Recent Advances in Non-Precious Transition Metal/Nitrogen-doped Carbon for Oxygen Reduction Electrocatalysts in PEMFCs
Abstract
:1. Introduction
2. Brief Introduction of the ORR Mechanism
3. Previous M–NxC Catalysts
3.1. Non-Pyrolyzed Transition Metal Macrocyclic Compounds
3.2. Pyrolyzed Metal Macrocyclic Compounds
4. Recent Developed M–NxC Catalysts
4.1. Choices of Nitrogen and Carbon Sources
4.2. Types of Metal
5. Conclusions
- The study of the ORR catalytic mechanism. Understanding the character of M–NxC and the catalytic kinetics in the catalytic process is helpful to us to regulate the catalyst structure and composition accurately, therefore improving the catalytic performance.
- The study of active sites. Although there are already several researches on the identification of active structures, the true catalytic sites in M–NxC catalyst are still confusing which drastically hinder the development of catalysts with adequate activity and stability. It is suggested that the atom-by-atom structural and chemical analysis in graphene can be achieved by the gentle ADF-STEM, which is a promising pathway to explore the local environment of active sites [136,137].
- The exploration of new types of active sites. The aforementioned design of binuclear active sites, which are proven to be more positive than traditional M–N2 and M–N4 active sites, is a successful example to search for new kinds of active sites. It has inspired us greatly to enter a newly emerging but promising world to design satisfactory ORR catalysts.
Author Contributions
Funding
Conflicts of Interest
References
- Guo, J.J.; Mao, Z.; Yan, X.L.; Su, R.; Guan, P.F.; Xu, B.S.; Zhang, X.F.; Qin, G.W.; Pennycook, S.J. Ultrasmall Tungsten Carbide Catalysts Stabilized in Graphitic Layers for High-Performance Oxygen Reduction Reaction. Nano Energy 2016, 28, 261–268. [Google Scholar] [CrossRef] [Green Version]
- Zhu, C.Z.; Fu, S.F.; Xu, B.Z.; Song, J.H.; Shi, Q.R.; Engelhard, M.H.; Li, X.L.; Beckman, S.P.; Sun, J.M.; Du, D.; et al. Sugar Blowing-Induced Porous Cobalt Phosphide/Nitrogen-Doped Carbon Nanostructures with Enhanced Electrochemical Oxidation Performance toward Water and Other Small Molecules. Small 2017, 13, 1700796–1700804. [Google Scholar] [CrossRef]
- Song, M.X.; Song, Y.H.; Li, H.; Liu, P.Z.; Xu, B.S.; Wei, H.; Guo, J.J.; Wu, Y.C. Sucrose Leavening-Induced Hierarchically Porous Carbon Enhanced the Hydrogen Evolution Reaction Performance of Pt Nanoparticles. Electrochim. Acta 2019, 320, 134603–134609. [Google Scholar] [CrossRef]
- Liu, B.; Zhao, Y.F.; Peng, H.Q.; Zhang, Z.Y.; Sit, C.K.; Yuen, M.F.; Zhang, T.R.; Lee, C.S.; Zhang, W.J. Nickel-Cobalt Diselenide 3D Mesoporous Nanosheet Networks Supported on Ni Foam: An All-pH Highly Efficient Integrated Electrocatalyst for Hydrogen Evolution. Adv. Mater. 2017, 29, 1606521–1606528. [Google Scholar] [CrossRef] [PubMed]
- Ravikumar, C.H.; Nair, G.V.; Muralikrishna, S.; Nagaraju, D.H.; Balakrishna, R.G. Nanoflower Like Structures of MoSe2 and MoS2 as Efficient Catalysts for Hydrogen Evolution. Mater. Lett. 2018, 220, 133–135. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Z.X.; Lei, T.; Ai, Y.F.; Peng, Z.K.; Yan, X.Y.; Li, H.; Zhang, J.J.; Wang, Z.M.M.; Chueh, Y.-L. Hollow NiCo2S4 Nanospheres Hybridized with 3D Hierarchical Porous rGO/Fe2O3 Composites toward High Performance Energy Storage Device. Adv. Energy Mater. 2018, 8, 1703453–1703463. [Google Scholar]
- Habas, S.E.; Platt, H.A.S.; van Hest, M.F.A.M.; Ginley, D.S. Low-Cost Inorganic Solar Cells: From Ink to Printed Device. Chem. Rev. 2010, 110, 6571–6594. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Yang, Z.R.; Li, X.W.; Sun, Q.J.; Yang, R.Z. Phosphorus-Doped Porous Carbons as Efficient Electrocatalysts for Oxygen Reduction. J. Mater. Chem. A 2013, 1, 9889–9896. [Google Scholar] [CrossRef] [Green Version]
- Steele, B.C.H.; Heinzel, A. Materials for Fuel-Cell Technologies. Nature 2001, 414, 345–352. [Google Scholar] [CrossRef]
- Liu, H.S.; Song, C.J.; Zhang, L.; Zhang, J.J.; Wang, H.J.; Wilkinson, D.P. A Review of Anode Catalysis in the Direct Methanol Fuel Cell. J. Power Sources 2006, 155, 95–110. [Google Scholar] [CrossRef]
- Shao, M.H.; Chang, Q.W.; Dodelet, J.P.; Chenitz, R. Recent Advances in Electrocatalysts for Oxygen Reduction Reaction. Chem. Rev. 2016, 116, 3594–3657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antolini, E. Structural Parameters of Supported Fuel Cell Catalysts: The Effect of Particle Size, Inter-Particle Distance and Metal Loading on Catalytic Activity and Fuel Cell Performance. Appl. Catal. B Environ. 2016, 181, 298–313. [Google Scholar] [CrossRef]
