Optofluidics in Microstructured Optical Fibers
Abstract
1. Introduction
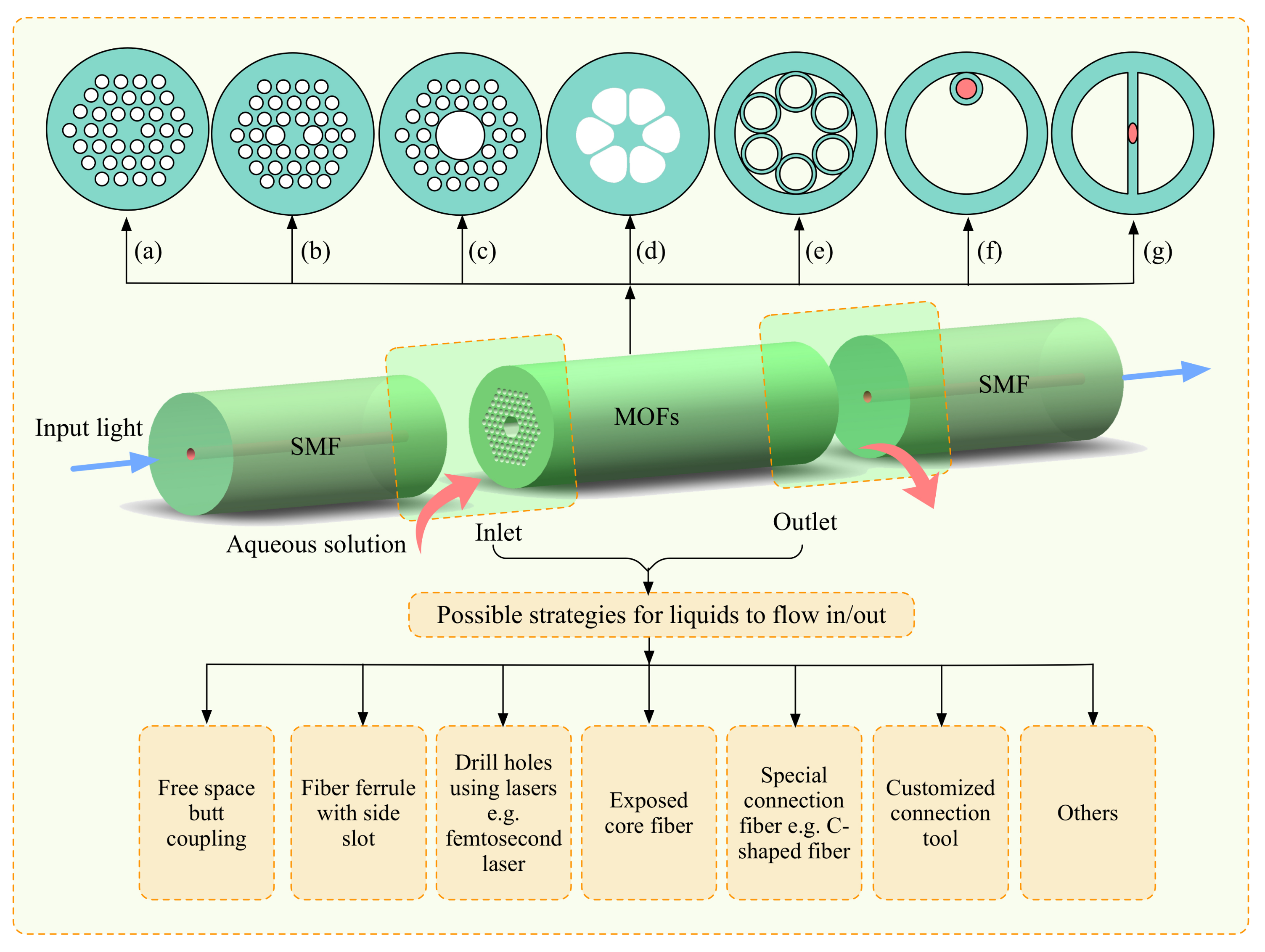
2. Implementation of Optofluidics Based on Microstructured Optical Fibers
3. Applications
3.1. Microfluidic Sensing Mechanism
3.2. Microfluidic Detection Applications
3.2.1. Physical Parameters Detection inside Fluids
Microfluidic Flow Rate Detection
Refractive Index Measurement
3.2.2. Chemosensing
PH Sensing
Gas Monitoring
Organic Chemicals and Solute Detection
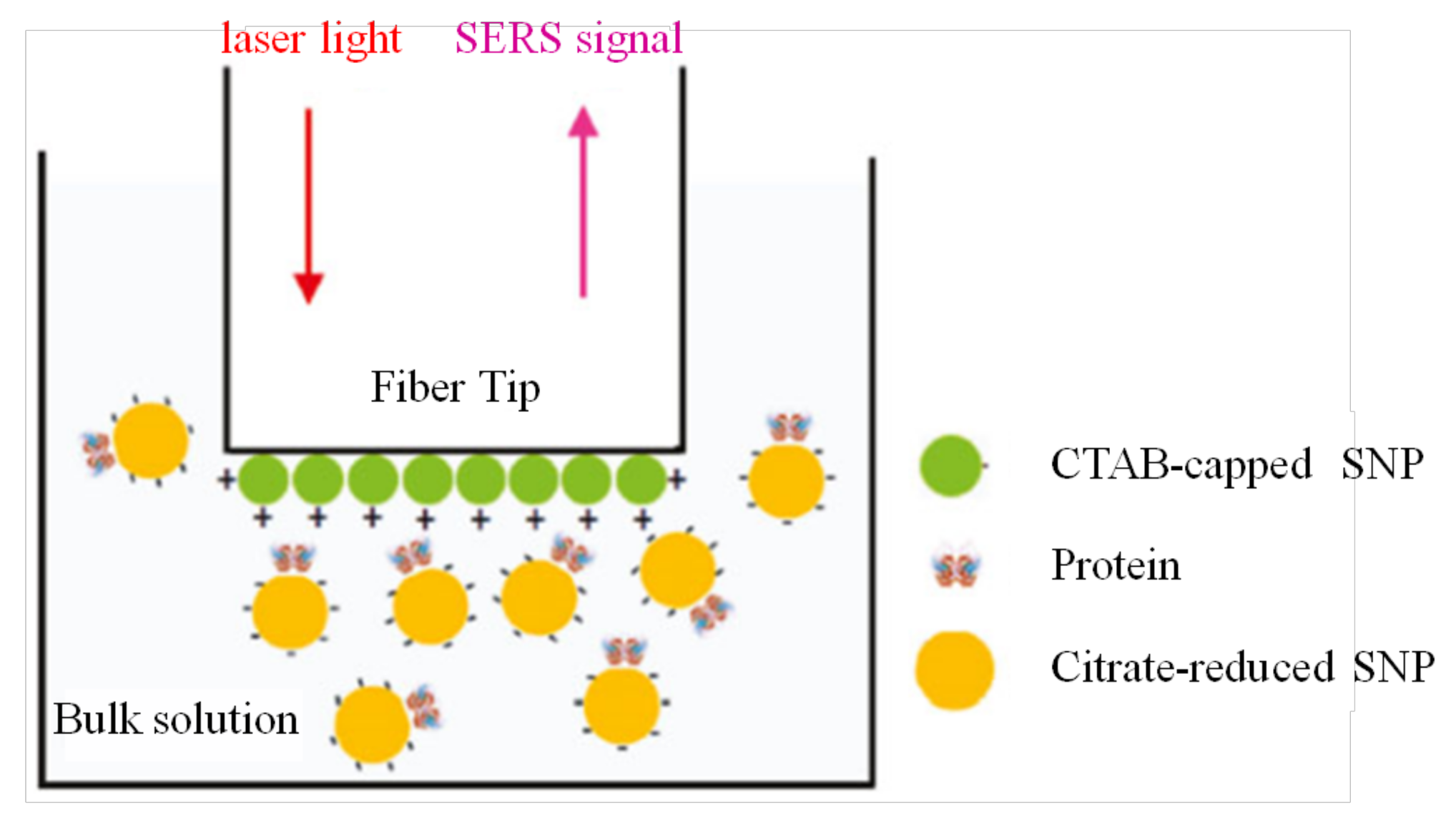
3.2.3. Biomedical Sensing inside Fluids
3.3. Other Applications
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Whitesides, G.M. The origins and the future of microfluidics. Nature 2006, 442, 368. [Google Scholar] [CrossRef] [PubMed]
- Bykov, D.S.; Schmidt, O.A. Flying particle sensors in hollow-core photonic crystal fibre. Nat. Photonics 2015, 9, 461. [Google Scholar] [CrossRef]
- Unterkofler, S.; Mcquitty, R.J. Microfluidic integration of photonic crystal fibers for online photochemical reaction analysis. Opt. Lett. 2012, 37, 1952–1954. [Google Scholar] [CrossRef] [PubMed]
- Warrensmith, S.C.; Ebendorffheidepriem, H. Exposed-core microstructured optical fibers for real-time fluorescence sensing. Opt. Express 2009, 17, 18533–18542. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Guo, T. Electrochemical Surface Plasmon Resonance Fiber-Optic Sensor: In Situ Detection of Electroactive Biofilms. Anal. Chem. 2016, 88, 7609–7616. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhang, A.P. Microfluidic device integrated with FBG in Co2+-doped fiber to measure flow rate with nL/s sensitivity. In Proceedings of the 23rd International Conference on Optical Fibre Sensors, Santander, Spain, 2–6 June 2014; SPIE: Bellingham, WA, USA, 2014; Volume 9157, p. 91573I. [Google Scholar]
- Lien, V.; Vollmer, F. Microfluidic flow rate detection based on integrated optical fiber cantilever. Lab Chip 2007, 7, 1352. [Google Scholar] [CrossRef] [PubMed]
- Minzioni, P.; Osellame, R. Roadmap for optofluidics. J. Opt. 2017, 19, 093003. [Google Scholar] [CrossRef]
- Haeberle, S.; Zengerle, R. Microfluidic platforms for lab-on-a-chip applications. Lab Chip 2007, 7, 1094–1110. [Google Scholar] [CrossRef] [PubMed]
- Cusano, A.; Ricciardi, A. Lab-on-Fiber Technology; Springer: Basel Switzerland, 2015; Volume 56. [Google Scholar]
