Bulk, Surface and Interface Promotion of Co3O4 for the Low-Temperature N2O Decomposition Catalysis
Abstract
:1. Introduction
2. Results
2.1. N2O Performance of Promoted Cobalt Spinel-Based Catalysts
2.2. Physicochemical Characterization
2.2.1. Bulk Promoted Catalysts
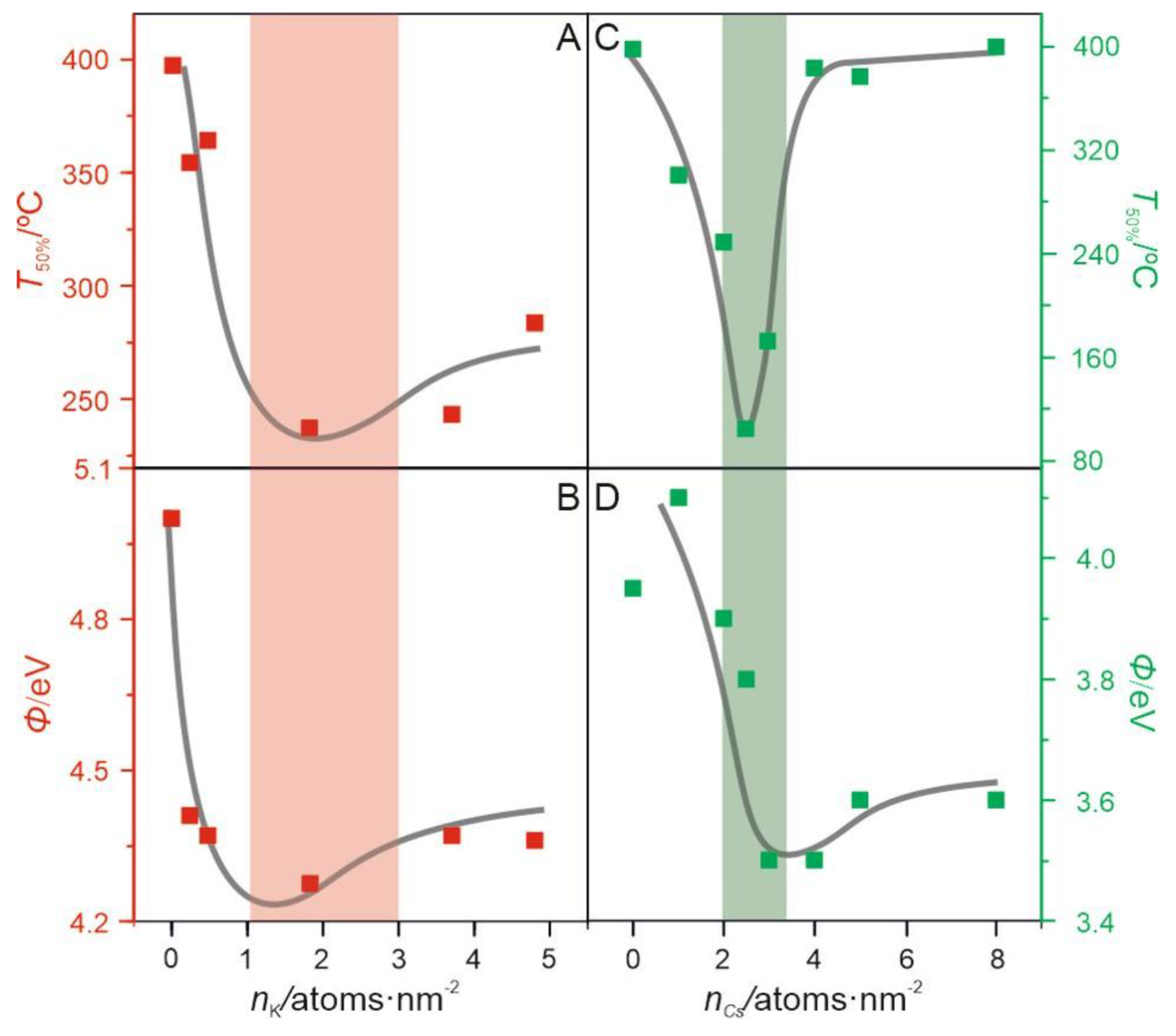
2.2.2. Surface Promotion
2.2.3. Interface Promotion
3. Discussion
4. Materials and Methods
4.1. Co3O4—Based Promoted Catalysts
4.2. Catalyst Characterization
4.3. N2O Decomposition Tests
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Rico-Pérez, V.; De Lecea, C.S.M.; Bueno-López, A. Preparation of RhOx/CeyPr1-yO2 N2O decomposition catalysts by rhodium nitrate impregnation with different solvents. Appl. Catal. A Gen. 2014, 472, 134–142. [Google Scholar] [CrossRef] [Green Version]
- Zabilskiy, M.; Djinović, P.; Tchernychova, E.; Tkachenko, O.P.; Kustov, L.M.; Pintar, A. Nanoshaped CuO/CeO2 Materials: Effect of the Exposed Ceria Surfaces on Catalytic Activity in N2O Decomposition Reaction. ACS Catal. 2015, 5, 5357–5365. [Google Scholar] [CrossRef]
- Obalová, L.; Pacultová, K.; Balabánová, J.; Jirátová, K.; Bastl, Z.; Valášková, M.; Lacný, Z.; Kovanda, F. Effect of Mn/Al ratio in Co-Mn-Al mixed oxide catalysts prepared from hydrotalcite-like precursors on catalytic decomposition of N2O. Catal. Today 2007, 119, 233–238. [Google Scholar] [CrossRef]
- Santiago, M.; Pérez-Ramírez, J. Decomposition of N2O over hexaaluminate catalysts. Environ. Sci. Technol. 2007, 41, 1704–1709. [Google Scholar] [CrossRef]
- Rutkowska, M.; Chmielarz, L.; Macina, D.; Piwowarska, Z.; Dudek, B.; Adamski, A.; Witkowski, S.; Sojka, Z.; Obalová, L.; Van Oers, C.J.; et al. Catalytic decomposition and reduction of N2O over micro-mesoporous materials containing Beta zeolite nanoparticles. Appl. Catal. B Environ. 2014, 146, 112–122. [Google Scholar] [CrossRef]
- Konsolakis, M. Recent Advances on Nitrous Oxide (N2O) Decomposition over Non-Noble-Metal Oxide Catalysts: Catalytic Performance, Mechanistic Considerations, and Surface Chemistry Aspects. ACS Catal. 2015, 5, 6397–6421. [Google Scholar] [CrossRef]
- Pietrogiacomi, D.; Campa, M.C.; Carbone, L.R.; Tuti, S.; Occhiuzzi, M. N2O decomposition on CoOx, CuOx, FeOx or MnOx supported on ZrO2: The effect of zirconia doping with sulfates or K+ on catalytic activity. Appl. Catal. B Environ. 2016, 187, 218–227. [Google Scholar] [CrossRef]
