Rapid Removal of Azophloxine via Catalytic Degradation by a Novel Heterogeneous Catalyst under Visible Light
Abstract
:1. Introduction
2. Results and Discussions
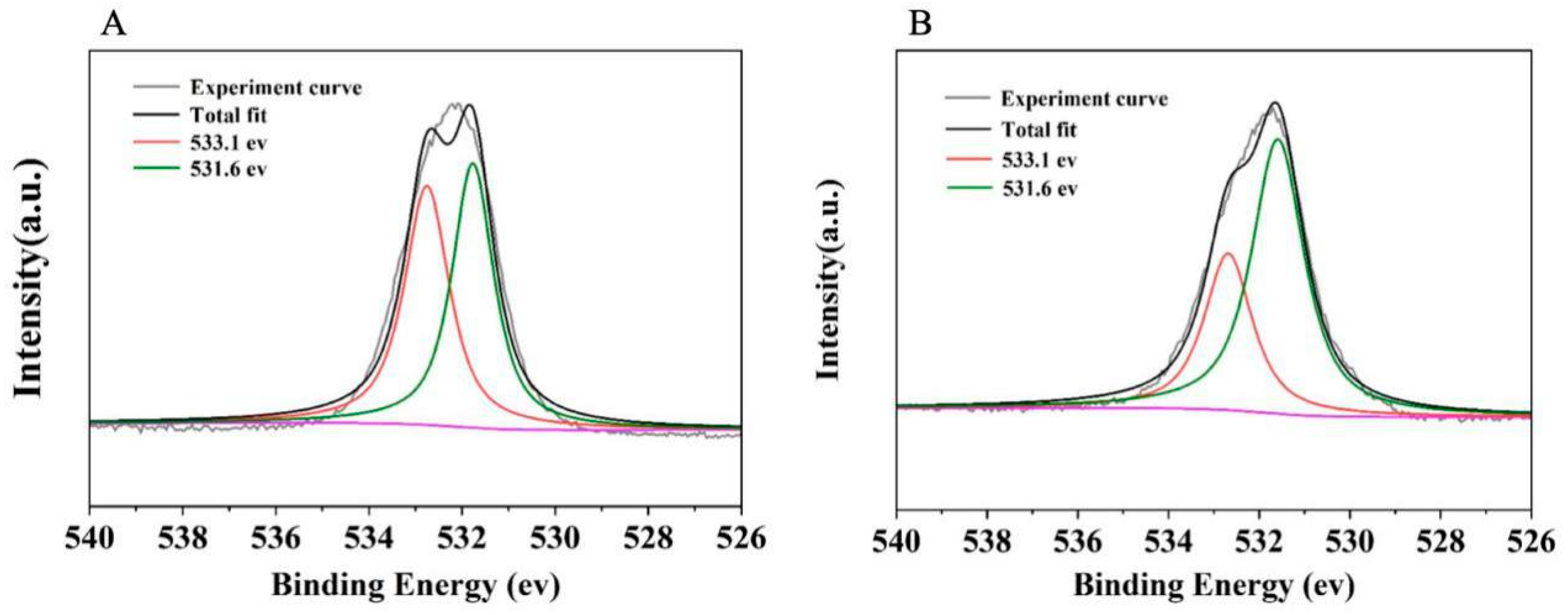
2.1. Characteristics of the As-Prepared Catalyst
2.2. Dye Removal by Fe-Alg Catalyst
2.3. Effect of Reaction Conditions
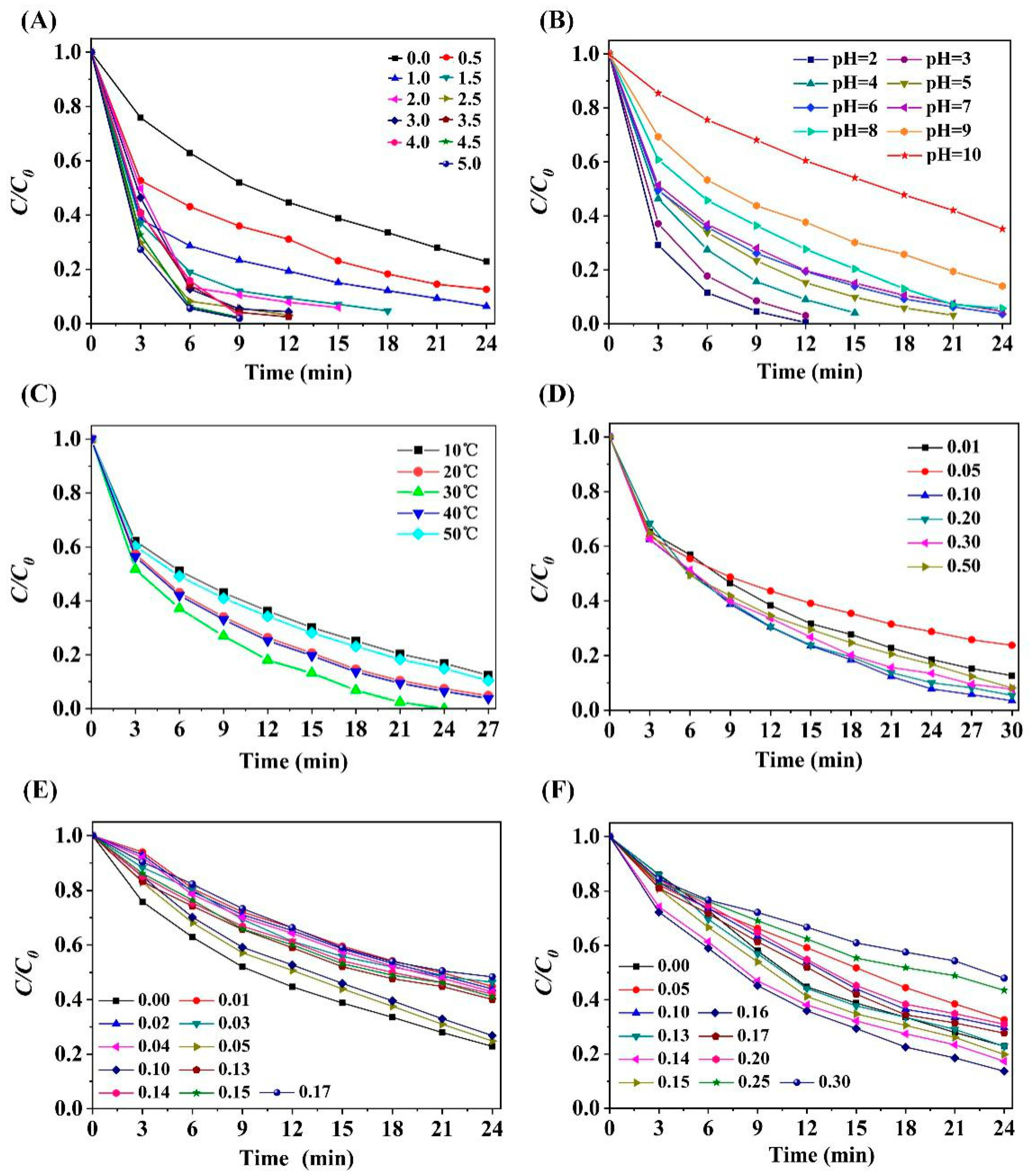
2.3.1. Effect of PMS Dosage
2.3.2. Effect of Initial pH Value
2.3.3. Effect of Treatment Temperature
2.3.4. Impact of Ion Strength
2.4. Comparison between the FeCit-ACFs/PMS/Visible Light and FeAlg-ACFs/PMS/Visible Light Systems
2.5. Reaction Kinetics of Catalytic Oxidation by the FeAlg-ACF/PMS System
2.6. Mechanism of Catalytic Oxidation by the FeAlg-ACF/PMS System
3. Experimental
3.1. Materials
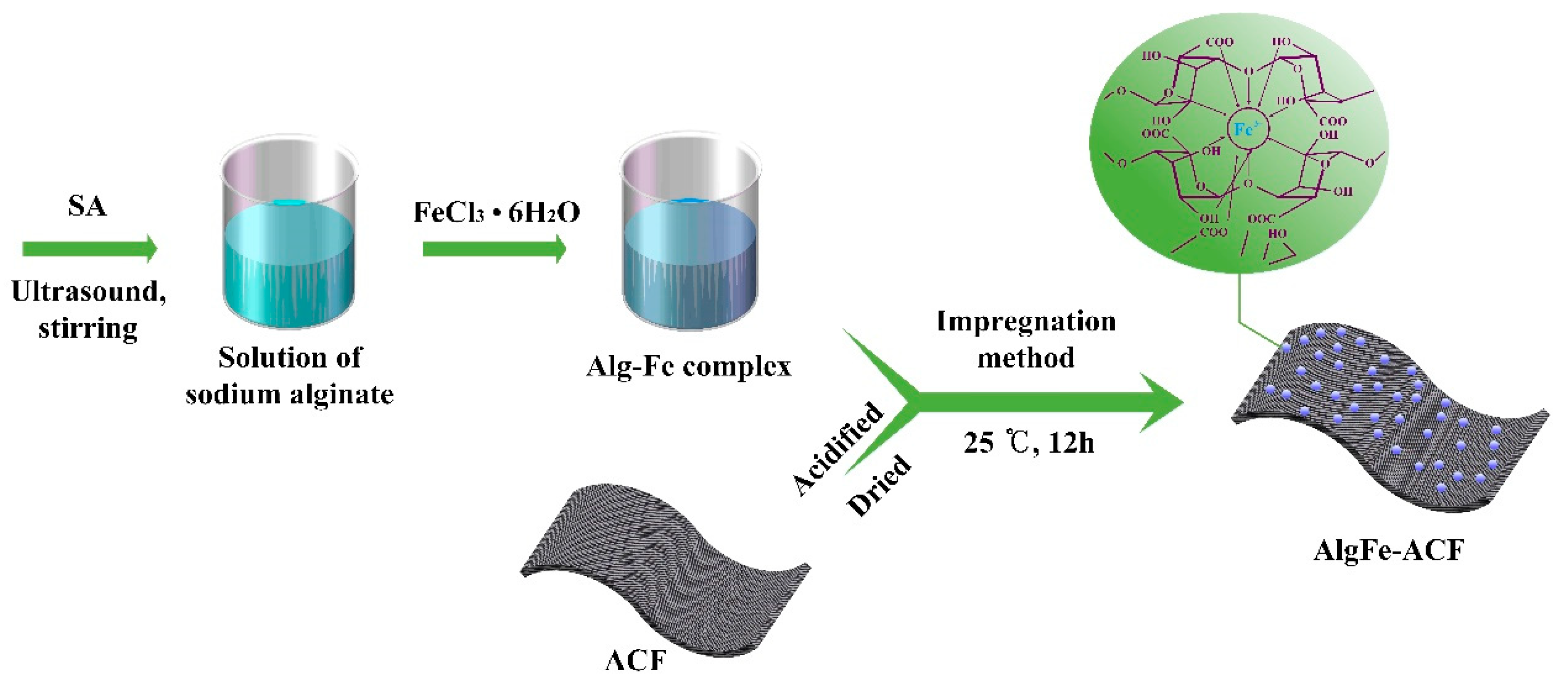
3.2. Sample Preparation
3.3. Dye Removal by FeAlg-ACF
3.4. Characterization
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sakaue, S.; Tsubakino, T.; Nishiyama, Y.; Ishii, Y.; Org, J. Oxidation of aromatic amines with hydrogen peroxide catalyzed by cetylpyridinium heteropolyoxometalates. J. Organ. Chem. 1993, 58, 3633–3638. [Google Scholar] [CrossRef]
- Dong, C.; Lu, J.; Qiu, B.; Shen, B.; Xing, M.; Zhang, J. Developing stretchable and graphene-oxide-based hydrogel for the removal of organic pollutants and metal ions. Appl. Catal. B Environ. 2018, 222, 146–156. [Google Scholar] [CrossRef]
- Ahmad, M.; Yousaf, M.; Nasir, A.; Bhatti, I.A.; Mahmood, A.; Fang, X.; Jian, X.; Kalantar-Zadeh, K.; Mahmood, N. Porous eleocharis@ MnPE layered hybrid for synergistic adsorption and catalytic biodegradation of toxic Azo dyes from industrial wastewater. Environ. Sci. Technol. 2019, 53, 2161–2170. [Google Scholar] [CrossRef]
- Wang, Q.; Huang, L.; Quan, X. Sequential anaerobic and electro-Fenton processes mediated by W and Mo oxides for degradation/mineralization of azo dye methyl orange in photo assisted microbial fuel cells. Appl. Catal. B Environ. 2019, 245, 672–680. [Google Scholar] [CrossRef] [Green Version]
