Heterodimeric Plasmonic Nanogaps for Biosensing
Abstract
1. Introduction
2. Experimental Section
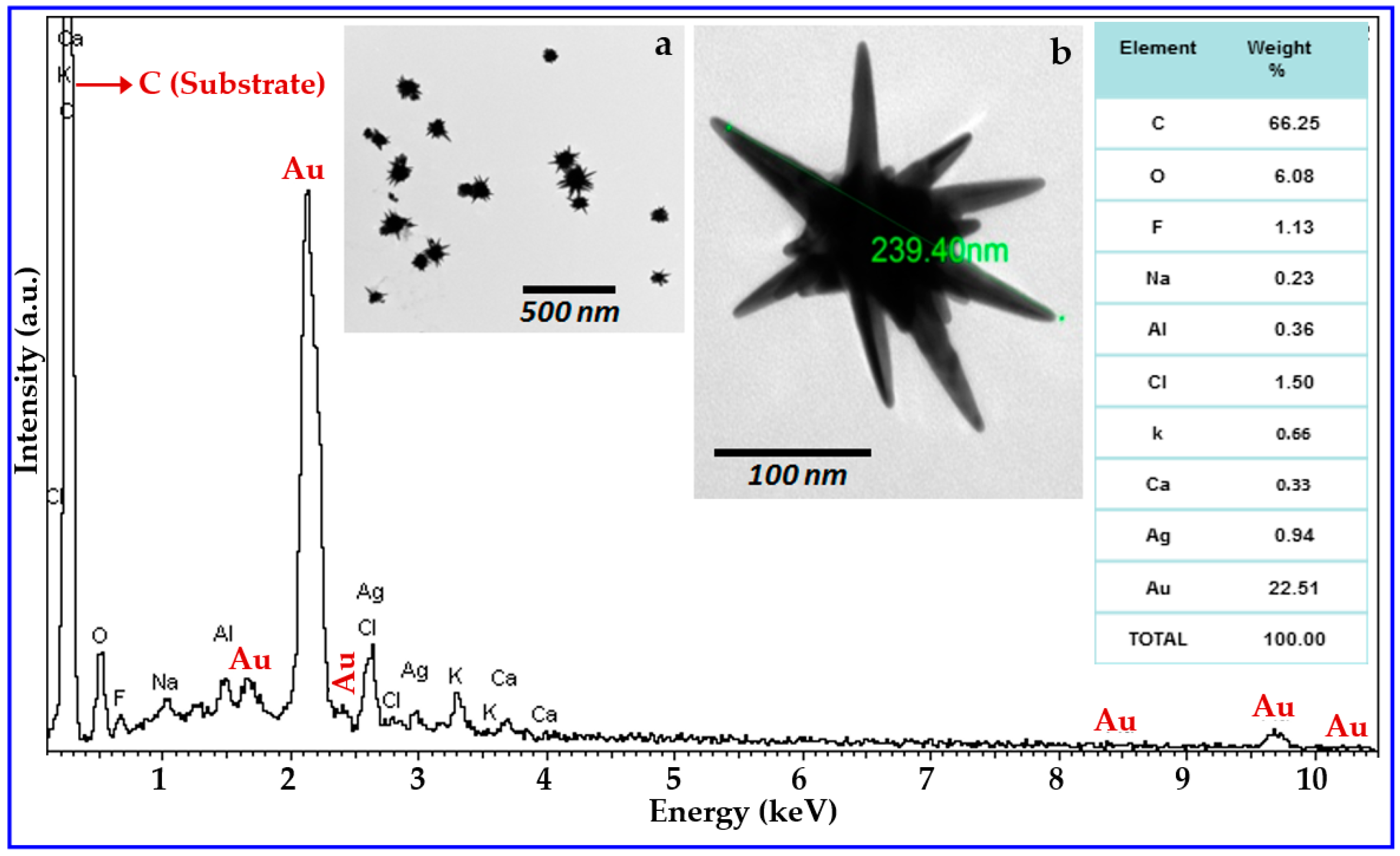
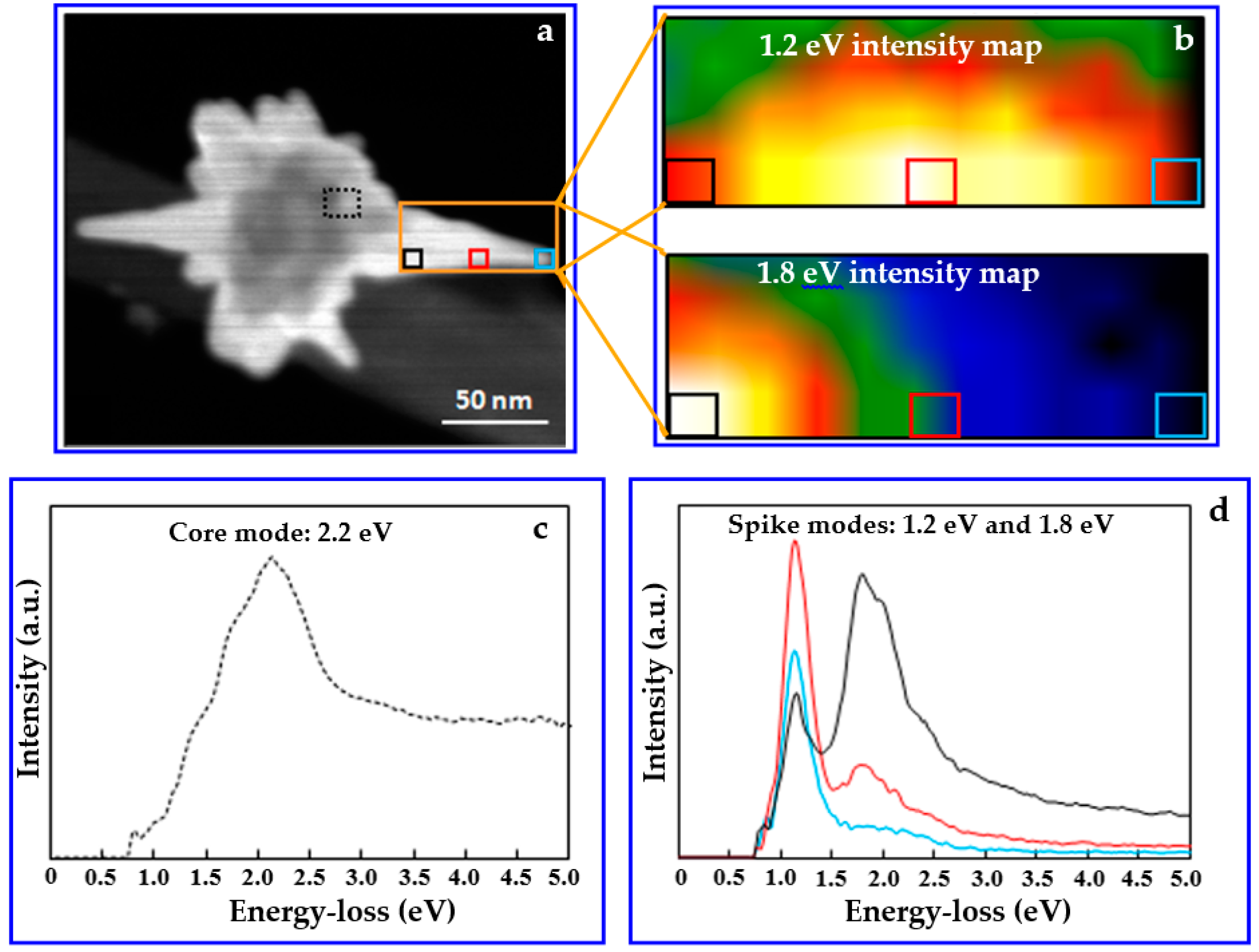
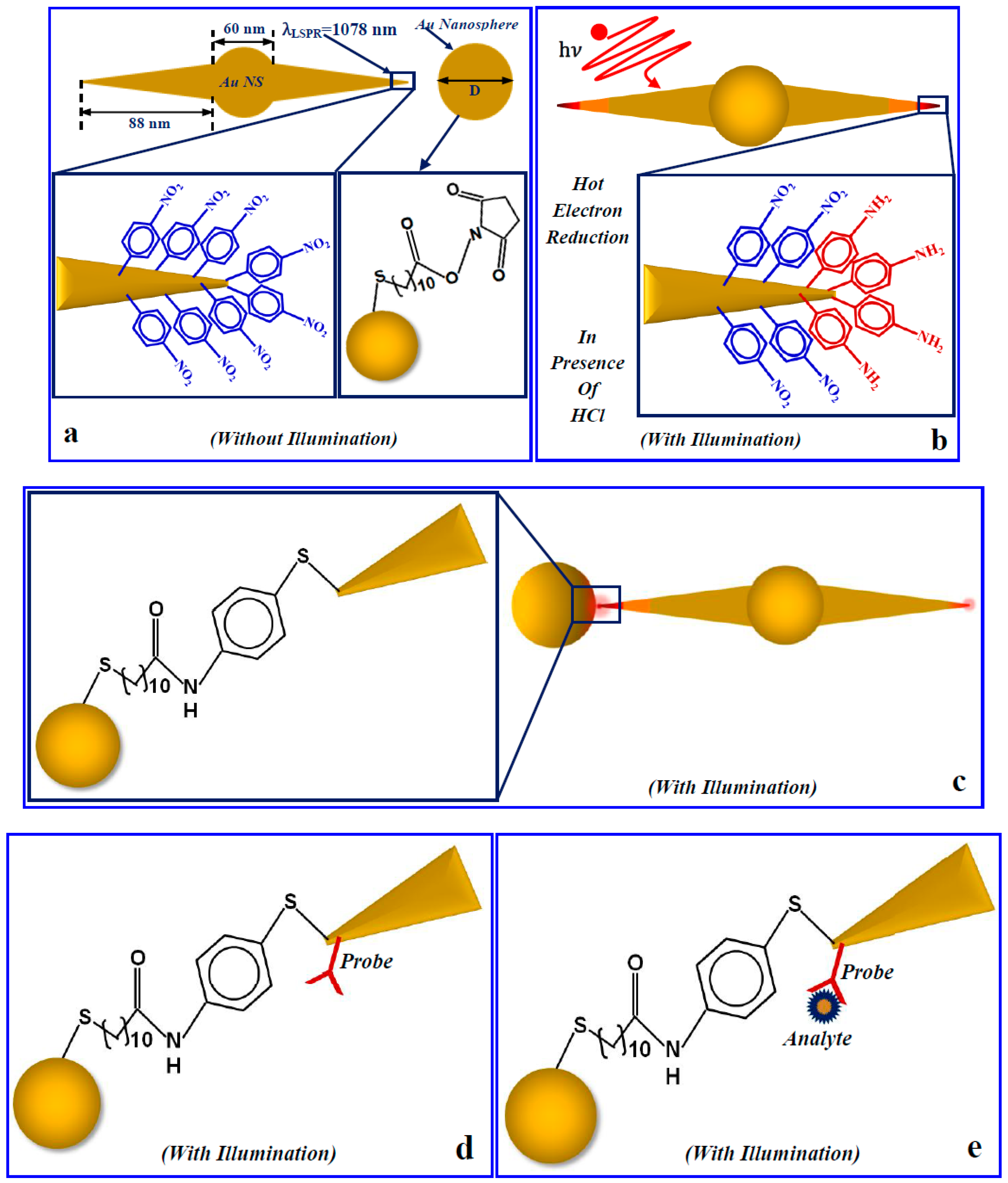
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Rezaei, N. Cancer Immunology; Springer: New York, NY, USA, 2014. [Google Scholar]
- Prasad, P.N. Introduction to Biophotonics; John Wiley & Sons: Hoboken, NJ, USA, 2003. [Google Scholar]
- Schuller, J.A.; Barnard, E.S.; Cai, W.S.; Jun, Y.C.; White, J.S.; Brongersma, M.L. Plasmonics for Extreme Light Concentration and Manipulation. Nat. Mater. 2010, 9, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B.; Frontiera, R.R.; Henry, A.I.; Ringe, E.; Van Duyne, R.P. SERS: Materials, Applications, and The Future. Mater. Today 2012, 15, 16–25. [Google Scholar] [CrossRef]
- Nie, S.M.; Emery, S.R. Probing Single Molecules and Single Nanoparticles by Surface-Enhanced Raman Scattering. Science 1997, 275, 1102–1106. [Google Scholar] [CrossRef] [PubMed]
- Willets, K.A.; Van Duyne, R.P. Localized Surface Plasmon Resonance Spectroscopy and Sensing. Annu. Rev. Phys. Chem. 2007, 58, 267–297. [Google Scholar] [CrossRef] [PubMed]
- Sreekanth, K.V.; Alapan, Y.; ElKabbash, M.; Ilker, E.; Hinczewski, M.; Gurkan, U.A.; De Luca, A.; Strangi, G. Extreme Sensitivity Biosensing Platform Based on Hyperbolic Metamaterials. Nat. Mater. 2016, 15, 621–627. [Google Scholar] [CrossRef] [PubMed]
- Sekhon, J.S.; Malik, H.K.; Verma, S.S. DDA Simulations of Noble Metal and Alloy Nanocubes for Tunable Optical Properties in Biological Imaging and Sensing. RSC Adv. 2013, 3, 15427–15434. [Google Scholar] [CrossRef]
- Hou, W.B.; Cronin, S.B. A Review of Surface Plasmon Resonance Enhanced Photocatalysis. Adv. Funct. Mater. 2013, 23, 1612–1619. [Google Scholar] [CrossRef]
- Reineck, P.; Lee, G.P.; Brick, D.; Karg, M.; Mulvaney, P.; Bach, U. A Solid-State Plasmonic Solar Cell Via Metal Nanoparticle Self-Assembly. Adv. Mater. 2012, 24, 4750–4755. [Google Scholar] [CrossRef]
