Amasia, M.; Kim, N.; Kim, J.; Kim, H.; Madou, M.; Cho, Y.K. Flow-Enhanced Electrochemical Immunosensors on Centrifugal Microfluidic Platforms. Lab Chip 2013, 13, 3747–3754. [Google Scholar] [CrossRef] [PubMed]
- Abi-Samra, K.; Kim, T.H.; Park, D.K.; Kim, N.; Kim, J.; Kim, H.; Cho, Y.K.; Madou, M. Electrochemical Velocimetry on Centrifugal Microfluidic Platforms. Lab Chip 2013, 13, 3253–3260. [Google Scholar] [CrossRef] [PubMed]
- Drummond, T.G.; Hill, M.G.; Barton, J.K. Electrochemical DNA Sensors. Nat. Biotechnol. 2003, 21, 1192–1199. [Google Scholar] [CrossRef] [PubMed]
- Sassolas, A.; Leca-Bouvier, B.D.; Blum, L.J. DNA Biosensors and Microarrays. Chem. Rev. 2008, 108, 109–139. [Google Scholar] [CrossRef] [PubMed]
- Suye, S.; Matsuura, T.; Kimura, T.; Zheng, H.; Hori, T.; Amano, Y.; Katayama, H. Amperometric DNA Sensor Using Gold Electrode Modified with Polymerized Mediator by Layer-by-layer Adsorption. Microelectron. Eng. 2005, 81, 441–447. [Google Scholar] [CrossRef]
- Takenaka, S.; Yamashita, K.; Takagi, M.; Uto, Y.; Kondo, H. DNA Sensing on a DNA Probe-Modified Electrode Using Ferrocenylnaphthalene Diimide as the Electrochemically Active Ligand. Anal. Chem. 2000, 72, 1334–1341. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.S.; Jiang, J.H.; Shen, G.L.; Yu, R.Q. Highly Sensitive DNA Detection and Point Mutation Identification: An Electrochemical Approach Based on the Combined Use of Ligase and Reverse Molecular Beacon. Hum. Mutat. 2007, 28, 630–637. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Duarte, R.; Gorkin III, R.A.; Abi-Samra, K.; Madou, M.J. The Integration of 3D Carbon-Electrode Dielectrophoresis on a CD-Like Centrifugal Microfluidic Platform. Lab Chip 2010, 10, 1030–1043. [Google Scholar] [CrossRef] [PubMed]
- Altman, G.H.; Diaz, F.; Jakuba, C.; Calabro, T.; Horan, R.L.; Chen, J.; Lu, H.; Richmond, J.; Kaplan, D.L. Silk-Based Biomaterials. Biomaterials 2003, 24, 401–416. [Google Scholar] [CrossRef]
- Horrocks, N.P.; Vollrath, F.; Dicko, C. The Silkmoth Cocoon as Humidity Trap and Waterproof Barrier. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2013, 164, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Tulachan, B.; Meena, S.K.; Rai, R.K.; Mallick, C.; Kusurkar, T.S.; Teotia, A.K.; Sethy, N.K.; Bhargava, K.; Bhattacharya, S.; Kumar, A.; et al. Electricity from the Silk Cocoon Membrane. Sci. Rep. 2014, 4, 5434. [Google Scholar] [CrossRef] [PubMed]
- Mano, J.F. Biomimetic Approaches for Biomaterials Development; Wiley-VCH: Weinheim, Germany, 2013. [Google Scholar]
- Anderson, N.G. Computer Interfaced Fast Analyzers. Science 1969, 166, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Wikipedia: List of Microfluidics Research Groups. Available online: https://en.wikipedia.org/wiki/List of microfluidics research groups (accessed on 1 November 2015).