- Shukla, P.; Wang, S.; Sun, H.; Ang, H.; Tade, M. Adsorption and heterogeneous advanced oxidation of phenolic contaminants using Fe loaded mesoporous SBA-15 and H2O2. Chem. Eng. J. 2010, 164, 255–260. [Google Scholar] [CrossRef]
- Luo, W.; Zhu, L.; Wang, N.; Tang, H.; Cao, M.; She, Y. Efficient removal of organic pollutants with magnetic nanoscaled BiFeO3 as a reusable heterogeneous Fenton-like catalyst. Environ. Sci. Technol. 2020, 44, 1786–1791. [Google Scholar] [CrossRef]
- Gao, C.; Chen, S.; Quan, X.; Hong, Y.; Yao, Z. Enhanced Fenton-like catalysis by iron-based metal organic frameworks for degradation of organic pollutants. J. Catal. 2017, 356, 125–132. [Google Scholar] [CrossRef]
- Yang, X.; Xu, X.; Xu, J.; Han, Y. Iron oxychloride (FeOCl): An efficient Fenton-like catalyst for producing hydroxyl radicals in degradation of organic contaminants. J. Am. Chem. Soc. 2013, 135, 16058–16061. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Qi, C.Y.; Yang, Z.; Guo, Q.; Chen, W.Q.; Zeng, G.M.; Lei, C. Pd/Fe3O4 nanocatalysts for highly effective and simultaneous removal of humic acids and Cr (VI) by electro-Fenton with H2O2 in situ electro-generated on the catalyst surface. J. Catal. 2017, 352, 337–350. [Google Scholar] [CrossRef]
- Lachkov, P.T.; Chin, Y.H. Catalytic consequences of reactive oxygen species during C3H6 oxidation on Ag clusters. J. Catal. 2018, 366, 127–138. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Z.; Cai, R. A new role for Fe3+ in TiO2 hydrosol: Accelerated photodegradation of dyes under visible light. Environ. Sci. Technol. 2008, 42, 5759–5764. [Google Scholar] [CrossRef]
- He, W.; Jia, H.; Yang, D.; Xiao, P.; Fan, X.; Zheng, Z.; Kim, H.; Wamer, W.G.; Yin, J. Composition directed generation of reactive oxygen species in irradiated mixed metal sulfides correlated with their photocatalytic activities. ACS Appl. Mater. Inter. 2015, 7, 16440–16449. [Google Scholar] [CrossRef] [Green Version]