- Yun, W.; Chen, K.S.; Mishler, J.; Cho, S.C.; Adroher, X.C. A Review of Polymer Electrolyte Membrane Fuel Cells: Technology, Applications, and Needs on Fundamental Research. Appl. Energy 2011, 88, 981–1007. [Google Scholar]
- Nie, Y.; Li, L.; Wei, Z.D. Recent Advancements in Pt and Pt-Free Catalysts for Oxygen Reduction Reaction. Chem. Soc. Rev. 2015, 44, 2168–2201. [Google Scholar] [CrossRef]
- Kang, S.Y.; Kim, H.J.; Chung, Y.H. Recent Developments of Nano-Structured Materials as the Catalysts for Oxygen Reduction Reaction. Nano Converg. 2018, 5, 13. [Google Scholar] [CrossRef]
- Hung, A.-J.; Chen, Y.-H.; Sung, L.-Y.; Yu, C.-C. Cost Analysis of Proton Exchange Membrane Fuel Cell Systems. AIChE J. 2008, 54, 1798–1810. [Google Scholar] [CrossRef]
- Othman, R.; Dicks, A.L.; Zhu, Z.H. Non Precious Metal Catalysts for the PEM Fuel Cell Cathode. Int. J. Hydrogen Energy 2012, 37, 357–372. [Google Scholar] [CrossRef]
- El-Kharouf, A.; Chandan, A.; Hattenberger, M.; Pollet, B.G. Proton Exchange Membrane Fuel Cell Degradation and Testing: Review. J. Energy Inst. 2012, 85, 188–200. [Google Scholar] [CrossRef]
- Banham, D.; Ye, S.; Pei, K.; Ozaki, J.i.; Kishimoto, T.; Imashiro, Y. A Review of the Stability and Durability of Non-Precious Metal Catalysts for the Oxygen Reduction Reaction in Proton Exchange Membrane Fuel Cells. J. Power Sources 2015, 285, 334–348. [Google Scholar] [CrossRef]
- Ioroi, T.; Siroma, Z.; Yamazaki, S.-i.; Yasuda, K. Electrocatalysts for PEM Fuel Cells. Adv. Energy Mater. 2019, 9. [Google Scholar] [CrossRef]
- Lin, R.; Cai, X.; Zeng, H.; Yu, Z.P. Stability of High-Performance Pt-Based Catalysts for Oxygen Reduction Reactions. Adv. Mater. 2018, 30. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.J.; Zhang, X.; Yang, Q.F.; Chen, K.; Guo, J.; Zhou, D.Y.; Feng, L.; Slanina, Z. Highly Porous Defective Carbons Derived from Seaweed Biomass as Efficient Electrocatalysts for Oxygen Reduction in Both Alkaline and Acidic Media. Carbon 2018, 137, 93–103. [Google Scholar] [CrossRef]
- Wee, J.H.; Lee, K.-Y.; Kim, S.H. Fabrication Methods for Low-Pt-Loading Electrocatalysts in Proton Exchange Membrane Fuel Cell Systems. J. Power Sources 2007, 165, 667–677. [Google Scholar] [CrossRef]
- Sharma, S.; Pollet, B.G. Support Materials for PEMFC and DMFC Electrocatalysts-A Review. J. Power Sources 2012, 208, 96–119. [Google Scholar] [CrossRef]
- Yu, S.H.; Gu, C.; Hu, S.J.; Zheng, X.S.; Zhu, J.F. Synthesis of Sub-2 nm Fe-Doped NiSe2 Nanowires and Their Surface Confined Oxidation for Oxygen Evolution Catalysis. Angew. Chem. Int. Ed. 2018, 57, 4020–4024. [Google Scholar]
- Wu, M.J.; Zhang, E.G.; Guo, Q.P.; Wang, Y.Z.; Qiao, J.L.; Li, K.X.; Pei, P.C. N/S-Me (Fe, Co, Ni) Doped Hierarchical Porous Carbons for Fuel Cell Oxygen Reduction Reaction with High Catalytic Activity and Long-term Stability. Appl. Energy 2016, 175, 468–478. [Google Scholar] [CrossRef]
- Stoyanov, S.R.; Titov, A.V.; Král, P. Transition Metal and Nitrogen Doped Carbon Nanostructures. Coord. Chem. Rev. 2009, 253, 2852–2871. [Google Scholar] [CrossRef]
- Masa, J.; Xia, W.; Muhler, M.; Schuhmann, W. On the Role of Metals in Nitrogen-Doped Carbon Electrocatalysts for Oxygen Reduction. Angew. Chem. Int. Ed. 2015, 54, 10102–10120. [Google Scholar] [CrossRef]
- Jaouen, F.; Proietti, E.; Lefevre, M.; Chenitz, R.; Zelenay, P. Recent Advances in Non-Precious Metal Catalysis for Oxygen-Reduction Reaction in Polymer Electrolyte Fuel Cells. Energy Environ. Sci. 2010, 4, 114–130. [Google Scholar] [CrossRef]
- Wu, G.; Zelenay, P. Nanostructured Nonprecious Metal Catalysts for Oxygen Reduction Reaction. Acc. Chem. Res. 2013, 46, 1878–1889. [Google Scholar] [CrossRef]
- Zeng, M.; Li, Y.G. Recent Advances in Heterogeneous Electrocatalysts for the Hydrogen Evolution Reaction. J. Mater. Chem. A 2015, 3, 14942–14962. [Google Scholar] [CrossRef]
- Liu, M.L.; Zhao, Z.P.; Duan, X.F.; Huang, Y. Nanoscale Structure Design for High-Performance Pt-Based ORR Catalysts. Adv. Mater. 2019, 31. [Google Scholar] [CrossRef]
- Liu, S.R.; Pu, Q.S.; Gao, L.; Korzeniewski, C.; Matzke, C. From Nanochannel-Induced Proton Conduction Enhancement to a Nanochannel-Based Fuel Cell. Nano Lett. 2005, 5, 1389–1393. [Google Scholar] [CrossRef]
- Arbizzani, C.; Righi, S.; Soavi, F.; Mastragostino, M. Graphene and Carbon Nanotube Structures Supported on Mesoporous Xerogel Carbon as Catalysts for Oxygen Reduction Reaction in Proton-Exchange-Membrane Fuel Cells. Int. J. Hydrogen Energy 2011, 36, 5038–5046. [Google Scholar] [CrossRef]
