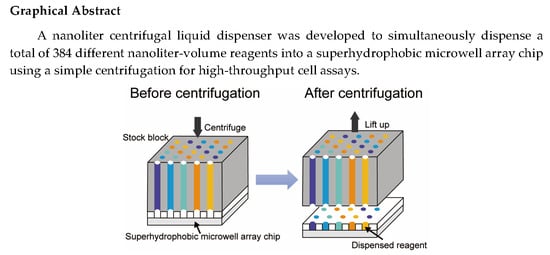
Nanoliter Centrifugal Liquid Dispenser Coupled with Superhydrophobic Microwell Array Chips for High-Throughput Cell Assays
Abstract
:1. Introduction
2. Materials and Methods
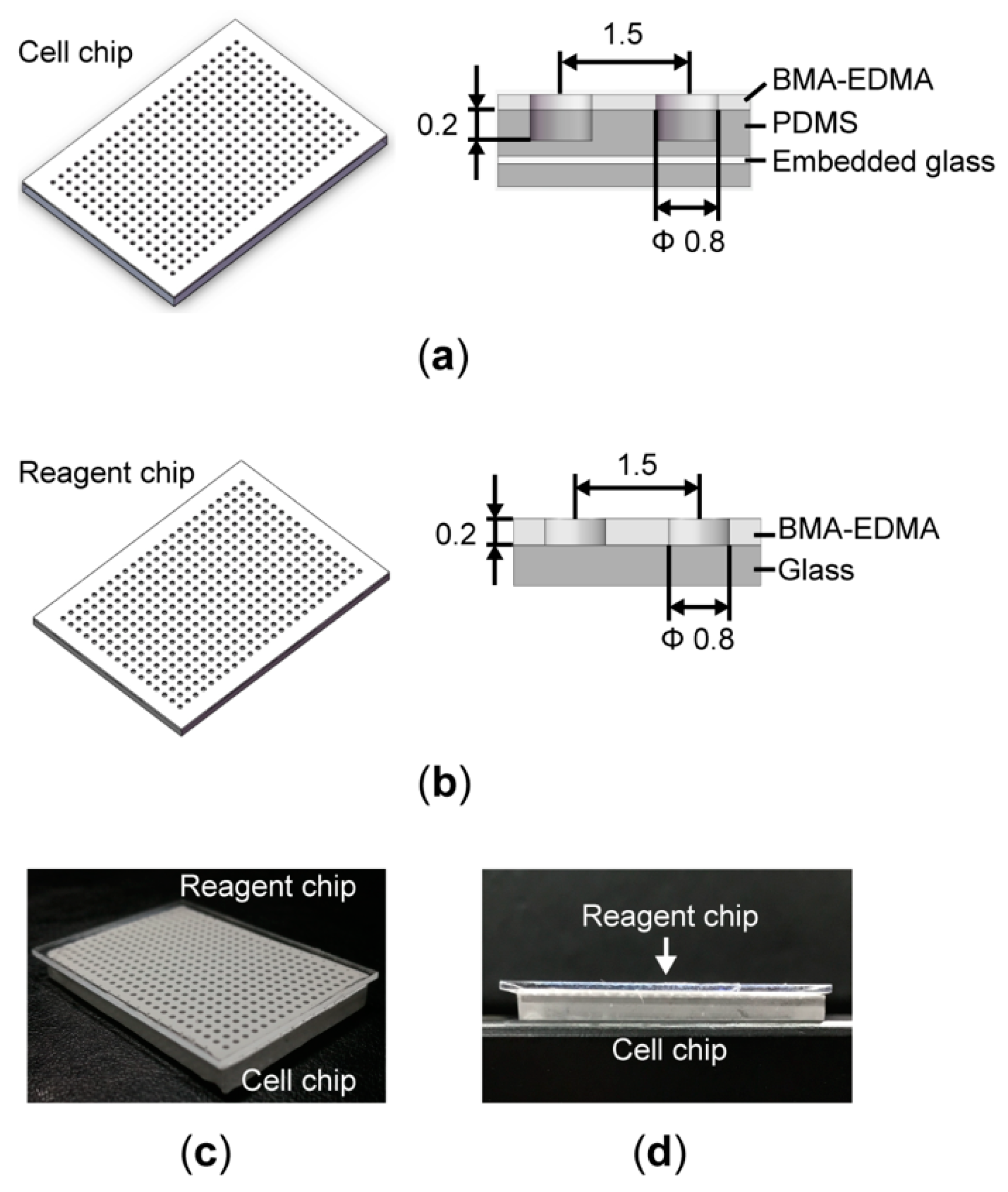
2.1. Superhydrophobic Microwell Array Chip
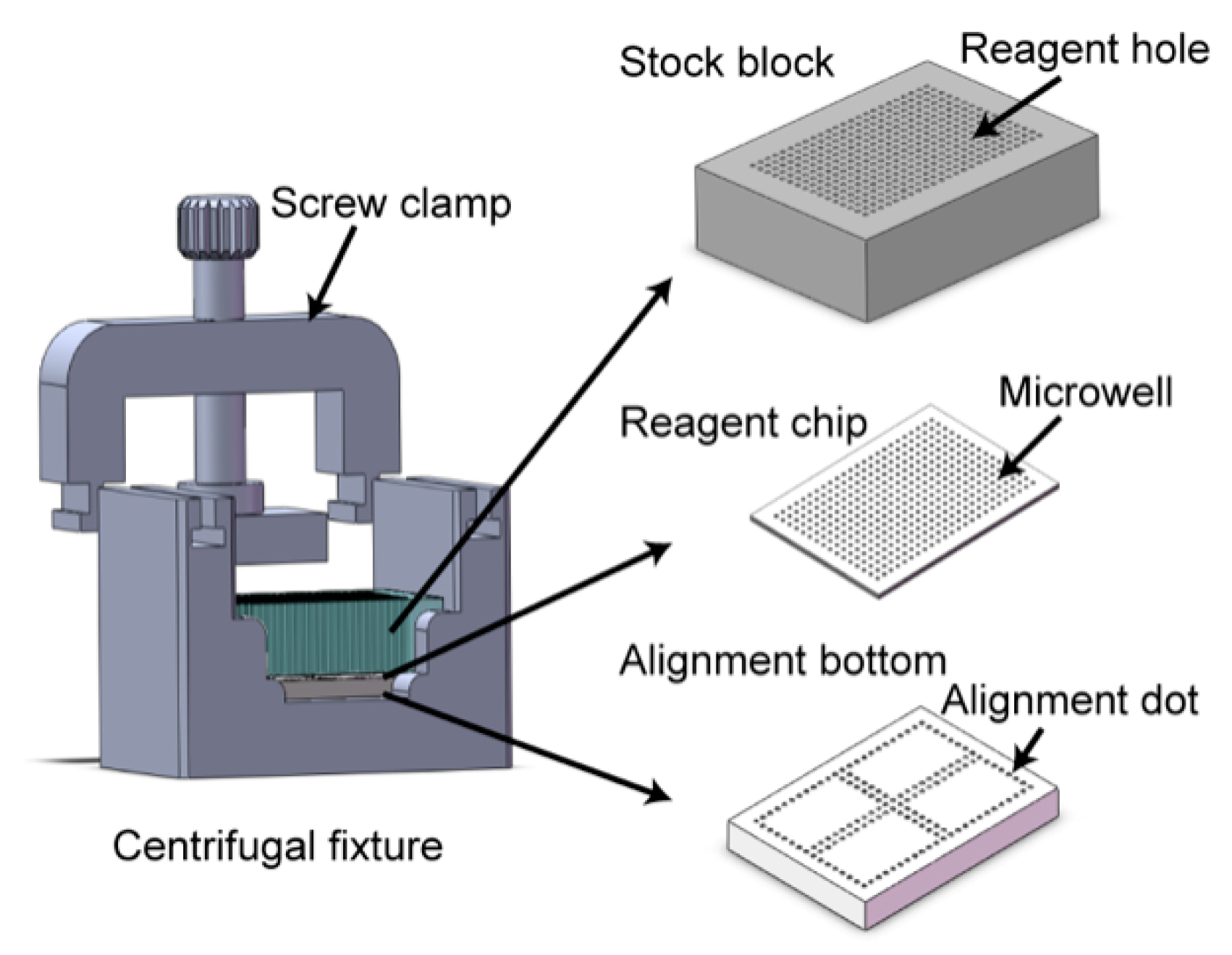
2.2. Nanoliter Centrifugal Liquid Dispenser
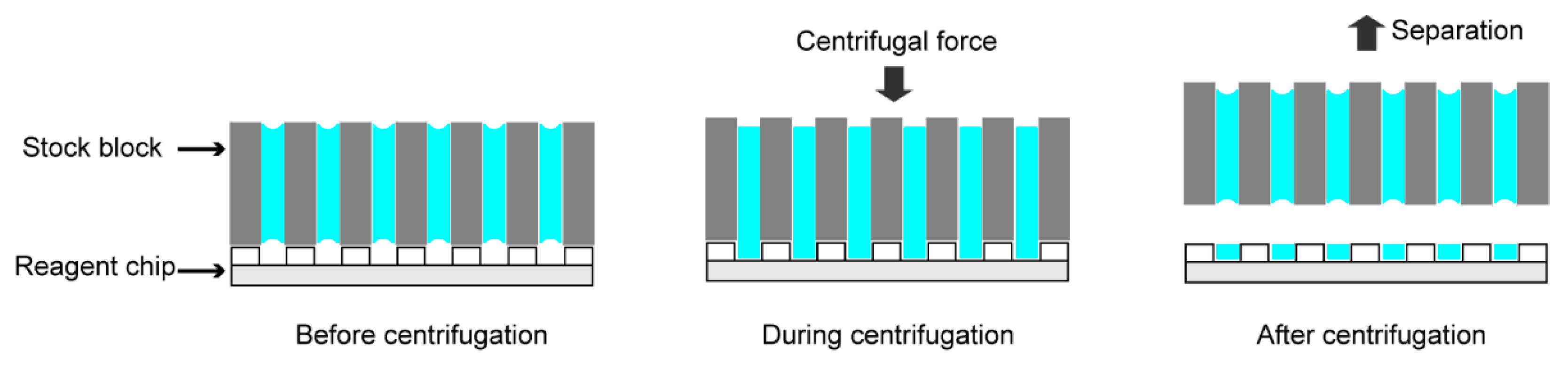
2.3. Procedure of Liquid Dispensing
2.4. On-Chip Cell Culture
2.5. Drug-Induced Cell Apoptosis
3. Results and Discussion
3.1. Nanoliter-Scale Liquid Handling
3.2. Characterization of the Liquid Dispensing
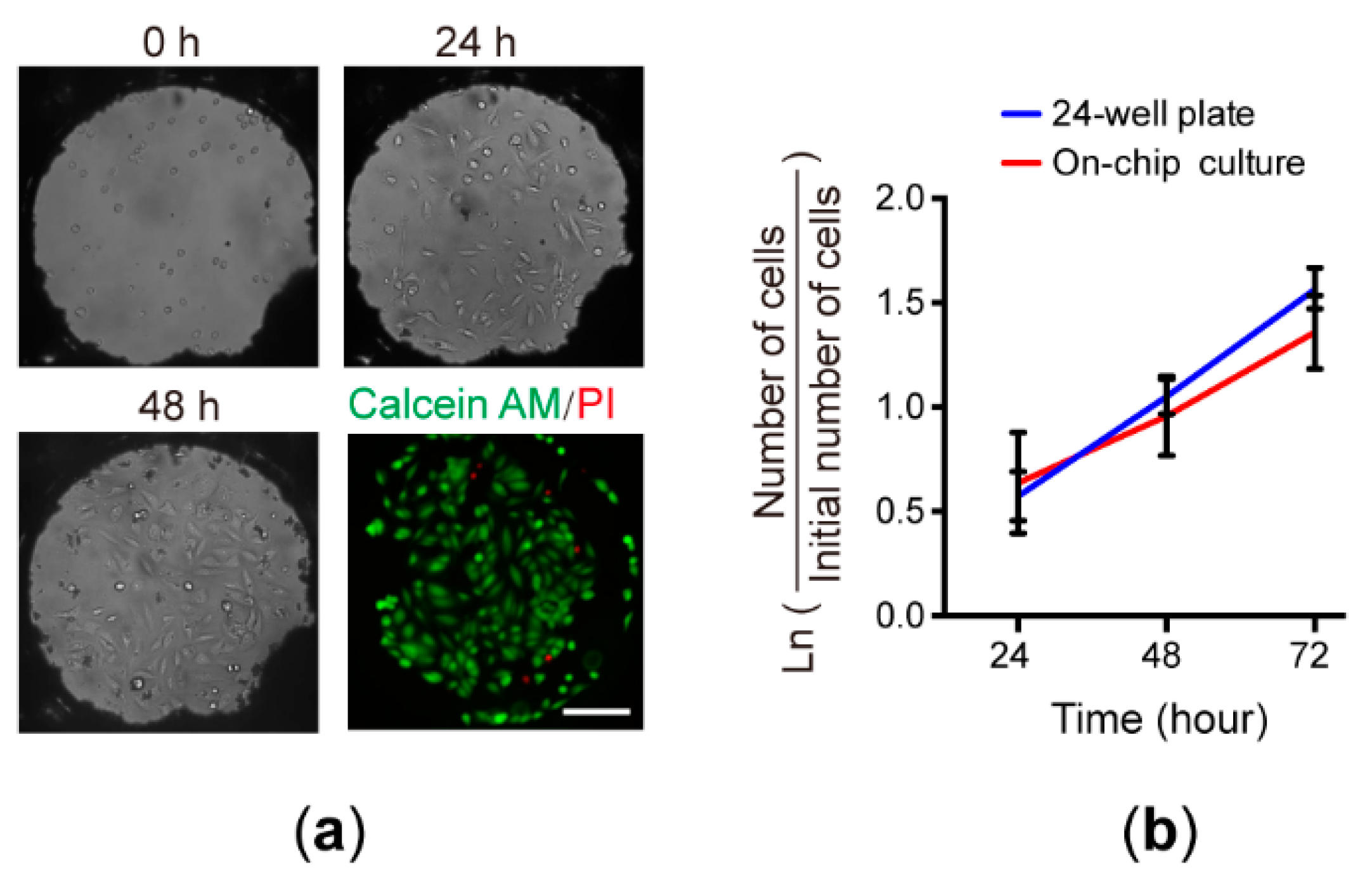
3.3. Cell Culture on the Chip
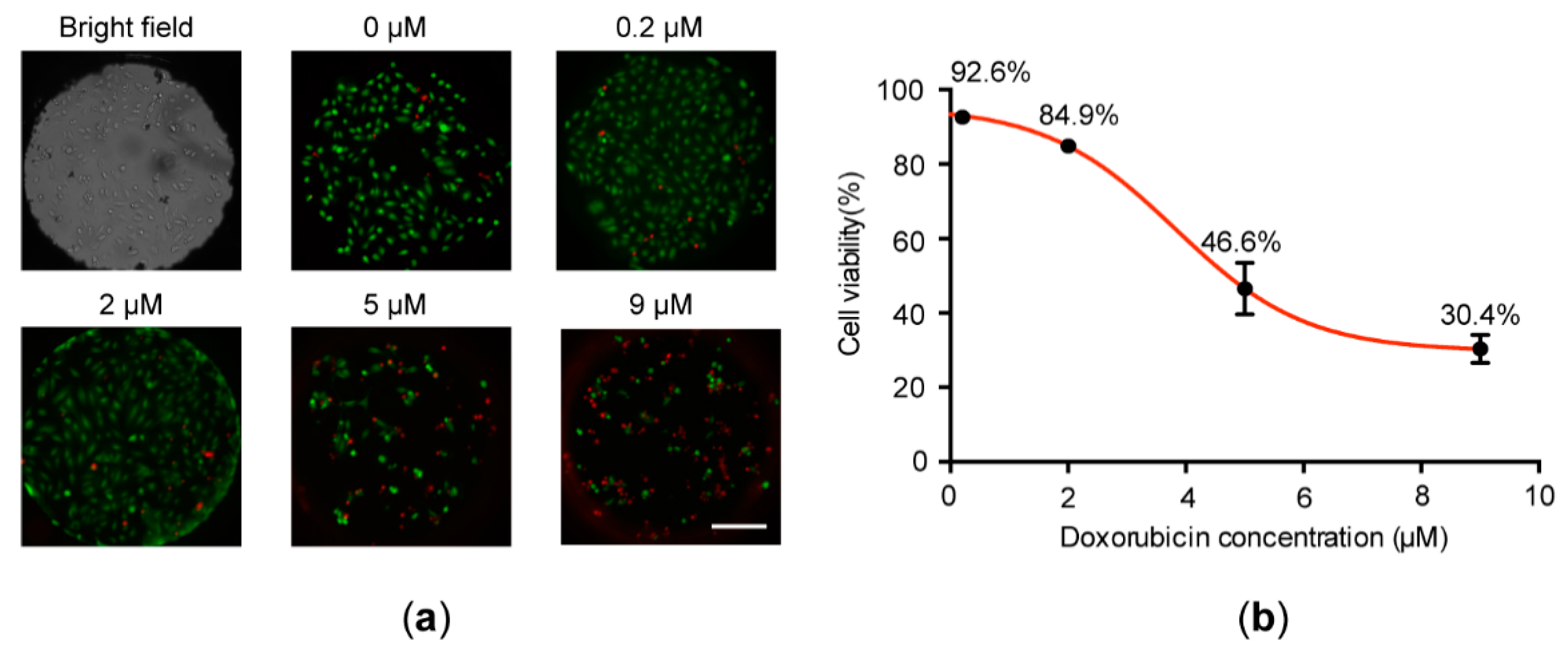
3.4. Doxorubicin-Induced Cell Apoptosis
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Schneider, G. Automating drug discovery. Nat. Rev. Drug Discov. 2018, 17, 97–113. [Google Scholar] [CrossRef] [PubMed]
- Moffat, J.G.; Rudolph, J.; Bailey, D. Phenotypic screening in cancer drug discovery—Past, present and future. Nat. Rev. Drug Discov. 2014, 13, 588–602. [Google Scholar] [CrossRef] [PubMed]