- Klegova, A.; Inayat, A.; Indyka, P.; Gryboś, J.; Sojka, Z.; Pacultová, K.; Schwieger, W.; Volodarskaja, A.; Kuśtrowski, P.; Rokicińska, A.; et al. Cobalt mixed oxides deposited on the SiC open-cell foams for nitrous oxide decomposition. Appl. Catal. B Environ. 2019, 255, 117745. [Google Scholar] [CrossRef]
- Inayat, A.; Ayoub, M.; Abdullah, A.Z.; Ullah, S.; Naqvi, S.R. Decomposition of N2O at low temperature over Co3O4 prepared by different methods. Environ. Prog. Sustain. Energy 2019, 38, 13129. [Google Scholar] [CrossRef]
- Grzybek, G.; Wójcik, S.; Ciura, K.; Gryboś, J.; Indyka, P.; Oszajca, M.; Stelmachowski, P.; Witkowski, S.; Inger, M.; Wilk, M.; et al. Influence of preparation method on dispersion of cobalt spinel over alumina extrudates and the catalyst deN2O activity. Appl. Catal. B Environ. 2017, 210, 34–44. [Google Scholar] [CrossRef]
- Chromčáková, Ž.; Obalová, L.; Kovanda, F.; Legut, D.; Titov, A.; Ritz, M.; Fridrichová, D.; Michalik, S.; Kuśtrowski, P.; Jirátová, K. Effect of precursor synthesis on catalytic activity of Co3O4 in N2O decomposition. Catal. Today 2015, 257, 18–25. [Google Scholar] [CrossRef]
- Russo, N.; Fino, D.; Saracco, G.; Specchia, V. N2O catalytic decomposition over various spinel-type oxides. Catal. Today 2007, 119, 228–232. [Google Scholar] [CrossRef]
- Stelmachowski, P.; Maniak, G.; Kotarba, A.; Sojka, Z. Strong electronic promotion of Co3O4 towards N2O decomposition by surface alkali dopants. Catal. Commun. 2009, 10, 1062–1065. [Google Scholar] [CrossRef]
- Gudyka, S.; Grzybek, G.; Gryboś, J.; Indyka, P.; Leszczyński, B.; Kotarba, A.; Sojka, Z. Enhancing the deN2O activity of the supported Co3O4|α-Al2O3 catalyst by glycerol-assisted shape engineering of the active phase at the nanoscale. Appl. Catal. B Environ. 2017, 201, 339–347. [Google Scholar] [CrossRef]
- Zasada, F.; Piskorz, W.; Sojka, Z. Cobalt Spinel at Various Redox Conditions: DFT+U Investigations into the Structure and Surface Thermodynamics of the (100) Facet. J. Phys. Chem. C 2015, 119, 19180–19191. [Google Scholar] [CrossRef]
- Pacultová, K.; Karásková, K.; Kovanda, F.; Jirátová, K.; Šrámek, J.; Kustrowski, P.; Kotarba, A.; Chromčáková, Z.; Kočí, K.; Obalová, L. K-Doped Co-Mn-Al Mixed Oxide Catalyst for N2O Abatement from Nitric Acid Plant Waste Gases: Pilot Plant Studies. Ind. Eng. Chem. Res. 2016, 55, 7076–7084. [Google Scholar] [CrossRef]