- Ghosh, A.; Chandran, P.; Ramaprabhu, S. Palladium-Nitrogen Coordinated Cobalt Alloy towards Hydrogen Oxidation and Oxygen Reduction Reactions with High Catalytic Activity in Renewable Energy Generations of Proton Exchange Membrane Fuel Cell. Appl. Energy 2017, 208, 37–48. [Google Scholar] [CrossRef]
- Seh, Z.W.; Kibsgaard, J.; Dickens, C.F.; Chorkendorff, I.B.; Norskov, J.K.; Jaramillo, T.F. Combining Theory and Experiment in Electrocatalysis: Insights Into Materials Design. Science 2017, 355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zou, X.; Zhang, Y. Noble Metal-Free Hydrogen Evolution Catalysts for Water Splitting. Chem. Soc. Rev. 2015, 44, 5148–5180. [Google Scholar] [CrossRef] [PubMed]
- Hung, T.-F.; Chen, S.-H.; Tu, M.-H.; Lu, Z.-H.; Chen, C.K.; Liu, R.-S.; Greer, H.F.; Zhou, W.Z.; Lo, M.-Y. Advances in Carbon-Incorporated Non-Noble Transition Metal Catalysts for Oxygen Reduction Reaction in Polymer Electrolyte Fuel Cells. J. Chin. Chem. Soc. 2014, 61, 93–100. [Google Scholar] [CrossRef]
- Wu, G.; Santandreu, A.; Kellogg, W.; Gupta, S.; Ogoke, O.; Zhang, H.G.; Wang, H.-L.; Dai, L.M. Carbon Nanocomposite Catalysts for Oxygen Reduction and Evolution Reactions: From Nitrogen Doping to Transition-Metal Addition. Nano Energy 2016, 29, 83–110. [Google Scholar] [CrossRef] [Green Version]
- Liu, D.D.; Tao, L.; Yan, D.F.; Zou, Y.Q.; Wang, S.Y. Recent Advances on Non-precious Metal Porous Carbon-based Electrocatalysts for Oxygen Reduction Reaction. Chemelectrochem 2018, 5, 1775–1785. [Google Scholar] [CrossRef]
- Singh, K.; Razmjooei, F.; Yu, J.-S. Active Sites and Factors Influencing Them for Efficient Oxygen Reduction Reaction in Metal-N Coordinated Pyrolyzed and Non-Pyrolyzed Catalysts: A Review. J. Mater. Chem. A 2017, 5, 20095–20119. [Google Scholar] [CrossRef]
- Zhu, C.Z.; Shi, Q.R.; Xu, B.Z.; Fu, S.F.; Wan, G.; Yang, C.; Yao, S.Y.; Song, J.H.; Zhou, H.; Du, D.; et al. Hierarchically Porous M-N-C (M = Co and Fe) Single-Atom Electrocatalysts with Robust MNx Active Moieties Enable Enhanced ORR Performance. Adv. Energy Mater. 2018, 8. [Google Scholar] [CrossRef]
- Li, B.; Chen, Y.; Ge, X.M.; Chai, J.W.; Zhang, X.; Hor, T.S.A.; Du, G.J.; Liu, Z.L.; Zhang, H.; Zong, Y. Mussel-Inspired One-Pot Synthesis of Transition Metal and Nitrogen Co-Doped Carbon (M/N-C) as Efficient Oxygen Catalysts for Zn-air Batteries. Nanoscale 2016, 8, 5067–5075. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, L.Q.; Chen, X.; Lu, Y.L.; Yang, W.S.; Duan, X. A Co-N/C Hollow-Sphere Electrocatalyst Derived from A Metanilic CoAl Layered Double Hydroxide for the Oxygen Reduction Reaction, and Its Active Sites in Various pH Media. Nano Res. 2017, 10, 2508–2518. [Google Scholar] [CrossRef]
- Yang, Y.; Mao, K.T.; Gao, S.Q.; Huang, H.; Xia, G.L.; Lin, Z.Y.; Jiang, P.; Wang, C.L.; Wang, H.; Chen, Q.W. O-, N-Atoms-Coordinated Mn Cofactors within a Graphene Framework as Bioinspired Oxygen Reduction Reaction Electrocatalysts. Adv. Mater. 2018, 30, e1801732. [Google Scholar] [CrossRef]
- Wang, T.; Yang, R.; Shi, N.E.; Yang, J.; Yan, H.Y.; Wang, J.Y.; Ding, Z.; Huang, W.; Luo, Q.; Lin, Y.; et al. Cu,N-Codoped Carbon Nanodisks with Biomimic Stomata-Like Interconnected Hierarchical Porous Topology as Efficient Electrocatalyst for Oxygen Reduction Reaction. Small 2019, 15. [Google Scholar] [CrossRef] [Green Version]
- Yang, T.T.; Wang, M.; Ju, X.P.; Zhao, J.S.; Fu, C.G. The Efficient Oxygen Reduction Catalysts Based on the Non-Noble Metal and Conducting Polymers. Int. J. Electrochem. Sci. 2017, 12, 12125–12139. [Google Scholar] [CrossRef]
- Gu, W.L.; Hu, L.Y.; Li, J.; Wang, E. Recent Advancements in Transition Metal-Nitrogen-Carbon Catalysts for Oxygen Reduction Reaction. Electroanalysis 2018, 30, 1217–1228. [Google Scholar] [CrossRef]
- Peera, S.G.; Balamurugan, J.; Kim, N.H.; Lee, J.H. Sustainable Synthesis of Co@NC Core Shell Nanostructures from Metal Organic Frameworks via Mechanochemical Coordination Self-Assembly: An Efficient Electrocatalyst for Oxygen Reduction Reaction. Small 2018, 14, e1800441. [Google Scholar] [CrossRef]
- Jasinski, R. A New Fuel Cell Cathode Catalyst. Nature 1964, 201, 1212–1213. [Google Scholar] [CrossRef]
- Veen, J.A.R.V.; Visser, C. Oxygen Reduction on Monomeric Transition Metal Phthalocyanines in Acid Electrolyte. Electrochim. Acta 1979, 24, 921–928. [Google Scholar] [CrossRef]
