Biodegradation of 1,2,3-Trichloropropane to Valuable (S)-2,3-DCP Using a One-Pot Reaction System
Abstract
:1. Introduction
2. Results and Discussion
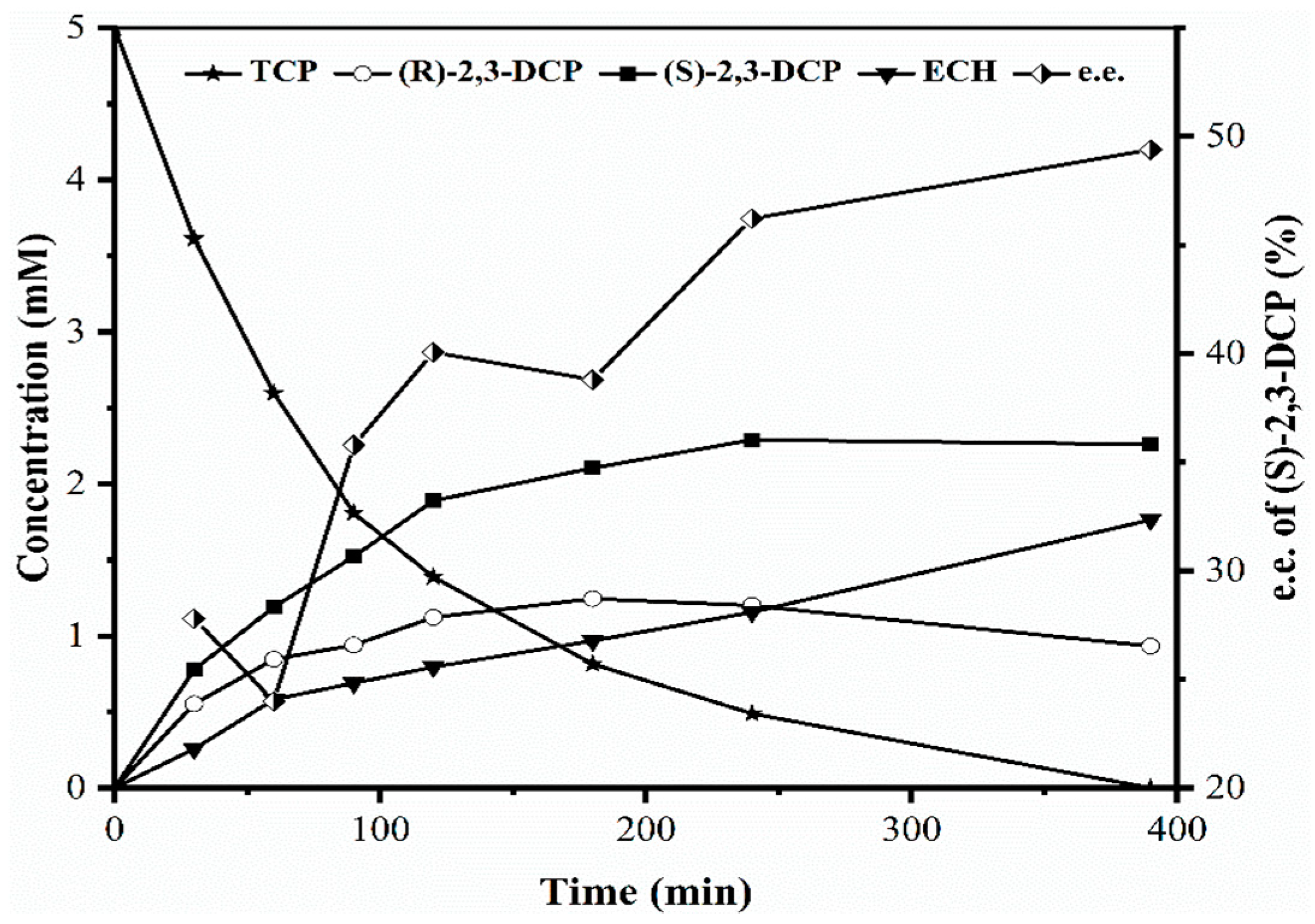
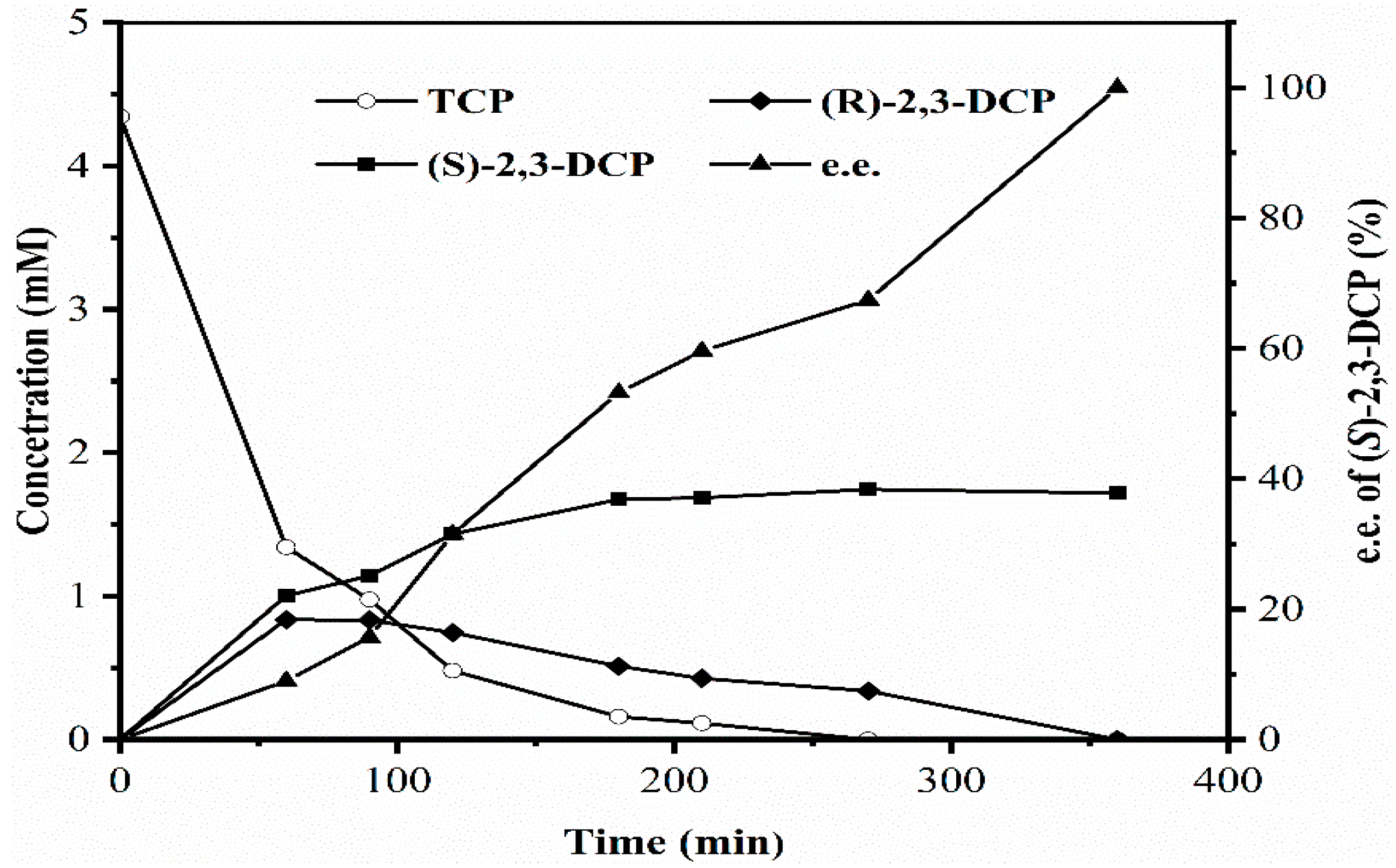
2.1. Identification of Bottlenecks in Degradation of TCP to Produce (S)-2,3-DCP
2.2. Optimization of Resin-Integrated One-Pot Degradation System
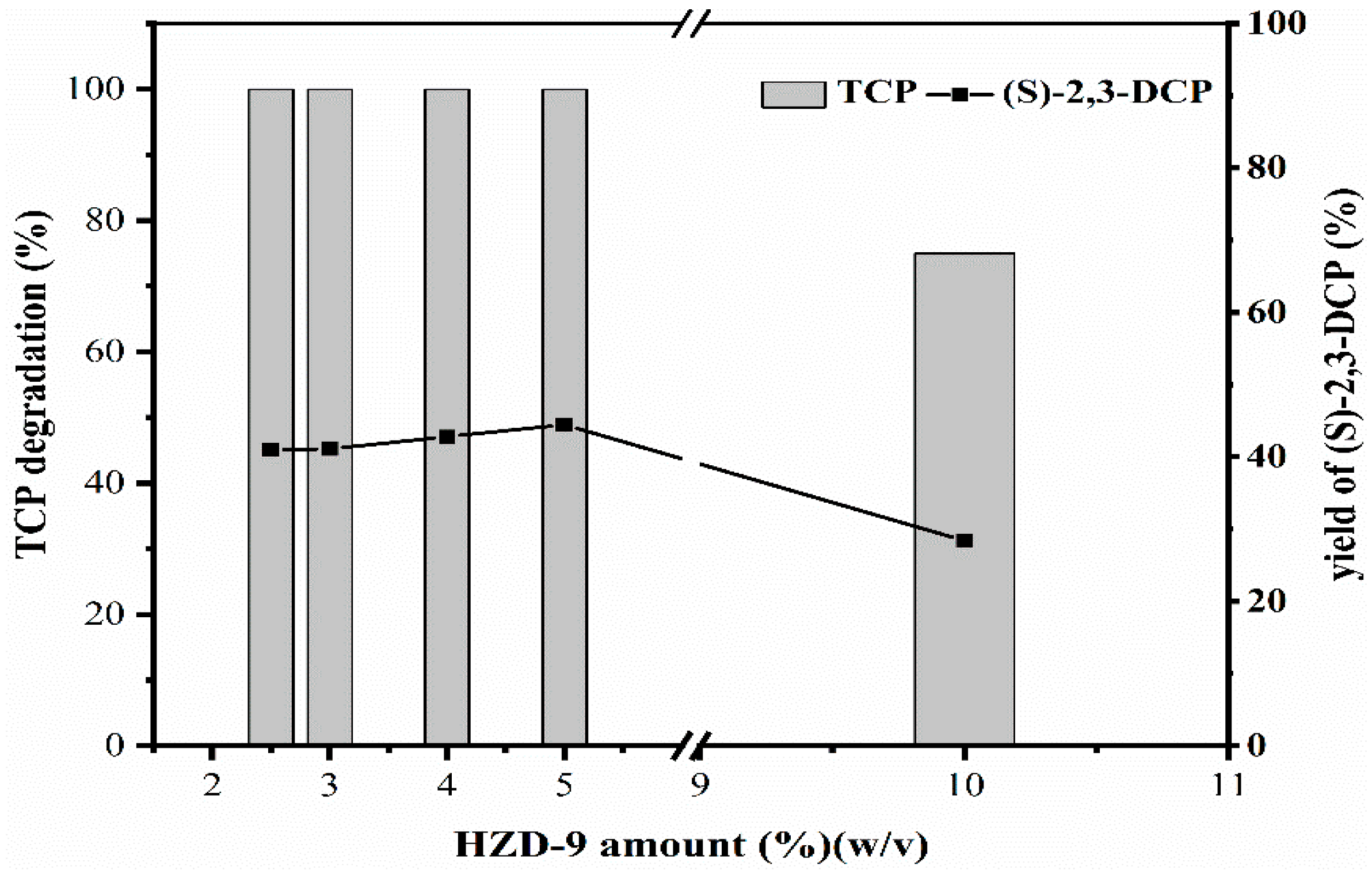
2.2.1. Effect of HZD-9 Concentration on Biotransformation
2.2.2. Effect of Temperature on Biotransformation
2.2.3. Effect of pH on Biotransformation
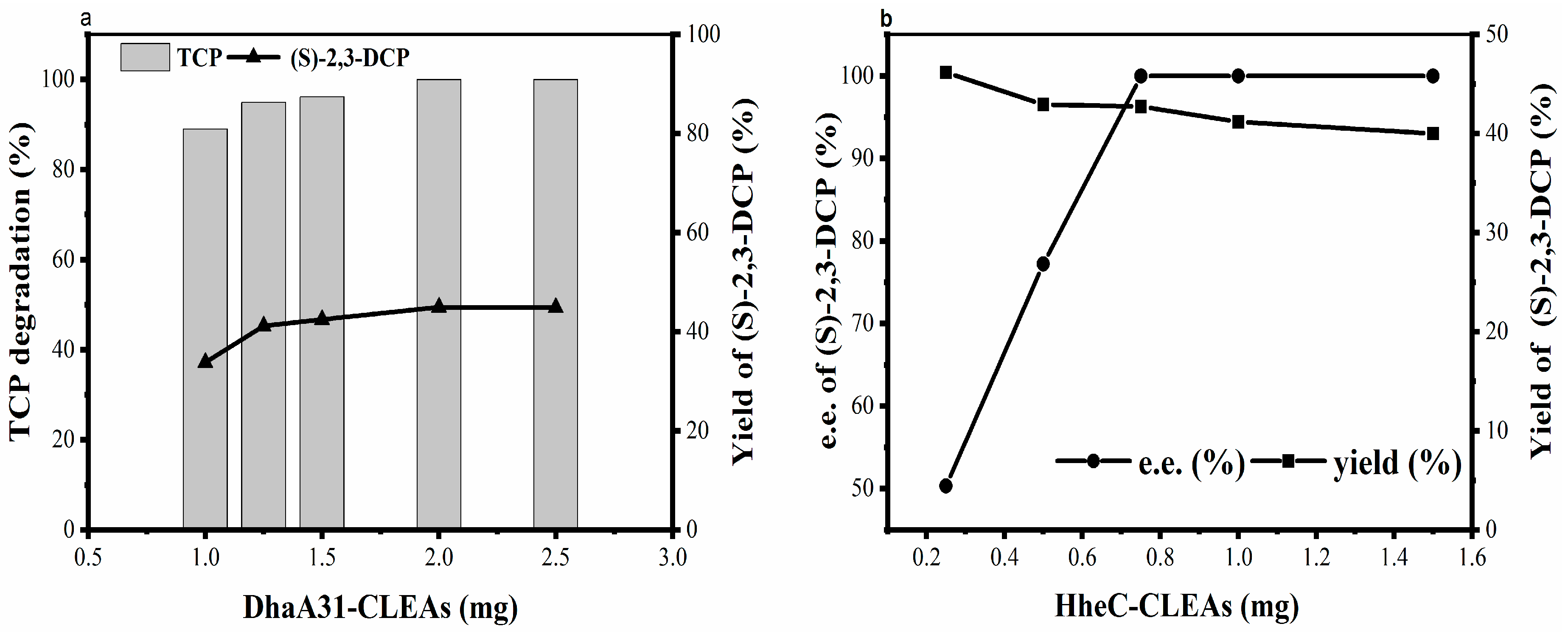
2.2.4. Effect of Enzyme Concentration on Biotransformation
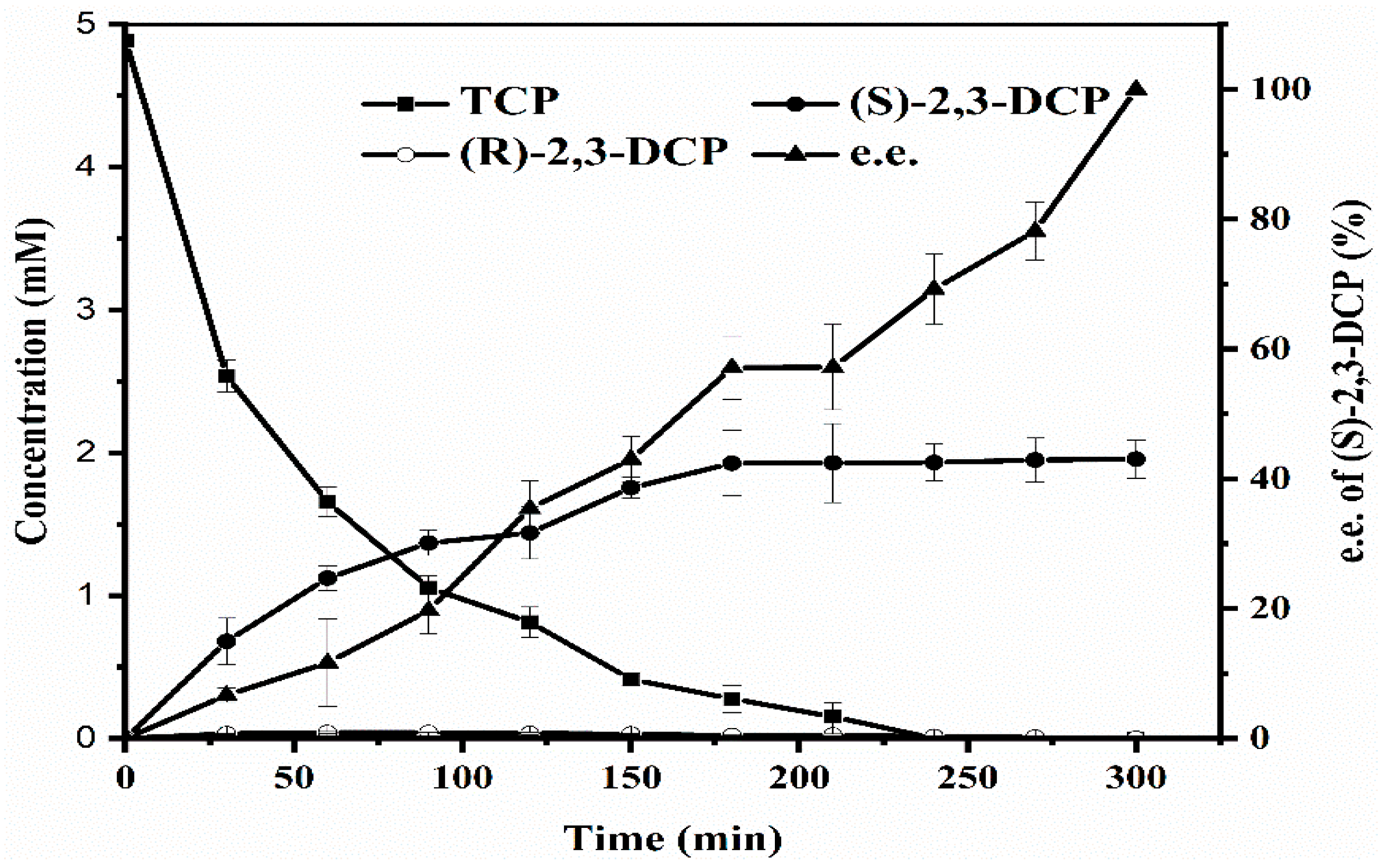
2.2.5. Resin-Integrated One-Pot Biotransformation Performed at The Optimized Conditions
2.2.6. Scaled Up Batch Biodegradation of TCP to (S)-2,3-DCP
2.2.7. Reusability of DhaA31-CLEAs and HheC-CLEAs
3. Materials and Methods
3.1. Materials
3.2. Protein Expression and Extraction