- Consales, M.; Ricciardi, A. Lab-on-Fiber Technology: Toward Multifunctional Optical Nanoprobes. ACS Nano 2012, 6, 3163. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Hao, P. High-visibility in-line fiber-optic optofluidic Fabry–Pérot cavity. Appl. Phys. Lett. 2017, 111, 191102. [Google Scholar] [CrossRef]
- Zhang, C.L.; Gong, Y. Microbubble-Based Fiber Optofluidic Interferometer for Sensing. J. Lightwave Technol. 2017, 35, 2514–2519. [Google Scholar] [CrossRef]
- Domachuk, P.; Littler, I.C.M. Compact resonant integrated microfluidic refractometer. Appl. Phys. Lett. 2006, 88, 330A. [Google Scholar] [CrossRef]
- Monat, C.; Domachuk, P. Integrated optofluidics: A new river of light. Nat. Photonics 2007, 1, 106–114. [Google Scholar] [CrossRef]
- Leite, I.T.; Turtaev, S. Three-dimensional holographic optical manipulation through a high-numerical-aperture soft-glass multimode fibre. Nat. Photonics 2018, 12, 33. [Google Scholar] [CrossRef]
- Cubillas, A.M.; Unterkofler, S. Photonic crystal fibres for chemical sensing and photochemistry. Chem. Soc. Rev. 2013, 42, 8629–8648. [Google Scholar] [CrossRef] [PubMed]
- Cubillas, A.M.; Schmidt, M. Ultra-Low Concentration Monitoring of Catalytic Reactions in Photonic Crystal Fiber. Chem. A Eur. J. 2012, 18, 1586–1590. [Google Scholar] [CrossRef] [PubMed]
- Jensen, J.B.; Pedersen, L.H. Photonic crystal fiber based evanescent-wave sensor for detection of biomolecules in aqueous solutions. Opt. Lett. 2004, 29, 1974–1976. [Google Scholar] [CrossRef] [PubMed]
- Ertman, S.; Lesiak, P. Optofluidic photonic crystal fiber-based sensors. J. Lightwave Technol. 2017, 35, 3399–3405. [Google Scholar] [CrossRef]
- Knight, J.C.; Birks, T.A. All-silica single-mode optical fiber with photonic crystal cladding. Opt. Lett. 1996, 21, 1547. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Tam, H.Y. Fabrication, Characterization, and Sensing Applications of a High-Birefringence Suspended-Core Fiber. J. Lightwave Technol. 2014, 32, 2113–2122. [Google Scholar]
- Monro, T.M.; Ebendorff-Heidepriem, H. Sensing in suspended-core optical fibers. Opt. Fiber Technol. 2010, 16, 343–356. [Google Scholar] [CrossRef]
- Russell, P.S.J.; Knight, J.C. Photonic crystal fibres. Nature 2003, 424, 847–851. [Google Scholar]
- Litchinitser, N.M.; Abeeluck, A.K. Antiresonant reflecting photonic crystal optical waveguides. Opt. Lett. 2002, 27, 1592. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.K.; Kuhlmey, B.T. Ultrasensitive photonic crystal fiber refractive index sensor. Opt. Lett. 2009, 34, 322–324. [Google Scholar] [CrossRef] [PubMed]
