Determinants of Pre-Surgical Treatment in Primary Rectal Cancer: A Population-Based Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Staging and Treatment
2.3. Statistics
3. Results
3.1. Patient Characteristics
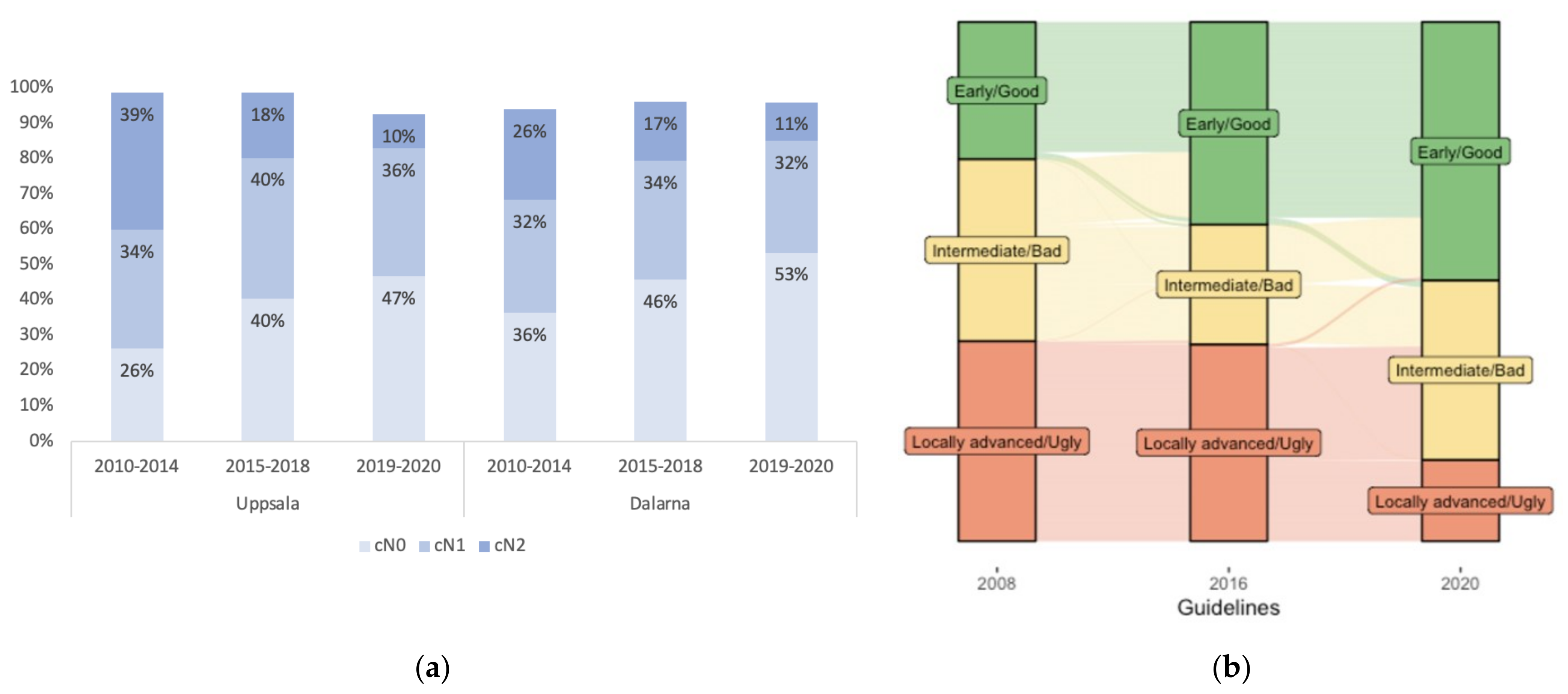
3.2. Changes in cT- and cN-Stages and Other MRI Characteristics with Time
3.3. Risk Grouping According to the “Good–Bad–Ugly” Concept
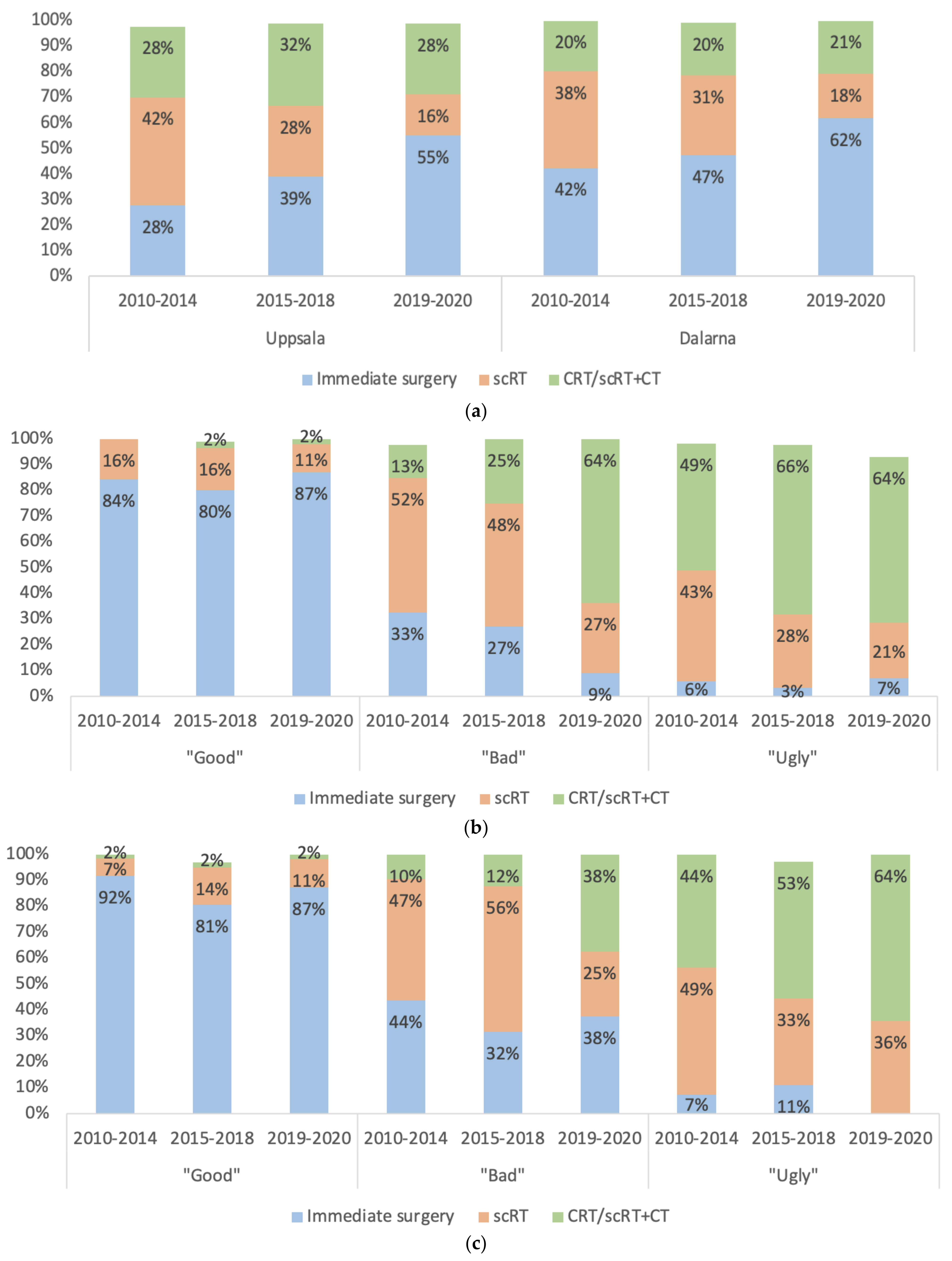
3.4. Selection to Different Treatments
3.5. Correlations between Clinical and Pathological Stage
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Glynne-Jones, R.; Wyrwicz, L.; Tiret, E.; Brown, G.; Rodel, C.; Cervantes, A.; Arnold, D.; Committee, E. Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2017, 28 (Suppl. 4), iv22–iv40. [Google Scholar] [CrossRef] [PubMed]
- Braendengen, M.; Tveit, K.M.; Berglund, Å.; Birkemeyer, E.; Frykholm, G.; Påhlman, L.; Wiig, J.N.; Byström, P.; Bujko, K.; Glimelius, B. A randomized phase III study (LARCS) comparing preoperative radiotherapy alone versus chemoradiotherapy in non-resectable rectal cancer. J. Clin. Oncol. 2008, 26, 3687–3694. [Google Scholar] [CrossRef] [PubMed]
- Glimelius, B. Multidisciplinary treatment of patients with rectal cancer: Development during the past decades and plans for the future. Ups. J. Med. Sci. 2012, 117, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Minsky, B.D. Emerging trends in the treatment of rectal cancer. Acta Oncol. 2019, 58, 1343–1351. [Google Scholar] [CrossRef] [PubMed]
- Swedish Rectal Cancer Trial. Improved survival with preoperative radiotherapy in resectable rectal cancer. N. Engl. J. Med. 1997, 336, 980–987. [Google Scholar] [CrossRef]
- Kapiteijn, E.; Marijnen, C.A.M.; Nagtegaal, I.D.; Putter, H.; Steup, W.H.; Wiggers, T.; Rutten, H.J.T.; Leer, J.W.H.; van Krieken, J.H.J.M.; Påhlman, L.; et al. Preoperative radiotherapy in combination with total mesorectal excision improves local control in resectable rectal cancer. Report from a multicenter randomized trial. N. Engl. J. Med. 2001, 345, 638–646. [Google Scholar] [CrossRef]
- Sauer, R.; Becker, H.; Hohenberger, W.; Rodel, C.; Wittekind, C.; Fietkau, R.; Martus, P.; Tschmelitsch, J.; Hager, E.; Hess, C.F.; et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N. Engl. J. Med. 2004, 351, 1731–1740. [Google Scholar] [CrossRef]
- Bosset, J.F.; Collette, L.; Calais, G.; Mineur, L.; Maingon, P.; Radosevic-Jelic, L.; Daban, A.; Bardet, E.; Beny, A.; Ollier, J.C.; et al. Chemotherapy with preoperative radiotherapy in rectal cancer. N. Engl. J. Med. 2006, 355, 1114–1123. [Google Scholar] [CrossRef]