3.3. Preparation of CLEAs
3.4. One-Pot Degradation of TCP in Batch System
3.5. Reusability of DhaA31-CLEAs and HheC-CLEAs
3.6. Analytical Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Wang, B.; Chu, K.H. Cometabolic biodegradation of 1,2,3-trichloropropane by propane-oxidizing bacteria. Chemosphere 2017, 168, 1494–1497. [Google Scholar] [CrossRef] [PubMed]
- Dvorak, P.; Bidmanova, S.; Damborsky, J.; Prokop, Z. Immobilized synthetic pathway for biodegradation of toxic recalcitrant pollutant 1,2,3-trichloropropane. Environ. Sci. Technol. 2014, 48, 6859–6866. [Google Scholar] [CrossRef] [PubMed]
- Samin, G.; Janssen, D.B. Transformation and biodegradation of 1,2,3-trichloropropane (TCP). Environ. Sci. Pollut. Res. 2012, 19, 3067–3078. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burow, K.R.; Floyd, W.D.; Landon, M.K. Factors affecting 1,2,3-trichloropropane contamination in groundwater in California. Sci. Total Environ. 2019, 672, 324–334. [Google Scholar] [CrossRef] [PubMed]
- Bosma, T.; Damborský, J.; Stucki, G.; Janssen, D.B. Biodegradation of 1,2,3-Trichloropropane through Directed Evolution and Heterologous Expression of a Haloalkane Dehalogenase Gene. Appl. Environ. Microbiol. 2002, 68, 3582–3587. [Google Scholar] [CrossRef] [Green Version]
- Pavlova, M.; Klvana, M.; Prokop, Z.; Chaloupkova, R.; Banas, P.; Otyepka, M.; Wade, R.C.; Tsuda, M.; Nagata, Y.; Damborsky, J. Redesigning dehalogenase access tunnels as a strategy for degrading an anthropogenic substrate. Nat. Chem. Biol. 2009, 5, 727–733. [Google Scholar] [CrossRef]
- Kurumbang, N.P.; Dvorak, P.; Bendl, J.; Brezovsky, J.; Prokop, Z.; Damborsky, J. Computer assisted engineering of the synthetic pathway for biodegradation of a toxic persistent pollutant. ACS Synth. Biol. 2014, 3, 172–181. [Google Scholar] [CrossRef]
- Zou, S.P.; Zheng, Y.G.; Du, E.H.; Hu, Z.C. Enhancement of (S)-2,3-dichloro-1-propanol production by recombinant whole-cell biocatalyst in n-heptane-aqueous biphasic system. J. Biotechnol. 