- Vasudevan, P.; Mann, N.; Tyagi, S. Transition Metal Complexes of Porphyrins and Phthalocyanines as Electrocatalysts for Dioxygen Reduction. Transit. Met. Chem. 1990, 15, 81–90. [Google Scholar] [CrossRef]
- Shi, C.N.; Anson, F.C. Catalytic Pathways for The Electroreduction of Oxygen by Iron Tetrakis(4-N-methylpyridyl)Porphyrin or Iron Tetraphenylporphyrin Adsorbed on Edge Plane Pyrolytic Graphite Electrodes. Inorg. Chem. 1990, 29, 4298–4305. [Google Scholar] [CrossRef]
- Ouyang, J.; Shigehara, K.; Yamada, A.; Anson, F.C. Hexadecafluoro- and Octacyano Phthalocyanines as Electrocatalysts for the Reduction of Dioxygen. J. Electroanal. Chem. Interfacial Electrochem. 1991, 297, 489–498. [Google Scholar] [CrossRef]
- Tanabe, H.; Ohno, K. Electrocatalysis of Metal Phthalocyanine Thin Film Prepared by the Plasma-Assisted Deposition on a Glassy Carbon in the Reduction of Carbon Dioxide. Electrochim. Acta 1987, 32, 1121–1124. [Google Scholar] [CrossRef]
- Shi, C.; Steiger, B.; Yuasa, M.; Anson, F.C. Electroreduction of O2 to H2O at Unusually Positive Potentials Catalyzed by the Simplest of the Cobalt Porphyrins. Inorg. Chem. 1997, 36, 4294–4295. [Google Scholar] [CrossRef]
- Randin, J.P. Interpretation of the Relative Electrochemical Activity of Various Metal Phthalocyanines for the Oxygen Reduction Reaction. Electrochim. Acta 1974, 19, 83–85. [Google Scholar] [CrossRef]
- Zagal, J.H. Metallophthalocyanines as Catalysts in Electrochemical Reactions. Coord. Chem. Rev. 1992, 119, 89–136. [Google Scholar] [CrossRef]
- Veen, J.A.R.V.; Colijn, H.A. Oxygen Reduction on Transition-Metal Porphyrins in Acid Electrolyte II. Stability. Ber. Der Bunsenges. Phys. Chem. 2010, 85, 700–704. [Google Scholar] [CrossRef]
- Alt, H.; Binder, H.; Lindner, W.; Sandstede, G. Metal Chelates as Electrocatalysts for Oxygen Reduction in Acid Electrolytes. J. Electroanal. Chem. 1971, 31, A19–A22. [Google Scholar] [CrossRef]
- Song, E.; Shi, C.; Anson, F.C. Comparison of the Behavior of Several Cobalt Porphyrins as Electrocatalysts for the Reduction of O2 at Graphite Electrodes. Langmuir 1998, 14, 4315–4321. [Google Scholar] [CrossRef]
- Wiesener, K.; Ohms, D.; Neumann, V.; Franke, R. N4 Macrocycles as Electrocatalysts for the Cathodic Reduction of Oxygen. Mater. Chem. Phys. 1989, 22, 457–475. [Google Scholar] [CrossRef]
- Putten, A.V.D.; Elzing, A.; Visscher, W.; Barendrecht, E. Oxygen Reduction on Vacuum-Deposited and Absorbed Transition-Metal Phthalocyanine Films. J. Electroanal. Chem. Interfacial Electrochem. 1986, 214, 523–533. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, N.; Janda, P.; Lever, A.B.P. Cathodic Reduction of Oxygen and Hydrogen Peroxide at Cobalt and Iron Crowned Phthalocyanines Adsorbed on Highly Oriented Pyrolytic Graphite Electrodes. Inorg. Chem. 1992, 31, 5172–5177. [Google Scholar] [CrossRef]
- Alt, H.; Binder, H.; Sandstede, G. Mechanism of the Electrocatalytic Reduction of Oxygen on Metal Chelates. J. Catal. 1973, 28, 8–19. [Google Scholar] [CrossRef]
- Jahnke, H.; Schonborn, M.; Zimmermann, G. Organic Dyestuffs as Catalysts for Fuel Cells. Top. Curr. Chem. 1976, 61, 133–181. [Google Scholar]
- Lefevre, M.; Proietti, E.; Jaouen, F.; Dodelet, J.-P. Iron-Based Catalysts with Improved Oxygen Reduction Activity in Polymer Electrolyte Fuel Cells. Science 2009, 324, 71–74. [Google Scholar] [CrossRef]
- Proietti, E.; Jaouen, F.; Lefevre, M.; Larouche, N.; Tian, J.; Herranz, J.; Dodelet, J.-P. Iron-Based Cathode Catalyst with Enhanced Power Density in Polymer Electrolyte Membrane Fuel Cells. Nat. Commun. 2011, 2, 416. [Google Scholar] [CrossRef]
- Iliev, I.; Gamburzev, S.; Kaisheva, A. Optimization of the Pyrolysis Temperature of Active Carbon-CoTMPP Catalysts for Air Electrodes in Alkaline Media. J. Power Sources 1986, 17, 345–352. [Google Scholar] [CrossRef]
- Faubert, G.; Lalande, G.; Côté, R.; Guay, D.; Dodelet, J.P.; Weng, L.T.; Bertrand, P.; Dénès, G. Heat-treated Iron and Cobalt Tetraphenylporphyrins Adsorbed on Carbon Black: Physical Characterization and Catalytic Properties of These Materials for the Reduction of Oxygen in Polymer Electrolyte Fuel Cells. Electrochim. Acta 1996, 41, 1689–1701. [Google Scholar] [CrossRef]
- Lalande, G.; Cote, R.; Tamizhmani, G.; Guay, D.; Dodelet, J.P.; Dignardbailey, L.; Weng, L.T.; Bertrand, P. Physical, Chemical and Electrochemical Characterization of Heat-Treated Tetracarboxylic Cobalt Phthalocyanine Adsorbed on Carbon-Black as Electrocatalyst for Oxygen Reduction in Polymer Electrolyte Fuel-Cells. Electrochim. Acta 1995, 40, 2635–2646. [Google Scholar] [CrossRef]
