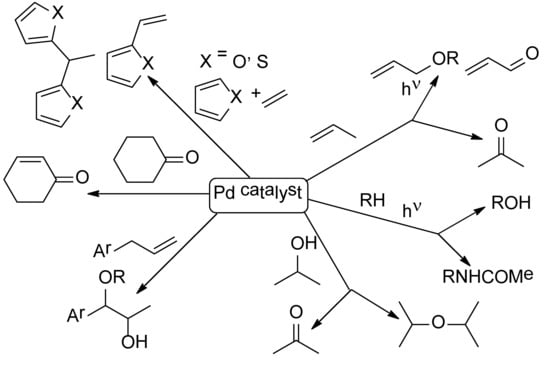
The Reims Journey Towards Discovery and Understanding of Pd-Catalyzed Oxidations
Abstract
:1. Introduction
2. UV-Light-Assisted Oxidation
2.1. Ethylenic Compounds



2.2. Alkanes


3. Allylic Oxidation
3.1. 1-(p-Toluenesulfonyl)-2-Propene and 1-(Trimethylsilyl)-1-(p-Toluenesulfonyl)-2-Propene


3.2. Terminal Alkenes


4. Alcohol Oxidation
4.1. With Sodium Percarbonate

4.2. With 1,2-Dichloroethane





4.3. With Aryl Bromide

4.4. Dehydrogenation

5. Allylphenols Oxidation


6. Wacker Oxidation

7. Cyclohexanone Dehydrogenation

8. Dehydrogenative Heck Reaction







9. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Muzart, J.; Pale, P.; Pète, J.-P. Preparation of conjugated carbonyl compounds by photolysis of η3-allylpalladium complexes. J. Chem. Soc. Chem. Commun. 1981, 14, 668–669. [Google Scholar] [CrossRef]
- Trost, B.M.; Metzner, P.J. Reaction of olefins with palladium trifluoroacetate. J. Am. Chem. Soc. 1980, 102, 3572–3577. [Google Scholar] [CrossRef]
- Muzart, J.; Pale, P.; Pète, J.-P. Photoactivation of alkenes oxidation by molecular oxygen in the presence of palladium. Tetrahedron Lett. 1982, 23, 3577–3578. [Google Scholar] [CrossRef]
- Muzart, J.; Pale, P.; Pète, J.-P. Catalyse par le palladium et la lumière ultra-violette de l’oxydation d’alcènes par l’oxygène moléculaire. J. Organomet. Chem. 1988, 353, 267–273. [Google Scholar] [CrossRef]
- Sen, A.; Lai, T.-W. Catalytic isomerization of alkenes by palladium(II) compounds. An alternative mechanistic view. Inorg. Chem. 1981, 20, 4036–4038. [Google Scholar] [CrossRef]
- Sen, A.; Lai, T.-W. Mechanism of palladium(II)-catalyzed carbon-carbon double bond isomerization in olefins. Inorg. Chem. 1984, 23, 3257–3258. [Google Scholar] [CrossRef]
- Muzart, J.; Riahi, A.; Pète, J.-P. Oxydation régiosélective de sulfones allyliques catalysée par le palladium et la lumière: Formation d’aldéhydes et alcools α,β-éthyléniques β-sulfonylés. J. Organomet. Chem. 1985, 280, 269–279. [Google Scholar] [CrossRef]
- Riahi, A.; Cossy, J.; Muzart, J.; Pète, J.-P. Palladium catalyzed oxidation of allylsilanes with U.V. light and molecular oxygen. Tetrahedron Lett. 1985, 26, 839–842. [Google Scholar] [CrossRef]
- Muzart, J.; Riahi, A. Oxygenation under UV light of allylsilanes catalyzed by palladium(II) and of (.eta. 3-allyl)palladium complexes: A mechanistic approach. Organometallics 1992, 11, 3478–3481. [Google Scholar] [CrossRef]
- Hütter, P.; Butters, T.; Winter, W.; Handschuh, D.; Woelter, W. Die kristall- und molekülstruktur des dimeren π-allylpalladiumchlorid-Complexes von testosteron. Liebigs Ann. Chem. 1982, 1111–1120. [Google Scholar] [CrossRef]
- Hughes, R.P.; Day, C.S. Steric blocking of η3 → η1 → η3 isomerizations of an η3-allylic ligand. Crystal and molecular structures of 1,3-chloropalladation products of cis-9-methylenebicyclo [6.1.0]nonane and cis-7-methylenebicyclo[4.1.0]heptane. Organometallics 1982, 1, 1221–1225. [Google Scholar] [CrossRef]
- Keinan, E.; Roth, Z. Regioselectivity in organo-transition-metal chemistry. A new indicator substrate for classification of nucleophiles. J. Org. Chem. 1983, 48, 1769–1772. [Google Scholar] [CrossRef]
- Walling, C.; Zavitsas, A.A. The copper-catalyzed reaction of peresters with hydrocarbons. J. Am. Chem. Soc. 1963, 85, 2084–2090. [Google Scholar] [CrossRef]