- Gerard, J.P.; Conroy, T.; Bonnetain, F.; Bouche, O.; Chapet, O.; Closon-Dejardin, M.T.; Untereiner, M.; Leduc, B.; Francois, E.; Maurel, J.; et al. Preoperative radiotherapy with or without concurrent fluorouracil and leucovorin in T3-4 rectal cancers: Results of FFCD 9203. J. Clin. Oncol. 2006, 24, 4620–4625. [Google Scholar] [CrossRef]
- Sebag-Montefiore, D.; Stephens, R.J.; Steele, R.; Monson, J.; Grieve, R.; Khanna, S.; Quirke, P.; Couture, J.; de Metz, C.; Myint, A.S.; et al. Preoperative radiotherapy versus selective postoperative chemoradiotherapy in patients with rectal cancer (MRC CR07 and NCIC-CTG C016): A multicentre, randomised trial. Lancet 2009, 373, 811–820. [Google Scholar] [CrossRef] [Green Version]
- Erlandsson, J.; Holm, T.; Pettersson, D.; Berglund, A.; Cedermark, B.; Radu, C.; Johansson, H.; Machado, M.; Hjern, F.; Hallbook, O.; et al. Optimal fractionation of preoperative radiotherapy and timing to surgery for rectal cancer (Stockholm III): A multicentre, randomised, non-blinded, phase 3, non-inferiority trial. Lancet Oncol. 2017, 18, 336–346. [Google Scholar] [CrossRef]
- Tiv, M.; Puyraveau, M.; Mineur, L.; Calais, G.; Maingon, P.; Bardet, E.; Mercier, M.; Bosset, J.F. Long-term quality of life in patients with rectal cancer treated with preoperative (chemo)-radiotherapy within a randomized trial. Cancer Radiother. 2010, 14, 530–534. [Google Scholar] [CrossRef]
- Braendengen, M.; Tveit, K.M.; Hjermstad, M.J.; Johansson, H.; Berglund, A.; Brandberg, Y.; Glimelius, B. Health-related quality of life (HRQoL) after multimodal treatment for primarily non-resectable rectal cancer. Long-term results from a phase III study. Eur. J. Cancer 2012, 48, 813–819. [Google Scholar] [CrossRef] [PubMed]
- Dijkstra, E.A.; Hospers, G.A.P.; Kranenbarg, E.M.; Fleer, J.; Roodvoets, A.G.H.; Bahadoer, R.R.; Guren, M.G.; Tjalma, J.J.J.; Putter, H.; Crolla, R.; et al. Quality of life and late toxicity after short-course radiotherapy followed by chemotherapy or chemoradiotherapy for locally advanced rectal cancer—The RAPIDO trial. Radiother. Oncol. 2022, 171, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Birgisson, H.; Pahlman, L.; Gunnarsson, U.; Glimelius, B. Late adverse effects of radiation therapy for rectal cancer—a systematic overview. Acta Oncol. 2007, 46, 504–516. [Google Scholar] [CrossRef] [PubMed]
- Martling, A.; Smedby, K.E.; Birgisson, H.; Olsson, H.; Granath, F.; Ekbom, A.; Glimelius, B. Risk of second primary cancer in patients treated with radiotherapy for rectal cancer. Br. J. Surg. 2017, 104, 278–287. [Google Scholar] [CrossRef]