- Bron, M.; Fiechter, S.; Hilgendorff, M.; Bogdanoff, P. Catalysts for Oxygen Reduction from Heat-Treated Carbon-Supported Iron Phenantroline Complexes. J. Appl. Electrochem. 2002, 32, 211–216. [Google Scholar] [CrossRef]
- Weng, L.T.; Bertrand, P.; Lalande, G.; Guay, D.; Dodelet, J.P. Surface Characterization by Time-of-Flight SIMS of A Catalyst for Oxygen Electroreduction: Pyrolyzed Cobalt Phthalocyanine-on-Carbon Black. Appl. Surf. Sci. 1995, 84, 9–21. [Google Scholar] [CrossRef]
- Alves, M.C.M.; Dodelet, J.P.; Guay, D.; Ladouceur, M.; Tourillon, G. Origin of the Electrocatalytic Properties for Oxygen Reduction of Some Heat-Hreated Polyacrylonitrile and Phthalocyanine Cobalt Compounds Adsorbed on Carbon Black as Probed by Electrochemistry and X-ray Absorption Spectroscopy. J. Phys. Chem. B 1992, 96, 10898–10905. [Google Scholar] [CrossRef]
- Li, X.; Liu, G.; Popov, B.N. Activity and Stability of Non-Precious Metal Catalysts for Oxygen Reduction in Acid and Alkaline Electrolytes. J. Power Sources 2010, 195, 6373–6378. [Google Scholar] [CrossRef]
- Veen, J.A.R.V.; Baar, J.F.V.; Kroese, K.J. Effect of Heat Treatment on the Performance of Carbon-Supported Transition-Metal Chelates in the Electrochemical Reduction of Oxygen. J. Chem. Soc. Faraday Trans. 1981, 77, 2827–2843. [Google Scholar] [CrossRef]
- Scherson, D.A.; Gupta, S.L.; Fierro, C.; Yeager, E.B.; Kordesch, M.E.; Eldridge, J.; Hoffman, R.W.; Blue, J. Cobalt Tetramethoxyphenyl Porphyrin—Emission Mossbauer Spectroscopy and O2 Reduction Electrochemical Studies. Electrochim. Acta 1983, 28, 1205–1209. [Google Scholar] [CrossRef]
- Putten, A.V.D.; Elzing, A.; Visscher, W.; Barendrecht, E. Redox Potential and Electrocatalysis of O2 Reduction on Transition Metal Chelates. J. Electroanal. Chem. 1987, 221, 95–104. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Wei, Z.D. Recent Progress in Non-Precious Metal Catalysts for Oxygen Reduction Reaction. Acta Phys. Chim. Sin. 2017, 33, 886–902. [Google Scholar]
- Gupta, S.L.; Tryk, D.; Bae, I.; Aldred, W.; Yeager, E.B. Heat-Treated Polyacrylonitrile-Based Catalysts for Oxygen Electroreduction. J. Appl. Electrochem. 1989, 19, 19–27. [Google Scholar] [CrossRef]
- Bouwkamp-Wijnoltz, A.L.; Visscher, W.; Veen, J.A.R.V.; Tang, S.C. Electrochemical Reduction of Oxygen: An Alternative Method to Prepare Active CoN4 Catalysts. Electrochim. Acta 1999, 45, 379–386. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Yue, X.P.; Li, K.X.; Qiao, J.L.; Wilkinson, D.P.; Zhang, J.J. PEM Fuel Cell Electrocatalysts Based on Transition Metal Macrocyclic Compounds. Coord. Chem. Rev. 2016, 315, 153–177. [Google Scholar] [CrossRef]
- Zhong, H.X.; Zhang, H.M.; Liang, Y.M.; Zhang, J.L.; Wang, M.R.; Wang, X.L. A Novel Non-Noble Electrocatalyst for Oxygen Reduction in Proton Exchange Membrane Fuel Cells. J. Power Sources 2007, 164, 572–577. [Google Scholar] [CrossRef]
- Wu, G.; Chen, Z.W.; Artyushkova, K.; Garzon, F.H.; Zelenay, P. Polyaniline-Derived Non-Precious Catalyst for the Polymer Electrolyte Fuel Cell Cathode. ECS Trans. 2008, 16, 159–170. [Google Scholar]
- Wu, G.; Johnston, C.M.; Mack, N.H.; Artyushkova, K.; Ferrandon, M.; Nelson, M.; Lezama-Pacheco, J.S.; Conradson, S.D.; More, K.L.; Myers, D.J. Synthesis–Structure–Performance Correlation for Polyaniline–Me–C Non-Precious Metal Cathode Catalysts for Oxygen Reduction in Fuel Cells. J. Mater. Chem. 2011, 21, 11392–11405. [Google Scholar] [CrossRef]
- Bashyam, R.; Zelenay, P. A Class of Non-Precious Metal Composite Catalysts for Fuel Cells. Nature 2006, 443, 63–66. [Google Scholar] [CrossRef]
- Han, S.J.; Jung, H.J.; Shim, J.H.; Kim, H.-C.; Sung, S.-J.; Yoo, B.; Lee, D.H.; Lee, C.; Lee, Y. Non-Platinum Oxygen Reduction Electrocatalysts Based on Carbon-Supported Metal-Polythiophene Composites. J. Electroanal. Chem. 2011, 655, 39–44. [Google Scholar] [CrossRef]
- Choi, J.Y.; Hsu, R.S.; Chen, Z.W. Highly Active Porous Carbon-Supported Nonprecious Metal-N Electrocatalyst for Oxygen Reduction Reaction in PEM Fuel Cells. J. Phys. Chem. C 2010, 114, 8048–8053. [Google Scholar] [CrossRef]
- Chung, H.T.; Johnston, C.M.; Artyushkova, K.; Ferrandon, M.; Myers, D.J.; Zelenay, P. Cyanamide-Derived Non-Precious Metal Catalyst for Oxygen Reduction. Electrochem. Commun. 2010, 12, 1792–1795. [Google Scholar] [CrossRef]
- Liu, Y.J.; Yu, L.M.; Jiang, X.H.; Li, X.; Yan, X.F. In Situ Self-Template Synthesis of Cobalt/Nitrogen-Doped Nanocarbons with Controllable Shapes for Oxygen Reduction Reaction and Supercapacitors. Int. J. Energy Res. 2019, 43, 4217–4228. [Google Scholar] [CrossRef]