- Corey, E.J.; Walker, J.C. Organoiron-mediated oxygenation of allylic organotin compounds. A possible chemical model for enzymatic lipoxygenation. J. Am. Chem. Soc. 1987, 109, 8108–8109. [Google Scholar] [CrossRef]
- Kliegman, J.M. The palladium(II) cleavage of allylsilanes. J. Organomet. Chem. 1971, 29, 73–77. [Google Scholar] [CrossRef]
- Crozet, M.P.; Muzart, J.; Pale, P.; Tordo, P. Photolyse des complexes η3--allylpalladium: Étude par résonnance paramagnétique électronique des nitroxydes allyliques formés en présence de nitrosodurène. J. Organomet. Chem. 1983, 244, 191–200. [Google Scholar] [CrossRef]
- Vermeersch, G.; Marko, J.; Muzart, J. Photoreactivity of η3-allylpalladium complexes studied by CINDP. J. Chem. Soc. Perkin Trans. 1986, 383–389. [Google Scholar] [CrossRef]
- Muzart, J.; Hénin, F. Activation de liaisons carbone-hydrogène d’alcanes par l’association de complexes métalliques électrophiles et de lumière ultra-violette. C. R. Acad. Sci. Paris Série II 1988, 307, 479–482. [Google Scholar]
- Barton, D.H.R.; Boivin, J.; Gastiger, M.; Morzycki, J.; Hay-Motherwell, R.S.; Motherwell, W.B.; Ozbalik, N.; Schwartzentruber, K.M. Functionalization of saturated hydrocarbons. Part 4. The Gif system for selective oxidation using molecular oxygen. J. Chem. Soc. Perkin Trans. 1986, 947–955. [Google Scholar] [CrossRef]
- Muzart, J.; Nizova, G.V.; Riahi, A.; Shul’pin, G.B. Oxidation of alkanes by peroxide complexes of palladium. J. Gen. Chem. USSR 1992, 62, 964. [Google Scholar]
- Muzart, J.; Pète, J.-P.; Riahi, A. Palladium-catalyzed allylic oxidation of 1-(p-toluenesulfonyl)-2-propene and 1-(trimethylsilyl)-1-(p-toluenesulfonyl)-2-propene. J. Organomet. Chem. 1987, 331, 113–119. [Google Scholar] [CrossRef]
- Mimoun, H.; Charpentier, R.; Mitschler, A.; Fischer, J.; Weiss, R. Palladium(II) tert-butyl peroxide carboxylates. New reagents for the selective oxidation of terminal olefins to methyl ketones. On the role of peroxymetalation in selective oxidative processes. J. Am. Chem. Soc. 1980, 102, 1047–1057. [Google Scholar] [CrossRef]
- Muzart, J. Oxydation d’alcènes: Induction par les métaux de la formation de liaison C-O en position allylique. Bull. Soc. Chim. Fr. 1986, 1, 65–77. [Google Scholar]
- Beccalli, E.M.; Broggini, G.; Martinelli, M.; Sottocornola, S. C-C, C-O, C-N bond formation on sp2 carbon by Pd(II)-catalyzed reactions involving oxidant agents. Chem. Rev. 2007, 107, 5318–5365. [Google Scholar] [CrossRef] [PubMed]
- Thiery, E.; Aouf, C.; Belloy, J.; Harakat, D.; Le Bras, J.; Muzart, J. Palladium-catalyzed allylic acyloxylation of terminal alkenes in the presence of a base. J. Org. Chem. 2010, 75, 1771–1774. [Google Scholar] [CrossRef] [PubMed]
- Skapski, A.C.; Smart, M.L. The crystal structure of trimeric palladium(II) acetate. J. Chem. Soc. Chem. Commun. 1970, 11, 658–659. [Google Scholar] [CrossRef]
- Muzart, J. Palladium-catalysed oxidation of primary and secondary alcohols. Tetrahedron 2003, 59, 5789–5816. [Google Scholar] [CrossRef]
- Muzart, J. Sodium perborate and sodium percarbonate in organic synthesis. Synthesis 1995, 1995, 1325–1347. [Google Scholar] [CrossRef]
- McKillop, W.R. Sanderson, Sodium perborate and sodium percarbonate: Cheap, safe and versatile oxidising agents for organic synthesis. Tetrahedron 1995, 51, 6145–6166. [Google Scholar] [CrossRef]
- Adogen 464 is a registered trademark of Ashland Chemical Co. for methyltrialky1(C8-C10) ammonium chloride. CAS Number 72749-59-8.