- Joseph, K.; Ibrahim, F.; Cho, J.; Thio, T.H.G.; Al-Faqheri, W.; Madou, M. Design and Development of Micro-Power Generating Device for Biomedical Applications of Lab-on-a-Disc. PLoS ONE 2015, 10, e0136519. [Google Scholar] [CrossRef] [PubMed]
- Chin, C.; Chin, S.; Laksanasopin, T.; Sia, S. Low-Cost Microdevices for Point-of-Care Testing. In Point-of-Care Diagnostics on a Chip; Biological and Medical Physics, Biomedical Engineering; Issadore, D., Westervelt, R.M., Eds.; Springer: Berlin, Germany, 2013; pp. 3–21. [Google Scholar]
- Siegrist, J.; Peytavi, R.; Bergeron, M.; Madou, M. Microfluidics for IVD Analysis: Triumphs and Hurdles of CD Platforms. IVD Technol. 2010, 22–26. [Google Scholar]
- Mark, D.; von Stetten, F.; Zengerle, R. Microfluidic Apps for Off-the-Shelf Instruments. Lab Chip 2012, 12, 2464–2468. [Google Scholar] [CrossRef] [PubMed]
- Thorsen, T.; Maerkl, S.J.; Quake, S.R. Microfluidic Large-Scale Integration. Science 2002, 298, 580–584. [Google Scholar] [CrossRef] [PubMed]
- Honda, N.; Lindberg, U.; Andersson, P.; Hoffmann, S.; Takei, H. Simultaneous Multiple Immunoassays in a Compact Disc-Shaped Microfluidic Device Based on Centrifugal Force. Clin. Chem. 2005, 51, 1955–1961. [Google Scholar] [CrossRef] [PubMed]
- Czilwik, G.; Messinger, T.; Strohmeier, O.; Wadle, S.; von Stetten, F.; Paust, N.; Roth, G.; Zengerle, R.; Saarinen, P.; Niittymäki, J.; et al. Rapid and Fully Automated Bacterial Pathogen Detection on a Centrifugal-Microfluidic LabDisk using Highly Sensitive Nested PCR with Integrated Sample Preparation. Lab Chip 2015, 15, 3749–3759. [Google Scholar] [CrossRef] [PubMed]
- Glynn, M.; Kirby, D.; Chung, D.; Kinahan, D.J.; Kijanka, G.; Ducrée, J. Centrifugo-Magnetophoretic Purification of CD4+ Cells from Whole Blood Toward Future HIV/AIDS Point-of-Care Applications. J. Lab. Autom. 2014, 19, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Schembri, C.; Burd, T.L.; Kopf-Sill, A.R.; Shea, L.R.; Braynin, B. Centrifugation and Capillarity Integrated into a Multiple Analyte Whole Blood Analyser. J. Anal. Methods Chem. 1995, 17, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.E.; Burd, E.M.; Kraft, C.S.; Ryan, E.L.; Duncan, A.; Winkler, A.M.; Cardella, J.C.; Ritchie, J.C.; Parslow, T.G. Laboratory Test Support for Ebola Patients Within a High-Containment Facility. Lab. Med. 2014, 45, e109–e111. [Google Scholar] [CrossRef] [PubMed]
- Owen, W.E.; Caron, J.E.; Genzen, J.R. Liver Function Testing on the Abaxis Piccolo Xpress: Use in Ebola Virus Disease Protocols. Clin. Chim. Acta 2015, 446, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Samsung LABGEO IB10. Available online: http://www.samsung.com/global/business/healthcare /healthcare/in-vitro-diagnostics/BCA-IB10/DE (accessed on 8 November 2015).
- GenePOC. Available online: http://www.genepoc-diagnostics.com/en/ (accessed on 8 November 2015).
- POC Medical Systems Inc. Available online: http://www.pocmedicalsystems.com/ (accessed on 8 November 2015).
- Biosurfit SA. Available online: http://biosurfit.com/ (accessed on 5 January 2016).
- Million Death Study Collaborators. Causes of neonatal and child mortality in India: A nationally representative mortality survey. Lancet 2010, 376, 1853–1860. [Google Scholar]
- Steigert, J.; Grumann, M.; Dube, M.; Streule, W.; Riegger, L.; Brenner, T.; Koltay, P.; Mittmann, K.; Zengerle, R.; Ducrée, J. Direct Hemoglobin Measurement on a Centrifugal Microfluidic Platform for Point-of-Care Diagnostics. Sens. Actuator A Phys. 2006, 130–131, 228–233. [Google Scholar] [CrossRef]
- Riegger, L.; Grumann, M.; Steigert, J.; Lutz, S.; Steinert, C.P.; Mueller, C.; Viertel, J.; Prucker, O.; Rühe, J.; Zengerle, R.; et al. Single-Step Centrifugal Hematocrit Determination on a 10-$ Processing Device. Biomed. Microdevices 2007, 9, 795–799. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.J.; Huang, C.W.; Chen, Z.W.; Chou, J.Y.; Hsiue, G.H. Design of Microfludic Chip for Blood Typing System. In Proceedings of the International Conference System Science and Engineering (ICSSE), Macau, China, 8–10 June 2011; pp. 521–524.