- Wu, G.; More, K.L.; Johnston, C.M.; Zelenay, P. High-Performance Electrocatalysts for Oxygen Reduction Derived from Polyaniline, Iron, and Cobalt. Science 2011, 332, 443–447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, D.; Li, J.C.; Shi, Q.R.; Feng, S.; Lyu, Z.Y.; Ding, S.C.; Hao, L.D.; Zhang, Q.; Wang, C.H.; Xu, M.J.; et al. Atomically Isolated Iron Atom Anchored on Carbon Nanotubes for Oxygen Reduction Reaction. ACS Appl. Mater. Int. 2019, 11, 39820–39826. [Google Scholar] [CrossRef] [PubMed]
- Huang, N.; Yang, L.; Zhang, M.Y.; Yan, S.F.; Ding, Y.Y.; Sun, P.P.; Sun, X.H. Cobalt-Embedded N-Doped Carbon Arrays Derived In Situ as Trifunctional Catalyst Toward Hydrogen and Oxygen Evolution, and Oxygen Reduction. Chemelectrochem 2019, 6, 4522–4532. [Google Scholar] [CrossRef]
- Guo, J.J.; Yang, X.W.; Yao, Y.L.; Wang, X.M.; Liu, X.G.; Xu, B.S. Pt/onion-Like Fullerenes as Catalyst for Direct Methanol Fuel Cell. Rare Met. 2006, 25, 305–308. [Google Scholar] [CrossRef]
- Yan, X.L.; Duan, P.; Zhang, F.W.; Li, H.; Zhang, H.X.; Zhao, M.; Zhang, X.M.; Xu, B.S.; Pennycook, S.J.; Guo, J.J. Stable Single-Atom Platinum Catalyst Trapped in Carbon Onion Graphitic Shells for Improved Chemoselective Hydrogenation of Nitroarenes. Carbon 2019, 143, 378–384. [Google Scholar] [CrossRef]
- Fan, Y.C.; Ida, S.; Staykov, A.; Akbay, T.; Hagiwara, H.; Matsuda, J.; Kaneko, K.; Ishihara, T. Ni-Fe Nitride Nanoplates on Nitrogen-Doped Graphene as a Synergistic Catalyst for Reversible Oxygen Evolution Reaction and Rechargeable Zn-Air Battery. Small 2017, 13. [Google Scholar] [CrossRef]
- Lim, K.H.; Kim, H. Nitrogen-Doped Carbon Catalysts Derived from Ionic Liquids in the Presence of Transition Metals for the Oxygen Reduction Reaction. Appl. Catal. B-Environ. 2014, 158, 355–360. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Jiang, H.L.; Zhu, Y.H.; Yang, X.L.; Li, C.Z. Transition Metals (Fe, Co, and Ni) Encapsulated in Nitrogen-Doped Carbon Nanotubes as Bifunctional Catalysts for Oxygen Electrode Reactions. J. Mater. Chem. A 2016, 4, 1694–1701. [Google Scholar] [CrossRef]
- Liu, W.J.; Ru, Q.X.; Zuo, S.X.; Yang, S.; Han, J.; Yao, C. Controllable Synthesis of Nitrogen-Doped Carbon Nanotubes Derived from Halloysite-Templated Polyaniline Towards Nonprecious ORR Catalysts. Appl. Surf. Sci. 2019, 469, 269–275. [Google Scholar] [CrossRef]
- Zhao, Y.F.; Zhang, J.Q.; Li, K.F.; Ao, Z.M.; Wang, C.Y.; Liu, H.; Sun, K.N.; Wang, G.X. Electrospun Cobalt Embedded Porous Nitrogen Doped Carbon Nanofibers as An Efficient Catalyst for Water Splitting. J. Mater. Chem. A 2016, 4, 12818–12824. [Google Scholar] [CrossRef]
- Yin, J.; Qiu, Y.J.; Yu, J. Enhanced Electrochemical Activity for Oxygen Reduction Reaction from Nitrogen-Doped Carbon Nanofibers by Iron Doping. ECS Solid State Lett. 2013, 2, M37–M39. [Google Scholar] [CrossRef]
- Huang, X.H.; Yin, X.L.; Yu, X.Q.; Tian, J.; Wu, W. Preparation of Nitrogen-Doped Carbon Materials Based on Polyaniline Fiber and their Oxygen Reduction Properties. Colloids Surf. A-Physicochem. Eng. Asp. 2018, 539, 163–170. [Google Scholar] [CrossRef]
- Yan, X.L.; Li, H.; Sun, J.T.; Liu, P.Z.; Zhang, H.X.; Xu, B.S.; Guo, J.J. Pt Nanoparticles Decorated High-Defective Graphene Nanospheres as Highly Efficient Catalysts for the Hydrogen Evolution Reaction. Carbon 2018, 137, 405–410. [Google Scholar] [CrossRef]
- Guo, J.J.; Morris, J.R.; Ihm, Y.; Contescu, C.I.; Gallego, N.C.; Duscher, G.; Pennycook, S.J.; Chisholm, M.F. Topological Defects: Origin of Nanopores and Enhanced Adsorption Performance in Nanoporous Carbon. Small 2012, 8, 3283–3288. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Zhou, R.F.; Chen, X.M.; Tang, Y.H.; Qiao, S.Z. Fe-N Decorated Hybrids of CNTs Grown on Hierarchically Porous Carbon for High-Performance Oxygen Reduction. Adv. Mater. 2014, 26, 6074. [Google Scholar] [CrossRef]
- Kim, M.; Yoo, J.M.; Ahn, C.-Y.; Jang, J.-H.; Son, Y.J.; Shin, H.; Kang, J.; Kang, Y.S.; Yoo, S.J.; Lee, K.S.; et al. Rational Generation of Fe-Nx Active Sites in Fe-N-C Electrocatalysts Facilitated by Fe-N Coordinated Precursors for the Oxygen Reduction Reaction. Chemcatchem 2019, 11, 5982–5988. [Google Scholar] [CrossRef]
- Park, M.J.; Lee, J.H.; Hembram, K.; Lee, K.R.; Han, S.S.; Yoon, C.W.; Nam, S.W.; Kim, J.Y. Oxygen Reduction Electrocatalysts Based on Coupled Iron Nitride Nanoparticles with Nitrogen-Doped Carbon. Catalysts 2016, 6, 11. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.; Zhang, H.Y.; Zhong, H.W.; Zhang, S.M.; Chen, S.L. N-doped Graphene/Carbon Composite as Non-Precious Metal Electrocatalyst for Oxygen Reduction Reaction. Electrochim. Acta 2012, 81, 313–320. [Google Scholar] [CrossRef]