- Song, K.; Lu, M.; Xu, S.; Chen, C.; Zhan, Y.; Li, D.; Au, C.; Jiang, L.; Tomishige, K. Effect of alloy composition on catalytic performance and coke-resistance property of Ni-Cu/Mg (Al) O catalysts for dry reforming of methane. Appl. Catal. B Environ. 2018, 239, 324–333. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, Y.; Teng, Y. Electrospun magnetic cobalt–carbon nanofiber composites with axis-sheath structure for efficient peroxymonosulfate activation. Appl. Surf. Sci. 2018, 452, 443–450. [Google Scholar] [CrossRef]
- Luo, J.; Wang, Y.; Cao, D.; Xiao, K.; Guo, T.; Zhao, X. Enhanced photoelectrocatalytic degradation of 2, 4-dichlorophenol by TiO2/Ru-IrO2 bifacial electrode. Chem. Eng. J. 2018, 343, 69–77. [Google Scholar] [CrossRef]
- Liu, S.; Zhao, X.; Wang, Y.; Shao, H.; Qiao, M.; Wang, Y.; Zhao, S. Peroxymonosulfate enhanced photoelectrocatalytic degradation of phenol activated by Co3O4 loaded carbon fiber cathode. J. Catal. 2017, 355, 167–175. [Google Scholar] [CrossRef]
- Fan, Z.; Wang, Z.; Shi, J.; Gao, C.; Gao, G.; Wang, B.; Wang, Y.; Chen, X.; He, C.; Niu, C. Charge-redistribution-induced new active sites on (0 0 1) facets of α-Mn2O3 for significantly enhanced selective catalytic reduction of NOx by NH3. J. Catal. 2019, 370, 30–37. [Google Scholar] [CrossRef]
- Luo, L.; Wu, D.; Dai, D.; Yang, Z.; Chen, L.; Liu, Q.; He, J.; Yao, Y. Synergistic effects of persistent free radicals and visible radiation on peroxymonosulfate activation by ferric citrate for the decomposition of organic contaminants. Appl. Catal. B Environ. 2017, 205, 404–411. [Google Scholar] [CrossRef]
- Wang, X.N.; Dong, W.B.; Brigante, M.; Mailhot, G. C60 Fullerol Promoted Fe (III)/H2O2 Fenton Oxidation: Role of Photosensitive Fe (III)-Fullerol Complex. Appl. Catal. B Environ. 2019, 245, 271–278. [Google Scholar] [CrossRef]
- Rastogi, A.; Al-Abed, S.R.; Dionysiou, D.D. Effect of inorganic, synthetic and naturally occurring chelating agents on Fe (II) mediated advanced oxidation of chlorophenols. Water Res. 2009, 43, 684–694. [Google Scholar] [CrossRef]
- Ling, L.; Zhang, D.P.; Fang, J.Y.; Fan, C.H.; Shang, C. A novel Fe (II)/citrate/UV/peroxymonosulfate process for micropollutant degradation: Optimization by response surface methodology and effects of water matrices. Chemosphere 2017, 184, 417–428. [Google Scholar] [CrossRef] [PubMed]