- Aït-Mohand, S.; Lunak, S.; Muzart, J. Chlorides and acetylacetonates of transition metals as catalysts for the oxidation of 1-indanol by sodium percarbonate. Chem. Ber. 1997, 130, 1655–1658. [Google Scholar] [CrossRef]
- Aït-Mohand, S.; Hénin, F.; Muzart, J. Palladium(II)-mediated oxidation of alcohols using 1,2-dichloroethane as Pd(0) reoxidant. Tetrahedron Lett. 1995, 36, 2473–2476. [Google Scholar] [CrossRef]
- Rothenberg, G.; Humbel, S.; Muzart, J. Palladium-catalyzed oxidation of alcohols to carbonyl compounds with 1,2-dichloroethane as the primary oxidant: A theoretical study. J. Chem. Soc. Perkin Trans. 2001, 10, 1998–2004. [Google Scholar] [CrossRef]
- Bouquillon, S.; du Moulinet d’Hardemare, A.; Averbuch-Pouchot, M.-T.; Hénin, F.; Muzart, J. Synthesis and characterization of monomeric and dimeric palladium(II)-ammonium complexes; Their use for the catalytic oxidation of alcohols. Polyhedron 1999, 18, 3511–3516. [Google Scholar] [CrossRef]
- Aït-Mohand, S.; Muzart, J. Palladium-catalyzed oxidative cyclization of 1,4-and 1,5-diols in 1,2-dichloroethane. J. Mol. Catal. A Chem. 1998, 129, 135–139. [Google Scholar] [CrossRef]
- Aït-Mohand, S. Catalyse de réactions d’oxydation: utilisation du percarbonate de sodium et régénération d’espèces palladiées actives par le 1,2-dichloroéthane. Doctoral Thesis, Reims University, Reims, France, 1997. [Google Scholar]
- Taniguchi, T.; Ogasawara, K. Specific asymmetric mono-epoxidation of meso 2,3-syn-bis-allylic alcohols having a bicyclo[2.2.1]heptane framework. Tetrahedron Lett. 1997, 38, 433–436. [Google Scholar] [CrossRef]
- Taniguchi, T.; Goto, Y.; Ogasawara, K. Preparation of a promising cyclobutanone chiral building block: Utilization its stereochemistry and utilization. Synlett 1997, 1997, 707–709. [Google Scholar] [CrossRef]
- Jaeschke, G.; Seebach, D. Highly enantioselective ring opening of cyclic meso-anhydrides to isopropyl hemiesters with Ti-TADDOLates: An alternative to hydrolytic enzymes? J. Org. Chem. 1998, 63, 1190–1197. [Google Scholar] [CrossRef]
- March, J. Advanced Organic Chemistry, 4th ed.; John Wiley: New York, NY, USA, 1992; p. 393. [Google Scholar]
- Aït-Mohand, S.; Hénin, F.; Muzart, J. Palladium-catalyzed oxidations: Inhibition of a Pd-H elimination by coordination of a remote carbon carbon double bond. Organometallics 2001, 20, 1683–1686. [Google Scholar] [CrossRef]
- Chung, K.; Banik, S.M.; De Crisci, A.G.; Pearson, D.M.; Blake, T.R.; Olsson, J.V.; Ingram, A.J.; Zare, R.N.; Waymouth, R.M. Chemoselective Pd-catalyzed oxidation of polyols: Synthetic scope and mechanistic studies. J. Am. Chem. Soc. 2013, 135, 7593–7602. [Google Scholar] [CrossRef]
- Li, Y.; Lin, Z. Understanding the reaction mechanisms of Pd-catalysed oxidation of alcohols and domino oxidation-arylation reactions using phenyl chloride as an oxidant. Org. Chem. Front. 2014, 1, 1188–1196. [Google Scholar] [CrossRef]
- Corey, E.J.; Palani, A. A method for the selective oxidation of 1,4-diols to lactols. Tetrahedron Lett. 1995, 36, 3485–3488. [Google Scholar] [CrossRef]
