High-Precision and Low-Cost Wireless 16-Channel Measurement System for Malachite Green Detection
Abstract
:1. Introduction
2. Method and Architecture of the Measurement System
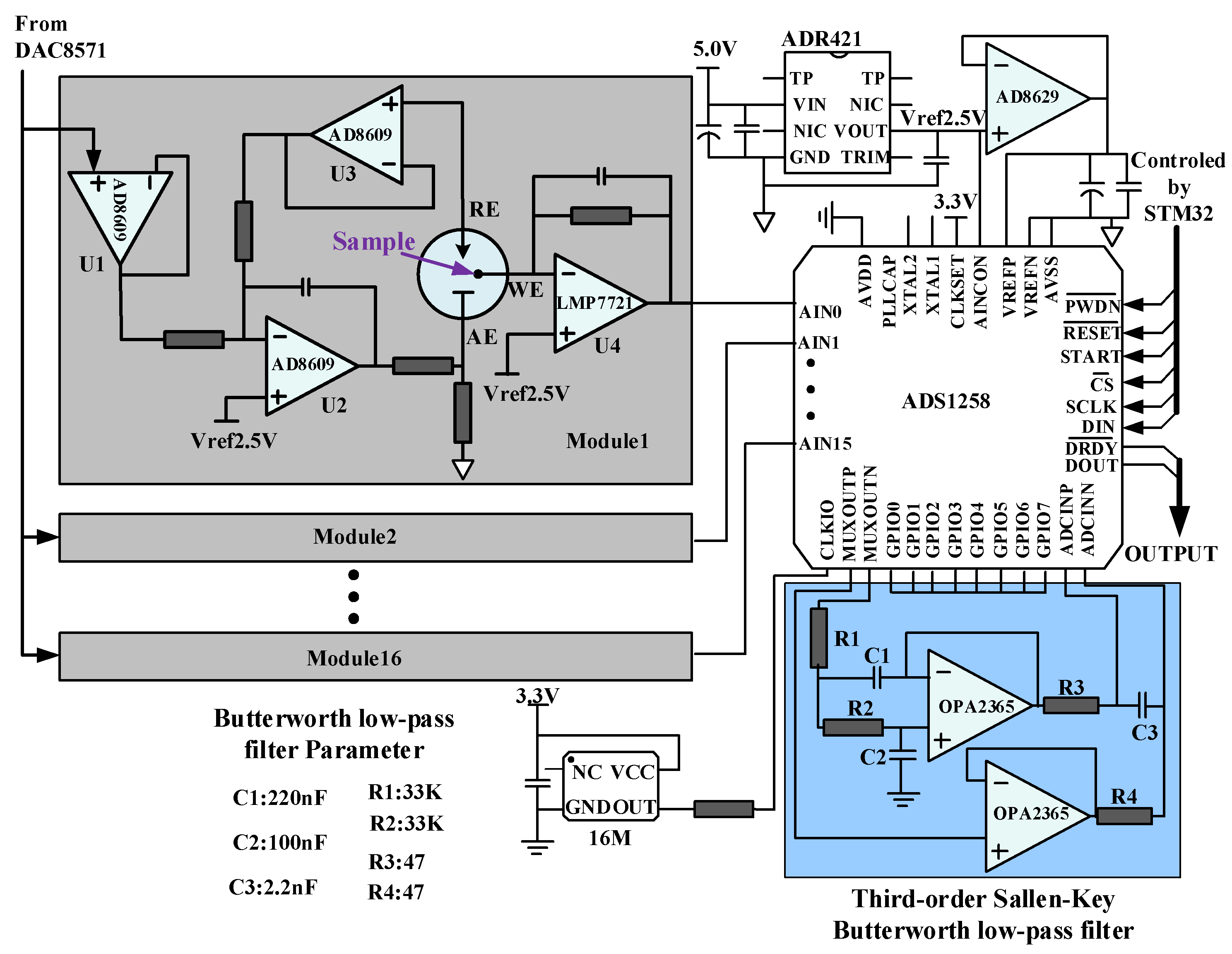
2.1. Construction of the DNA Biosensor
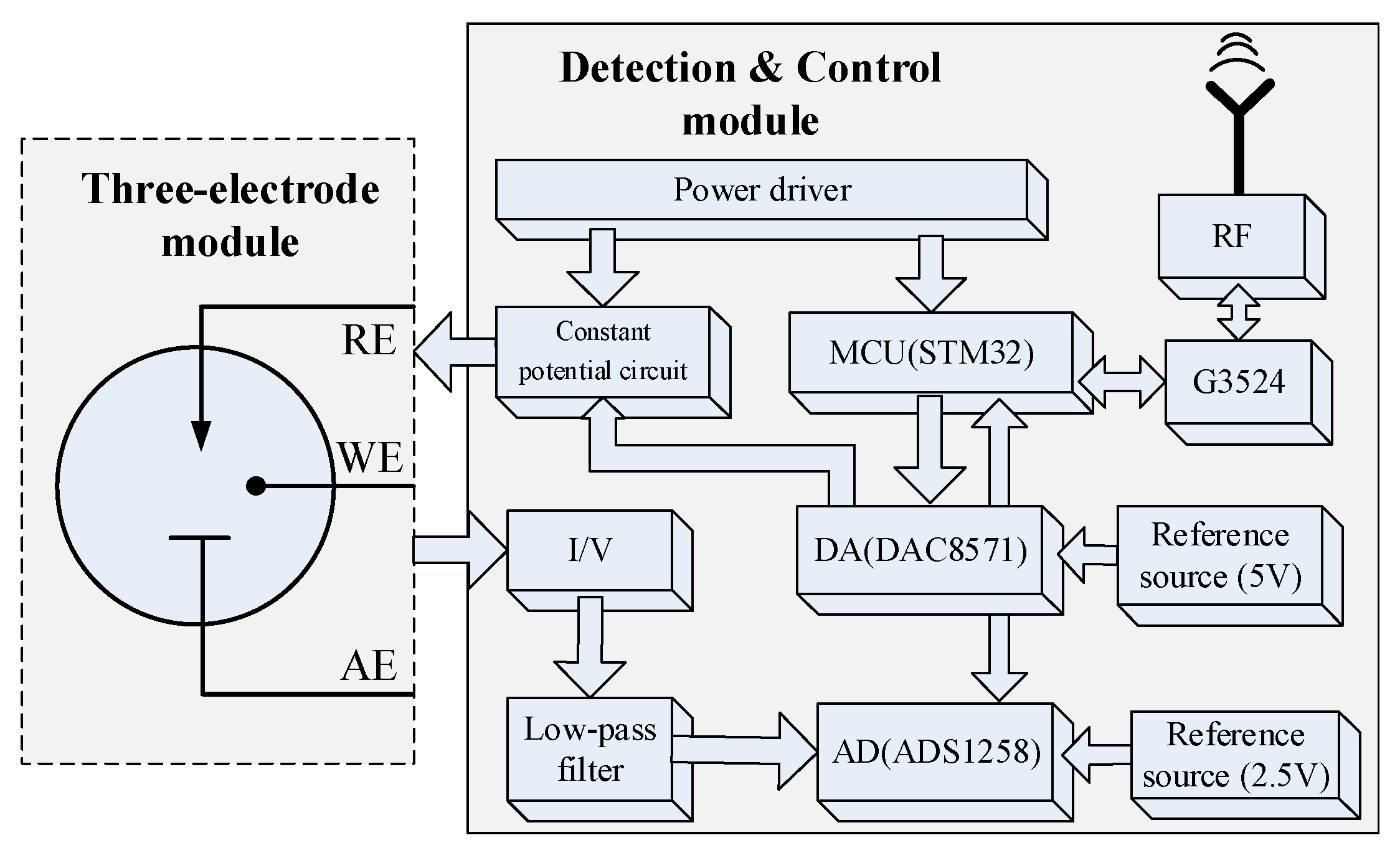
2.2. Hand-Held Electrochemical Instrument
3. Characteristics of Measurement System
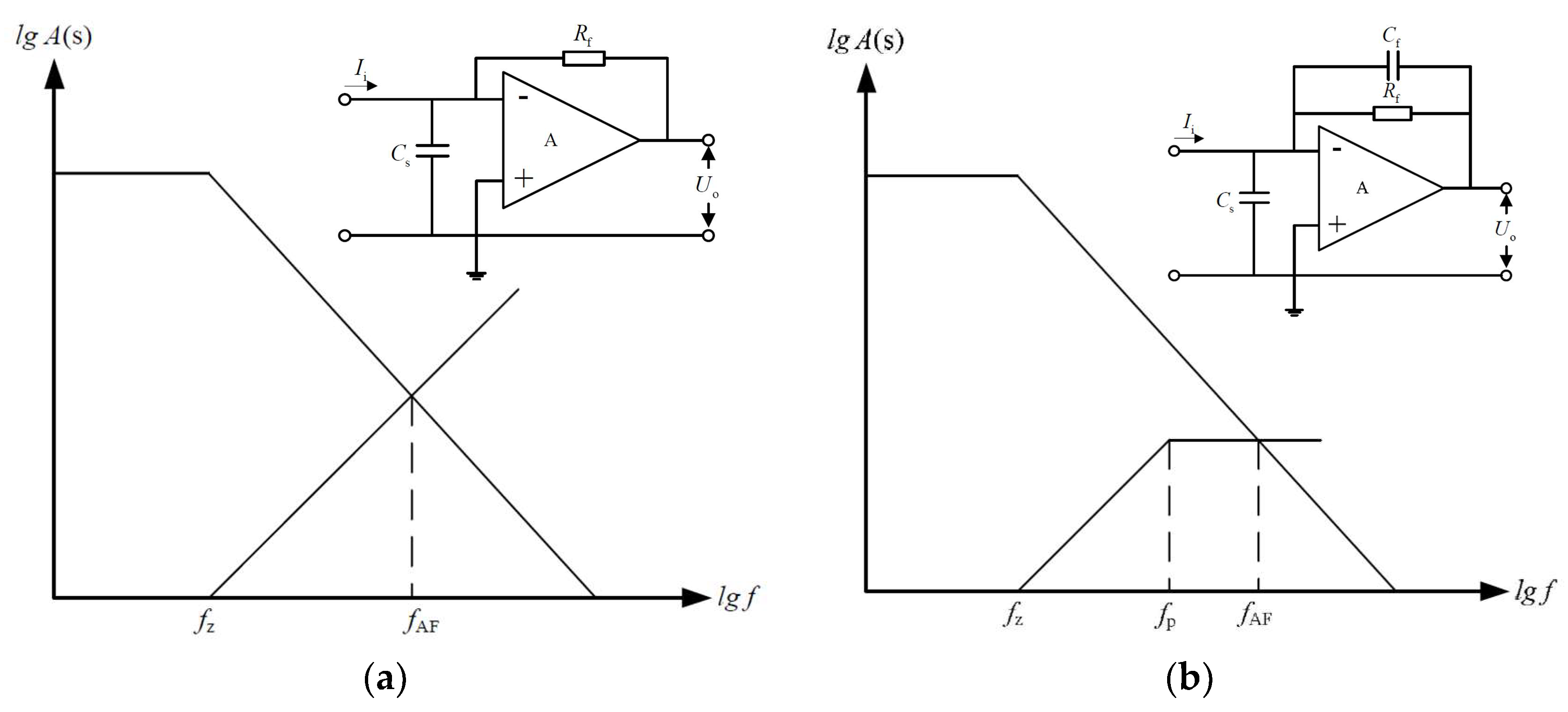
3.1. High Detection Accuracy
3.2. Multichannel Measurement Method
4. Results and Discussion
4.1. Multichannel Precision Test
4.2. Biosensor Test
4.3. Actual Samples Test
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kagalkar, A.N.; Jadhav, M.U.; Bapat, V.A.; Govindwar, S.P. Phytodegradation of the triphenylmethane dye Malachite Green mediated by cell suspension cultures of Blumea malcolmii Hook. Bioresour. Technol. 2011, 102, 10312–10318. [Google Scholar] [CrossRef] [PubMed]
- Pierrard, M.A.; Kestemont, P.; Phuong, N.T.; Tran, M.P.; Delaive, E.; Thezenas, M.L.; Dieu, M.; Raes, M.; Silvestre, F. Proteomic analysis of blood cells in fish exposed to chemotherapeutics: Evidence for long term effects. J. Proteomics 2012, 75, 2454–2467. [Google Scholar] [CrossRef] [PubMed]
- Posyniak, A.; Zmudzki, J.; Mitrowska, K. Dispersive solid-phase extraction for the determination of sulfonamides in chicken muscle by liquid chromatography. J. Chromatogr. A 2005, 1087, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Fallah, A.A.; Barani, A. Determination of malachite green residues in farmed rainbow trout in Iran. Food Control 2014, 40, 100–105. [Google Scholar] [CrossRef]