- Han, D.H.; Wan, J.Q.; Ma, Y.W.; Wang, Y.; Li, Y.; Li, D.Y.; Guan, Z.Y. New insights into the role of organic chelating agents in Fe (II) activated persulfate processes. Chem. Eng. J. 2015, 269, 425–433. [Google Scholar] [CrossRef]
- Xu, Z.; Gao, G.; Pan, B.; Zhang, W.; Lv, L. A new combined process for efficient removal of Cu (II) organic complexes from wastewater: Fe (III) displacement/UV degradation/alkaline precipitation. Water Res. 2015, 87, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Chen, X.; Ji, H.; Ma, W.; Chen, C.; Zhao, J. Photochemical cycling of iron mediated by dicarboxylates: Special effect of malonate. Environ. Sci. Technol. 2010, 44, 263–268. [Google Scholar] [CrossRef]
- Pang, H.; Zhang, Q.; Wang, H.; Cai, D.; Ma, Y.; Li, L.; Li, K.; Lu, X.; Chen, H.; Yang, X.; et al. Photochemical Aging of Guaiacol by Fe (III)–Oxalate Complexes in Atmospheric Aqueous Phase. Environ. Sci. Technol. 2019, 53, 127–136. [Google Scholar] [CrossRef]
- Chen, J.; Browne, W.R. Photochemistry of iron complexes. Chem. Rev. 2018, 374, 15–35. [Google Scholar] [CrossRef]
- Ruales-Lonfat, C.; Barona, J.F.; Sienkiewicz, A.; Vélez, J.; Benítez, L.N.; Pulgarín, C. Bacterial inactivation with iron citrate complex: A new source of dissolved iron in solar photo-Fenton process at near-neutral and alkaline pH. Appl. Catal. B Environ. 2016, 180, 379–390. [Google Scholar] [CrossRef]
- Li, L.; Fang, Y.; Vreeker, R.; Appelqvist, I.; Mendes, E. Reexamining the egg-box model in calcium− alginate gels with X-ray diffraction. Biomacromolecules 2007, 8, 464–468. [Google Scholar] [CrossRef]
- Dong, Y.; Dong, W.; Cao, Y.; Han, Z.; Ding, Z. Preparation and catalytic activity of Fe alginate gel beads for oxidative degradation of azo dyes under visible light irradiation. Catal. Today 2011, 175, 346–355. [Google Scholar] [CrossRef]
- Fernandez, J.; Dhananjeyan, M.R.; Kiwi, J.; Senuma, Y.; Hilborn, J. Evidence for Fenton photoassisted processes mediated by encapsulated Fe ions at biocompatible pH values. J. Phys. Chem. B 2000, 104, 5298–5301. [Google Scholar] [CrossRef]
