Photocatalytic Oxidation of Methyl Tert-Butyl Ether in Presence of Various Phase Compositions of TiO2
Abstract
:1. Introduction
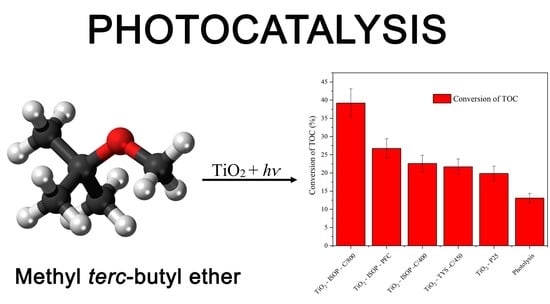
2. Results and Discussion
Reaction Mechanism
3. Materials and Methods
3.1. Photocatalysts Preparation Method
3.2. Characterization of TiO2 Photocatalysts
3.3. Photocatalytic Degradation of MTBE
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Levchuk, I.; Bhatnagar, A.; Sillanpää, M. Overview of technologies for removal of methyl tert-butyl ether (MTBE) from water. Sci. Total Environ. 2014, 476, 415–433. [Google Scholar] [CrossRef] [PubMed]
- U.S. Environmental Protection Agency (EPA). Methyl Tertiary Butyl Ether (MTBE)—Overview. Available online: https://archive.epa.gov/mtbe/web/html/faq.html (accessed on 16 October 2018).
- Media, A. Argus MTBE Annual 2017; Argus Media: London, UK, 2017; pp. 1–6. [Google Scholar]
- Park, S.E.; Joo, H.; Kang, J.W. Photodegradation of methyl tertiary butyl ether (MTBE) vapor with immobilized titanium dioxide. Sol. Energy Mater. Sol. Cells 2003, 80, 73–84. [Google Scholar] [CrossRef]
- Zang, Y.; Farnood, R. Photocatalytic decomposition of methyl tert-butyl ether in aqueous slurry of titanium dioxide. Appl. Catal. B 2005, 57, 275–282. [Google Scholar] [CrossRef]
- Orlov, A.; Jefferson, D.A.; Tikhov, M.; Lambert, R.M. Enhancement of MTBE photocatalytic degradation by modification of TiO2 with gold nanoparticles. Catal. Commun. 2007, 8, 821–824. [Google Scholar] [CrossRef]
- Rodríguez-González, V.; Zanella, R.; Del Angel, G.; Gómez, R. MTBE visible-light photocatalytic decomposition over Au/TiO2 and Au/TiO2–Al2O3 sol–gel prepared catalysts. J. Mol. Catal. A Chem. 2008, 281, 93–98. [Google Scholar] [CrossRef]
- Mirhoseini, F.; Salabat, A. Removal of methyl tert-butyl ether as a water pollutant by photodegradation over a new type of poly(methyl methacrylate)/TiO2 nanocomposite. Polym. Compos. 2018, 39, 1248–1254. [Google Scholar] [CrossRef]
- Seddigi, Z.S.; Ahmed, S.A.; Bumajdad, A.; Danish, E.Y.; Shawky, A.M.; Gondal, M.A.; Soylak, M. The efficient photocatalytic degradation of methyl tert-butyl ether under Pd/ZnO and visible light irradiation. Photochem. Photobiol. 2015, 91, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Seddigi, Z.S.; Ahmed, S.A.; Bumajdad, A.; Gonadal, M.A.; Danish, E.Y.; Shawky, A.M.; Yarkandi, N.H. Photocatalytic degradation oftert-butyl alcohol andtert-butyl formate using palladium-doped zinc oxide nanoparticles with UV irradiation. Desalin. Water Treat. 2015, 1–10. [Google Scholar] [CrossRef]
- Klauson, D.; Gromyko, I.; Dedova, T.; Pronina, N.; Krichevskaya, M.; Budarnaja, O.; Oja Acik, I.; Volobujeva, O.; Sildos, I.; Utt, K. Study on photocatalytic activity of ZnO nanoneedles, nanorods, pyramids and hierarchical structures obtained by spray pyrolysis method. Mater. Sci. Semicond. Process. 2015, 31, 315–324. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, R.A.; Rouquerol, J.; Siemieniewska, T. Reporting Physisorption Data for Gas/Solid Systems. Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Reli, M.; Kobielusz, M.; Matějová, L.; Daniš, S.; Macyk, W.; Obalová, L.; Kuśtrowski, P.; Rokicińska, A.; Kočí, K. TiO2 Processed by pressurized hot solvents as a novel photocatalyst for photocatalytic reduction of carbon dioxide. Appl. Surf. Sci. 2017, 391, 282–287. [Google Scholar] [CrossRef]