- Nebot, C.; Iglesias, A.; Barreiro, R.; Manuel, J.; Beatriz, M.; Carlos, V.; Franco, M.; Cepeda, A. A simple and rapid method for the identification and quantification of malachite green and its metabolite in hake by HPLC–MS/MS. Food Control 2013, 31, 102–107. [Google Scholar] [CrossRef]
- Sacara, A.M.; Nairi, V.; Salis, A.; Turdean, G.L.; Muresan, L.M. Silica-modified Electrodes for Electrochemical Detection of Malachite Green. Electroanalysis 2017, 29, 2602–2609. [Google Scholar] [CrossRef]
- Sacara, A.M.; Cristea, C.; Muresan, L.M. Electrochemical detection of Malachite Green using glassy carbon electrodes modified with CeO2, nanoparticles and Nafion. J. Electroanal. Chem. 2017, 792, 23–30. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, Y.; Trombly, N.; Yang, C.; Mason, A. Electrochemical array microsystem with integrated potentiostat. IEEE Sens. 2005. [Google Scholar] [CrossRef]
- Turner, R.F.B.; Harrison, D.J.; Baltes, H.P. A CMOS potentiostat for amperometric chemical sensors. IEEE J. Solid-State Circuits 1987, 22, 473–478. [Google Scholar] [CrossRef]
- Shi, Y.; Dou, H.; Zhou, A.; Chen, Y.Q. Design and fabrication of a miniaturized electrochemical instrument and its preliminary evaluation. Sens. Actuators B Chem. 2008, 131, 516–524. [Google Scholar] [CrossRef]
- Lin, Z.; Huang, H.; Xu, Y.; Gao, X.; Qiu, B.; Chen, X.; Chen, G. Determination of microcystin-LR in water by a label-free aptamer based electrochemical impedance biosensor. Talanta 2013, 103, 371–374. [Google Scholar] [CrossRef]