- Chen, H.C.; Chou, S.W.; Tseng, W.H.; Chen, I.W.P.; Liu, C.C.; Liu, C.; Liu, C.L.; Chen, C.H.; Wu, C.I.; Chou, P.T. Large Au-Ag Alloy Nanoparticles Synthesized in Organic Media Using a One-Pot Reaction: Their Applications for High-Performance Bulk Heterojunction Solar Cells. Adv. Funct. Mater. 2012, 22, 3975–3984. [Google Scholar] [CrossRef]
- Kelkar, S.S.; Reineke, T.M. Theranostics: Combining Imaging and Therapy. Bioconj. Chem. 2011, 22, 1879–1903. [Google Scholar] [CrossRef]
- Barnes, W.L.; Dereux, A.; Ebbesen, T.W. Surface Plasmon Subwavelength Optics. Nature 2003, 424, 824–830. [Google Scholar] [CrossRef] [PubMed]
- Ozbay, E. Plasmonics: Merging Photonics and Electronics at Nanoscale Dimensions. Science 2006, 311, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Kelly, K.L.; Coronado, E.; Zhao, L.L.; Schatz, G.C. The Optical Properties of Metal Nanoparticles: The Influence of Size, Shape, and Dielectric Environment. J. Phys. Chem. B 2003, 107, 668–677. [Google Scholar] [CrossRef]
- Scholl, J.A.; Koh, A.L.; Dionne, J.A. Quantum Plasmon Resonances of Individual Metallic Nanoparticles. Nature 2012, 483, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Baniukevic, J.; Hakki Boyaci, I.; Goktug Bozkurt, A.; Tamer, U.; Ramanavicius, A.; Ramanaviciene, A. Magnetic gold nanoparticles in SERS-based sandwich immunoassay for antigen detection by well oriented antibodies. Biosensors and Bioelectronics 2013, 43, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Nelayah, J.; Kociak, M.; Stephan, O.; Garcia de Abajo, F.J.; Tence, M.; Henrard, L.; Taverna, D.; Pastoriza-Santos, I.; Liz-Marzan, L.M.; Colliex, C. Mapping Surface Plasmons on a Single Metallic Nanoparticle. Nat. Phys. 2007, 3, 348–353. [Google Scholar] [CrossRef]
- Rycenga, M.; Xia, X.; Moran, C.H.; Zhou, F.; Qin, D.; Li, Z.-Y.; Xia, Y. Generation of Hot Spots with Silver Nanocubes for Single-Molecule Detection by Surface-Enhanced Raman Scattering. Angew. Chem. Int. Ed. 2011, 50, 5473–5477. [Google Scholar] [CrossRef]