- Matějová, L.; Šihor, M.; Lang, J.; Troppová, I.; Ambrožová, N.; Reli, M.; Brunátová, T.; Čapek, L.; Kotarba, A.; Kočí, K. Investigation of low Ce amount doped-TiO2 prepared by using pressurized fluids in photocatalytic N2O decomposition and CO2 reduction. J. Sol-Gel Sci. Technol. 2017, 84, 158–168. [Google Scholar] [CrossRef]
- Matějová, L.; Matěj, Z.; Fajgar, R.; Cajthaml, T.; Šolcová, O. TiO2 powders synthesized by pressurized fluid extraction and supercritical drying: Effect of water and methanol on structural properties and purity. Mater. Res. Bull. 2012, 47, 3573–3579. [Google Scholar] [CrossRef]
- Araña, J.; Peña Alonso, A.; Doña Rodríguez, J.M.; Herrera Melián, J.A.; González Díaz, O.; Pérez Peña, J. Comparative study of MTBE photocatalytic degradation with TiO2 and Cu-TiO2. Appl. Catal. B 2008, 78, 355–363. [Google Scholar] [CrossRef]
- Mohebali, S. Degradation of methyl t-butyl ether (MTBE) by photochemical process in nanocrystalline TiO2 slurry: Mechanism, by-products and carbonate ion effect. J. Environ. Chem. Eng. 2013, 1, 1070–1078. [Google Scholar] [CrossRef]
- Hu, Q.; Zhang, C.; Wang, Z.; Chen, Y.; Mao, K.; Zhang, X.; Xiong, Y.; Zhu, M. Photodegradation of methyl tert-butyl ether (MTBE) by UV/H2O2 and UV/TiO2. J. Hazard. Mater. 2008, 154, 795–803. [Google Scholar] [CrossRef]
- Selli, E.; Letizia Bianchi, C.; Pirola, C.; Bertelli, M. Degradation of methyl tert-butyl ether in water: Effects of the combined use of sonolysis and photocatalysis. Ultrason. Sonochem. 2005, 12, 395–400. [Google Scholar] [CrossRef]
- Prieto-Mahaney, O.-O.; Murakami, N.; Abe, R.; Ohtani, B. Correlation between Photocatalytic Activities and Structural and Physical Properties of Titanium(IV) Oxide Powders. Chem. Lett. 2009, 38, 238–239. [Google Scholar] [CrossRef]
- Bai, S.; Jiang, J.; Zhang, Q.; Xiong, Y. Steering charge kinetics in photocatalysis: Intersection of materials syntheses, characterization techniques and theoretical simulations. Chem. Soc. Rev. 2015, 44, 2893–2939. [Google Scholar] [CrossRef]
- Zhang, Y.; Gan, H.; Zhang, G. A novel mixed-phase TiO2/kaolinite composites and their photocatalytic activity for degradation of organic contaminants. Chem. Eng. J. 2011, 172, 936–943. [Google Scholar] [CrossRef]
- Huang, K.C.; Couttenye, R.A.; Hoag, G.E. Kinetics of heat-assisted persulfate oxidation of methyl tert-butyl ether (MTBE). Chemosphere 2002, 49, 413–420. [Google Scholar] [CrossRef]
- Hetflejš, J.; Šabata, S.; Kuncová, G. terc-butylmethylether a jeho degradace oxidačními procesy. Chem. Listy 2007, 101, 1011–1019. [Google Scholar]
- Buchalska, M.; Kobielusz, M.; Matuszek, A.; Pacia, M.; Wojtyla, S.; Macyk, W. On Oxygen Activation at Rutile- and Anatase-TiO2. ACS Catal. 2015, 5, 7424–7431. [Google Scholar] [CrossRef]
- Barreto, R.D.; Gray, K.A.; Anders, K. Photocatalytic degradation of methyl-tert-butyl ether in TiO2 slurries: A proposed reaction scheme. Water Res. 1995, 29, 1243–1248. [Google Scholar] [CrossRef]