- Van den Broek, C.B.; van Gijn, W.; Bastiaannet, E.; Moller, B.; Johansson, R.; Elferink, M.A.; Wibe, A.; Pahlman, L.; Iversen, L.H.; Penninckx, F.; et al. Differences in pre-operative treatment for rectal cancer between Norway, Sweden, Denmark, Belgium and the Netherlands. Eur. J. Surg. Oncol. 2014, 40, 1789–1796. [Google Scholar] [CrossRef] [PubMed]
- Morris, E.J.; Finan, P.J.; Spencer, K.; Geh, I.; Crellin, A.; Quirke, P.; Thomas, J.D.; Lawton, S.; Adams, R.; Sebag-Montefiore, D. Wide Variation in the Use of Radiotherapy in the Management of Surgically Treated Rectal Cancer Across the English National Health Service. Clin. Oncol. 2016, 28, 522–531. [Google Scholar] [CrossRef]
- Hammarstrom, K.; Imam, I.; Korsavidou Hult, N.; Ekstrom, J.; Sjoblom, T.; Glimelius, B. Determining the use of preoperative (chemo)radiotherapy in primary rectal cancer according to national and international guidelines. Radiother. Oncol. 2019, 136, 106–112. [Google Scholar] [CrossRef]
- Detering, R.; de Neree Tot Babberich, M.P.M.; Bos, A.; Dekker, J.W.T.; Wouters, M.; Bemelman, W.A.; Beets-Tan, R.G.H.; Marijnen, C.A.M.; Hompes, R.; Tanis, P.J.; et al. Nationwide analysis of hospital variation in preoperative radiotherapy use for rectal cancer following guideline revision. Eur. J. Surg. Oncol. 2020, 46, 486–494. [Google Scholar] [CrossRef]
- Bakx, R.; Emous, M.; Legemate, D.A.; Zoetmulder, F.A.; van Tienhoven, G.; Bemelman, W.A.; van Lanschot, J.J. Harm and benefits of short-term pre-operative radiotherapy in patients with resectable rectal carcinomas. Eur. J. Surg. Oncol. 2006, 32, 520–526. [Google Scholar] [CrossRef] [PubMed]
- Poulsen, L.O.; Yilmaz, M.K.; Ljungmann, K.; Jespersen, N.; Wille-Jorgensen, P.; Petersen, L.N.; Falkmer, U.G. Local recurrence rate in a national Danish patient cohort after curative treatment for rectal cancer. Acta Oncol. 2018, 57, 1639–1645. [Google Scholar] [CrossRef] [PubMed]
- Banwell, V.C.; Phillips, H.A.; Duff, M.J.; Speake, D.; McLean, C.; Williams, L.J.; He, Y.; Paterson, H.M. Five-year oncological outcomes after selective neoadjuvant radiotherapy for resectable rectal cancer. Acta Oncol. 2019, 58, 1267–1272. [Google Scholar] [CrossRef] [PubMed]
- Kakizoe, M.; Watanabe, J.; Goto, K.; Suwa, Y.; Nakagawa, K.; Suwa, H.; Ozawa, M.; Ishibe, A.; Ota, M.; Kunisaki, C.; et al. Identification of Patients with Locally Advanced Rectal Cancer in Whom Preoperative Radiotherapy Can Be Omitted: A Multicenter Retrospective Study at Yokohama Clinical Oncology Group (YCOG1307). J. Anus. Rectum. Colon. 2021, 5, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Habr-Gama, A.; Perez, R.O.; Nadalin, W.; Sabbaga, J.; Ribeiro, U., Jr.; Silva e Sousa, A.H., Jr.; Campos, F.G.; Kiss, D.R.; Gama-Rodrigues, J. Operative versus nonoperative treatment for stage 0 distal rectal cancer following chemoradiation therapy: Long-term results. Ann. Surg. 2004, 240, 711–717. [Google Scholar] [CrossRef] [PubMed]