- Chemnitz, M.; Gebhardt, M. Hybrid soliton dynamics in liquid-core fibres. Nat. Commun. 2017, 8, 42. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Tse, M.L.V. In-line microfluidic refractometer based on C-shaped fiber assisted photonic crystal fiber Sagnac interferometer. Opt. Lett. 2013, 38, 3283–3286. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, S. Selective-Fluid-Filling Technique of Microstructured Optical Fibers. J. Lightwave Technol. 2010, 28, 3193–3196. [Google Scholar]
- Gao, R.; Lu, D. Simultaneous measurement of refractive index and flow rate using graphene-coated optofluidic anti-resonant reflecting guidance. Opt. Express 2017, 25, 28731. [Google Scholar] [CrossRef]
- Yang, X.; Guo, X. Lab-on-fiber electrophoretic trace mixture separating and detecting an optofluidic device based on a microstructured optical fiber. Opt. Lett. 2016, 41, 1873. [Google Scholar] [CrossRef] [PubMed]
- Tian, F.; Min, J. Lab-on-fiber optofluidic platform for in situ monitoring of drug release from therapeutic eluting polyelectrolyte multilayers. Opt. Express 2015, 23, 20132–20142. [Google Scholar] [CrossRef] [PubMed]
- Jelger, P.; Laurell, F. An In-Fibre Microcavity. In Proceedings of the 2007 Conference on Lasers and Electro-Optics, Baltimore, MD, USA, 6–11 May 2007. [Google Scholar]
- Sudirman, A.; Margulis, W. All-Fiber Optofluidic Component to Combine Light and Fluid. IEEE Photonics Technol. Lett. 2014, 26, 1031–1033. [Google Scholar] [CrossRef]
- Tian, F.; Kanka, J. Photonic crystal fiber for layer-by-layer assembly and measurements of polyelectrolyte thin films. Opt. Lett. 2012, 37, 4299–4301. [Google Scholar] [CrossRef] [PubMed]
- Nissen, M.; Doherty, B. UV Absorption Spectroscopy in Water-Filled Antiresonant Hollow Core Fibers for Pharmaceutical Detection. Sensors 2018, 18, 478. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, V.S.; Kalluru, R.R. Fiber optic Raman sensor to monitor the concentration ratio of nitrogen and oxygen in a cryogenic mixture. Appl. Opt. 2007, 46, 3345–3351. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Motz, J.T.; Hunter, M. Optical Fiber Probe for Biomedical Raman Spectroscopy. Appl. Opt. 2004, 43, 542. [Google Scholar] [CrossRef] [PubMed]
- Rouvie, A.; Roosen, G. Stimulated Raman scattering in an ethanol core microstructured optical fiber. Opt. Express 2005, 13, 4786–4791. [Google Scholar]