- Sun, Y.G.; Xia, Y.N. Shape-Controlled Synthesis of Gold and Silver Nanoparticles. Science 2002, 298, 2176–2179. [Google Scholar] [CrossRef]
- Chen, H.J.; Shao, L.; Li, Q.; Wang, J.F. Gold Nanorods and Their Plasmonic Properties. Chem. Soc. Rev. 2013, 42, 2679–2724. [Google Scholar] [CrossRef]
- Lee, S.J.; Moskovits, M. Remote Sensing by Plasmonic Transport. J. Am. Chem. Soc. 2012, 134, 11384–11387. [Google Scholar] [CrossRef]
- Rodríguez-Lorenzo, L.; Alvarez-Puebla, R.A.; Pastoriza-Santos, I.; Mazzucco, S.; Stéphan, O.; Kociak, M.; Liz-Marzán, L.M.; García de Abajo, F.J. Zeptomol Detection through Controlled Ultrasensitive Surface-Enhanced Raman Scattering. J. Am. Chem. Soc. 2009, 131, 4616–4618. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.-G.; Jiang, Y.-X.; Zhou, Z.-Y.; Chen, S.-P.; Sun, S.-G. Shape-Controlled Synthesis of Gold Nanoparticles in Deep Eutectic Solvents for Studies of Structure-Functionality Relationships in Electrocatalysis. Angew. Chem. Int. Ed. 2008, 47, 9100–9103. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.-L.; Chen, C.-H.; Huang, M.H. Seed-Mediated Synthesis of Branched Gold Nanocrystals Derived from the Side Growth of Pentagonal Bipyramids and the Formation of Gold Nanostars. Chem. Mater. 2008, 21, 110–114. [Google Scholar] [CrossRef]
- Liu, X.-L.; Wang, J.-H.; Liang, S.; Yang, D.-J.; Nan, F.; Ding, S.-J.; Zhou, L.; Hao, Z.-H.; Wang, Q.-Q. Tuning Plasmon Resonance of Gold Nanostars for Enhancements of Nonlinear Optical Response and Raman Scattering. J. Phys. Chem. C 2014, 118, 9659–9664. [Google Scholar] [CrossRef]
- Zhang, J.; Gao, Y.; Alvarez-Puebla, R.A.; Buriak, J.M.; Fenniri, H. Synthesis and SERS Properties of Nanocrystalline Gold Octahedra Generated from Thermal Decomposition of HAuCl4 in Block Copolymers. Adv. Mater. 2006, 18, 3233–3237. [Google Scholar] [CrossRef]
- Li, C.R.; Lu, N.P.; Mei, J.; Dong, W.J.; Zheng, Y.Y.; Gao, L.; Tsukamoto, K.; Cao, Z.X. Polyhedral to Nearly Spherical Morphology Transformation of Silver Microcrystals Grown from Vapor Phase. J. Cryst. Growth 2011, 314, 324–330. [Google Scholar] [CrossRef]