- Dou, Z.; Zhang, H.; Pan, Y.; Xu, X. Catalytic decomposition of N2O over potassium-modified Cu-Co spinel oxides. J. Fuel Chem. Technol. 2014, 42, 238–245. [Google Scholar] [CrossRef]
- Maniak, G.; Stelmachowski, P.; Stanek, J.J.; Kotarba, A.; Sojka, Z. Catalytic properties in N2O decomposition of mixed cobalt-iron spinels. Catal. Commun. 2011, 15, 127–131. [Google Scholar] [CrossRef]
- Inger, M.; Wilk, M.; Saramok, M.; Grzybek, G.; Grodzka, A.; Stelmachowski, P.; Makowski, W.; Kotarba, A.; Sojka, Z. Cobalt spinel catalyst for N2O abatement in the pilot plant operation-long-term activity and stability in tail gases. Ind. Eng. Chem. Res. 2014, 53, 10335–10342. [Google Scholar] [CrossRef]
- Abu-Zied, B.M.; Soliman, S.A.; Abdellah, S.E. Pure and Ni-substituted Co3O4 spinel catalysts for direct N2O decomposition. Chin. J. Catal. 2014, 35, 1105–1112. [Google Scholar] [CrossRef]
- Yu, H.; Tursun, M.; Wang, X.; Wu, X. Pb0.04Co catalyst for N2O decomposition in presence of impurity gases. Appl. Catal. B Environ. 2016, 185, 110–118. [Google Scholar] [CrossRef]
- Tursun, M.; Wang, X.; Zhang, F.; Yu, H. Bi-Co3O4 catalyzing N2O decomposition with strong resistance to CO2. Catal. Commun. 2015, 65, 1–5. [Google Scholar] [CrossRef]
- Abu-Zied, B.M.; Asiri, A.M. The role of alkali promoters in enhancing the direct N2O decomposition reactivity over NiO catalysts. Chin. J. Catal. 2015, 36, 1837–1845. [Google Scholar] [CrossRef]
- Grzybek, G.; Wójcik, S.; Indyka, P.; Ciura, K.; Sojka, Z.; Kotarba, A. Optimization of cesium and potassium promoter loading in alkali-doped Zn0.4Co2.6O4|Al2O3 catalysts for N2O abatement. React. Kinet. Mech. Catal. 2017, 121, 645–655. [Google Scholar]
- Abu-Zied, B.; Bawaked, S.; Kosa, S.; Schwieger, W.; Abu-Zied, B.M.; Bawaked, S.M.; Kosa, S.A.; Schwieger, W. Rare Earth-Promoted Nickel Oxide Nanoparticles as Catalysts for N2O Direct Decomposition. Catalysts 2016, 6, 70. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Xia, L.; Li, J.; Liu, X.; Sun, J.; Wang, H.; Chi, Y.; Li, C.; Song, Y. Effect of alkaline earth metal doping on the catalytic performance of cobalt-based spinel composite metal oxides in N2O decomposition. J. Fuel Chem. Technol. 2018, 46, 1377–1385. [Google Scholar] [CrossRef]
- Yu, H.; Wang, X.; Li, Y. Strong impact of cobalt distribution on the activity for Co3O4/CaCO3 catalyzing N2O decomposition. Catal. Today 2020, 339, 274–280. [Google Scholar] [CrossRef]