- Li, Y.; Bao, J.; Han, M.; Dai, Z.; Wang, H. A simple assay to amplify the electrochemical signal by the aptamer based biosensor modified with CdS hollow nanospheres. Biosens. Bioelectron. 2011, 26, 3531–3535. [Google Scholar] [CrossRef]
- Lao, R.; Song, S.; Wu, H.; Wang, L.; Zhang, Z.; He, L.; Fan, C. Electrochemical interrogation of DNA monolayers on gold surfaces. Anal. Chem. 2005, 77, 6475–6480. [Google Scholar] [CrossRef]
- Sun, W.; Qin, P.; Gao, H.; Li, G.; Jiao, K. Electrochemical DNA biosensor based on chitosan/nano-V2O5/MWCNTs composite film modified carbon ionic liquid electrode and its application to the LAMP product of Yersinia enterocolitica gene sequence. Biosens. Bioelectron. 2010, 25, 1264–1270. [Google Scholar] [CrossRef]
- Wan, Y.; Su, Y.; Zhu, X.; Yang, S.; Lu, J.; Gao, J.; Fan, C.; Huang, Q. Ultrasensitive electrochemical DNA sensor based on the target induced structural switching and surface-initiated enzymatic polymerization. Biosens. Bioelectron. 2014, 55, 231–236. [Google Scholar] [CrossRef]
- Savas, S.; Ersoy, A.; Gulmez, Y.; Kilic, S.; Levent, B.; Altintas, Z. Nanoparticle Enhanced Antibody and DNA Biosensors for Sensitive Detection of Salmonella. Materials 2018, 11, 1541. [Google Scholar] [CrossRef]
- Wang, H.; Wang, Y.; Liu, S.; Yu, J.; Xu, W.; Guo, Y.; Huang, J. An RNA aptamer-based electrochemical biosensor for sensitive detection of malachite green. RSC Adv. 2014, 4, 60987–60994. [Google Scholar] [CrossRef]