- Li, T.; Fan, Y.; Cheng, Y.; Yang, J. An Electrochemical Lab-on-a-CD System for Parallel Whole Blood Analysis. Lab Chip 2013, 13, 2634–2640. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Okuda, S.; Sawai, A.; Suzuki, S. Development of a N-Acetyl-β-D-glucosaminidase (NAG) Assay on a Centrifugal Lab-on-a-Compact-Disc (Lab-CD) Platform. Anal. Sci. 2012, 28, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.S.; Lee, J.N.; Park, J.M.; Lee, J.G.; Kim, S.; Cho, Y.K.; Ko, C. A Fully Automated Immunoassay from Whole Blood on a Disc. Lab Chip 2009, 9, 1548–1555. [Google Scholar] [CrossRef] [PubMed]
- Ramachandraiah, H.; Amasia, M.; Cole, J.; Sheard, P.; Pickhaver, S.; Walker, C.; Lione, R.; Russom, A. Centrifugal microfluidic system for rapid, low-cost HIV diagnosis: CD4+ T-cell counting using an integrated DVD platform. In Proceedings of the 16th International Conference Miniaturized Systems for Chemistry and Life Sciences (MicroTAS 2012), Okinawa, Japan, 28 October–1 November 2012.
- Strohmeier, O.; Kanat, B.; Bär, D.; Patel, P.; Drexler, J.; Weidmann, M.; van Oordt, T.; Roth, G.; Mark, D.; Zengerle, R.; von Stetten, F. DNA Based Sample to Answer Detection of Bacterial Pathogens on a Centrifugal Microfluidic Foil Cartridge. In Proceedings of the 16th International Conference Miniaturized Systems for Chemistry and Life Sciences (MicroTAS 2012), Okinawa, Japan, 28 October–1 November 2012.
- Salvi, S.; Apte, K.; Madas, S.; Barne, M.; Chhowala, S.; Sethi, T.; Aggarwal, K.; Agrawal, A.; Gogtay, J. Symptoms and Medical Conditions in 204 912 Patients Visiting Primary Health-Care Practitioners in India: A 1-Day Point Prevalence Study (the POSEIDON Study). Lancet Glob Health 2015, 3, e776–e784. [Google Scholar] [CrossRef]
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smith, S.; Mager, D.; Perebikovsky, A.; Shamloo, E.; Kinahan, D.; Mishra, R.; Torres Delgado, S.M.; Kido, H.; Saha, S.; Ducrée, J.; et al. CD-Based Microfluidics for Primary Care in Extreme Point-of-Care Settings. Micromachines 2016, 7, 22. https://doi.org/10.3390/mi7020022
Smith S, Mager D, Perebikovsky A, Shamloo E, Kinahan D, Mishra R, Torres Delgado SM, Kido H, Saha S, Ducrée J, et al. CD-Based Microfluidics for Primary Care in Extreme Point-of-Care Settings. Micromachines. 2016; 7(2):22. https://doi.org/10.3390/mi7020022
Chicago/Turabian StyleSmith, Suzanne, Dario Mager, Alexandra Perebikovsky, Ehsan Shamloo, David Kinahan, Rohit Mishra, Saraí M. Torres Delgado, Horacio Kido, Satadal Saha, Jens Ducrée, and et al. 2016. "CD-Based Microfluidics for Primary Care in Extreme Point-of-Care Settings" Micromachines 7, no. 2: 22. https://doi.org/10.3390/mi7020022
APA StyleSmith, S., Mager, D., Perebikovsky, A., Shamloo, E., Kinahan, D., Mishra, R., Torres Delgado, S. M., Kido, H., Saha, S., Ducrée, J., Madou, M., Land, K., & Korvink, J. G. (2016). CD-Based Microfluidics for Primary Care in Extreme Point-of-Care Settings. Micromachines, 7(2), 22. https://doi.org/10.3390/mi7020022