- Corey, E.J.; Palani, A. A mechanistic model for the selective oxidation of 1,4-diols to γ-lactols by o-iodoxybenzoic acid. Tetrahedron Lett. 1995, 36, 7945–7948. [Google Scholar] [CrossRef]
- Lieb, F.; Niewöhner, U.; Wendisch, D. 6-(3-Carbamoylbicyclo[2.2.1]hept-2-yl)hexansäuren, eine neue klasse von TxA2-antagonisten. Liebigs Ann. Chem. 1987, 1987, 607–615. [Google Scholar] [CrossRef]
- For the introduction of this expression and its use, see, respectively: Berson, J.A.; Willcott, M.R. Bredt rule Interdictions of cyclopropane rearrangements.The vinylnortricyclenes. J. Org. Chem. 1965, 30, 3569–3572. [CrossRef]
- Baldwin, J.E.; Dunmire, D.A. The “No Reaction” reaction of 1-vinylnortricyclene to tricyclo[4.2.1.03,7]non-3-ene. J. Org. Chem. 2000, 65, 6791–6794. [Google Scholar] [CrossRef] [PubMed]
- Bouquillon, S.; Hénin, F.; Muzart, J. The critical role of the coordination environment of palladium dichloride on the course of its reaction with secondary benzylic alcohols; selective oxidation or etherification catalysts. Organometallics 2000, 19, 1434–1437. [Google Scholar] [CrossRef]
- Sen, A.; Lai, T.-W. Catalysis by solvated transition-metal cations. Novel catalytic transformations of alkenes by tetrakis(acetonitrile)palladium ditetrafluoroborate. Evidence for the formation of incipient carbonium ions as intermediates. J. Am. Chem. Soc. 1981, 103, 4627–4629. [Google Scholar] [CrossRef]
- Sen, A.; Lai, T.-W. Oligomerization and isomerization of olefins by η3-allyl complexes of palladium. The role of the allyl group. Organometallics 1983, 2, 1059–1060. [Google Scholar] [CrossRef]
- Lipshutz, B.H.; Pollart, D.; Monforte, J.; Kotsuki, H. Pd(II)-catalyzed acetal/ketal hydrolysis/exchange reactions. Tetrahedron Lett. 1985, 26, 705–708. [Google Scholar] [CrossRef]
- Tenaglia, A.; Kammerer, F. Palladium(II)-catalyzed dehydrative cyclization of cis-4-alkylcycloalken-2-ols. Synthesis of tricyclic spiroketals in a one-pot sequence. Synlett 1996, 1996, 576–578. [Google Scholar] [CrossRef]
- Salehi, P.; Iranpoor, N.; Behbahani, F.K. Selective and efficient alcoholyses of allylic, secondary-and tertiary benzylic alcohols in the presence of iron (III). Tetrahedron 1998, 54, 943–948. [Google Scholar] [CrossRef]
- Tamaru, Y.; Yamamoto, Y.; Yamada, Y.; Yoshida, Z. Palladium catalyzed oxidations of secondary alcohols. Tetrahedron Lett. 1979, 20, 1401–1404. [Google Scholar] [CrossRef]
- Tamaru, Y.; Yamada, Y.; Inoue, K.; Yamamoto, Y.; Yoshida, Z. Oxidation of primary and secondary alcohols by the catalysis of palladium. J. Org. Chem. 1983, 48, 1286–1292. [Google Scholar] [CrossRef]
- Bessmertnykh, A.; Hénin, F.; Muzart, J. Palladium-catalyzed oxidation of benzylated aldose hemiacetals to lactones. Carbohydr. Res. 2004, 339, 1377–1380. [Google Scholar] [CrossRef] [PubMed]
- Welton, T. Room-temperature ionic liquids. Solvents for synthesis and catalysis. Chem. Rev. 1999, 99, 2071–2083. [Google Scholar] [CrossRef] [PubMed]
- Dupont, J.; de Souza, R.F.; Suarez, P.A.Z. Ionic liquid (molten salt) phase organometallic catalysis. Chem. Rev. 2002, 102, 3667–3692. [Google Scholar] [CrossRef] [PubMed]