- Hwang, S.; Huling, S.G.; Ko, S. Fenton-like degradation of MTBE: Effects of iron counter anion and radical scavengers. Chemosphere 2010, 78, 563–568. [Google Scholar] [CrossRef]
- Troppová, I.; Šihor, M.; Reli, M.; Ritz, M.; Praus, P.; Kočí, K. Unconventionally prepared TiO2/g-C3N4 photocatalysts for photocatalytic decomposition of nitrous oxide. Appl. Surf. Sci. 2018, 430, 335–347. [Google Scholar] [CrossRef]
- Reli, M.; Troppová, I.; Šihor, M.; Pavlovský, J.; Praus, P.; Kočí, K. Photocatalytic decomposition of N2O over g-C3N4/BiVO4 composite. Appl. Surf. Sci. 2019, 469, 181–191. [Google Scholar] [CrossRef]
- Reli, M.; Svoboda, L.; Šihor, M.; Troppová, I.; Pavlovský, J.; Praus, P.; Kočí, K. Photocatalytic decomposition of N2O over g-C3N4/WO3 photocatalysts. Environ. Sci. Pollut. Res. Int. 2017, 1–12. [Google Scholar] [CrossRef]







| Photocatalyst Labeling | Physisorption | UV–Vis | |
|---|---|---|---|
| SBET (m2 g−1) | Vne (cm3liq g−1) | Band Gap Energy (eV) | |
| TiO2–TYS–C/450 | 137 | 0.226 | 3.18 |
| TiO2–ISOP–C/400 | 80 | 0.120 | 3.04 |
| TiO2–ISOP–PFC | 171 | 0.265 | 3.11 |
| TiO2–ISOP–C/800 | 0.99 | ---- | 2.90 |
| TiO2–P25 | 44 | 0.208 | 3.22 |
| Photocatalyst Labeling | Phase Composition (wt.%) | Crystallite-Size (nm) | Facets (hkl) |
|---|---|---|---|
| TiO2–TYS–C/450 | Anatase | 7.6 | (101) (200) |
| TiO2–ISOP–C/400 | Anatase | 10.3 | (101) (200) |
| TiO2–ISOP–PFC | 79% Anatase | 6.5 | (101) (200) |
| 21% Brookite | 5.2 | (211) | |
| TiO2–ISOP–C/800 | 75% Anatase | 112 | (110) (101) (200) |
| 25% Rutile | 356 | (101) (200) | |
| TiO2–P25 | 85% Anatase | 24 | (110) (101) (200) |
| 15% Rutile | 43 | (101) (200) |
| Photocatalysts Labeling | Preparation | Processing | ||
|---|---|---|---|---|
| Method | Precursor | Method | Conditions | |
| TiO2–TYS–C/450 | Thermal hydrolysis | Titanyl sulphate | Calcination | 450 °C (2 h), 3 °C min−1 |
| TiO2–ISOP–C/400 | Sol-gel | Titanium (IV) isopropoxide | Calcination | 400 °C (4 h), 10 °C min−1 |
| TiO2–ISOP–PFC | Sol-gel | Titanium (IV) isopropoxide | Pressurized hot fluids crystallization | 200 °C, 10 MPa, 1.5 L H2O + 0.25 L CH3OH + 0.1 L H2O, 3.5–4.5 mL min−1 |
| TiO2–ISOP–C/800 | Sol-gel | Titanium (IV) isopropoxide | Calcination | 800 °C (4 h), 5 °C min−1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šihor, M.; Reli, M.; Vaštyl, M.; Hrádková, K.; Matějová, L.; Kočí, K. Photocatalytic Oxidation of Methyl Tert-Butyl Ether in Presence of Various Phase Compositions of TiO2. Catalysts 2020, 10, 35. https://doi.org/10.3390/catal10010035
Šihor M, Reli M, Vaštyl M, Hrádková K, Matějová L, Kočí K. Photocatalytic Oxidation of Methyl Tert-Butyl Ether in Presence of Various Phase Compositions of TiO2. Catalysts. 2020; 10(1):35. https://doi.org/10.3390/catal10010035
Chicago/Turabian StyleŠihor, Marcel, Martin Reli, Michal Vaštyl, Květoslava Hrádková, Lenka Matějová, and Kamila Kočí. 2020. "Photocatalytic Oxidation of Methyl Tert-Butyl Ether in Presence of Various Phase Compositions of TiO2" Catalysts 10, no. 1: 35. https://doi.org/10.3390/catal10010035