- Caiazzo, M.; Okawa, Y.; Ranga, A.; Piersigilli, A.; Tabata, Y.; Lutolf, M.P. Defined three-dimensional microenvironments boost induction of pluripotency. Nat. Mater. 2016, 15, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Ranga, A.; Gobaa, S.; Okawa, Y.; Mosiewicz, K.; Negro, A.; Lutolf, M.P. 3D niche microarrays for systems-level analyses of cell fate. Nat. Commun. 2014, 5, 4324. [Google Scholar] [CrossRef] [PubMed]
- Agrotis, A.; Ketteler, R. A new age in functional genomics using CRISPR/Cas9 in arrayed library screening. Front. Genet. 2015, 6, 300. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, D.; Dittrich, P.S. Advances in microfluidics for drug discovery. Expert Opin. Drug Discov. 2010, 5, 1081–1094. [Google Scholar] [CrossRef] [PubMed]
- Du, G.S.; Fang, Q.; den Toonder, J.M.J. Microfluidics for cell-based high throughput screening platformsd-A review. Anal. Chim. Acta 2016, 903, 36–50. [Google Scholar] [CrossRef] [PubMed]
- Neuzil, P.; Giselbrecht, S.; Lange, K.; Huang, T.J.; Manz, A. Revisiting lab-on-a-chip technology for drug discovery. Nat. Rev. Drug Discov. 2012, 11, 620–632. [Google Scholar] [CrossRef] [PubMed]
- Lecault, V.; Vaninsberghe, M.; Sekulovic, S.; Knapp, D.J.; Wohrer, S.; Bowden, W.; Viel, F.; McLaughlin, T.; Jarandehei, A.; Miller, M.; et al. High-throughput analysis of single hematopoietic stem cell proliferation in microfluidic cell culture arrays. Nat. Methods 2011, 8, 581–586. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Taylor, D.; Agrawal, N.; Wang, H.; Kim, H.; Han, A.; Rege, K.; Jayaraman, A. A programmable microfluidic cell array for combinatorial drug screening. Lab Chip 2012, 12, 1813–1822. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.X.; Zhang, X.M.; Yang, Y.S.; Zeng, S.R.; Wei, J.F.; Wang, Y.H.; Li, Y.J. An integrated microfludic device for culturing and screening of Giardia lamblia. Exp. Parasitol. 2014, 137, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Kasuya, J.; Jeon, J.; Chung, S.; Kamm, R.D. A quantitative microfluidic angiogenesis screen for studying anti-angiogenic therapeutic drugs. Lab Chip 2015, 15, 301–310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jakiela, S.; Kaminski, T.S.; Cybulski, O.; Weibel, D.B.; Garstecki, P. Bacterial growth and adaptation in microdroplet chemostats. Angew. Chem. Int. Ed. 2013, 52, 8908–8911. [Google Scholar] [CrossRef] [PubMed]
- Shembekar, N.; Chaipan, C.; Utharala, R.; Merten, C.A. Droplet-based microfluidics in drug discovery, transcriptomics and high-throughput molecular genetics. Lab Chip 2016, 16, 1314–1331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sesen, M.; Alan, T.; Neild, A. Droplet control technologies for microfluidic high throughput screening (μHTS). Lab Chip 2017, 17, 2372–2394. [Google Scholar] [CrossRef] [PubMed]