- Cox, F.M.; Argyros, A. Surface enhanced Raman scattering in a hollow core microstructured optical fiber. Opt. Express 2007, 15, 13675–13681. [Google Scholar] [CrossRef] [PubMed]
- Amezcua-Correa, A.; Yang, J. Surface-Enhanced Raman Scattering Using Microstructured Optical Fiber Substrates. Adv. Funct. Mater. 2010, 17, 2024–2030. [Google Scholar] [CrossRef]
- Yan, H.; Liu, J. Novel index-guided photonic crystal fiber surface-enhanced Raman scattering probe. Opt. Express 2008, 16, 8300–8305. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Liu, J. Gas Raman sensing with multi-opened-up suspended core fiber. Appl. Opt. 2011, 50, 6026. [Google Scholar] [CrossRef] [PubMed]
- Afshar, V.S.; Ruan, Y. Enhanced fluorescence sensing using microstructured optical fibers: a comparison of forward and backward collection modes. Opt. Lett. 2008, 33, 1473–1475. [Google Scholar] [CrossRef]
- Warren-Smith, S.C.; Afshar, S. Theoretical study of liquid-immersed exposed-core microstructured optical fibers for sensing. Opt. Express 2008, 16, 9034. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.H.; Hardwick, S.A. Intrinsic Fluorescence-Based Optical Fiber Sensor for Cocaine Using a Molecularly Imprinted Polymer as the Recognition Element. IEEE Sens. J. 2011, 12, 255–260. [Google Scholar] [CrossRef]
- Chu, F.; Tsiminis, G. Explosives detection by fluorescence quenching of conjugated polymers in suspended core optical fibers. Sens. Actuators B Chem. 2014, 199, 22–26. [Google Scholar] [CrossRef]
- Yuan, L.; Yang, J. A Compact Fiber-Optic Flow Velocity Sensor Based on a Twin-Core Fiber Michelson Interferometer. IEEE Sens. J. 2008, 8, 1114–1117. [Google Scholar] [CrossRef]
- Lu, P.; Chen, Q. Fiber Bragg grating sensor for simultaneous measurement of flow rate and direction. Meas. Sci. Technol. 2008, 19, 1169–1175. [Google Scholar] [CrossRef]
- Li, Y.; Yan, G. Microfluidic flowmeter based on micro “hot-wire” sandwiched Fabry-Perot interferometer. Opt. Express 2015, 23, 9483. [Google Scholar] [CrossRef] [PubMed]
- Christodoulides, P.; Florides, G.A. 1–Microfluidics flow and heat transfer in microstructured fibers of circular and elliptical geometry. In Optofluidics Sensors & Actuators in Microstructured Optical Fibers; Elsevier: Amsterdam, The Netherlands, 2015; pp. 3–27. [Google Scholar]
- Wang, G.; Liu, J. Fluidic sensor based on the side-opened and suspended dual-core fiber. Appl. Opt. 2012, 51, 3096. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Wang, C. Side-Opened Suspended Core Fiber-Based Surface Plasmon Resonance Sensor. IEEE Sens. J. 2015, 15, 4086–4092. [Google Scholar] [CrossRef]