- Sarid, D.; Challener, W. Modern Introduction to Surface Plasmons: Theory, Mathematica Modeling, and Applications; Cambridge University Press: Cambridge, UK, 2010. [Google Scholar]
- Giannini, V.; Rodríguez-Oliveros, R.; Sánchez-Gil, J.A. Surface Plasmon Resonances of Metallic Nanostars/ Nanoflowers for Surface-Enhanced Raman Scattering. Plasmonics 2010, 5, 99–104. [Google Scholar] [CrossRef]
- Alvarez-Puebla, R.; Liz-Marzan, L.M.; García de Abajo, F.J. Light Concentration at the Nanometer Scale. J. Phys. Chem. Lett. 2010, 1, 2428–2434. [Google Scholar] [CrossRef]
- Etchegoin, P.G.; Le Ru, E.C. Surface Enhanced Raman Spectroscopy: Analytical, Biophysical and Life Science Applications. Chapter I—“Basic Electromagnetic Theory of SERS”; WILEY-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2010. [Google Scholar]
- Bohren, C.F.; Huffman, D.R. Adsorption and Scattering of Light by Small Particles; Wiley-VCH: New York, NY, USA, 1983. [Google Scholar]
- Jackson, J.D. Classical Electrodynamics; Wiley: New York, NY, USA, 1999. [Google Scholar]
- Maier, S.A.; Atwater, H.A. Plasmonics: Localization and Guiding of Electromagnetic Energy in Metal/Dielectric Structures. J. Appl. Phys. 2005, 98, 0111011. [Google Scholar] [CrossRef]
- Wang, H. Brandl, D.W.; Le, F.; Nordlander, P.; Halas, N.J. Nanorice: A Hybrid Plasmonic Nanostructure. Nano Lett. 2006, 6, 827–832. [Google Scholar] [CrossRef] [PubMed]
- Liaw, J.W.; Kuo, M.K.; Liao, C.N. Plasmon Resonances of Spherical and Ellipsoidal Nanoparticles. J. Electromagn. Waves Appl. 2005, 19, 1787–1794. [Google Scholar] [CrossRef]
- Mohamed, M.B.; Volkov, V.; Link, S.; El-Sayed, M.A. The ‘Lightning’ Gold Nanorods: Fluorescence Enhancement of over a Million Compared to the Gold Metal. Chem. Phys. Lett. 2000, 317, 517–523. [Google Scholar] [CrossRef]
- Colleen, L.N.; Liao, H.; Hafner, J.H. Optical Properties of Star-Shaped Gold Nanoparticles. Nano Lett. 2006, 6, 683–688. [Google Scholar]