- Li, B.; Dong, Y.; Zou, C.; Xu, Y. Iron (III)–alginate fiber complex as a highly effective and stable heterogeneous fenton photocatalyst for mineralization of organic dye. Ind. Eng. Chem. Res. 2014, 53, 4199–4206. [Google Scholar] [CrossRef]
- Narayanan, R.P.; Melman, G.; Letourneau, N.J.; Mendelson, N.L.; Melman, A. Photodegradable iron (III) cross-linked alginate gels. Biomacromolecules 2012, 13, 2465–2471. [Google Scholar] [CrossRef]
- Titouhi, H.; Belgaied, J.E. Removal of ofloxacin antibiotic using heterogeneous Fenton process over modified alginate beads. J. Environ. Sci. 2016, 45, 84–93. [Google Scholar] [CrossRef]
- Iglesias, O.; Gómez, J.; Pazos, M.; Sanromán, M.Á. Electro-fenton oxidation of imidacloprid by fe alginate gel beads. Appl. Catal. B Environ. 2014, 144, 416–424. [Google Scholar] [CrossRef]
- Zhang, W.F.; Liu, F.; Sun, Y.G.; Zhang, J.; Hao, Z.P. Simultaneous redox conversion and sequestration of chromate (VI) and arsenite (III) by iron (III)-alginate based photocatalysis. Appl. Catal. B Environ. 2019, 259, 118046. [Google Scholar] [CrossRef]
- Tao, X.; Su, J.; Wang, L. A new heterogeneous catalytic system for wastewater treatment: Fe-immobilized polyelectrolyte microshells for accumulation and visible light-assisted photooxidative degradation of dye pollutants. J. Mol. Catal. A Chem. 2008, 280, 186–193. [Google Scholar] [CrossRef]
- Lim, S.; Zheng, Y.; Zou, S.; Chen, J.P. Characterization of copper adsorption onto an alginate encapsulated magnetic sorbent by a combined FT-IR, XPS, and mathematical modeling study. Environ. Sci. Technol. 2008, 42, 2551–2556. [Google Scholar] [CrossRef]
- Gonzalez-Olmos, R.; Martin, M.J.; Georgi, A.; Kopinke, F.D.; Oller, I.; Malato, S. Fe-zeolites as heterogeneous catalysts in solar fenton-like reactions at neutral ph. Appl. Catal. B Environ. 2012, 125, 51–58. [Google Scholar] [CrossRef]