- Ionic Liquids in Synthesis; Wasserscheid, P.; Welton, T. (Eds.) Wiley: Weinheim, Germany, 2003. [Google Scholar]
- Muzart, J. Ionic liquids as solvents for catalyzed oxidations of organic compounds. Adv. Synth. Catal. 2006, 348, 275–295. [Google Scholar] [CrossRef]
- Betza, D.; Altmann, P.; Cokoja, M.; Herrmann, W.A.; Kuhn, F.E. Recent advances in oxidation catalysis using ionic liquids as solvents. Coord. Chem. Rev. 2011, 255, 1518–1540. [Google Scholar] [CrossRef]
- Dai, C.; Zhang, J.; Huang, C.; Lei, Z. Ionic liquids in selective oxidation: Catalysts and solvents. Chem. Rev. 2017, 117, 6929–6983. [Google Scholar] [CrossRef]
- Bouquillon, S.; Ganchegui, B.; Estrine, B.; Hénin, F.; Muzart, J. Heck arylation of allylic alcohols in molten salts. J. Organomet. Chem. 2001, 634, 153–156. [Google Scholar] [CrossRef]
- Keresszegi, C.; Mallat, T.; Baiker, A. Selective transfer dehydrogenation of aromatic alcohols on supported palladium. New J. Chem. 2001, 25, 1163–1167. [Google Scholar] [CrossRef]
- Murahashi, S.; Shimura, T.; Moritani, I. Conversion of alcohols into unsymmetrical secondary or tertiary amines by a palladium catalyst. Synthesis of N-substituted pyrroles. Chem. Commun. 1974, 22, 931–932. [Google Scholar] [CrossRef]
- Ganchegui, B.; Bouquillon, S.; Hénin, F.; Muzart, J. Palladium-catalyzed dehydrogenation of benzylic alcohols in molten ammonium salts, a recyclable system. Tetrahedron Lett. 2002, 43, 6641–6644. [Google Scholar] [CrossRef]
- Ganchegui, B. Transformations palladocatalysées et utilisation d’un sel fondu comme solvant. Doctoral Thesis, Reims University, Reims, France, 2004. [Google Scholar]
- Li, C.-J. Organic reactions in aqueous media—with a focus on carbon-carbon bond formation. Chem. Rev. 1993, 93, 2023–2035. [Google Scholar] [CrossRef]
- Li, C.-J.; Chen, L. Organic chemistry in water. Chem. Soc. Rev. 2006, 35, 68–82. [Google Scholar] [CrossRef] [PubMed]
- Hailes, H.C. Reaction solvent selection: The potential of water as a solvent for organic transformations. Org. Process Res. Dev. 2007, 11, 114–120. [Google Scholar] [CrossRef]
- Chanda, A.; Fokin, V.V. Organic synthesis “on water”. Chem. Rev. 2009, 109, 725–748. [Google Scholar] [CrossRef] [Green Version]
- Lamblin, M.; Nassar-Hardy, L.; Hierso, J.-C.; Fouquet, E.; Felpin, F.-X. Recyclable heterogeneous palladium catalysts in pure water: Sustainable developments in Suzuki, Heck, Sonogashira and Tsuji–Trost reactions. Adv. Synth. Catal. 2010, 352, 33–79. [Google Scholar] [CrossRef]
- Le Bras, J.; Muzart, J. From metal-catalyzed reactions with hydrosoluble ligands to reactions in and on water. Curr. Org. Synth. 2011, 8, 330–334. [Google Scholar] [CrossRef]
- Harry, N.A.; Radhika, S.; Neetha, M.; Anilkumar, G. Recent advances and prospects of organic reactions “on water”. ChemistrySelect 2019, 4, 12337–12355. [Google Scholar] [CrossRef]
- Le Bras, J.; Muzart, J. Water-soluble and reusable copper catalyst for the allylic benzoyloxylation of olefins. Tetrahedron Lett. 2002, 43, 431–433. [Google Scholar] [CrossRef]
