Low Temperature Dehydration of Glycerol to Acrolein in Vapor Phase with Hydrogen as Dilution: From Catalyst Screening via TPSR to Real-Time Reaction in a Fixed-Bed
Abstract
:1. Introduction
2. Result and Discussion
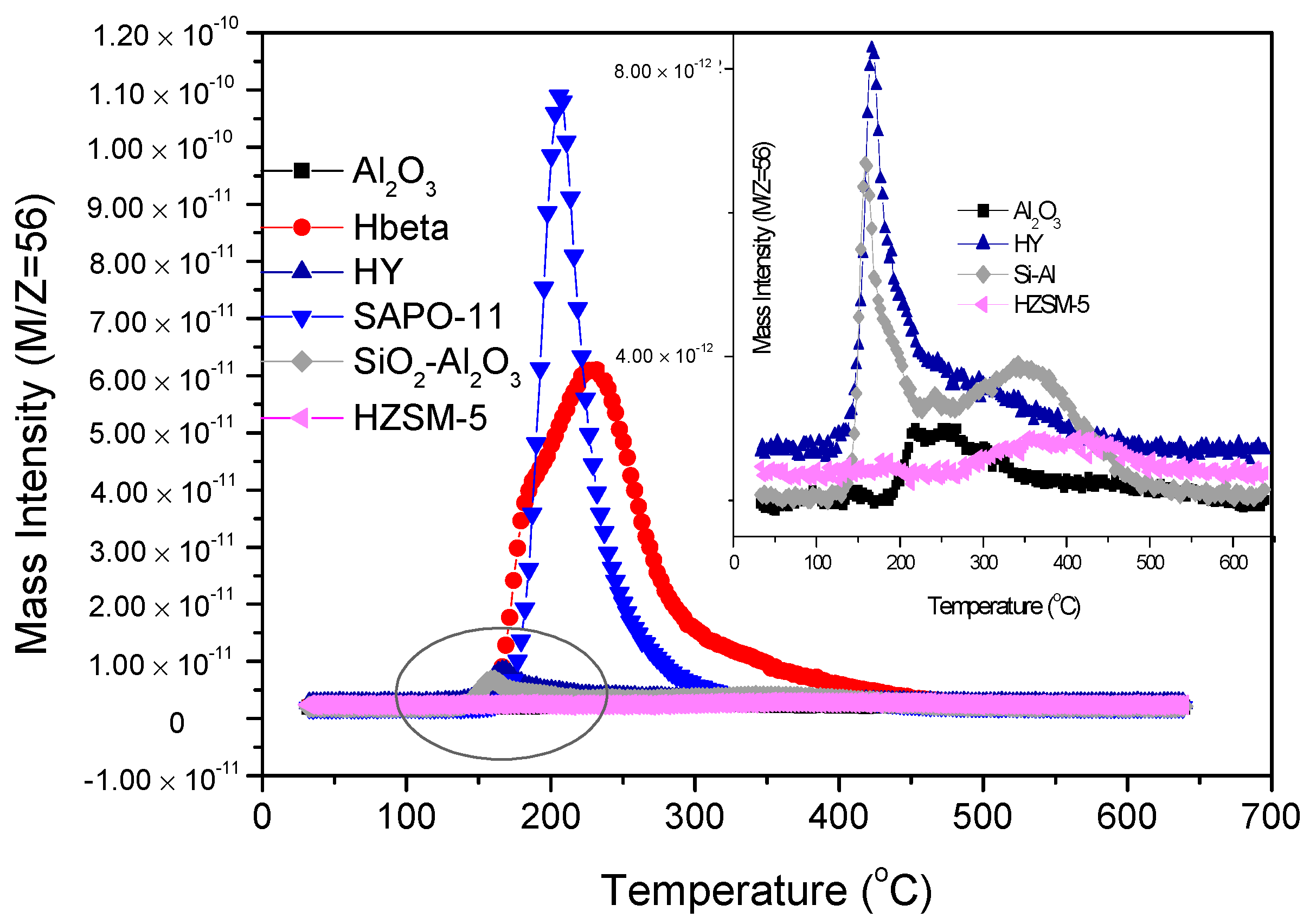
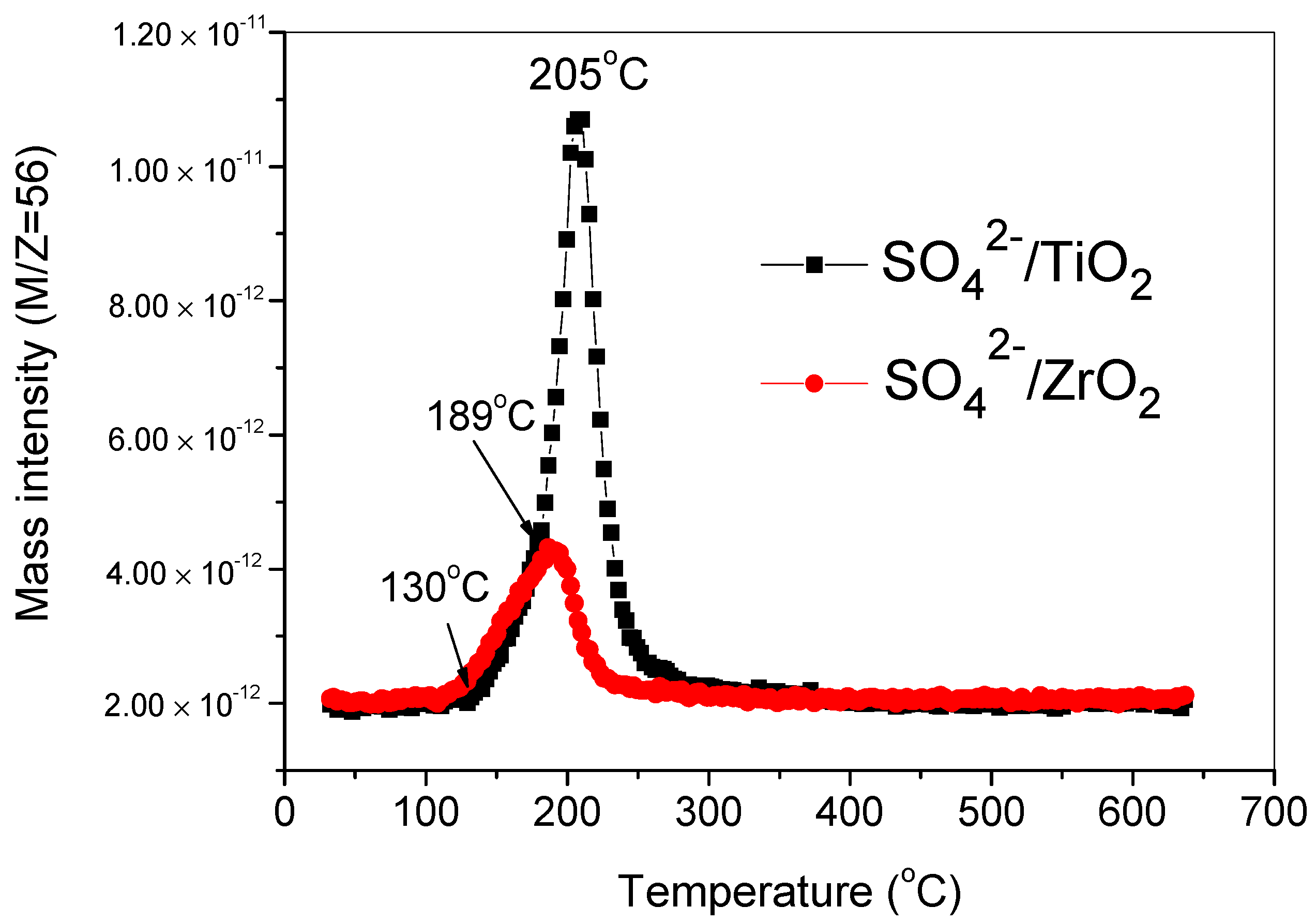
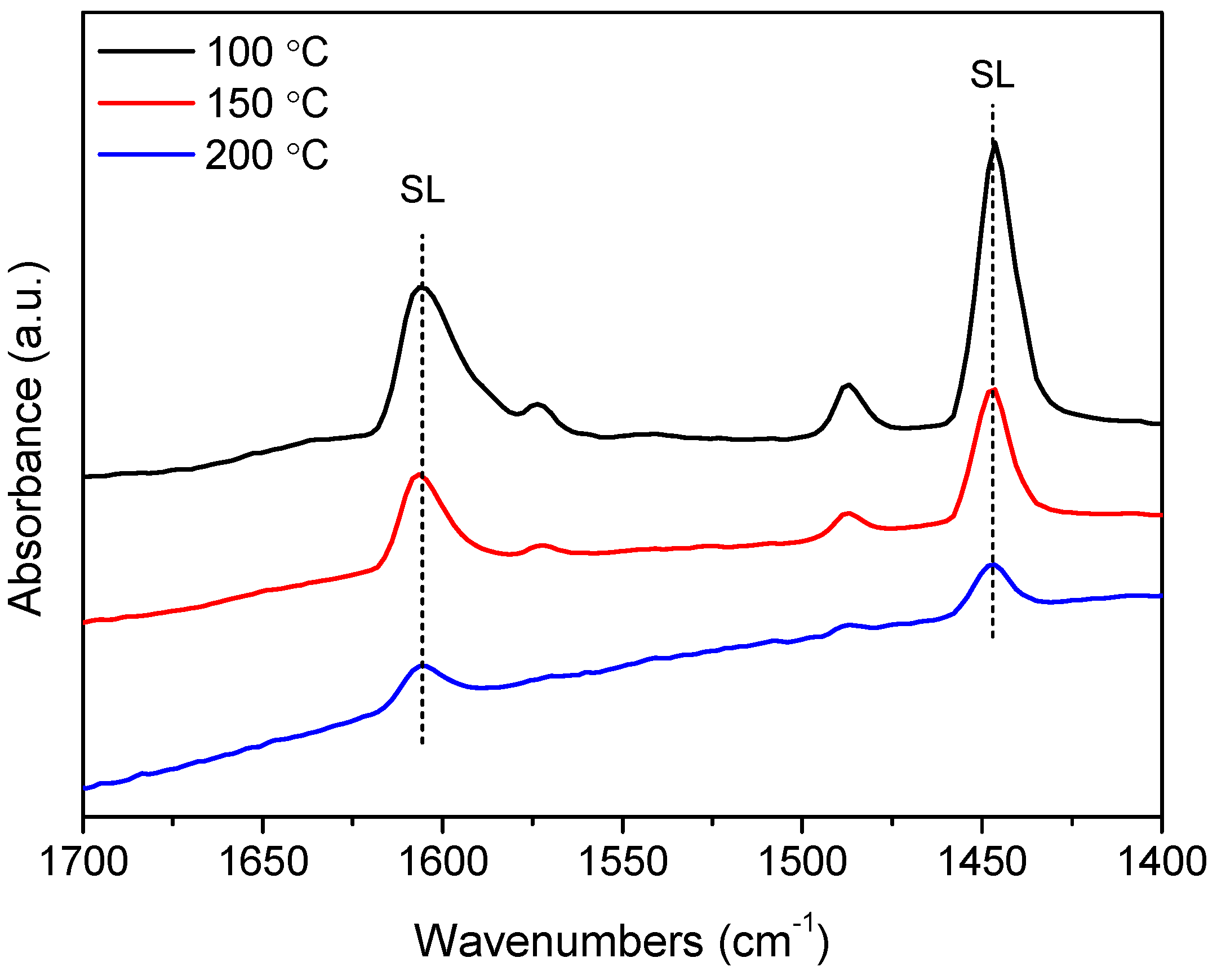
2.1. TPSR Results
2.2. Catalytic Performance of Different Catalysts and Fixed-Bed Reactions
3. Experimental
3.1. Catalysts
3.2. TPSR
3.3. Catalyst Activity
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Kim, Y.C.; Moon, D.J. Sustainable process for the synthesis of value-added products using glycerol as a useful raw material. Catal. Surv. Asia 2019, 23, 10–22. [Google Scholar] [CrossRef]
- Wang, Z.; Zhuge, J.; Fang, H.; Prior, B.A. Glycerol production by microbial fermentation: A review. Biotechnol. Adv. 2001, 19, 201–223. [Google Scholar] [CrossRef]
- Adeniyi, A.G.; Ighalo, J.O. A review of steam reforming of glycerol. Chem. Pap. 2019, 73, 2619–2635. [Google Scholar] [CrossRef]
- Corma, A.; Huber, G.W.; Sauvanaud, L.; O’Connor, P. Biomass to chemicals: Catalytic conversion of glycerol/water mixtures into acrolein, reaction network. J. Catal. 2008, 257, 163–171. [Google Scholar] [CrossRef]