- Van der Valk, M.J.M.; Hilling, D.E.; Bastiaannet, E.; Meershoek-Klein Kranenbarg, E.; Beets, G.L.; Figueiredo, N.L.; Habr-Gama, A.; Perez, R.O.; Renehan, A.G.; van de Velde, C.J.H.; et al. Long-term outcomes of clinical complete responders after neoadjuvant treatment for rectal cancer in the International Watch & Wait Database (IWWD): An international multicentre registry study. Lancet 2018, 391, 2537–2545. [Google Scholar] [CrossRef]
- Hammarstrom, K.; Imam, I.; Mezheyeuski, A.; Ekstrom, J.; Sjoblom, T.; Glimelius, B. A Comprehensive Evaluation of Associations Between Routinely Collected Staging Information and The Response to (Chemo)Radiotherapy in Rectal Cancer. Cancers 2020, 13, 16. [Google Scholar] [CrossRef]
- Jankowski, M.; Pietrzak, L.; Rupinski, M.; Michalski, W.; Holdakowska, A.; Paciorek, K.; Rutkowski, A.; Olesinski, T.; Cencelewicz, A.; Szczepkowski, M.; et al. Watch-and-wait strategy in rectal cancer: Is there a tumour size limit? Results from two pooled prospective studies. Radiother. Oncol. 2021, 160, 229–235. [Google Scholar] [CrossRef]
- Al-Sukhni, E.; Milot, L.; Fruitman, M.; Beyene, J.; Victor, J.C.; Schmocker, S.; Brown, G.; McLeod, R.; Kennedy, E. Diagnostic accuracy of MRI for assessment of T category, lymph node metastases, and circumferential resection margin involvement in patients with rectal cancer: A systematic review and meta-analysis. Ann. Surg. Oncol. 2012, 19, 2212–2223. [Google Scholar] [CrossRef]
- Beets-Tan, R.G.; Beets, G.L. MRI for assessing and predicting response to neoadjuvant treatment in rectal cancer. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 480–488. [Google Scholar] [CrossRef]
- Beets-Tan, R.G.H.; Lambregts, D.M.J.; Maas, M.; Bipat, S.; Barbaro, B.; Curvo-Semedo, L.; Fenlon, H.M.; Gollub, M.J.; Gourtsoyianni, S.; Halligan, S.; et al. Magnetic resonance imaging for clinical management of rectal cancer: Updated recommendations from the 2016 European Society of Gastrointestinal and Abdominal Radiology (ESGAR) consensus meeting. Eur. Radiol. 2018, 28, 1465–1475. [Google Scholar] [CrossRef] [PubMed]
- RCC. Nationella kvalitetsregister Cancer. 2020. Available online: https://cancercentrum.se/samverkan/cancerdiagnoser/tjocktarm-andtarm-och-anal/tjock--och-andtarm/kvalitetsregister/ (accessed on 6 March 2022).