- Du, G.S.; Pan, J.Z.; Zhao, S.P.; Zhu, Y.; den Toonder, J.M.J.; Fang, Q. Cell-based drug combination screening with a microfluidic droplet array system. Anal. Chem. 2013, 85, 6740–6747. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.W.; Choi, Y.S.; Seo, Y.J.; Lee, M.Y.; Jeon, S.Y.; Ku, B.; Kim, S.; Yi, S.H.; Nam, D.H. High-throughput screening (HTS) of anticancer drug efficacy on a micropillar/microwell chip platform. Anal. Chem. 2014, 86, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.E.; Kim, J.; Oh, D.Y.; Song, Y.; Lee, S.H.; Min, S.; Kwon, S. One-step pipetting and assembly of encoded chemical-laden microparticles for high-throughput multiplexed bioassays. Nat. Commun. 2014, 5, 3468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwon, S.J.; Lee, D.W.; Shah, D.A.; Ku, B.; Jeon, S.Y.; Solanki, K.; Ryan, J.D.; Clark, D.S.; Dordick, J.S.; Lee, M.Y. High-throughput and combinatorial gene expression on a chip for metabolism-induced toxicology screening. Nat. Commun. 2014, 5, 3739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwon, C.H.; Wheeldon, I.; Kachouie, N.N.; Lee, S.H.; Bae, H.; Sant, S.; Fukuda, J.; Kang, J.W.; Khademhosseini, A. Drug-eluting microarrays for cell-based screening of chemical-induced apoptosis. Anal. Chem. 2011, 83, 4118–4125. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Zhang, Y.X.; Cai, L.F.; Fang, Q. Sequential operation droplet array: An automated microfluidic platform for picoliter-scale liquid handling, analysis, and screening. Anal. Chem. 2013, 85, 6723–6731. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Zhang, J.; Bian, S.; Chen, Z.; Hu, Y.; Hu, R.; Li, J.; Cheng, Y.; Zhang, X.; Zhou, Y.; et al. High-throughput superhydrophobic microwell arrays for investigating multifactorial stem cell niches. Lab Chip 2016, 16, 2996–3006. [Google Scholar] [CrossRef] [PubMed]
- Kong, F.W.; Yuan, L.; Zheng, Y.F.; Chen, W.D. Automatic liquid handling for life science: A critical review of the current state of the art. JALA-J. Lab Autom. 2012, 17, 169–185. [Google Scholar] [CrossRef] [PubMed]
- Koltay, P.; Steger, R.; Bohl, B.; Zengerle, R. The dispensing well plate: A novel nanodispenser for the multiparallel delivery of liquids (DWP Part I). Sens. Actuator A-Phys. 2004, 116, 483–491. [Google Scholar] [CrossRef]
- Ellson, R.; Mutz, M.; Browning, B.; Lee, L., Jr.; Miller, M.; Papen, R., Jr. Transfer of low nanoliter volumes between microplates using focused acoustics-automation considerations. JALA-J. Lab. Autom. 2003, 8, 29–34. [Google Scholar] [CrossRef]