- Maniak, G.; Stelmachowski, P.; Zasada, F.; Piskorz, W.; Kotarba, A.; Sojka, Z. Guidelines for optimization of catalytic activity of 3d transition metal oxide catalysts in N2O decomposition by potassium promotion. Proc. Catal. Today Elsevier 2011, 176, 369–372. [Google Scholar] [CrossRef]
- Maniak, G.; Stelmachowski, P.; Kotarba, A.; Sojka, Z.; Rico-Pérez, V.; Bueno-López, A. Rationales for the selection of the best precursor for potassium doping of cobalt spinel based deN2O catalyst. Appl. Catal. B Environ. 2013, 136–137, 302–307. [Google Scholar] [CrossRef]
- Obalová, L.; Maniak, G.; Karásková, K.; Kovanda, F.; Kotarba, A. Electronic nature of potassium promotion effect in Co-Mn-Al mixed oxide on the catalytic decomposition of N2O. Catal. Commun. 2011, 12, 1055–1058. [Google Scholar] [CrossRef] [Green Version]
- Grzybek, G.; Wójcik, S.; Legutko, P.; Gryboś, J.; Indyka, P.; Leszczyński, B.; Kotarba, A.; Sojka, Z. Thermal stability and repartition of potassium promoter between the support and active phase in the K-Co2.6Zn0.4O4|α-Al2O3 catalyst for N2O decomposition: Crucial role of activation temperature on catalytic performance. Appl. Catal. B Environ. 2017, 205, 597–604. [Google Scholar] [CrossRef]
- Zasada, F.; Kaczmarczyk, J.; Indyka, P.; Sojka, Z.; Kotarba, A.; Piskorz, W.; Janas, J. Thermodynamic Stability, Redox Properties, and Reactivity of Mn3O4, Fe3O4, and Co3O4 Model Catalysts for N2O Decomposition: Resolving the Origins of Steady Turnover. ACS Catal. 2016, 6, 1235–1246. [Google Scholar]
- Zasada, F.; Gryboś, J.; Indyka, P.; Piskorz, W.; Kaczmarczyk, J.; Sojka, Z. Surface structure and morphology of M[CoM′] O4 (M = Mg, Zn, Fe, Co and M′ = Ni, Al, Mn, Co) spinel nanocrystals-DFT+U and TEM screening investigations. J. Phys. Chem. C 2014, 118, 19085–19097. [Google Scholar] [CrossRef]
- Lavina, B.; Salviulo, G.; Della Giusta, D. Cation distribution and structure modelling of spinel solid solutions. Phys. Chem. Miner. 2002, 29, 10–18. [Google Scholar] [CrossRef]
- Hill, R.J.; Craig, J.R.; Gibbs, G.V. Systematics of the spinel structure type. Phys. Chem. Miner. 1979, 4, 317–339. [Google Scholar] [CrossRef]
- Kim, J.-G.; Pugmire, D.L.; Battaglia, D.; Langell, M.A. Analysis of the NiCo2O4 spinel surface with Auger and X-ray photoelectron spectroscopy. Appl. Surf. Sci. 2000, 165, 70–84. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Wang, X. MgO Modifying Al2O3 to Load Cobalt Oxide for Catalytic N2O Decomposition. Catal. Lett. 2019, 149, 1856–1863. [Google Scholar] [CrossRef]
