How [18F]-FDG-PET/CT Affects Clinical Management of Patients with Germ Cell Tumors in the Real World
Abstract
:Simple Summary
Abstract
1. Introduction
2. Methods
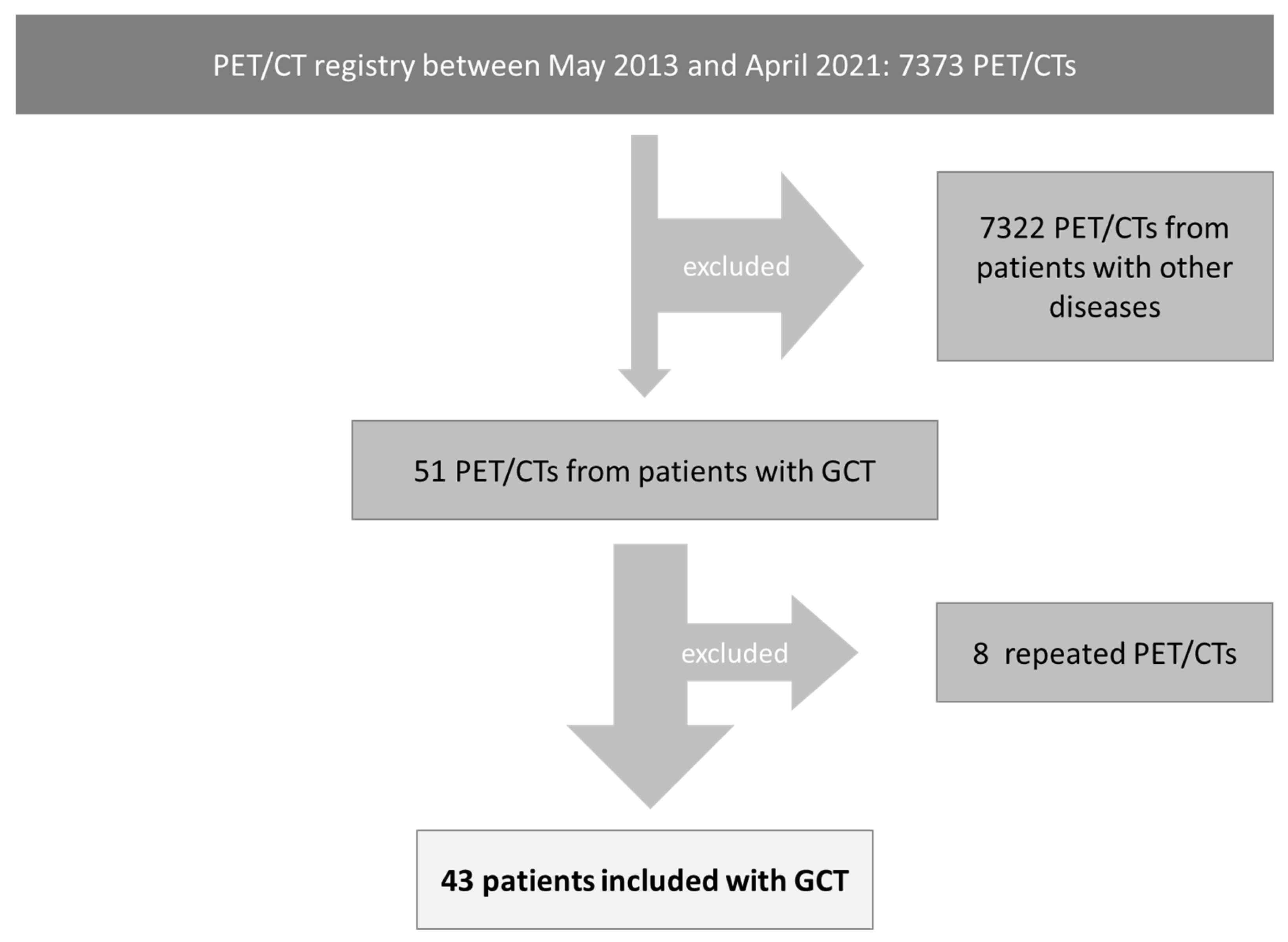
2.1. Patient Cohort
2.2. PET/CT Imaging
2.3. Patient Management
2.4. Data Analysis
- -
- Therapy, i.e., changes in intended treatment goals (curative, palliative) and intended treatment modalities (e.g., chemotherapy, surgery, and radiotherapy).