- Teplitsky, E.; Joshi, K.; Ericson, D.L.; Scalia, A.; Mullen, J.D.; Sweet, R.M.; Soares, A.S. High throughput screening using acoustic droplet ejection to combine protein crystals and chemical libraries on crystallization plates at high density. J. Struct. Biol. 2015, 191, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Pang, Y.; Huang, Y. Openly accessible microfluidic liquid handlers for automated high-throughput nanoliter cell culture. Anal. Chem. 2012, 84, 2576–2584. [Google Scholar] [CrossRef] [PubMed]
- Bian, S.; Zhou, Y.; Hu, Y.; Cheng, J.; Chen, X.; Xu, Y.; Liu, P. High-throughput in situ cell electroporation microsystem for parallel delivery of single guide rnas into mammalian cells. Sci. Rep. 2017, 7, 42512. [Google Scholar] [CrossRef] [PubMed]
- Popova, A.A.; Schillo, S.M.; Demir, K.; Ueda, E.; Nesterov-Mueller, A.; Levkin, P.A. Droplet-array (DA) sandwich chip: A versatile platform for high-throughput cell screening based on superhydrophobic-superhydrophilic micropatterning. Adv. Mater. 2015, 27, 5217–5222. [Google Scholar] [CrossRef] [PubMed]
- Markarian, S.A.; Terzyan, A.M. Surface tension and refractive index of dialkylsulfoxide+ water mixtures at several temperatures. J. Chem. Eng. Data 2007, 52, 1704–1709. [Google Scholar] [CrossRef]