- Kumar, P.S.; Pastoriza-Santos, I.; Rodriguez-Gonzalez, B.; García de Abajo, F.J.; Javier, F.; Liz-Marzan, L.M. High-Yield Synthesis and Optical Response of Gold Nanostars. Nanotechnology 2008, 19, 0156061. [Google Scholar]
- Chen, Q.; Kaneko, T.; Hatakeyama, R. Green Tea Induced Gold Nanostar Synthesis Mediated by Ag(I) Ions. Condens. Mater. 2014, 1585, 1401–1417. [Google Scholar]
- Moukarzel, W.; Fitremann, J.; Marty, J.D. Seed-Less Amino-Sugar Mediated Synthesis of Gold Nanostars. Nanoscale 2011, 3, 3285–3290. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Khoury, C.G.; Hwang, H.; Wilson, C.M.; Grant, G.A.; Vo-Dinh, T. Gold Nanostars: Surfactant-Free Synthesis, 3D Modelling, and Two-Photon Photoluminescence Imaging. Nanotechnology 2012, 23, 075102. [Google Scholar] [CrossRef]
- Kedia, A.; Kumar, P.S. Controlled Reshaping and Plasmon Tuning Mechanism of Gold Nanostars. J. Mater. Chem. C 2013, 1, 4540–4549. [Google Scholar] [CrossRef]
- Minati, L.; Benetti, F.; Chiappini, A.; Speranza, G. One-Step Synthesis of Star-Shaped Gold Nanoparticles. Physicochem. Eng. Asp. 2014, 441, 623–628. [Google Scholar] [CrossRef]
- Chatterjee, S.; Ringane, A.B.; Arya, A.; Das, G.M.; Dantham, V.R.; Laha, R.; Hussain, S. A High-Yield, One-Step Synthesis of Surfactant-Free Gold Nanostars and Numerical Study for Single Molecule SERS Application. J. Nanopart. Res. 2016, 18, 242–249. [Google Scholar] [CrossRef]
- Krug, M.K.; Reisecker, M.; Hohenau, A.; Ditlbacher, H.; Trugler, A.; Hohenester, U.; Krenn, J.R. Probing Plasmonic Breathing Modes Optically. Appl. Phys. Lett. 2014, 105, 1711031–1711033. [Google Scholar] [CrossRef]
- Chu, M.W.; Myroshnychenko, V.; Chen, C.H.; Deng, J.P.; Mou, C.Y.; García de Abajo, F.J. Probing Bright and Dark Surface-Plasmon Modes in Individual and Coupled Noble Metal Nanoparticles Using an Electron Beam. Nano Lett. 2009, 9, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wu, Y.; Lassiter, B.; Nehl, C.L.; Hafner, J.H.; Nordlander, P.; Halas, N.J. Symmetry Breaking in Individual Plasmonic Nanoparticles. Proc. Natl. Acad. Sci. USA 2006, 103, 10856–10860. [Google Scholar] [CrossRef] [PubMed]