- -
- Diagnostic procedures. i.e., changes in intended diagnostic procedures (e.g., biopsy and additional imaging).
2.5. Statistical Analysis
3. Results
3.1. Patient Cohort
3.2. Indications and Findings of PET/CT
3.3. Impact of PET/CT on Clinical Patient Management
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gilligan, T.D.; Seidenfeld, J.; Basch, E.M.; Einhorn, L.H.; Fancher, T.; Smith, D.C.; Stephenson, A.J.; Vaughn, D.J.; Cosby, R.; Hayes, D.F. American Society of Clinical Oncology Clinical Practice Guideline on Uses of Serum Tumor Markers in Adult Males with Germ Cell Tumors. J. Clin. Oncol. 2010, 28, 3388–3404. [Google Scholar] [CrossRef] [PubMed]
- Brunereau, L.; Bruyère, F.; Linassier, C.; Baulieu, J.-L. The role of imaging in staging and monitoring testicular cancer. Diagn. Interv. Imaging 2012, 93, 310–318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Treglia, G.; Sadeghi, R.; Annunziata, S.; Caldarella, C.; Bertagna, F.; Giovanella, L. Diagnostic performance of fluorine-18-fluorodeoxyglucose positron emission tomography in the postchemotherapy management of patients with seminoma: Systematic review and meta-analysis. BioMed Res. Int. 2014, 2014, 852681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pfannenberg, C.; Aschoff, P.; Dittmann, H.; Mayer, F.; Reischl, G.; von Weyhern, C.; Kanz, L.; Claussen, C.D.; Bares, R.; Hartmann, J.T. PET/CT with 18F-FLT: Does it improve the therapeutic management of metastatic germ cell tumors? J. Nucl. Med. 2010, 51, 845–853. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonkat, G.; Pickard, R.; Bartoletti, R. EAU Guidelines. In Proceedings of the 33rd EAU Annual Congress, Copenhagen, Denmark, 16–20 March 2018; EAU Guidelines Office: Arnhem, The Netherlands, 2018. Available online: http://uroweb.org/guidelines/compilations-of-all-guidelines (accessed on 21 May 2023).
- Onkologie, L. S3-Leitlinie Diagnostik, Therapie und Nachsorge der Keimzelltumoren des Hodens, Langversion 1.0, 2019, AWMF-Registernummer: 043/049OL. 2020. Available online: https://www.leitlinienprogramm-onkologie.de/fileadmin/user_upload/Downloads/Leitlinien/Hodentumoren/LL_Hodentumoren_Langversion_1.0_.pdf (accessed on 21 May 2023).