2014, 188, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Spelberg, J.H.L.; Vlieg, J.E.T.V.; Bosma, T.; Kellogg, R.M.; Janssen, D.B. A tandem enzyme reaction to produce optically active halohydrins, epoxides and diols. Tetrahedron Asymmetry 1999, 10, 2863–2870. [Google Scholar] [CrossRef]
- van Leeuwen, J.G.; Wijma, H.J.; Floor, R.J.; van der Laan, J.M.; Janssen, D.B. Directed Evolution Strategies for Enantiocomplementary Haloalkane Dehalogenases: From Chemical Waste to Enantiopure Building Blocks. ChemBioChem 2012, 13, 137–148. [Google Scholar] [CrossRef] [Green Version]
- Jin, H.X.; Hu, Z.C.; Liu, Z.Q.; Zheng, Y.G. Nitrite-mediated synthesis of chiral epichlorohydrin using halohydrin dehalogenase from Agrobacterium radiobacter AD1. Biotechnol. Appl. Biochem. 2012, 59, 170–177. [Google Scholar] [CrossRef]
- Majerić Elenkov, M.; Tang, L.; Hauer, B.; Janssen, D.B. Sequential kinetic resolution catalyzed by halohydrin dehalogenase. Org. Lett. 2006, 8, 4227–4229. [Google Scholar] [CrossRef] [Green Version]