- Hassani, A.; Kabashin, A. Photonic bandgap fiber-based Surface Plasmon Resonance sensors. Opt. Express 2007, 15, 11413. [Google Scholar]
- Yuan, W.; Town, G.E. Ultrasensitive refractive index sensor based on twin-core photonic bandgap fibers. In Proceedings of the 20th International Conference on Optical Fibre Sensors, Edinburgh, UK, 5–9 October 2009; Volume 7503, p. 75035A. [Google Scholar]
- Yu, X.; Shum, P. Highly Sensitive Photonic Crystal Fiber-Based Refractive Index Sensing Using Mechanical Long-Period Grating. IEEE Photonics Technol. Lett. 2008, 20, 1688–1690. [Google Scholar] [CrossRef]
- Lin, W.; Zhang, H. Microstructured optical fiber for multichannel sensing based on Fano resonance of the whispering gallery modes. Opt. Express 2017, 25, 994. [Google Scholar] [CrossRef] [PubMed]
- Luan, N.; Ding, C. A Refractive Index and Temperature Sensor Based on Surface Plasmon Resonance in an Exposed-Core Microstructured Optical Fiber. IEEE Photonics J. 2016, 8, 1–8. [Google Scholar] [CrossRef]
- Cox, F.M.; Lwin, R. Opening up optical fibres. Opt. Express 2007, 15, 11843–11848. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.H.; Wang, L.L. Fluorescence pH probe based on microstructured polymer optical fiber. Opt. Express 2007, 15, 16478–16483. [Google Scholar] [CrossRef] [PubMed]
- Hoo, Y.L.; Jin, W. Evanescent-wave gas sensing using microstructure fiber. Opt. Eng. 2002, 41, 8–9. [Google Scholar] [CrossRef]
- Fini, J.M. Microstructure fibres for optical sensing in gases and liquids. Meas. Sci. Technol. 2004, 15, 1120–1128. [Google Scholar] [CrossRef]
- Li, S.G.; Liu, S.Y. Study of the sensitivity of gas sensing by use of index-guiding photonic crystal fibers. Appl. Opt. 2007, 46, 5183. [Google Scholar] [CrossRef] [PubMed]
- Ritari, T.; Tuominen, J. Gas sensing using air-guiding photonic bandgap fibers. Opt. Express 2004, 12, 4080–4087. [Google Scholar] [CrossRef] [PubMed]
- Hoo, Y.L.; Jin, W. Gas diffusion measurement using hollow-core photonic bandgap fiber. Sens. Actuators B Chem. 2005, 105, 183–186. [Google Scholar] [CrossRef]
- Pawłat, J.; Sugiyama, T. PBG Fibers for Gas Concentration Measurement. Plasma Process. Polym. 2007, 4, 743–752. [Google Scholar] [CrossRef]
- Gayraud, N.; Kornaszewski, Ł.W. Mid-infrared gas sensing using a photonic bandgap fiber. Appl. Opt. 2008, 47, 1269–1277. [Google Scholar] [CrossRef] [PubMed]