- Glimelius, B.; Melin, B.; Enblad, G.; Alafuzoff, I.; Beskow, A.; Ahlstrom, H.; Bill-Axelson, A.; Birgisson, H.; Bjor, O.; Edqvist, P.H.; et al. U-CAN: A prospective longitudinal collection of biomaterials and clinical information from adult cancer patients in Sweden. Acta Oncol. 2018, 57, 187–194. [Google Scholar] [CrossRef] [PubMed]
- MERCURY Study Group. Extramural depth of tumor invasion at thin-section MR in patients with rectal cancer: Results of the MERCURY study. Radiology 2007, 243, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Hammarstrom, K.; Mezheyeuski, A.; Korsavidou Hult, N.; Sjoblom, T.; Glimelius, B. Stage distribution utilizing magnetic resonance imaging in an unselected population of primary rectal cancers. Eur. J. Surg. Oncol. 2018, 44, 1858–1864. [Google Scholar] [CrossRef] [PubMed]
- Martling, A.; Holm, T.; Rutqvist, L.E.; Johansson, H.; Moran, B.J.; Heald, R.J.; Cedermark, B. Impact of a surgical training programme on rectal cancer outcomes in Stockholm. Br. J. Surg. 2005, 92, 225–229. [Google Scholar] [CrossRef]
- Bhoday, J.; Balyasnikova, S.; Wale, A.; Brown, G. How Should Imaging Direct/Orient Management of Rectal Cancer? Clin. Colon. Rectal. Surg. 2017, 30, 297–312. [Google Scholar] [CrossRef] [PubMed]
- Blomqvist, L.; Glimelius, B. The ‘good’, the ‘bad’, and the ‘ugly’ rectal cancers. Acta Oncol. 2008, 47, 5–8. [Google Scholar] [CrossRef]
- Regionalt Cancercentrum. Nationellt Vårdprogram Tjock- Och Ändtarmscancer. 2016. Available online: http://www.cancercentrum.se/samverkan/cancerdiagnoser/tjocktarm-andtarm-och-anal/tjock--och-andtarm/vardprogram (accessed on 18 January 2019).
- RCC. National Care Programme. 2020. Available online: https://kunskapsbanken.cancercentrum.se/diagnoser/tjock-och-andtarmscancer/vardprogram/ (accessed on 27 October 2020).
- Bahadoer, R.R.; Dijkstra, E.A.; van Etten, B.; Marijnen, C.A.M.; Putter, H.; Kranenbarg, E.M.; Roodvoets, A.G.H.; Nagtegaal, I.D.; Beets-Tan, R.G.H.; Blomqvist, L.K.; et al. Short-course radiotherapy followed by chemotherapy before total mesorectal excision (TME) versus preoperative chemoradiotherapy, TME, and optional adjuvant chemotherapy in locally advanced rectal cancer (RAPIDO): A randomised, open-label, phase 3 trial. Lancet Oncol. 2021, 22, 29–42. [Google Scholar] [CrossRef]
- Glimelius, B. Short Course Radiation Therapy Followed by Pre-Operative Chemotherapy and Surgery in High-Risk Rectal Cancer (LARCT-US). Uppsala University/Akademiska Sjukhuset. 2018. Available online: https://clinicaltrials.gov/ct2/show/NCT03729687 (accessed on 20 June 2022).