- Koh, A.L.; Bao, K.; Khan, I.; Smith, W.E.; Kothleitner, G.; Nordlander, P.; Maier, S.A.; McComb, D.W. Electron Energy-Loss Spectroscopy (EELS) of Surface Plasmons in Single Silver Nanoparticles and Dimers: Influence of Beam Damage and Mapping of Dark Modes. ACS Nano 2009, 3, 3015–3022. [Google Scholar] [CrossRef] [PubMed]
- Morla-Folch, J.; Guerrini, L.; Pazos-Perez, N.; Arenal, R.; Alvarez-Puebla, R.A. Synthesis and Optical Properties of Homogeneous Nanoshurikens. ACS Photonics 2014, 1, 1237–1244. [Google Scholar] [CrossRef]
- Wu, Y.; Li, G.; Cherqui, C.; Bigelow, N.W.; Thakkar, N.; Masiello, D.J.; Camden, J.P.; Rack, P.D. Electron Energy Loss Spectroscopy Study of the Full Plasmonic Spectrum of Self-Assembled Au–Ag Alloy Nanoparticles: Unraveling Size, Composition, and Substrate Effects. ACS Photonics 2016, 3, 130–138. [Google Scholar] [CrossRef]
- Mazzucco, S.; Stéphan, O.; Colliex, C.; Pastoriza-Santos, I.; Liz-Marzan, L.M.; García de Abajo, F.J.; Kociak, M. Spatially Resolved Measurements of Plasmonic Eigenstates in Complex-Shaped, Asymmetric Nanoparticles: Gold Nanostars. Eur. Phys. J. Appl. Phys. 2011, 54, 335121–335129. [Google Scholar] [CrossRef]
- García de Abajo, F.J.; Kociak, M. Probing the Photonic Local Density of States with Electron Energy Loss Spectroscopy. Phys. Rev. Lett. 2008, 100, 1068041–1068044. [Google Scholar] [CrossRef]
- Myroshnychenko, V.; Nelayah, J.; Adamo, G.; Geuquet, N.; Rodriguez-Fernandez, J.; Pastoriza-Santos, I.; MacDonald, K.F.; Henrard, L.; Liz-Marzan, L.M.; Zheludev, N.I.; et al. Plasmon Spectroscopy and Imaging of Individual Gold Nanodecahedra: A Combined Optical Microscopy, Cathodoluminescence, and Electron Energy-Loss Spectroscopy Study. Nano Lett. 2012, 12, 4172–4180. [Google Scholar] [CrossRef]
- Losquin, A.; Zagonel, L.F.; Myroshnychenko, V.; Rodriguez-Gonzalez, B.; Tencé, M.; Scarabelli, L.; Förstner, J.; Liz-Marzan, L.M.; García de Abajo, F.J.; Stephan, O.; Kociak, M. Unveiling Nanometer Scale Extinction and Scattering Phenomena through Combined Electron Energy Loss Spectroscopy and Cathodoluminescence Measurements. Nano Lett. 2015, 15, 1229–1237. [Google Scholar] [CrossRef]