- Hoo, Y.L.; Liu, S. Fast Response Microstructured Optical Fiber Methane Sensor with Multiple Side-Openings. IEEE Photonics Technol. Lett. 2010, 22, 296–298. [Google Scholar] [CrossRef]
- Kassani, S.H.; Khazaeinezhad, R. Suspended Ring-Core Photonic Crystal Fiber Gas Sensor with High Sensitivity and Fast Response. IEEE Photonics J. 2015, 7, 1–9. [Google Scholar] [CrossRef]
- Zhou, X.; Dai, Y. Microstructured FBG hydrogen sensor based on Pt-loaded WO3. Opt. Express 2017, 25, 8777. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Lu, Y. Broad spectral photonic crystal fiber surface enhanced Raman scattering probe. Appl. Phys. B 2009, 95, 751–755. [Google Scholar] [CrossRef]
- Zhang, Y.; Shi, C. Liquid core photonic crystal fiber sensor based on surface enhanced Raman scattering. Appl. Phys. Lett. 2007, 90, 193504. [Google Scholar] [CrossRef]
- Yan, H.; Gu, C. Hollow core photonic crystal fiber surface-enhanced Raman probe. Appl. Phys. Lett. 2006, 89, 1102. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, A.Y. Direct molecule-specific glucose detection by Raman spectroscopy based on photonic crystal fiber. Anal. Bioanal. Chem. 2012, 402, 687–691. [Google Scholar] [CrossRef] [PubMed]
- Cordeiro, C.M.B.; Franco, M.A.R. Microstructured-core optical fibre for evanescent sensing applications. Opt. Express 2006, 14, 13056–13066. [Google Scholar] [CrossRef] [PubMed]
- Zheltikov, A.M.; Scalora, M. Photonic-crystal fiber as a multifunctional optical sensor and sample collector. Opt. Express 2005, 13, 3454–3459. [Google Scholar]
- Cordeiro, C.M.; Dos Santos, E.M. Lateral access to the holes of photonic crystal fibers—Selective filling and sensing applications. Opt. Express 2006, 14, 8403–8412. [Google Scholar] [CrossRef] [PubMed]
- Williams, G.; Euser, T.G. Spectrofluorimetry with attomole sensitivity in photonic crystal fibres. Methods Appl. Fluoresc. 2013, 1, 015003. [Google Scholar] [CrossRef] [PubMed]
- Martelli, C.; Canning, J. Water-soluble porphyrin detection in a pure-silica photonic crystal fiber. Opt. Lett. 2006, 31, 2100–2102. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Sun, Y. Evanescent Field Absorption Sensor Using a Pure-Silica Defected-Core Photonic Crystal Fiber. IEEE Photonics Technol. Lett. 2008, 20, 336–338. [Google Scholar] [CrossRef]