- Martling, A.; Granath, F.; Cedermark, B.; Johansson, R.; Holm, T. Gender differences in the treatment of rectal cancer: A population based study. Eur. J. Surg. Oncol. 2009, 35, 427–433. [Google Scholar] [CrossRef]
- Olsson, L.I.; Granstrom, F.; Glimelius, B. Socioeconomic inequalities in the use of radiotherapy for rectal cancer: A nationwide study. Eur. J. Cancer 2011, 47, 347–353. [Google Scholar] [CrossRef]
- RCC. “Nationell Kvalitetsrapport För år 2020 Från Svenska Kolorektalcancerregistret”—National Quality Report for Year 2020 from the Swedish Colorectal Cancer Registry (SCRCR). Available online: https://cancercentrum.se/globalassets/cancerdiagnoser/tjock--och-andtarm-anal/kvalitetsregister/tjock--och-andtarm-2021/rektalrapport.pdf (accessed on 17 August 2021).
- Noren, A.; Sandstrom, P.; Gunnarsdottir, K.; Ardnor, B.; Isaksson, B.; Lindell, G.; Rizell, M. Identification of Inequalities in the Selection of Liver Surgery for Colorectal Liver Metastases in Sweden. Scand. J. Surg. 2018, 107, 294–301. [Google Scholar] [CrossRef] [PubMed]
- Gietelink, L.; Wouters, M.; Marijnen, C.A.M.; van Groningen, J.; van Leersum, N.; Beets-Tan, R.G.H.; Tollenaar, R.; Tanis, P.J.; Dutch Surgical Colorectal Cancer Audit, G. Changes in nationwide use of preoperative radiotherapy for rectal cancer after revision of the national colorectal cancer guideline. Eur. J. Surg. Oncol. 2017, 43, 1297–1303. [Google Scholar] [CrossRef] [PubMed]
- Read, B.; Sylla, P. Aggressive Colorectal Cancer in the Young. Clin. Colon Rectal. Surg. 2020, 33, 298–304. [Google Scholar] [CrossRef]
- Collaborative, R.; Zaborowski, A.M.; Abdile, A.; Adamina, M.; Aigner, F.; d’Allens, L.; Allmer, C.; Alvarez, A.; Anula, R.; Andric, M.; et al. Characteristics of Early-Onset vs Late-Onset Colorectal Cancer: A Review. JAMA Surg. 2021, 156, 865–874. [Google Scholar] [CrossRef]
- Lord, A.C.; D’Souza, N.; Shaw, A.; Rokan, Z.; Moran, B.; Abulafi, M.; Rasheed, S.; Chandramohan, A.; Corr, A.; Chau, I.; et al. MRI-Diagnosed Tumour Deposits and EMVI Status Have Superior Prognostic Accuracy to Current Clinical TNM Staging in Rectal Cancer. Ann. Surg. 2020, 276, 334–344. [Google Scholar] [CrossRef]
- Yu, S.K.; Chand, M.; Tait, D.M.; Brown, G. Magnetic resonance imaging defined mucinous rectal carcinoma is an independent imaging biomarker for poor prognosis and poor response to preoperative chemoradiotherapy. Eur. J. Cancer 2014, 50, 920–927. [Google Scholar] [CrossRef] [PubMed]
- McCawley, N.; Clancy, C.; O’Neill, B.D.; Deasy, J.; McNamara, D.A.; Burke, J.P. Mucinous Rectal Adenocarcinoma Is Associated with a Poor Response to Neoadjuvant Chemoradiotherapy: A Systematic Review and Meta-analysis. Dis. Colon Rectum. 2016, 59, 1200–1208. [Google Scholar] [CrossRef]
- Kim, N.K.; Kim, M.J.; Park, J.K.; Park, S.I.; Min, J.S. Preoperative staging of rectal cancer with MRI: Accuracy and clinical usefulness. Ann. Surg. Oncol. 2000, 7, 732–737. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, Z.; Qin, Q.; Zhang, C.; Sun, X. Assessment of T and N staging with MRI3T in lower and middle rectal cancer and impact on clinical strategy. J. Int. Med. Res. 2020, 48, 300060520928685. [Google Scholar] [CrossRef]