- Myroshnychenko, V.; Rodríguez-Fernández, J.; Pastoriza-Santos, I.; Funston, A.M.; Novo, C.; Mulvaney, P.; Liz-Marzán, L.M.; García de Abajo, F.J. Modelling the Optical Response of Gold Nanoparticles. Chem. Soc. Rev. 2008, 37, 1792–1805. [Google Scholar] [CrossRef] [PubMed]
- Novikov, S.M.; Sánchez-Iglesias, A.; Schmidt, M.K.; Chuvilin, A.; Aizpurua, J.; Grzelczak, M.; Liz-Marzán, L.M. Gold Spiky Nanodumbbells: Anisotropy in Gold Nanostars. Part. Part. Syst. Charact. 2014, 31, 77–80. [Google Scholar] [CrossRef]
- Koh, A.L.; Fernández-Domínguez, A.I.; McComb, D.W.; Maier, S.A.; Yang, J.K.W. High-Resolution Mapping of Electron-Beam-Excited Plasmon Modes in Lithographically Defined Gold Nanostructures. Nano Lett. 2011, 11, 1323–1330. [Google Scholar] [CrossRef] [PubMed]
- Cube, F.V.; Niegemann, J.; Irsen, S.; Bell, D.C.; Linden, S. Angular-Resolved Electron Energy Loss Spectroscopy on a Split-Ring Resonator. Phys. Rev. B 2014, 89, 1154341–1154345. [Google Scholar]
- Cortes, E.; Xie, W.; Cambiasso, J.; Jermyn, A.S.; Sundararaman, R.; Narang, P.; Schlucker, S.; Maier, S.A. Plasmonic Hot Electron Transport Drives Nano-Localized Chemistry. Nat. Commun. 2017, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Stoger-Pollach, M. Optical Properties and Bandgaps from Low Loss EELS: Pitfalls and Solutions. Micron 2008, 39, 1092–1110. [Google Scholar] [CrossRef] [PubMed]
- Deitz, J.I.; Karki, S.; Marsillac, S.X.; Grassman, T.J.; McComb, D.W. Bandgap Profiling in CIGS Solar Cells via Valence Electron Energy-Loss Spectroscopy. J. Appl. Phys. 2018, 123, 1157031–1157036. [Google Scholar] [CrossRef]
- Contreras, M.; Tuttle, J.; Du, D.; Qi, Y.; Swartzlander, A.; Tennant, A.; Noufi, R. Graded Band-Gap Cu(In,Ga)Se2 Thin-Film Solar Cell Absorber with Enhanced Open Circuit Voltage. Appl. Phys. Lett. 1993, 63, 1824–1826. [Google Scholar] [CrossRef]