- Zou, S.P.; Du, E.H.; Hu, Z.C.; Zheng, Y.G. Enhanced biotransformation of 1,3-dichloro-2-propanol to epichlorohydrin via resin-based in situ product removal process. Biotechnol. Lett. 2013, 35, 937–942. [Google Scholar] [CrossRef] [PubMed]
- Zou, S.P.; Gu, K.; Zheng, Y.G. Covalent immobilization of halohydrin dehalogenase for efficient synthesis of epichlorohydrin in an integrated bioreactor. Biotechnol. Prog. 2018, 34, 784–792. [Google Scholar] [CrossRef] [PubMed]
- Liao, Q.; Du, X.; Jiang, W.; Tong, Y.; Zhao, Z.; Fang, R.; Feng, J.; Tang, L. Cross-linked enzyme aggregates (CLEAs) of halohydrin dehalogenase from Agrobacterium radiobacter AD1reparation, characterization and application as a biocatalyst. J. Biotechnol. 2018, 272–273, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Philips, R.S. Temperature modulation of the stereochemistry of enzymatic catalysisrospects for exploitation. Trends Biotechnol. 1996, 14, 13–16. [Google Scholar] [CrossRef]
- Zhang, Z.J.; Pan, J.; Liu, J.F.; Xu, J.H.; He, Y.C.; Liu, Y.Y. Significant enhancement of (R)-mandelic acid production by relieving substrate inhibition of recombinant nitrilase in toluene-water biphasic system. J. Biotechnol. 2011, 152, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Dvorak, P.; Kurumbang, N.P.; Bendl, J.; Brezovsky, J.; Prokop, Z.; Damborsky, J. Maximizing the Efficiency of Multienzyme Process by Stoichiometry Optimization. ChemBioChem 2014, 15, 1891–1895. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Chen, Y.; Zheng, Y.; Zhang, W.; Tao, Y.; Feng, J.; Tang, L. Exploring the enantioselective mechanism of halohydrin dehalogenase from Agrobacterium radiobacter AD1 by iterative saturation mutagenesis. Appl. Environ. Microbiol. 2015, 81, 2919–2926. [Google Scholar] [CrossRef] [PubMed] [Green Version]




© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bogale, T.F.; Gul, I.; Wang, L.; Deng, J.; Chen, Y.; Majerić-Elenkov, M.; Tang, L. Biodegradation of 1,2,3-Trichloropropane to Valuable (S)-2,3-DCP Using a One-Pot Reaction System. Catalysts 2020, 10, 3. https://doi.org/10.3390/catal10010003
Bogale TF, Gul I, Wang L, Deng J, Chen Y, Majerić-Elenkov M, Tang L. Biodegradation of 1,2,3-Trichloropropane to Valuable (S)-2,3-DCP Using a One-Pot Reaction System. Catalysts. 2020; 10(1):3. https://doi.org/10.3390/catal10010003
Chicago/Turabian StyleBogale, Tadesse Fantaye, Ijaz Gul, Le Wang, Jiao Deng, Yong Chen, Maja Majerić-Elenkov, and Lixia Tang. 2020. "Biodegradation of 1,2,3-Trichloropropane to Valuable (S)-2,3-DCP Using a One-Pot Reaction System" Catalysts 10, no. 1: 3. https://doi.org/10.3390/catal10010003
APA StyleBogale, T. F., Gul, I., Wang, L., Deng, J., Chen, Y., Majerić-Elenkov, M., & Tang, L. (2020). Biodegradation of 1,2,3-Trichloropropane to Valuable (S)-2,3-DCP Using a One-Pot Reaction System. Catalysts, 10(1), 3. https://doi.org/10.3390/catal10010003