- Brouwer, N.P.M.; Stijns, R.C.H.; Lemmens, V.; Nagtegaal, I.D.; Beets-Tan, R.G.H.; Futterer, J.J.; Tanis, P.J.; Verhoeven, R.H.A.; de Wilt, J.H.W. Clinical lymph node staging in colorectal cancer; a flip of the coin? Eur. J. Surg. Oncol. 2018, 44, 1241–1246. [Google Scholar] [CrossRef]
- Beresford, M.; Glynne-Jones, R.; Richman, P.; Makris, A.; Mawdsley, S.; Stott, D.; Harrison, M.; Osborne, M.; Ashford, R.; Grainger, J.; et al. The reliability of lymph-node staging in rectal cancer after preoperative chemoradiotherapy. Clin. Oncol. (R Coll. Radiol.) 2005, 17, 448–455. [Google Scholar] [CrossRef] [PubMed]
- Bown, E.J.; Lloyd, G.M.; Boyle, K.M.; Miller, A.S. Rectal cancer: Prognostic indicators of long-term outcome in patients considered for surgery. Int. J. Colorectal. Dis. 2014, 29, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Imam, I.; Hammarstrom, K.; Sjoblom, T.; Glimelius, B. Neoadjuvant rectal (NAR) score: Value evaluating the efficacy of neoadjuvant therapy and prognostic significance after surgery? Radiother. Oncol. 2021, 157, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Stijns, R.C.H.; Philips, B.W.J.; Nagtegaal, I.D.; Polat, F.; de Wilt, J.H.W.; Wauters, C.A.P.; Zamecnik, P.; Futterer, J.J.; Scheenen, T.W.J. USPIO-enhanced MRI of lymph nodes in rectal cancer: A node-to-node comparison with histopathology. Eur. J. Radiol. 2021, 138, 109636. [Google Scholar] [CrossRef]

| Characteristics | Uppsala (C-Region) n = 542 | Dalarna (W-Region) n = 608 | p-Value |
| Age, median | 70 (32–96) | 73 1 (26–96) | <0.001 |
| Men | 314 (58) | 357 (59) | 0.788 |
| cT-stage 2 | 0.228 | ||
| T1 | 40 (7) | 49 (8) | |
| T2 | 104 (19) | 131 (22) | |
| T3 | 275 (51) | 291 (48) | |
| a | 68 (25) | 49 (17) | |
| b | 111 (40) | 118 (41) | |
| c | 74 (27) | 76 (26) | |
| d | 15 (6) | 17 (6) | |
| T4 | 109 (20) | 112 (18) | |
| a | 20 (18) | 43 (38) | |
| b | 83 (76) | 67 (60) | |
| cN-stage | 0.003 | ||
| N0 | 193 (36) | 260 (43) | |
| N1 | 199 (37) | 198 (33) | |
| N2 | 137 (25) | 121 (20) | |
| MRF+ (in cT3 tumours 3) | 107 (39) | 81 (28) | 0.005 |
| EMVI+ | 147 (28) | 134 (23) | 0.045 |
| Tumour level | 0.205 | ||
| Low (0–4 cm) | 119 (22) | 150 (25) | |
| Mid (5–9 cm) | 191 (35) | 219 (36) | |
| High (10–15 cm) | 230 (42) | 238 (39) | |
| Treatment | <0.001 | ||
| Immediate surgery | 196 (39) | 278 (46) | |
| scRT | 170 (31) | 186 (31) | |
| CRT/scRT + CT | 157 (31) | 118 (21) | |
| Risk group 4 | 0.007 | ||
| Early/good | 169 (31) | 221 (36) | |
| Intermediate/bad | 151 (28) | 172 (28) | |
| Locally advanced/ugly | 210 (39) | 187 (31) |
| Treatments | Immediate Surgery n = 475 | scRT 1 n = 355 | CRT/scRT + CT 1 n = 275 | p-Value |
|---|---|---|---|---|
| Age | <0.001 | |||
| <65 years | 118 (25) | 67 (19) | 122 (44) | |
| 65–79 year | 255 (54) | 158 (45) | 149 (54) | |
| ≥80 years | 102 (21) | 130 (37) | 4 (1) | |
| Men | 276 (58) | 208 (58) | 162 (59) | 0.966 |
| cT-stage | <0.001 | |||
| T1 | 80 (17) | 4 (1) | 1 (0.4) | |
| T2 | 168 (35) | 54 (15) | 9 (3) | |
| T3 | 194 (41) | 225 (63) | 133 (48) | |
| a | 63 (32) | 47 (21) | 10 (8) | |
| b | 96 (50) | 103 (46) | 35 (26) | |
| c | 29 (15) | 57 (25) | 68 (51) | |