- Markos, C.; Yuan, W. Label-free biosensing with high sensitivity in dual-core microstructured polymer optical fibers. Opt. Express 2011, 19, 7790. [Google Scholar] [CrossRef] [PubMed]
- Rindorf, L.; Jensen, J.B. Photonic crystal fiber long-period gratings for biochemical sensing. Opt. Express 2006, 14, 8224. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.R.; Akter, S. Spiral Photonic Crystal Fiber Based Dual- Polarized Surface Plasmon Resonance Biosensor. IEEE Sens. J. 2017, 18, 133–140. [Google Scholar] [CrossRef]
- Li, Z.; Liao, C.; Chen, D.; Song, J.; Jin, W.; Peng, G.D.; Zhu, F.; Wang, Y.; He, J.; Wang, Y. Label-free detection of bovine serum albumin based on an in-fiber Mach-Zehnder interferometric biosensor. Opt. Express 2017, 25, 17105. [Google Scholar] [CrossRef] [PubMed]
- Popescu, V.A. Application of a Plasmonic Biosensor for Detection of Human Blood Groups. Plasmonics 2017, 12, 1733–1739. [Google Scholar] [CrossRef]
- Sun, J.; Chan, C.C. High-resolution photonic bandgap fiber-based biochemical sensor. J. Biomed. Opt. 2007, 12, 044022. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Li, K. Ultra-sensitive chemical and biological analysis via specialty fibers with built-in microstructured optofluidic channels. Lab Chip 2018. [Google Scholar] [CrossRef] [PubMed]
- Jensen, J.; Hoiby, P. Selective detection of antibodies in microstructured polymer optical fibers. Opt. Express 2005, 13, 5883–5889. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Gu, C. Highly Sensitive Detection of Proteins and Bacteria in Aqueous Solution Using Surface-Enhanced Raman Scattering and Optical Fibers. Anal. Chem. 2011, 83, 5888–5894. [Google Scholar] [CrossRef] [PubMed]
- Dinish, U.S.; Fu, C.Y.; Soh, K.S.; Ramaswamy, B.; Kumar, A.; Olivo, M. Highly sensitive SERS detection of cancer proteins in low sample volume using hollow core photonic crystal fiber. Biosens. Bioelectron. 2012, 33, 293. [Google Scholar]
- Rindorf, L.; Høiby, P.E. Towards biochips using microstructured optical fiber sensors. Anal. Bioanal. Chem. 2006, 385, 1370–1375. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Nguyen, N.T. Enhanced electrophoretic DNA separation in photonic crystal fiber. Anal. Bioanal. Chem. 2009, 394, 1707–1710. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Thakur, H.V.; Nalawade, S.M. Photonic crystal fiber injected with Fe3O4 nanofluid for magnetic field detection. Appl. Phys. Lett. 2011, 99, 161101. [Google Scholar] [CrossRef]