- Katryniok, B.; Paul, S.; Belliere-Baca, V.; Rey, P.; Dumeignil, F. Glycerol dehydration to acrolein in the context of new uses of glycerol. Green Chem. 2010, 12, 2079–2098. [Google Scholar] [CrossRef]
- Saxena, R.K.; Anand, P.; Saran, S.; Isar, J. Microbial production of 1,3-propanediol: Recent developments and emerging opportunities. Biotechnol. Adv. 2009, 27, 895–913. [Google Scholar] [CrossRef]
- Chai, S.H.; Wang, H.P.; Liang, Y.; Xu, B.Q. Sustainable production of acrolein: Investigation of solid acid–base catalysts for gas-phase dehydration of glycerol. Green Chem. 2007, 9, 1130–1136. [Google Scholar] [CrossRef]
- Tao, L.Z.; Chai, S.H.; Zuo, Y.; Zheng, W.T.; Liang, Y.; Xu, B.Q. Sustainable production of acrolein: Acidic binary metal oxide catalysts for gas-phase dehydration of glycerol. Catal. Today 2010, 158, 310–316. [Google Scholar] [CrossRef]
- Tsukuda, E.; Sato, S.; Takahashi, R.; Sodesawa, T. Production of acrolein from glycerol over silica-supported heteropoly acids. Catal. Commun. 2007, 8, 1349–1353. [Google Scholar] [CrossRef]
- Qureshi, B.A.; Lan, X.; Arslan, M.T.; Wang, T. Highly active and selective nano H-ZSM-5 catalyst with short channels along b-Axis for glycerol dehydration to acrolein. Ind. Eng. Chem. Res. 2019, 58, 12611–12622. [Google Scholar] [CrossRef]