- Gyawali, B.; Griffiths, R.; Robinson, A.G.; McInnes, M.D.; Bedard, P.L.; Booth, C.M. Use of Imaging for Active Surveillance in Testicular Cancer: Is Real-World Practice Concordant with Guidelines? Can. Urol. Assoc. J. 2022, 16, 26. [Google Scholar]
- de Wit, M.; Brenner, W.; Hartmann, M.; Kotzerke, J.; Hellwig, D.; Lehmann, J.; Franzius, C.; Kliesch, S.; Schlemmer, M.; Tatsch, K.; et al. 18F-FDG-PET in clinical stage I/II non-seminomatous germ cell tumours: Results of the German multicentre trial. Ann. Oncol. 2008, 19, 1619–1623. [Google Scholar] [CrossRef]
- Huddart, R.A.; O’Doherty, M.J.; Padhani, A.; Rustin, G.J.S.; Mead, G.M.; Vasey, J.K.J.; Harland, S.J.; Logue, J.; Daugaard, G.; Hain, S.F.; et al. 18fluorodeoxyglucose positron emission tomography in the prediction of relapse in patients with high-risk, clinical stage I nonseminomatous germ cell tumors: Preliminary report of MRC Trial TE22—The NCRI Testis Tumour Clinical Study Group. J. Clin. Oncol. 2007, 25, 3090–3095. [Google Scholar] [CrossRef]
- Cook, G.J.; Sohaib, A.; Huddart, R.A.; Dearnaley, D.P.; Horwich, A.; Chua, S. The Role of 18F-FDG PET/CT in the Management of Testicular Cancers: Wolters Kluwer. Nucl. Med. Commun. 2015, 36, 702–708. [Google Scholar] [CrossRef]
- Conduit, C.; Koh, T.T.; Hofman, M.S.; Toner, G.C.; Goad, J.; Lawrentschuk, N.; Tai, K.-H.; Lewin, J.H.; Ben Tran, B. Two decades of FDG-PET/CT in seminoma: Exploring its role in diagnosis, surveillance and follow-up. Cancer Imaging 2022, 22, 58. [Google Scholar] [CrossRef]
- Rossen, P.B.; Pedersen, A.F.; Zachariae, R.; von der Maase, H. Health-related quality of life in long-term survivors of testicular cancer. J. Clin. Oncol. 2009, 27, 5993–5999. [Google Scholar] [CrossRef]
- Schepisi, G.; de Padova, S.; de Lisi, D.; Casadei, C.; Meggiolaro, E.; Ruffilli, F.; Rosti, G.; Lolli, C.; Ravaglia, G.; Conteduca, V.; et al. Psychosocial Issues in Long-Term Survivors of Testicular Cancer. Front. Endocrinol. 2019, 10, 113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pfannenberg, C.; Gueckel, B.; Wang, L.; Gatidis, S.; Olthof, S.-C.; Vach, W.; Reimold, M.; la Fougere, C.; Nikolaou, K.; Martus, P. Practice-based evidence for the clinical benefit of PET/CT-results of the first oncologic PET/CT registry in Germany. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Hillner, B.E.; Siegel, B.A.; Liu, D.; Shields, A.F.; Gareen, I.F.; Hanna, L.; Stine, S.H.; Coleman, R.E. Impact of Positron Emission Tomography/Computed Tomography and Positron Emission Tomography (PET) Alone on Expected Management of Patients with cancer: Initial results from the National Oncologic PET Registry. J. Clin. Oncol. 2008, 26, 2155–2161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, P.; Jain, T.K.; Parida, G.K.; Karunanithi, S.; Patel, C.; Sharma, A.; Thulkar, S.; Julka, P.K.; Bal, C.; Kumar, R. Diagnostic accuracy of integrated (18)F-FDG PET/CT for restaging patients with malignant germ cell tumours. Br. J. Radiol. 2014, 87, 20140263. [Google Scholar] [CrossRef] [Green Version]
- De Santis, M.; Becherer, A.; Bokemeyer, C.; Stoiber, F.; Oechsle, K.; Sellner, F.; Lang, A.; Kletter, K.; Dohmen, B.M.; Dittrich, C.; et al. 2-18fluoro-deoxy-D-glucose positron emission tomography is a reliable predictor for viable tumor in postchemotherapy seminoma: An update of the prospective multicentric SEMPET trial. J. Clin. Oncol. 2004, 22, 1034–1039. [Google Scholar] [CrossRef]