- Ji, H.W.; Song, W.J.; Chen, C.; Yuan, H.; Ma, W.; Zhao, J. Anchored oxygen-donor coordination to iron for photodegradation of organic pollutants. Environ. Sci. Technol. 2007, 41, 5103–5107. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.; Ma, W.H.; Chen, C.; Yao, J.; Zhao, J. Photocatalytic degradation of organic pollutants catalyzed by layered iron(ii) bipyridine complex–clay hybrid under visible irradiation. Appl. Catal. B Environ. 2006, 65, 217–226. [Google Scholar] [CrossRef]
- Feng, J.Y.; Hu, X.J.; Yue, P.L. Novel bentonite clay-based Fe−nanocomposite as a heterogeneous catalyst for photo-fenton discoloration and mineralization of orange II. Environ. Sci. Technol. 2004, 38, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Duarte, F.; Maldonado-Hódar, F.J.; Madeira, L.M. Prussian blue onto activated carbon as a catalyst for heterogeneous fenton-like processes. Appl. Catal. B Environ. 2013, 129, 264–272. [Google Scholar] [CrossRef]
- Yang, S.; Yang, X.; Shao, X.; Niu, R.; Wang, L. Activated carbon catalyzed persulfate oxidation of Azo dye acid orange 7 at ambient temperature. J. Hazard. Mater. 2011, 186, 659–666. [Google Scholar] [CrossRef]
- Hu, L.; Yang, X.; Dang, S. An easily recyclable Co/SBA-15 catalyst: Heterogeneous activation of peroxymonosulfate for the degradation of phenol in water. Appl. Catal. B Environ. 2011, 102, 19–26. [Google Scholar] [CrossRef]
- Huang, Y.; Peng, L.; Liu, Y.; Zhao, G.; Chen, J.Y.; Yu, G. Biobased nano porous active carbon fibers for high-performance supercapacitors. ACS Appl. Mater. Inter. 2016, 8, 15205–15215. [Google Scholar] [CrossRef] [PubMed]
- Nabais, J.M.V.; Carrott, P.J.M. Chemical characterization of activated carbon fibers and activated carbons. J. Chem. Educ. 2006, 83, 436. [Google Scholar] [CrossRef]
- Yao, Y.; Wang, L.; Sun, L.; Zhu, S.; Huang, Z.; Mao, Y.; Lu, W.; Chen, W. Efficient removal of dyes using heterogeneous Fenton catalysts based on activated carbon fibers with enhanced activity. Chem. Eng. Sci. 2013, 101, 424–431. [Google Scholar] [CrossRef]