- Williams, D.B.; Carter, C.B. Transmission Electron Microscopy: A Textbook for Materials Science; Springer: New York, NY, USA, 2009. [Google Scholar]
- Digital Micrograph EELS Analysis User’s Guide—EELS Analysis User Guide; Gatan, Inc.: Pleasanton, CA, USA, 2003.
- Johnson, P.B.; Christy, R.W. Optical Constants of the Noble Metals. Phys. Rev. B 1972, 6, 4370–4379. [Google Scholar] [CrossRef]
- Dantham, V.R.; Holler, S.; Barbre, C.; Keng, D.; Kolchenko, V.; Arnold, S. Label-Free Detection of Single Protein using a Nanoplasmonic-Photonic Hybrid Microcavity. Nano Lett. 2013, 13, 3347–3351. [Google Scholar] [CrossRef]
- Ament, I.; Prasad, J.; Henkel, A.; Schmachtel, S.; Sönnichsen, C. Single Unlabeled Protein Detection on Individual Plasmonic Nanoparticles. Nano Lett. 2012, 12, 1092–1095. [Google Scholar] [CrossRef] [PubMed]
- Alexe-Ionescu, A.L.; Ionescu, A.T.; Scaramuzza, N.; Strangi, G.; Versace, C.; Barbero, G.; Bartolino, R. Liquid-crystal–electrochromic-material interface: A p-n-like electro-optic junction. Phys. Rev. E 2001, 64, 011708. [Google Scholar] [CrossRef] [PubMed]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chatterjee, S.; Ricciardi, L.; Deitz, J.I.; Williams, R.E.A.; McComb, D.W.; Strangi, G. Heterodimeric Plasmonic Nanogaps for Biosensing. Micromachines 2018, 9, 664. https://doi.org/10.3390/mi9120664
Chatterjee S, Ricciardi L, Deitz JI, Williams REA, McComb DW, Strangi G. Heterodimeric Plasmonic Nanogaps for Biosensing. Micromachines. 2018; 9(12):664. https://doi.org/10.3390/mi9120664
Chicago/Turabian StyleChatterjee, Sharmistha, Loredana Ricciardi, Julia I. Deitz, Robert E. A. Williams, David W. McComb, and Giuseppe Strangi. 2018. "Heterodimeric Plasmonic Nanogaps for Biosensing" Micromachines 9, no. 12: 664. https://doi.org/10.3390/mi9120664
APA StyleChatterjee, S., Ricciardi, L., Deitz, J. I., Williams, R. E. A., McComb, D. W., & Strangi, G. (2018). Heterodimeric Plasmonic Nanogaps for Biosensing. Micromachines, 9(12), 664. https://doi.org/10.3390/mi9120664