- Oldenburg, J.; Berney, D.M.; Bokemeyer, C.; Climent, M.A.; Daugaard, G.; Gietema, J.A.; De Giorgi, U.; Haugnes, H.S.; Huddart, R.A.; Leão, R.; et al. Testicular seminoma and non-seminoma: ESMO-EURACAN Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2022, 33, 362–375. [Google Scholar] [CrossRef]
- Heidenreich, A.; Paffenholz, P.; Nestler, T.; Pfister, D. Primary and Postchemotherapy Retroperitoneal Lymphadenectomy for Testicular Cancer. Oncol. Res. Treat. 2018, 41, 370–378. [Google Scholar] [CrossRef]
- Williams, S.B.; McDermott, D.W.; Winston, D.; Bahnson, E.; Berry, A.M.; Steele, G.S.; Richie, J.P. Morbidity of open retroperitoneal lymph node dissection for testicular cancer: Contemporary perioperative data. BJU Int. 2010, 105, 918–921. [Google Scholar] [CrossRef]
- Cary, K.C.; Beck, S.D.W.; Bihrle, R.; Foster, R.S. Clinical and pathological features predictive of nephrectomy at post-chemotherapy retroperitoneal lymph node dissection. J. Urol. 2013, 189, 812–817. [Google Scholar] [CrossRef]
- Dusaud, M.; Malavaud, B.; Bayoud, Y.; Sebe, P.; Hoepffner, J.L.; Salomon, L.; Houlgatte, A.; Pignot, G.; Rigaud, J.; Fléchon, A.; et al. Post-chemotherapy retroperitoneal teratoma in nonseminomatous germ cell tumors: Do predictive factors exist? Results from a national multicenter study. J. Surg. Oncol. 2016, 114, 992–996. [Google Scholar] [CrossRef]
- Hiester, A.; Nini, A.; Fingerhut, A.; Siemer, R.G.; Winter, C.; Albers, P.; Lusch, A. Preservation of Ejaculatory Function After Postchemotherapy Retroperitoneal Lymph Node Dissection (PC-RPLND) in Patients with Testicular Cancer: Template vs. Bilateral Resection. Front. Surg. 2018, 5, 80. [Google Scholar] [CrossRef]
- Cohn-Cedermark, G.; Stahl, O.; Tandstad, T. Surveillance vs. adjuvant therapy of clinical stage I testicular tumors—A review and the SWENOTECA experience. Andrology 2015, 3, 102–110. [Google Scholar] [CrossRef] [Green Version]
- Kiefer, L.S.; Sekler, J.; Gückel, B.; Kraus, M.S.; la Fougère, C.; Nikolaou, K.; Bitzer, M.; Gatidis, S.; Pfannenberg, C. Impact of 18F-FDG-PET/CT on Clinical Management in Patients with Cholangiocellular Carcinoma. BJR Open 2021, 3, 20210008. [Google Scholar] [CrossRef]
- Olthof, S.-C.; Forschner, A.; Martus, P.; Garbe, C.; Nikolaou, K.; la Fougère, C.; Gückel, B.; Vach, W.; Pfannenberg, C. Influence of 18F-FDG PET/CT on clinical management and outcome in patients with advanced melanoma not primarily selected for surgery based on a linked evidence approach. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 2313–2321. [Google Scholar] [CrossRef]
- Reinert, C.P.; Sekler, J.; Gani, C.; Nikolaou, K.; la Fougère, C.; Pfannenberg, C.; Gatidis, S. Impact of PET/CT on management of patients with esophageal cancer—Results from a PET/CT registry study. Eur. J. Radiol. 2021, 136, 109524. [Google Scholar] [CrossRef]
- Reinert, C.P.; Sekler, J.; La Fougère, C.; Pfannenberg, C.; Gatidis, S. Impact of PET/CT on clinical management in patients with cancer of unknown primary—A PET/CT registry study. Eur. Radiol. 2020, 30, 1325–1333. [Google Scholar] [CrossRef] [PubMed]