- Ivanova, Y.A.; Sutormina, E.F.; Isupova, L.A.; Rogov, V.A. Effect of the Composition of NixCo3-xO4 (x = 0-0.9) Oxides on Their Catalytic Activity in the Low-Temperature Reaction of N2O Decomposition. Kinet. Catal. 2018, 59, 365–370. [Google Scholar] [CrossRef]
- Stelmachowski, P.; Maniak, G.; Kaczmarczyk, J.; Zasada, F.; Piskorz, W.; Kotarba, A.; Sojka, Z. Mg and Al substituted cobalt spinels as catalysts for low temperature deN2O-Evidence for octahedral cobalt active sites. Appl. Catal. B Environ. 2014, 146, 105–111. [Google Scholar] [CrossRef]
- Stelmachowski, P.; Ciura, K.; Grzybek, G. Morphology-dependent reactivity of cobalt oxide nanoparticles in N2O decomposition. Catal. Sci. Technol. 2016, 6, 5554–5560. [Google Scholar] [CrossRef]
- Zasada, F.; Gryboś, J.; Budiyanto, E.; Janas, J.; Sojka, Z. Oxygen species stabilized on the cobalt spinel nano-octahedra at various reaction conditions and their role in catalytic CO and CH4 oxidation, N2O decomposition and oxygen isotopic exchange. J. Catal. 2019, 371, 224–235. [Google Scholar] [CrossRef]
- Grzybek, G.; Stelmachowski, P.; Gudyka, S.; Duch, J.; Ćmil, K.; Kotarba, A.; Sojka, Z. Insights into the twofold role of Cs doping on deN2O activity of cobalt spinel catalyst-towards rational optimization of the precursor and loading. Appl. Catal. B Environ. 2015, 168–169, 509–514. [Google Scholar] [CrossRef]
- Abu-Zied, B.M.; Soliman, S.A.; Asiri, A.M. Role of rubidium promotion on the nitrous oxide decomposition activity of nanocrystalline Co3O4-CeO2 catalyst. Appl. Surf. Sci. 2019, 479, 148–157. [Google Scholar] [CrossRef]
- Kapteijn, F.; Rodriguez-Mirasol, J.; Moulijn, J.A. Heterogeneous catalytic decomposition of nitrous oxide. Appl. Catal. B Environ. 1996, 9, 25–64. [Google Scholar] [CrossRef]
- Tolman, W.B. Binding and activation of N2O at transition-metal centers: Recent mechanistic insights. Angew. Chem. Int. Ed. 2010, 49, 1018–1024. [Google Scholar] [CrossRef] [Green Version]
- Somorjai, G.A.; Li, Y. Introduction to Surface Chemistry and Catalysis; Wiley: Hoboken, NJ, USA, 2010; ISBN 9780470508237. [Google Scholar]
- Zasada, F.; Stelmachowski, P.; Maniak, G.; Paul, J.F.; Kotarba, A.; Sojka, Z. Potassium promotion of cobalt spinel catalyst for N2O decomposition-accounted by work function measurements and DFT modelling. Catal. Lett. 2009, 127, 126–131. [Google Scholar] [CrossRef]