- Meulemans, T.M.; Kiers, N.H.; Feringa, B.L.; van Leeuwen, P.W.N.M. Catalytic oxidation of homoallylalcohols to α-alkoxytetrahydrofurans by a Pd-nitro complex and molecular oxygen. Tetrahedron Lett. 1994, 35, 455–458. [Google Scholar] [CrossRef] [Green Version]
- Roshchin, A.I.; Kel’chevski, S.M.; Bumagin, N.A. Synthesis of benzofurans via Pd2+-catalyzed oxidative cyclization of 2-allylphenols. J. Organomet. Chem. 1998, 560, 163–167. [Google Scholar] [CrossRef]
- Larock, R.C.; Wei, L.; Hightower, T.R. Synthesis of 2H-1-benzopyrans by Pd-catalyzed cyclization of o-allylic phenols. Synlett 1998, 522–524. [Google Scholar] [CrossRef]
- Chevrin, C.; Le Bras, J.; Hénin, F.; Muzart, J. One-pot catalytic synthesis of 2-(1,2-dihydroxypropyl)-phenol derivatives from 2-allylphenols in aqueous media. Synthesis 2005, 2005, 2615–2618. [Google Scholar] [CrossRef]
- Wahlen, J.; De Vos, D.E.; Jacobs, P.A. Activation of hydrogen peroxide through hydrogen-bonding interaction with acidic alcohols: epoxidation of alkenes in phenol. Org. Lett. 2003, 5, 1777–1780. [Google Scholar] [CrossRef] [PubMed]
- Hassam, M.; Taher, A.; Arnott, G.E.; Green, I.R.; van Otterlo, W.A.L. Isomerization of allylbenzenes. Chem. Rev. 2015, 115, 5462–5569. [Google Scholar] [CrossRef]
- Lattanzi, A.; Senatore, A.; Massa, A.; Scettri, A. Novel highly regioselective VO(acac)2/TBHP mediated oxidation of o-alkenyl phenols to o-hydroxybenzyl ketones. J. Org. Chem. 2003, 68, 3691–3694. [Google Scholar] [CrossRef]
- Muzart, J. Pd-mediated reactions of epoxides. Eur. J. Org. Chem. 2011, 2011, 4717–4741. [Google Scholar] [CrossRef]
- Thiery, E.; Chevrin, C.; Le Bras, J.; Harakat, D.; Muzart, J. Mechanism insights into the palladiumII-catalyzed hydroalkoxylation of 2-allylphenols. J. Org. Chem. 2007, 72, 1859–1862. [Google Scholar] [CrossRef]
- Tsuji, J. Synthetic applications of the palladium-catalyzed oxidation of olefins to ketones. Synthesis 1984, 1984, 369–384. [Google Scholar] [CrossRef]
- Keith, J.A.; Henry, P.M. The Mechanism of the Wacker reaction: A tale of two hydroxypalladations. Angew. Chem. Int. Ed. 2009, 48, 9038–9049. [Google Scholar] [CrossRef] [PubMed]
- Comas-Vives, A.; Stirling, A.; Lledós, A.; Ujaque, G. The Wacker process: Inner- or outer-sphere nucleophilic addition? New insights from ab initio molecular dynamics. Chem. Eur. J. 2010, 16, 8738–8747. [Google Scholar] [CrossRef] [PubMed]
- Anderson, B.J.; Keith, J.A.; Sigman, M.S. Experimental and computational study of a direct O2-coupled Wacker oxidation: Water dependence in the absence of Cu salts. J. Am. Chem. Soc. 2010, 132, 11872–11874. [Google Scholar] [CrossRef] [Green Version]
- Nair, N.N. Ligand exchanges and hydroxypalladation reactions of the Wacker process in aqueous solution at high Cl− concentration. J. Phys. Chem. B 2011, 115, 2312–2321. [Google Scholar] [CrossRef]
- Muzart, J. N,N-Dimethylformamide: Much more than a solvent. Tetrahedron 2009, 65, 8313–8323. [Google Scholar] [CrossRef]
- Le Bras, J.; Muzart, J. N,N-Dimethylformamide and N,N-dimethylacetamide as carbon, hydrogen, nitrogen and/or oxygen sources. In Solvents as Reagents in Organic Synthesis, Reactions and Applications; Wu, X.-F., Ed.; Wiley-VCH: Hoboken, NJ, USA, 2017; pp. 199–314. [Google Scholar]