- Chen, M.X.; Zhu, M.Z.; Zuo, M.; Chu, S.Q.; Zhang, J.; Wu, Y.; Liang, H.W.; Feng, X.L. Identification of Catalytic Sites for Oxygen Reduction in Metal/Nitrogen-Doped Carbons with Encapsulated Metal Nanoparticles. Angew. Chem. Int. Ed. 2019, 58. [Google Scholar] [CrossRef] [Green Version]
- Li, X.B.; Zou, R.Y.; Niu, Y.Y.; Sun, W.; Tian, X.L. Synthesis of A N-doped Mesoporous Carbon as An Efficient Electrocatalyst for Oxygen Reduction. Int. J. Hydrogen Energy 2018, 43, 21791–21797. [Google Scholar] [CrossRef]
- Liu, J.; Jin, Z.; Wang, X.; Ge, J.J.; Liu, C.P.; Xing, W. Recent Advances in Active Sites Identification and Regulation of M-N/C Electro-Catalysts towards ORR. Sci. China-Chem. 2019, 62, 669–683. [Google Scholar] [CrossRef]
- Xia, D.S.; Yang, X.; Xie, L.; Wei, Y.P.; Jiang, W.; Dou, M.; Li, X.N.; Li, J.; Gan, L.; Kang, F.Y. Direct Growth of Carbon Nanotubes Doped with Single Atomic Fe-N4 Active Sites and Neighboring Graphitic Nitrogen for Efficient and Stable Oxygen Reduction Electrocatalysis. Adv. Funct. Mater. 2019, 29, 10. [Google Scholar]
- Li, J.C.; Xiao, F.; Zhong, H.; Li, T.; Xu, M.J.; Ma, L.; Cheng, M.; Liu, D.; Feng, S.; Shi, Q.R.; et al. Secondary-Atom-Assisted Synthesis of Single Iron Atoms Anchored on N-Doped Carbon Nanowires for Oxygen Reduction Reaction. ACS Catal. 2019, 9, 5929–5934. [Google Scholar] [CrossRef]
- Yin, P.Q.; Yao, T.; Wu, Y.E.; Zheng, L.R.; Lin, Y.; Liu, W.; Ju, H.X.; Zhu, J.F.; Hong, X.; Deng, Z.X.; et al. Single Cobalt Atoms with Precise N-Coordination as Superior Oxygen Reduction Reaction Catalysts. Angew. Chem. Int. Ed. 2016, 55, 10800–10805. [Google Scholar] [CrossRef]
- Ao, X.; Zhang, W.; Li, Z.S.; Li, J.G.; Soule, L.; Huang, X.; Chiang, W.H.; Chen, H.M.; Wang, C.D.; Liu, M.L.; et al. Markedly Enhanced Oxygen Reduction Activity of Single-Atom Fe Catalysts via Integration with Fe Nanoclusters. ACS Nano 2019, 13, 11853–11862. [Google Scholar] [CrossRef]
- Xu, G.; Xu, G.C.; Ban, J.J.; Zhang, L.; Jia, D.Z. Cobalt and Cobalt Oxides N-Codoped Porous Carbon Derived from Metal-Organic Framework as Bifunctional Catalyst for Oxygen Reduction and Oxygen Evolution Reactions. J. Colloid Interface Sci. 2018, 521, 141–149. [Google Scholar] [CrossRef]
- Zhang, X.K.; Huang, X.B.; Hu, W.H.; Huang, Y.M. A Metal-Organic Framework-Derived Fe-N-C Electrocatalyst with Highly Dispersed Fe-Nx towards Oxygen Reduction Reaction. Int. J. Hydrogen Energy 2019, 44, 27379–27389. [Google Scholar] [CrossRef]
- Sun, W.; Du, L.; Tan, Q.; Zhou, J.G.; Hu, Y.F.; Du, C.Y.; Gao, Y.Z.; Yin, G.P. Engineering of Nitrogen Coordinated Single Cobalt Atom Moieties for Oxygen Electroreduction. ACS Appl. Mater. Inter. 2019, 11, 41258–41266. [Google Scholar] [CrossRef]
- Peng, P.; Shi, L.; Huo, F.; Mi, C.X.; Wu, X.H.; Zhang, S.J.; Xiang, Z.H. A Pyrolysis-Free Path toward Superiorly Catalytic Nitrogen-Coordinated Single Atom. Sci. Adv. 2019, 5, 7. [Google Scholar] [CrossRef] [Green Version]
- Kicinski, W.; Sek, J.P.; Matysiak-Brynda, E.; Miecznikowski, K.; Donten, M.; Budner, B.; Nowicka, A.M. Enhancement of PGM-free Oxygen Reduction Electrocatalyst Performance for Conventional and Enzymatic Fuel Cells: The Influence of An External Magnetic Field. Appl. Catal. B Environ. 2019, 258, 14. [Google Scholar] [CrossRef]
- Lee, S.H.; Kim, J.; Yoon, S.H.; Peck, D.H.; Kim, S.K.; Jung, D.H. Preparation of Chestnut-Like Carbon and Its Application for Electrodes with High Specific Capacitance. Appl. Catal. B Environ. 2014, 158–159, 308–313. [Google Scholar] [CrossRef]
- Deng, J.; Ren, P.J.; Deng, D.H.; Yu, L.; Yang, F.; Bao, X.H. Highly Active and Durable Non-Precious-Metal Catalysts Encapsulated in Carbon Nanotubes for Hydrogen Evolution Reaction. Energy Environ. Sci. 2014, 7, 1919–1923. [Google Scholar] [CrossRef]
- Strickland, K.; Miner, E.; Jia, Q.Y.; Tylus, U.; Ramaswamy, N.; Liang, W.T.; Sougrati, M.-T.; Jaouen, F.; Mukerjee, S. Highly Active Oxygen Reduction Non-Platinum Group Metal Electrocatalyst without Direct Metal-Nitrogen Coordination. Nat. Commun. 2015, 6, 7343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kramm, U.I.; Herrmann-Geppert, I.; Behrends, J.; Lips, K.; Fiechter, S.; Bogdanoff, P. On an Easy Way to Prepare Metal Nitrogen Doped Carbon with Exclusive Presence of MeN4-type Sites Active for the ORR. J. Am. Chem. Soc. 2016, 138, 635–640. [Google Scholar] [CrossRef]