| d | 1 (0.5) | 11 (5) | 20 (15) | |
| T4 | 9 (2) | 69 (19) | 131 (48) | |
| a | 7 (78) | 15 (22) | 39 (30) | |
| b | 2 (22) | 53 (77) | 92 (70) | |
| cN-stage | <0.001 | |||
| N0 | 312 (66) | 98 (28) | 27 (10) | |
| N1 | 121 (26) | 164 (46) | 101 (37) | |
| N2 | 16 (3) | 91 (26) | 147 (53) | |
| MRF+ (in cT3-tumours) | 15 (8) | 77 (34) | 89 (67) | <0.001 |
| EMVI+ | 32 (7) | 104 (29) | 140 (51) | <0.001 |
| Tumour level | <0.001 | |||
| Low (0–4 cm) | 62 (13) | 111 (31) | 84 (31) | |
| Mid (5–9 cm) | 150 (32) | 150 (42) | 93 (34) | |
| High (10–15 cm) | 261 (55) | 94 (26) | 98 (36) | |
| Risk group | <0.001 | |||
| Early/Good | 318 (67) | 48 (14) 2 | 6 (2) 2 | |
| Intermediate/Bad | 110 (23) | 154 (43) | 61 (22) | |
| Locally advanced/Ugly | 24 (5) 3 | 148 (42) | 207 (75) |
| (A) | ||||||
| Pre-Treatment or Not | Univariable Logistic Regression | Multivariable Logistic Regression | ||||
| OR | 95% CI | p | OR | 95% CI | p | |
| Sex (Male) | 0.968 | 0.761–1.233 | 0.795 | - | - | - |
| Age | 0.903 | 0.761–1.071 | 0.242 | - | - | - |
| Region (Dalarna) | 0.655 | 0.515–0.834 | <0.001 | 0.606 | 0.421–0.872 | 0.007 |
| Year of diagnosis | 0.625 | 0.527–0.741 | <0.001 | 0.621 | 0.483–0.798 | <0.001 |
| Tumour level | 0.478 | 0.405–0.564 | <0.001 | 0.211 | 0.159–0.281 | <0.001 |
| cT-stage | 5.961 | 4.702–7.557 | <0.001 | 4.550 | 3.327–6.225 | <0.001 |
| cT3-substage * | 4.240 | 2.722–6.605 | <0.001 | - | - | - |
| cN-stage | 5.766 | 4.615–7.204 | <0.001 | 3.720 | 2.784–4.970 | <0.001 |
| MRF+ (cT3 tumours) * | 9.683 | 5.481–17.109 | <0.001 | - | - | - |
| EMVI+ | 7.930 | 5.338–11.781 | <0.001 | 3.128 | 1.897–5.158 | <0.001 |
| (B) | ||||||
| CRT/scRT + CT or scRT alone | Univariable Logistic Regression | Multivariable Logistic Regression | ||||
| OR | 95% CI | p | OR | 95% CI | p | |
| Sex (Male) | 0.992 | 0.720–1.365 | 0.959 | - | - | - |
| Age | 0.247 | 0.187–0.326 | <0.001 | 0.179 | 0.125–0.255 | <0.001 |
| Region (Dalarna) | 0.687 | 0.500–0.943 | 0.020 | 0.638 | 0.426–0.957 | 0.030 |
| Year of diagnosis | 1.539 | 1.219–1.942 | <0.001 | 2.182 | 1.584–3.005 | <0.001 |
| Tumour level | 1.175 | 0.960–1.437 | 0.117 | - | - | - |
| cT-stage | 3.175 | 2.373–4.247 | <0.001 | 3.800 | 2.585–5.587 | <0.001 |
| cT3-substage * | 4.314 | 2.725–6.830 | <0.001 | - | - | - |
| cN-stage | 2.421 | 1.910–3.069 | <0.001 | 2.226 | 1.612–3.073 | <0.001 |
| MRF+ (cT3 tumours) * | 3.678 | 2.331–5.802 | <0.001 | - | - | - |
| EMVI+ | 2.347 | 1.683–3.272 | <0.001 | 1.376 | 0.892–2.123 | 0.149 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Imam, I.; Hammarström, K.; Glimelius, B. Determinants of Pre-Surgical Treatment in Primary Rectal Cancer: A Population-Based Study. Cancers 2023, 15, 1154. https://doi.org/10.3390/cancers15041154
Imam I, Hammarström K, Glimelius B. Determinants of Pre-Surgical Treatment in Primary Rectal Cancer: A Population-Based Study. Cancers. 2023; 15(4):1154. https://doi.org/10.3390/cancers15041154
Chicago/Turabian StyleImam, Israa, Klara Hammarström, and Bengt Glimelius. 2023. "Determinants of Pre-Surgical Treatment in Primary Rectal Cancer: A Population-Based Study" Cancers 15, no. 4: 1154. https://doi.org/10.3390/cancers15041154