- Le Bras, J.; Muzart, J. Recent uses of N,N-dimethylformamide and N,N-dimethylacetamide as reagents. Molecules 2018, 23, 1939. [Google Scholar] [CrossRef] [Green Version]
- Vasseur, A.; Muzart, J.; Le Bras, J. Ubiquitous benzoquinones, multitalented compounds for palladium-catalyzed oxidative reactions. Eur. J. Org. Chem. 2015, 2015, 4053–4059. [Google Scholar] [CrossRef]
- Harakat, D.; Muzart, J.; Le Bras, J. ESI-MS mechanistic studies of Wacker oxidation of alkenes: Dinuclear species as catalytic active intermediates. RSC Adv. 2012, 2, 3094–3099. [Google Scholar] [CrossRef]
- Muzart, J.; Pète, J.-P. Dehydrogenation of cyclohexanones catalyzed by palladium (II) trifluoroacetate. J. Mol. Catal. 1982, 15, 373–376. [Google Scholar] [CrossRef]
- Muzart, J. One-pot synthesis of α, β-unsaturated carbonyl compounds through palladium-mediated dehydrogenation of ketones, aldehydes, esters, lactones and amides. Eur. J. Org. Chem. 2010, 3779–3790. [Google Scholar] [CrossRef]
- Gligorich, K.M.; Sigman, M.S. Mechanistic questions about the reaction of molecular oxygen with palladium in oxidase catalysis. Angew. Chem. Int. Ed. 2006, 118, 6612–6615. [Google Scholar] [CrossRef] [PubMed]
- Muzart, J. Molecular oxygen to regenerate PdII active species. Chem. Asian J. 2006, 1, 508–515. [Google Scholar] [CrossRef] [PubMed]
- Gligorich, K.M.; Sigman, M.S. Recent advancements and challenges of palladiumII-catalyzed oxidation reactions with molecular oxygen as the sole oxidant. Chem. Commun. 2009, 26, 3854–3867. [Google Scholar] [CrossRef]
- Konnick, M.M.; Stahl, S.S. Reaction of molecular oxygen with a PdII-hydride to produce a PdII-hydroperoxide: Experimental evidence for an HX-reductive-elimination pathway. J. Am. Chem. Soc. 2008, 130, 5753–5762. [Google Scholar] [CrossRef]
- Le Bras, J.; Muzart, J. The Saegusa oxidation and related procedures. Org. React. 2019, 98, 1–172. [Google Scholar]
- Le Bras, J.; Muzart, J. Pd-catalyzed domino dehydrogenation/Heck-type reactions of carbonyl compounds. Adv. Synth. Catal. 2018, 360, 2411–2428. [Google Scholar] [CrossRef]
- The Mizoroki-Heck Reaction; Oestreich, M. (Ed.) Wiley: Chichester, UK, 2009. [Google Scholar]
- Fujiwara, Y.; Moritani, I.; Matsuda, M.; Teranishi, S. Aromatic substitution of olefin. IV Reaction with palladium metal and silver acetate. Tetrahedron Lett. 1968, 9, 3863–3865. [Google Scholar] [CrossRef]
- Ferreira, E.M.; Zhang, H.; Stoltz, B.M. C–H bond functionalizations with palladium(II): Intramolecular oxidative annulations of arenes. Tetrahedron 2008, 64, 5987–6001. [Google Scholar] [CrossRef] [Green Version]
- Trost, B.M. Atom economy—a challenge for organic synthesis: Homogeneous catalysis leads the way. Angew. Chem. Int. Ed. Engl. 1995, 34, 259–281. [Google Scholar] [CrossRef]
- Le Bras, J.; Muzart, J. Intermolecular dehydrogenative Heck reactions. Chem. Rev. 2011, 111, 1170–1214. [Google Scholar] [CrossRef] [PubMed]
- Aouf, C.; Thiery, E.; Le Bras, J.; Muzart, J. Palladium-catalyzed dehydrogenative coupling of furans with styrenes. Org. Lett. 2009, 11, 4096–4099. [Google Scholar] [CrossRef] [PubMed]