- Kobayashi, H.; Ito, S.; Hara, K.; Fukuoka, A. Conversion of glycerol to acrolein by mesoporous sulfated zirconia-silica catalyst. Chin. J. Catal. 2017, 38, 420–425. [Google Scholar] [CrossRef]
- Gambaro, L.A.; Briand, L.E. In situ quantification of the active acid sites of H6P2W18O62 center dot nH2O heteropoly-acid through chemisorption and temperature programmed surface reaction of isopropanol. Appl. Catal. A Gen. 2004, 264, 151–159. [Google Scholar] [CrossRef]
- Wang, H.; Liu, Z.M.; Shen, J.H.; Liu, H.C.; Zhang, J.L. High-throughput screening of HZSM-5 supported metal-oxides catalysts for the coupling of methane with CO to benzene and naphthalene. Catal. Commun. 2005, 6, 343–346. [Google Scholar] [CrossRef]
- Sakakini, B.H.; Verbrugge, A.S. Temperature-programmed surface reaction as a means of characterizing supported-metal catalysts and probing their surface reactivity. J. Chem. Soc. Trans. 1997, 93, 1637–1640. [Google Scholar] [CrossRef]
- Atia, H.; Armbruster, U.; Martin, A. Dehydration of glycerol in gas phase using heteropolyacid catalysts as active compounds. J. Catal. 2008, 258, 71–82. [Google Scholar] [CrossRef]
- Zhao, H.; Zhou, C.H.; Wu, L.M.; Lou, J.Y.; Li, N.; Yang, H.M.; Tong, D.S.; Yu, W.H. Catalytic dehydration of glycerol to acrolein over sulfuric acid-activated montmorillonite catalysts. Appl. Clay Sci. 2013, 74, 154–162. [Google Scholar] [CrossRef]