- Zitolo, A.; Ranjbar-Sahraie, N.; Mineva, T.; Li, J.K.; Jia, Q.Y.; Stamatin, S.; Harrington, G.F.; Lyth, S.M.; Krtil, P.; Mukerjee, S.; et al. Identification of Catalytic Sites in Cobalt-Nitrogen-Carbon Materials for the Oxygen Reduction Reaction. Nat. Commun. 2017, 8, 957. [Google Scholar] [CrossRef]
- Chung, H.T.; Cullen, D.A.; Higgins, D.; Sneed, B.T.; Holby, E.F.; More, K.L.; Zelenay, P. Direct Atomic-Level Insight into the Active Sites of a High-Performance PGM-free ORR Catalyst. Science 2017, 357, 479–483. [Google Scholar] [CrossRef] [Green Version]
- Zeng, X.J.; Shui, J.L.; Liu, X.F.; Liu, Q.T.; Li, Y.C.; Shang, J.X.; Zheng, L.R.; Yu, R.H. Single-Atom to Single-Atom Grafting of Pt1 onto Fe-N4 Center: Pt1@Fe-N-C Multifunctional Electrocatalyst with Significantly Enhanced Properties. Adv. Energy Mater. 2018, 8. [Google Scholar] [CrossRef]
- Shen, H.J.; Gracia-Espino, E.; Ma, J.Y.; Tang, H.D.; Mamat, X.; Wagberg, T.; Hu, G.Z.; Guo, S.J. Atomically FeN2 Moieties Dispersed on Mesoporous Carbon: A New Atomic Catalyst for Efficient Oxygen Reduction Catalysis. Nano Energy 2017, 35, 9–16. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, Y.J.; Zhou, K. Self-Adjusting Activity Induced by Intrinsic Reaction Intermediate in Fe-N-C Single-Atom Catalysts. J. Am. Chem. Soc. 2019, 141, 14115–14119. [Google Scholar] [CrossRef]
- Chen, L.Y.; Liu, X.F.; Zheng, L.R.; Li, Y.C.; Guo, X.; Wan, X.; Liu, Q.T.; Shang, J.X.; Shui, J.L. Insights into the Role of Active Site Density in the Fuel Cell Performance of Co-N-C Catalysts. Appl. Catal. B-Environ. 2019, 256, 117849. [Google Scholar] [CrossRef]
- Zhang, X.L.; Lu, Z.S.; Yang, Z.X. The Mechanism of Oxygen Reduction Reaction on CoN4 Embedded Graphene: A Combined Kinetic and Atomistic Thermodynamic Study. Int. J. Hydrogen Energy 2016, 41, 21212–21220. [Google Scholar] [CrossRef]
- Bruller, S.; Liang, H.W.; Kramm, U.I.; Krumpfer, J.W.; Feng, X.L.; Mullen, K. Bimetallic Porous Porphyrin Polymer-Derived Non-Precious Metal Electrocatalysts for Oxygen Reduction Reactions. J. Mater. Chem. A 2015, 3, 23799–23808. [Google Scholar] [CrossRef] [Green Version]
- Chen, P.Z.; Zhou, T.P.; Xing, L.L.; Xu, K.; Tong, Y.; Xie, H.; Zhang, L.D.; Yan, W.S.; Chu, W.S.; Wu, C.Z.; et al. Atomically Dispersed Iron-Nitrogen Species as Electrocatalysts for Bifunctional Oxygen Evolution and Reduction Reactions. Angew. Chem. Int. Ed. 2017, 56, 610–614. [Google Scholar] [CrossRef] [PubMed]
- Zhai, L.F.; Kong, S.Y.; Zhang, H.Y.; Tian, W.J.; Sun, M.; Sun, H.Q.; Wang, S.B. Facile Synthesis of Co-N-rGO Composites as An Excellent Electrocatalyst for Oxygen Reduction Reaction. Chem. Eng. Sci. 2019, 194, 45–53. [Google Scholar] [CrossRef]
- Xiao, M.L.; Zhang, H.; Chen, Y.T.; Zhu, J.B.; Gao, L.Q.; Jin, Z.; Ge, J.J.; Jiang, Z.; Chen, S.L.; Liu, C.P.; et al. Identification of Binuclear Co2N5 Active Sites for Oxygen Reduction Reaction with More than One Magnitude Higher Activity than Single Atom CoN4 Site. Nano Energy 2018, 46, 396–403. [Google Scholar] [CrossRef]
- Guo, J.J.; Lee, J.; Contescu, C.I.; Gallego, N.C.; Pantelides, S.T.; Pennycook, S.J.; Moyer, B.A.; Chisholm, M.F. Crown Ethers in Graphene. Nat. Commun. 2014, 5, 5389–5394. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.X.; Liu, W.W.; Zhang, Z.H.; Li, M.F.; Xu, B.S.; Guo, J.J. Direct Imaging of A Single Ni Atom Cutting Graphene to Form A Graphene Nanomesh. Phys. Chem. Chem. Phys. 2018, 20, 26814–26818. [Google Scholar] [CrossRef]




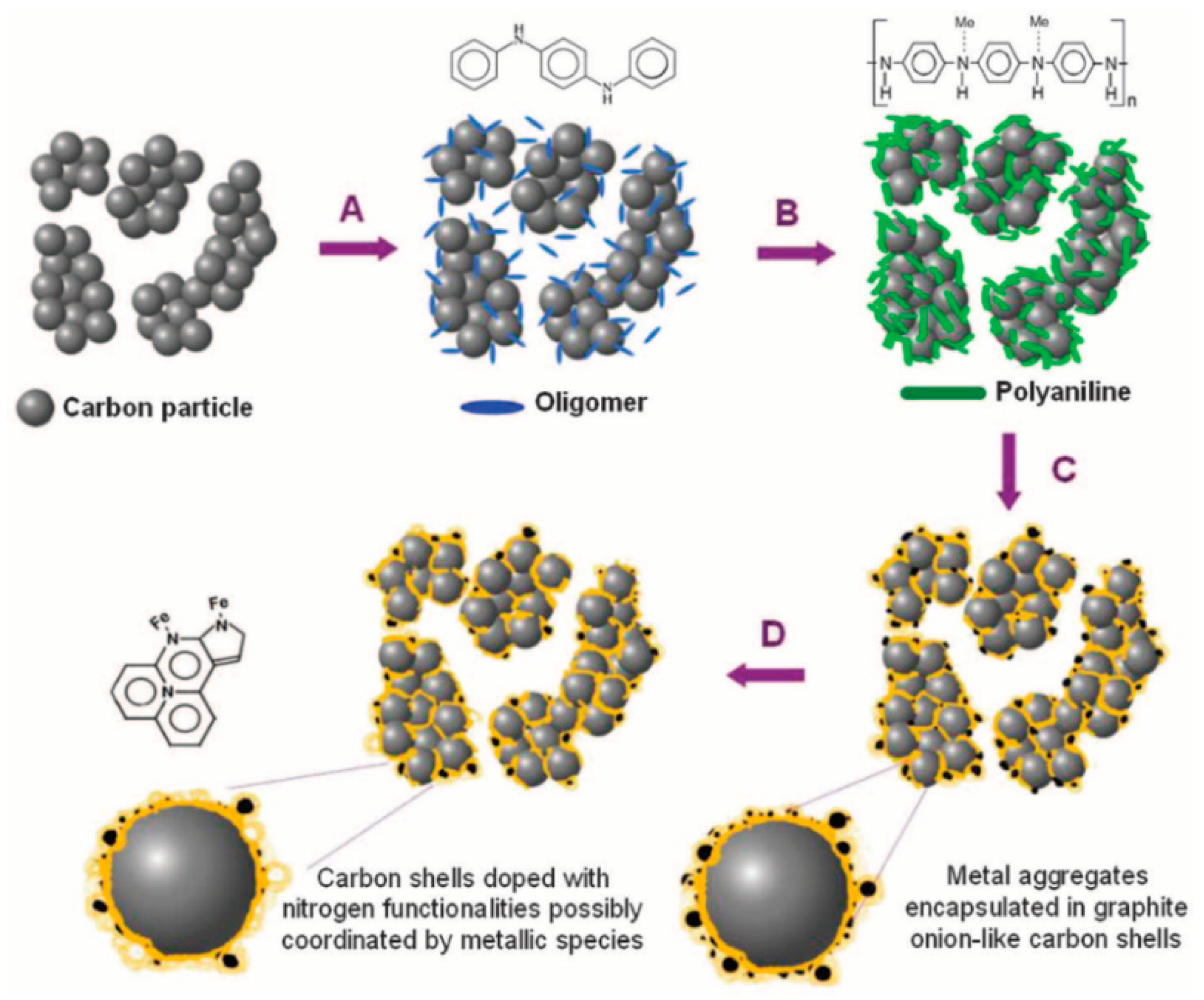





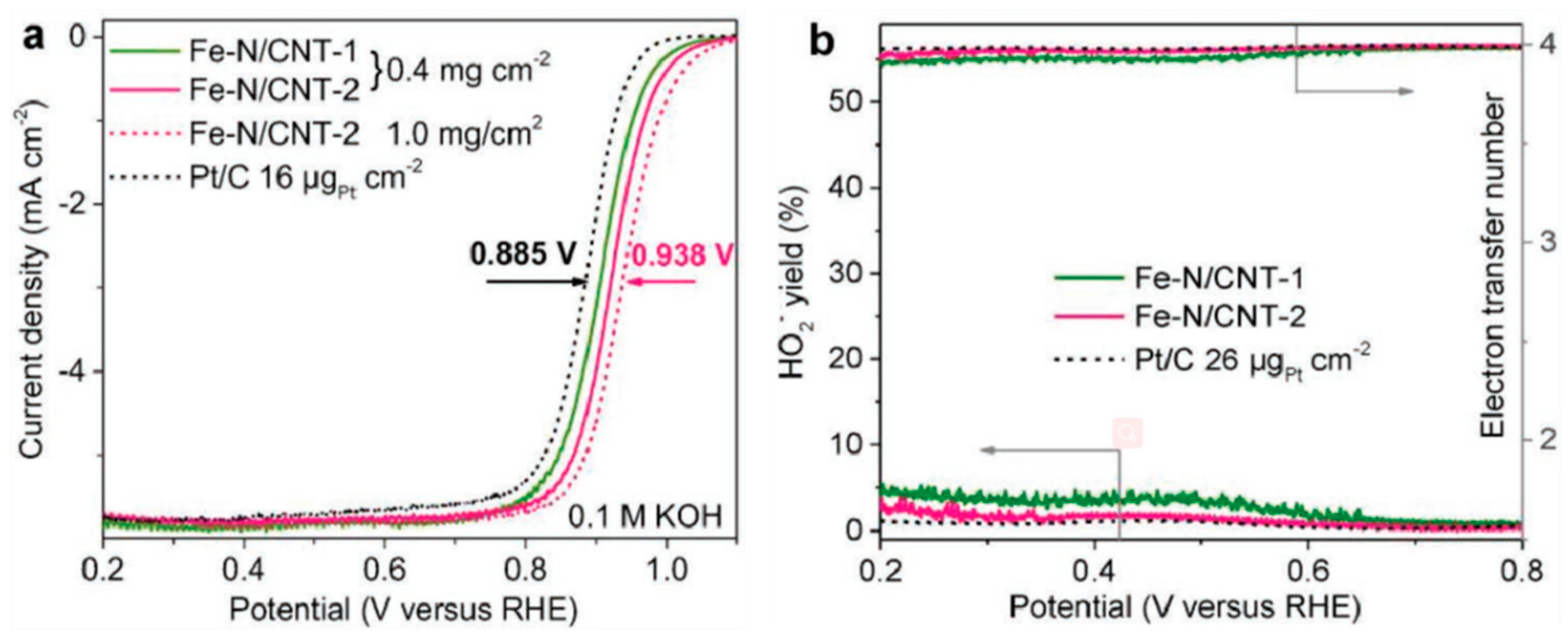









© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, M.; Song, Y.; Sha, W.; Xu, B.; Guo, J.; Wu, Y. Recent Advances in Non-Precious Transition Metal/Nitrogen-doped Carbon for Oxygen Reduction Electrocatalysts in PEMFCs. Catalysts 2020, 10, 141. https://doi.org/10.3390/catal10010141
Song M, Song Y, Sha W, Xu B, Guo J, Wu Y. Recent Advances in Non-Precious Transition Metal/Nitrogen-doped Carbon for Oxygen Reduction Electrocatalysts in PEMFCs. Catalysts. 2020; 10(1):141. https://doi.org/10.3390/catal10010141
Chicago/Turabian StyleSong, Meixiu, Yanhui Song, Wenbo Sha, Bingshe Xu, Junjie Guo, and Yucheng Wu. 2020. "Recent Advances in Non-Precious Transition Metal/Nitrogen-doped Carbon for Oxygen Reduction Electrocatalysts in PEMFCs" Catalysts 10, no. 1: 141. https://doi.org/10.3390/catal10010141
APA StyleSong, M., Song, Y., Sha, W., Xu, B., Guo, J., & Wu, Y. (2020). Recent Advances in Non-Precious Transition Metal/Nitrogen-doped Carbon for Oxygen Reduction Electrocatalysts in PEMFCs. Catalysts, 10(1), 141. https://doi.org/10.3390/catal10010141