- Grzybek, G.; Gudyka, S.; Stelmachowski, P.; Rico-Pérez, V.; Indyka, P.; Guillén-Hurtado, N.; Bueno-López, A.; Kotarba, A.; Sojka, Z. Strong dispersion effect of cobalt spinel active phase spread over ceria for catalytic N2O decomposition: The role of the interface periphery. Appl. Catal. B Environ. 2015, 180, 622–629. [Google Scholar] [CrossRef] [Green Version]
- Wójcik, S.; Indyka, P.; Sojka, Z.; Kotarba, A. Development of structured Co3O4-based catalyst for N2O removal from hospital ventilation systems. Catal. Today 2019. [CrossRef]
- You, Y.; Chang, H.; Ma, L.; Guo, L.; Qin, X.; Li, J.; Li, J. Enhancement of N2O decomposition performance by N2O pretreatment over Ce-Co-O catalyst. Chem. Eng. J. 2018, 347, 184–192. [Google Scholar] [CrossRef]
- Yan, L.; Ren, T.; Wang, X.; Gao, Q.; Ji, D.; Suo, J. Excellent catalytic performance of ZnxCo1−xCo2O4 spinel catalysts for the decomposition of nitrous oxide. Catal. Commun. 2003, 4, 505–509. [Google Scholar] [CrossRef]
- Amrousse, R.; Chang, P.-J.; Choklati, A.; Friche, A.; Rai, M.; Bachar, A.; Follet-Houttemane, C.; Hori, K. Catalytic decomposition of N2O over Ni and Mg-magnetite catalysts. Catal. Sci. Technol. 2013, 3, 2288–2294. [Google Scholar] [CrossRef]
- Kim, M.J.; Lee, S.J.; Ryu, I.S.; Moon, S.H.; Jeon, M.W.; Ko, C.H.; Jeon, S.G. Understanding the Effect of NO Adsorption on Potassium-Promoted Co3O4 for N2O Decomposition. Catal. Lett. 2017, 147, 2886–2892. [Google Scholar] [CrossRef]
- Pasha, N.; Lingaiah, N.; Babu, N.S.; Reddy, P.S.S.; Prasad, P.S.S. Studies on cesium doped cobalt oxide catalysts for direct N2O decomposition in the presence of oxygen and steam. Catal. Commun. 2008, 10, 132–136. [Google Scholar] [CrossRef]
- Su, D.; Dou, S.X.; Wang, G. Single crystalline Co3O4 nanocrystals exposed with different crystal planes for Li-o2 batteries. Sci. Rep. 2014, 4, 5767. [Google Scholar] [CrossRef] [Green Version]
- Xiong, S.; Chen, J.; Huang, N.; Yang, S.; Peng, Y.; Li, J. Balance between Reducibility and N2O Adsorption Capacity for the N2O Decomposition: CuxCoy Catalysts as an Example. Environ. Sci. Technol. 2019, 53, 10379–10386. [Google Scholar] [CrossRef]
- Wang, Y.; Zheng, K.; Hu, X.; Zhou, W.; Wei, X.; Zhao, Y. Y2O3 promoted Co3O4 catalyst for catalytic decomposition of N2O. Mol. Catal. 2019, 470, 104–111. [Google Scholar] [CrossRef]