- Quintero, S.M.M.; Martelli, C. Photonic crystal fiber sensor for magnetic field detection. In Proceedings of the Fourth European Workshop on Optical Fibre Sensors, Porto, Portugal, 8–10 September 2010; SPIE: Bellingham, WA, USA, 2010; Volume 7653. [Google Scholar]
- Bjarklev, A.; Xianyu, H. Frequency tunability of solid-core photonic crystal fibers filled with nanoparticle-doped liquid crystals. Opt. Express 2009, 17, 3754–3764. [Google Scholar]
- Budaszewski, D.; Srivastava, A.K. Photo-aligned photonic ferroelectric liquid crystal fibers. J. Soc. Inf. Disp. 2015, 23, 196–201. [Google Scholar] [CrossRef]
- Donvalkar, P.S.; Ramelow, S. Continuous generation of rubidium vapor in hollow-core photonic bandgap fibers. Opt. Lett. 2015, 40, 5379–5382. [Google Scholar] [CrossRef] [PubMed]







| Structure | Sensitivity | Limit of Detection | Reference/Year |
|---|---|---|---|
| Side-opened and suspended-dual-core fiber | 8360 rad/RIU* | 2.2 × 10−6 RIU | [52]/2012 |
| Side-opened suspended-core fiber with surface plasmon resonance (SPR) | 3500 nm/RIU | 2.3 × 10−5 RIU | [53]/2015 |
| Photonic bandgap fiber-with SPR | 13,750 nm/RIU | 7.2 × 10−6 RIU | [54]/2007 |
| Twin-core all-solid photonic bandgap fibers | 10−6 RIU | [55]/2009 | |
| Single-mode photonic crystal fiber (PCF) with long-period grating | 243 nm/RIU | 4.1 × 10−6 RIU | [56]/2008 |
| Photonic crystal fiber with selective hole(s) filled by fluid | 30,100 nm/RIU | 4.6 × 10−7 RIU | [26]/2009 |
| Exposed-core MOF with SPR | 1900 nm/RIU~12,500 nm/RIU | [57]/2016 | |
| Graphene coated hollow-core PCF | 1328 nm/RIU | [30]/2017 | |
| Capillary channels (different diameters) inside a tubular frame | 13.3181 nm/RIU~29.0557 nm/RIU | [58]/2017 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shao, L.; Liu, Z.; Hu, J.; Gunawardena, D.; Tam, H.-Y. Optofluidics in Microstructured Optical Fibers. Micromachines 2018, 9, 145. https://doi.org/10.3390/mi9040145
Shao L, Liu Z, Hu J, Gunawardena D, Tam H-Y. Optofluidics in Microstructured Optical Fibers. Micromachines. 2018; 9(4):145. https://doi.org/10.3390/mi9040145
Chicago/Turabian StyleShao, Liyang, Zhengyong Liu, Jie Hu, Dinusha Gunawardena, and Hwa-Yaw Tam. 2018. "Optofluidics in Microstructured Optical Fibers" Micromachines 9, no. 4: 145. https://doi.org/10.3390/mi9040145
APA StyleShao, L., Liu, Z., Hu, J., Gunawardena, D., & Tam, H.-Y. (2018). Optofluidics in Microstructured Optical Fibers. Micromachines, 9(4), 145. https://doi.org/10.3390/mi9040145