- Zhang, Y.; Tang, Y.; Qiu, Q.; Chen, Y.; Sun, Y.; Wan, P.; Yang, X.J. Electrochemically Enhanced Adsorption of Aluminum from Sodium Carbonate Solution by Activated Carbon Fibers. Ind. Eng. Chem. Res. 2013, 52, 14449–14455. [Google Scholar] [CrossRef]
- Li, H.Y.; Li, N.J.; Chen, D.Y. Polyethylene imine-grafted ACF@BiOI0.5Cl0.5 as a recyclable photocatalyst for high-efficient dye removal by adsorption-combined degradation. Appl. Surf. Sci. 2017, 403, 80–88. [Google Scholar] [CrossRef]
- Cihanoğlu, A.; Gündüz, G.; Dükkancı, M. Degradation of acetic acid by heterogeneous Fenton-like oxidation over iron-containing ZSM-5 zeolites. Appl. Catal. B Environ. 2015, 165, 687–699. [Google Scholar] [CrossRef]
- Aleksić, M.; Kušić, H.; Koprivanac, N.; Leszczynska, D.; Božić, A.L. Heterogeneous Fenton type processes for the degradation of organic dye pollutant in water-The application of zeolite assisted AOPs. Desalination 2010, 257, 22–29. [Google Scholar] [CrossRef]
- Gonzalez-Olmos, R.; Kopinke, F.D.; Mackenzie, K. Hydrophobic Fe-zeolites for removal of MTBE from water by combination of adsorption and oxidation. Environ. Sci. Technol. 2013, 47, 2353–2360. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.Q.; Zou, H.K.; Li, X.; Arowo, M.; Sun, B.C.; Chen, J.F.; Chu, G.W.; Shao, L. Degradation of phenol by ozone in the presence of Fenton reagent in a rotating packed bed. Chem. Eng. J. 2013, 229, 404–411. [Google Scholar] [CrossRef]
- Duesterberg, C.; Mylon, S.; Waite, T. PH effects on iron-catalyzed oxidation using Fenton’s reagent. Environ. Sci. Technol. 2008, 42, 8522–8527. [Google Scholar] [CrossRef]
- Chen, X.; Ma, W.H.; Li, J.; Wang, Z.H.; Chen, C.; Ji, H.W.; Zhao, J.C. Photocatalytic oxidation of organic pollutants catalyzed by an iron complex at biocompatible pH values: Using O2 as main oxidant in a Fenton-like reaction. J. Phys. Chem. C 2011, 115, 4089–4095. [Google Scholar] [CrossRef]