- Vasseur, A.; Muzart, J.; Le Bras, J. Dehydrogenative Heck reaction of furans and thiophenes with styrenes under mild conditions and influence of the oxidizing agent on the reaction rate. Chem. Eur. J. 2011, 17, 12556–12560. [Google Scholar] [CrossRef] [PubMed]
- Larock, R.C.; Hightower, T.R.J. Synthesis of unsaturated lactones via palladium-catalyzed cyclization of alkenoic acids. J. Org. Chem. 1993, 58, 5298–5300. [Google Scholar] [CrossRef]
- Muzart, J. Oxidation adjacent to oxygen of alcohols catalyzed by palladium/dimethyl sulfoxide. In Comprehensive Organic Synthesis, 2nd ed.; Molander, G.A., Knochel, P., Eds.; Elsevier: Amsterdam, The Netherlands; Oxford, UK, 2014; Volume 7, pp. 295–301. [Google Scholar]
- Stash, A.I.; Perepelkova, T.I.; Kravtsova, S.V.; Noskov, Y.G.; Romm, I.P. Palladium(II) diacetate complex with dimethyl sulfoxide: Structure and properties. Koord. Khim. 1998, 24, 40–43. Russ. J. Coord. Chem. 1998, 24, 36–39. [Google Scholar]
- Zierkiewicz, W.; Privalov, T. A theoretical study of the essential role of DMSO as a solvent/ligand in the Pd(OAc)2/DMSO catalyst system for aerobic oxidation. Organometallics 2005, 24, 6019–6028. [Google Scholar] [CrossRef]
- Vasseur, A.; Harakat, D.; Muzart, J.; Le Bras, J. ESI-MS studies of the dehydrogenative Heck reaction of furans with acrylates using benzoquinone as the reoxidant and DMSO as the solvent. J. Org. Chem. 2012, 77, 5751–5758. [Google Scholar] [CrossRef]
- Vasseur, A.; Harakat, D.; Muzart, J.; Le Bras, J. Aerobic dehydrogenative Heck reactions of heterocycles with styrenes: A negative effect of metallic co-oxidants. Adv. Synth. Catal. 2013, 355, 59–67. [Google Scholar] [CrossRef]
- Vasseur, A.; Laugel, C.; Harakat, D.; Muzart, J.; Le Bras, J. Ligand-promoted reactivity of alkenes in dehydrogenative Heck reactions of furans and thiophenes. Eur. J. Org. Chem. 2015, 2015, 944–948. [Google Scholar] [CrossRef]
- Thiery, E.; Harakat, D.; Le Bras, J.; Muzart, J. Pd-catalyzed oxidative coupling of 2-alkylfurans with olefins through C-H activation: Synthesis of difurylalkanes. Organometallics 2008, 27, 3996–4004. [Google Scholar] [CrossRef]
- Rulev, A.Y. Serendipity or the art of making discoveries. New J. Chem. 2017, 41, 4262–4268. [Google Scholar] [CrossRef]
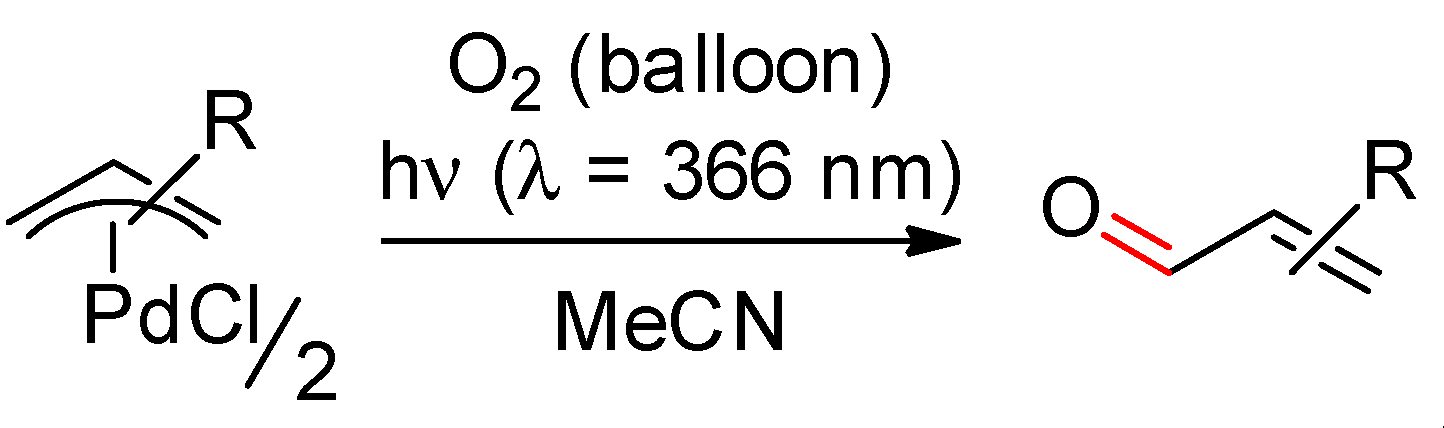












© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Le Bras, J.; Muzart, J. The Reims Journey Towards Discovery and Understanding of Pd-Catalyzed Oxidations. Catalysts 2020, 10, 111. https://doi.org/10.3390/catal10010111
Le Bras J, Muzart J. The Reims Journey Towards Discovery and Understanding of Pd-Catalyzed Oxidations. Catalysts. 2020; 10(1):111. https://doi.org/10.3390/catal10010111
Chicago/Turabian StyleLe Bras, Jean, and Jacques Muzart. 2020. "The Reims Journey Towards Discovery and Understanding of Pd-Catalyzed Oxidations" Catalysts 10, no. 1: 111. https://doi.org/10.3390/catal10010111
APA StyleLe Bras, J., & Muzart, J. (2020). The Reims Journey Towards Discovery and Understanding of Pd-Catalyzed Oxidations. Catalysts, 10(1), 111. https://doi.org/10.3390/catal10010111