- Ivanova, Y.A.; Sutormina, E.F.; Isupova, I.A.; Vovk, E.I. Catalytic Activity of the Oxide Catalysts Based on Ni0.75Co2.25O4 Modified with Cesium Cations in a Reaction of N2O Decomposition. Kinet. Catal. 2017, 58, 773–779. [Google Scholar] [CrossRef]








| Sample | Ea/kJ mol−1 | SSA */m2g−1 |
|---|---|---|
| Co3O4 | 63 | 30 |
| Zn0.4Co2.6O4 | 61 | 58 |
| Ni0.7Co2.3O4 | 60 | 53 |
| MgCo2O4 | 71 | 6 |
| CoAl2O4 | 117 | 34 |
| Catalyst | Conditions | T50% | Reference |
|---|---|---|---|
| Co3O4 | 5% N2O, GHSV = 7000 h−1 | 380 | This work |
| Co3O4 | 0.5% N2O, GHSV = 80,000 h−1 | 475 | [12] |
| Co3O4 | 0.1% N2O, GHSV = 15,000 h−1 | 340 | [51] |
| MgCo2O4 | 5% N2O, GHSV = 7000 h−1 | 460 | This work |
| MgCo2O4 | 0.5% N2O, GHSV = 80,000 h−1 | 440 | [12] |
| Co0.6Fe2.4O4 | Pure N2O, GHSV = 15,000 h−1 | 160 | [52] |
| Co2FeO4 | 5% N2O, GHSV = 7000 h−1 | 442 | This work |
| CoFe2O4 | 0.5% N2O, GHSV = 80,000 h−1 | 580 | [12] |
| Zn0.36Co2.64O4 | 0.1% N2O, GHSV = 15,000 h−1 | 130 | [51] |
| Zn0.4Co2.6O4 | 5% N2O, GHSV = 7000 h−1 | 320 | This work |
| ZnCo2O4 | 0.5% N2O, GHSV = 80,000 h−1 | 475 | [12] |
| K-Co3O4 | 5% N2O, GHSV = 7000 h−1 | 235 | This work |
| K-Co3O4 | 0.05% N2O, GHSV = 45,000 h−1 | 275 | [53] |
| Cs-Co3O4 | 5% N2O, GHSV = 7000 h−1 | 110 | This work |
| Cs-Co3O4 | 0.25% N2O, GHSV = 8500 h−1 | 210 | [54] |
| PbO2|Co3O4 | 5% N2O, GHSV = 7000 h−1 | 195 | This work |
| PbO2|Co3O4 | 0.2% N2O, GHSV = 20,000 h−1 | 180 | [21] |
| CeO2|Co3O4 | 5% N2O, GHSV = 7000 h−1 | 330 | This work |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wójcik, S.; Grzybek, G.; Stelmachowski, P.; Sojka, Z.; Kotarba, A. Bulk, Surface and Interface Promotion of Co3O4 for the Low-Temperature N2O Decomposition Catalysis. Catalysts 2020, 10, 41. https://doi.org/10.3390/catal10010041
Wójcik S, Grzybek G, Stelmachowski P, Sojka Z, Kotarba A. Bulk, Surface and Interface Promotion of Co3O4 for the Low-Temperature N2O Decomposition Catalysis. Catalysts. 2020; 10(1):41. https://doi.org/10.3390/catal10010041
Chicago/Turabian StyleWójcik, Sylwia, Gabriela Grzybek, Paweł Stelmachowski, Zbigniew Sojka, and Andrzej Kotarba. 2020. "Bulk, Surface and Interface Promotion of Co3O4 for the Low-Temperature N2O Decomposition Catalysis" Catalysts 10, no. 1: 41. https://doi.org/10.3390/catal10010041
APA StyleWójcik, S., Grzybek, G., Stelmachowski, P., Sojka, Z., & Kotarba, A. (2020). Bulk, Surface and Interface Promotion of Co3O4 for the Low-Temperature N2O Decomposition Catalysis. Catalysts, 10(1), 41. https://doi.org/10.3390/catal10010041