- Cuartero, M.; Crespo, G.; Cherubini, T.; Pankratova, N.; Confalonieri, F.; Massa, F.; Tercier-Waeber, M.; Abdou, M.; Schäfer, J.; Bakker, E. In situ detection of macronutrients and chloride in seawater by submersible electrochemical sensors. Anal. Chem. 2018, 90, 4702–4710. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.D.; Wang, D.; Ma, J.; Zhang, T.; Lu, X.H.; Chen, Z.Q. A superior active and stable spinel sulfide for catalytic peroxymonosulfate oxidation of bisphenol S. Appl. Catal. B Environ. 2018, 238, 557–567. [Google Scholar] [CrossRef]
- Page, S.E.; Sander, M.; Arnold, W.A.; McNeill, K. Hydroxyl radical formation upon oxidation of reduced humic acids by oxygen in the dark. Environ. Sci. Technol. 2012, 46, 1590–1597. [Google Scholar] [CrossRef]
- Zuorro, A.; Lavecchia, R.; Monaco, M.M.; Lervolino, G.; Vaiano, V. Photocatalytic degradation of azo dye Reactive Violet 5 on Fe-doped titania catalysts under visible light irradiation. Catalysts 2019, 9, 645. [Google Scholar] [CrossRef] [Green Version]


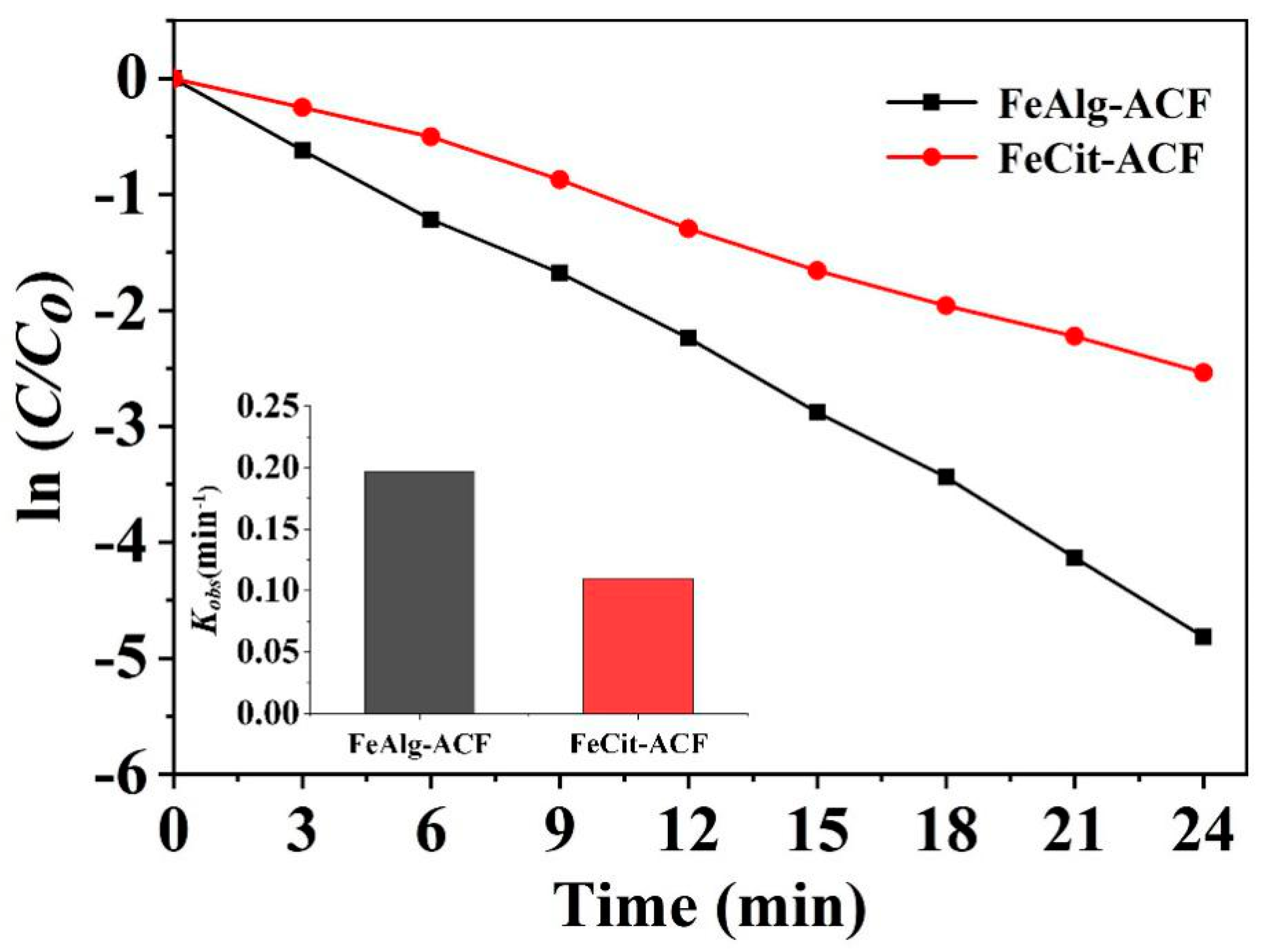


| Dye | Dynamic Equation Series | The Fitted Reaction Rate Equation | kobs | Adj. R-Square |
|---|---|---|---|---|
| azophloxine | pseudo-first-order kinetic model | −ln(Ct/C0) = −0.19694t − 0.04125 | 0.19694 | 0.99524 |
| pseudo-second-order kinetic model | 1/Ct = −0.09478t − 21.84025 | 0.09478 | 0.98076 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, D.; Xia, K.; Fang, C.; Chen, X.; Ye, Y. Rapid Removal of Azophloxine via Catalytic Degradation by a Novel Heterogeneous Catalyst under Visible Light. Catalysts 2020, 10, 138. https://doi.org/10.3390/catal10010138
Wu D, Xia K, Fang C, Chen X, Ye Y. Rapid Removal of Azophloxine via Catalytic Degradation by a Novel Heterogeneous Catalyst under Visible Light. Catalysts. 2020; 10(1):138. https://doi.org/10.3390/catal10010138
Chicago/Turabian StyleWu, Di, Kequan Xia, Chengzhu Fang, Xuegang Chen, and Ying Ye. 2020. "Rapid Removal of Azophloxine via Catalytic Degradation by a Novel Heterogeneous Catalyst under Visible Light" Catalysts 10, no. 1: 138. https://doi.org/10.3390/catal10010138
APA StyleWu, D., Xia, K., Fang, C., Chen, X., & Ye, Y. (2020). Rapid Removal of Azophloxine via Catalytic Degradation by a Novel Heterogeneous Catalyst under Visible Light. Catalysts, 10(1), 138. https://doi.org/10.3390/catal10010138