- Waterhouse, D.; Gelmon, K.; Klasa, R.; Chi, K.; Huntsman, D.; Ramsay, E.; Wasan, E.; Edwards, L.; Tucker, C.; Zastre, J. Development and assessment of conventional and targeted drug combinations for use in the treatment of aggressive breast cancers. Curr. Cancer Drug Targets 2006, 6, 455–489. [Google Scholar] [CrossRef] [PubMed]



© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Wu, Y.; Chen, Y.; Zhang, J.; Chen, X.; Liu, P. Nanoliter Centrifugal Liquid Dispenser Coupled with Superhydrophobic Microwell Array Chips for High-Throughput Cell Assays. Micromachines 2018, 9, 286. https://doi.org/10.3390/mi9060286
Wang Y, Wu Y, Chen Y, Zhang J, Chen X, Liu P. Nanoliter Centrifugal Liquid Dispenser Coupled with Superhydrophobic Microwell Array Chips for High-Throughput Cell Assays. Micromachines. 2018; 9(6):286. https://doi.org/10.3390/mi9060286
Chicago/Turabian StyleWang, Yuyi, Yushuai Wu, Yue Chen, Jianxiong Zhang, Xiaofang Chen, and Peng Liu. 2018. "Nanoliter Centrifugal Liquid Dispenser Coupled with Superhydrophobic Microwell Array Chips for High-Throughput Cell Assays" Micromachines 9, no. 6: 286. https://doi.org/10.3390/mi9060286
APA StyleWang, Y., Wu, Y., Chen, Y., Zhang, J., Chen, X., & Liu, P. (2018). Nanoliter Centrifugal Liquid Dispenser Coupled with Superhydrophobic Microwell Array Chips for High-Throughput Cell Assays. Micromachines, 9(6), 286. https://doi.org/10.3390/mi9060286