- Ambrosini, V.; Zucchini, G.; Nicolini, S.; Berselli, A.; Nanni, C.; Allegri, V.; Martoni, A.; Rubello, D.; Cricca, A.; Fanti, S. 18F-FDG PET/CT impact on testicular tumours clinical management. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 668–673. [Google Scholar] [CrossRef] [PubMed]
- Müller, J.; Schrader, A.J.; Jentzmik, F.; Schrader, M. Beurteilung von Residualtumoren nach Systemtherapie des metastasierten Seminoms: (18)F-2-Fluor-2-Deoxy-D-Glucose-Positronenemissionstomographie—Metaanalyse zur diagnostischen Wertigkeit. Urologe 2011, 50, 322–327. [Google Scholar] [CrossRef] [PubMed]
- Lumbreras, B.; Donat, L.; Hernández-Aguado, I. Incidental findings in imaging diagnostic tests: A systematic review. Br. J. Radiol. 2010, 83, 276–289. [Google Scholar] [CrossRef] [Green Version]
- Coursey Moreno, C.; Small, W.C.; Camacho, J.C.; Master, V.; Kokabi, N.; Lewis, M.; Hartman, M.; Mittal, P.K. Testicular tumors: What radiologists need to know--differential diagnosis, staging, and management. RadioGraphics 2015, 35, 400–415. [Google Scholar] [CrossRef] [Green Version]
- Jamal-Hanjani, M.; Karpathakis, A.; Kwan, A.; Mazhar, D.; Ansell, W.; Shamash, J.; Harper, P.; Rudman, S.; Powles, T.; Chowdhury, S. Bone metastases in germ cell tumours: Lessons learnt from a large retrospective study. BJU Int. 2013, 112, 176–181. [Google Scholar] [CrossRef] [Green Version]
- Xu, P.; Wang, J.; Abudurexiti, M.; Jin, S.; Wu, J.; Shen, Y.; Ye, D. Prognosis of Patients with Testicular Carcinoma Is Dependent on Metastatic Site. Front. Oncol. 2019, 9, 1495. [Google Scholar] [CrossRef] [PubMed]
- Levine, M.N.; Julian, J.A. Registries that show efficacy: Good, but not good enough. J. Clin. Oncol. 2008, 26, 5316–5319. [Google Scholar] [CrossRef] [PubMed]
- Gallach, M.; Lette, M.M.; Abdel-Wahab, M.; Giammarile, F.; Pellet, O.; Paez, D. Addressing global inequities in positron emission tomography-computed tomography (PET-CT) for cancer management: A statistical model to guide strategic planning. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2020, 26, e926544-1. [Google Scholar] [CrossRef] [PubMed]




| No. of Patients (n = 43) | % | |
|---|---|---|
| Sex | ||
| Male | 43 | 100 |
| Age (years) | ||
| Median | 46 | |
| Interquartile range (25–75) | 38.5–51.0 | |
| In-/outpatients | ||
| Inpatients | 43 | 100 |
| Outpatients | 0 | 0 |
| Patient follow-up time (years) | ||
| Mean | 4 | |
| Minimum | 0 | |
| Maximum | 9 | |
| Death rate | ||
| Documented cases of death | 3 | 7.0 |
| Tumor-related deaths | 1 | 2.3 |
| Histological diagnosis | ||
| Testicular GCTs | ||
| Seminoma | 30 | 74.4 |
| Non-seminoma | 9 | 20.9 |
| Embryonal carcinoma | 4 | 9.3 |
| Mixed germ cell tumor | 5 | 11.6 |
| Extratesticular GCTs | ||
| Mediastinal seminoma | 2 | 4.7 |
| Unknown | 2 | 4.7 |
| Clinical stage | ||
| I | 9 | 20.9 |
| II | 18 | 41.9 |
| III | 14 | 32.6 |
| Unknown | 2 | 4.7 |
| Preceding imaging (pre-PET/CT) | ||
| CT | 29 | 67.4 |
| MRI | 1 | 2.3 |
| PET/CT | 3 | 7.0 |
| Not documented | 10 | 23.3 |
| Days between preceding imaging and PET/CT | ||
| Median | 46 | |
| Interquartile range (25–75) | 20–117 | |
| No. of Patients (n = 43) | % | |
|---|---|---|
| PET/CT indication | ||