- Yao, W.; Hou, Y.; Wang, G.; Liu, H. The Research and Design of the Remote Monitoring Terminal Based the Wireless Communications Fault Indicator and GPRS. Electr. Eng. 2012, 3, 52–54. [Google Scholar]
- Suzuki, H.; Hirakawa, T.; Sasaki, S.; Karube, I. An integrated three-electrode system with a micromachined liquid-junction Ag/AgCl reference electrode. Anal. Chim. Acta 1999, 387, 103–112. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods: Fundamentals and Applications, 2nd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2001. [Google Scholar]
- Bonomo, M.; Gatti, D.; Barolo, C.; Dini, D. Effect of Sensitization on the Electrochemical Properties of Nanostructured NiO. Coatings 2018, 8, 232. [Google Scholar] [CrossRef]
- Laschuk, N.O.; Ebralidze, I.I.; Poisson, J.; Egan, J.G.; Quaranta, S.; Allan, J.T.; Cusden, H.; Gaspari, F.; Naumkin, F.Y.; Easton, E.B.; et al. Ligand Impact on Monolayer Electrochromic Material Properties. ACS Appl. Mater. Interfaces 2018, 10, 35334–35343. [Google Scholar] [CrossRef]
- Ferri, J.; Lidón-Roger, J.V.; Moreno, J.; Martinez, G.; Garcia-Breijo, E. A Wearable Textile 2D Touchpad Sensor Based on Screen-Printing Technology. Materials 2017, 10, 1450. [Google Scholar] [CrossRef]
- Zhou, T.; Dong, T.; Su, Y.; He, Y. High-precision and low-cost wireless 16-channel measurement system for multi-layer thin film characterization. Meas. J. Int. Meas. Confed. 2013, 46, 3600–3611. [Google Scholar] [CrossRef]