| Primary staging | 2 | 4.7 |
| Restaging | 28 | 65.0 |
| Evaluation residual tumor | 16 | 37.2 |
| Evaluation of treatment response | 6 | 14.0 |
| Evaluation of surgical resectability | 4 | 9.3 |
| Intermediate staging during therapy break | 2 | 4.7 |
| Assessment of dignity | 13 | 30.2 |
| No. of Patients (n = 43) | % | |
|---|---|---|
| Metastases | ||
| Lymph nodes | 36 | 83.7 |
| Visceral organs | 4 | 9.3 |
| Lung/pleura | 3 | 7.0 |
| Bone | 1 | 2.3 |
| Brain | 1 | 2.3 |
| Metastatic spread | ||
| 1 organ | 25 | 58.1 |
| 2–3 organs | 5 | 11.6 |
| >3 organs | 2 | 4.7 |
| New secondary diagnosis | 4 | 9.3 |
| Oncologic | 1 | 2.3 |
| Non-oncologic | 3 | 7.0 |
| Relapse | Upstaging | No Change | Downstaging | Not Clear | |
|---|---|---|---|---|---|
| Histopathology | |||||
| Testicular GCTs | |||||
| Seminoma | 4 | 4 | 15 | 6 | 1 |
| Non-seminoma | |||||
| Embryonal carcinoma | 0 | 0 | 2 | 2 | 0 |
| Mixed GCT | 0 | 2 | 3 | 0 | 0 |
| Extratesticular GCTs | |||||
| Mediastinal seminoma | 0 | 1 | 0 | 1 | 0 |
| Unknown | 1 | 0 | 0 | 1 | 0 |
| Indication | |||||
| Evaluation of residual tumor | 2 | 2 | 5 | 5 | 0 |
| Evaluation of treatment response | 0 | 0 | 3 | 2 | 0 |
| Evaluation of surgical resectability | 1 | 1 | 2 | 0 | 0 |
| Intermediate staging during therapy break | 0 | 0 | 1 | 1 | 0 |
| Assessment of dignity | 0 | 3 | 5 | 3 | 0 |
| Before PET/CT | After PET/CT | |||
|---|---|---|---|---|
| n | % | n | % | |
| Intended treatment concept | ||||
| Curative | 26 | 60.5 | 26 | 60.5 |
| Palliative | 0 | 0 | 2 | 4.7 |
| No information/unknown | 17 | 39.5 | 15 | 34.9 |
| Intended mode of therapeutic intervention | ||||
| Chemotherapy | 3 | 7.0 | 11 | 25.6 |
| Radiotherapy | 1 | 2.3 | 2 | 4.7 |
| Surgical resection | 11 | 25.6 | 2 | 4.7 |
| Intended invasive diagnostics | ||||
| Surgical biopsy | 9 | 20.9 | 1 | 2.3 |
| Intended additional imaging | ||||
| CT | 22 | 51.2 | 3 | 7.0 |
| MRI | 5 | 11.6 | 0 | 0.0 |
| Ultrasound | 1 | 2.3 | 0 | 0.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, C.; Sekler, J.; Gückel, B.; Pfannenberg, C.; Dittmann, H.; Seith, F.; Amend, B.; Nikolaou, K.; Reinert, C.P. How [18F]-FDG-PET/CT Affects Clinical Management of Patients with Germ Cell Tumors in the Real World. Cancers 2023, 15, 3652. https://doi.org/10.3390/cancers15143652
Liang C, Sekler J, Gückel B, Pfannenberg C, Dittmann H, Seith F, Amend B, Nikolaou K, Reinert CP. How [18F]-FDG-PET/CT Affects Clinical Management of Patients with Germ Cell Tumors in the Real World. Cancers. 2023; 15(14):3652. https://doi.org/10.3390/cancers15143652
Chicago/Turabian StyleLiang, Cecilia, Julia Sekler, Brigitte Gückel, Christina Pfannenberg, Helmut Dittmann, Ferdinand Seith, Bastian Amend, Konstantin Nikolaou, and Christian Philipp Reinert. 2023. "How [18F]-FDG-PET/CT Affects Clinical Management of Patients with Germ Cell Tumors in the Real World" Cancers 15, no. 14: 3652. https://doi.org/10.3390/cancers15143652
APA StyleLiang, C., Sekler, J., Gückel, B., Pfannenberg, C., Dittmann, H., Seith, F., Amend, B., Nikolaou, K., & Reinert, C. P. (2023). How [18F]-FDG-PET/CT Affects Clinical Management of Patients with Germ Cell Tumors in the Real World. Cancers, 15(14), 3652. https://doi.org/10.3390/cancers15143652