| Measured Value | Mean of Channels/A | Standard Deviation of Channels/A |
|---|---|---|
| 2 mA | 0.0020062 | 10782 × 10−6 |
| 700 μA | 0.0007025 | 9.203 × 10−7 |
| 200 μA | 0.0002002 | 5.999 × 10−7 |
| 70 μA | 6.995 × 10−5 | 1.542 × 10−7 |
| 20 μA | 1.996 × 10−5 | 6.725 × 10−8 |
| 7 μA | 6.986 × 10−6 | 1.087 × 10−8 |
| 2 μA | 2 × 10−6 | 6.205 × 10−9 |
| 700 nA | 7 × 10−7 | 1.426 × 10−9 |
| 200 nA | 2.003 × 10−7 | 6.715 × 10−10 |
| 80 nA | 8.037 × 10−8 | 7.706 × 10−10 |
| 12 nA | 1.21 × 10−8 | 1.32 × 10−10 |
| 1 nA | 1.055 × 10−9 | 1.02 × 10−10 |
| Adding Standard (μg/mL) | Measured Quantity (μg/mL) | ELISA Measured Result (μg/mL) |
|---|---|---|
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, T.; Zhou, T.; Wan, Y.; Su, Y. High-Precision and Low-Cost Wireless 16-Channel Measurement System for Malachite Green Detection. Micromachines 2018, 9, 646. https://doi.org/10.3390/mi9120646
Shen T, Zhou T, Wan Y, Su Y. High-Precision and Low-Cost Wireless 16-Channel Measurement System for Malachite Green Detection. Micromachines. 2018; 9(12):646. https://doi.org/10.3390/mi9120646
Chicago/Turabian StyleShen, Tong, Tong Zhou, Ying Wan, and Yan Su. 2018. "High-Precision and Low-Cost Wireless 16-Channel Measurement System for Malachite Green Detection" Micromachines 9, no. 12: 646. https://doi.org/10.3390/mi9120646
APA StyleShen, T., Zhou, T., Wan, Y., & Su, Y. (2018). High-Precision and Low-Cost Wireless 16-Channel Measurement System for Malachite Green Detection. Micromachines, 9(12), 646. https://doi.org/10.3390/mi9120646