- Liu, Q.; Cao, X.F.; Wang, T.J.; Wang, C.G.; Zhang, Q.; Ma, L.L. Synthesis of shape-controllable cobalt nanoparticles and their shape-dependent performance in glycerol hydrogenolysis. RSC Adv. 2015, 5, 4861–4871. [Google Scholar] [CrossRef]
- Castle, L.W.; Gross, M.L. The cycloaddition of the 1,3-butadiene radical cation with acrolein and methyl vinyl ketone. Org. Mass Spectrom. 1989, 24, 637–646. [Google Scholar] [CrossRef]
- Sherrill, A.B.; Idriss, H.; Barteau, M.A.; Chen, J.G. Adsorption and reaction of acrolein on titanium oxide single crystal surfaces: Coupling versus condensation. Catal. Today 2003, 85, 321–331. [Google Scholar] [CrossRef]
- Berteau, P.; Delmon, B. Modified aluminas: Relationship between activity in 1-butanol dehydration and acidity measured by NH3-TPD. Catal. Today 1989, 5, 121–137. [Google Scholar] [CrossRef]
- Varisli, D.; Dogu, T.; Dogu, G. Ethylene and diethyl-ether production by dehydration reaction of ethanol over different heteropolyacid catalysts. Chem. Eng. Sci. 2007, 62, 5349–5352. [Google Scholar] [CrossRef]
- Bertero, N.M.; Trasarti, A.F.; Apesteguía, C.R.; Marchi, A.J. Liquid-phase dehydration of 1-phenylethanol on solid acids: Influence of catalyst acidity and pore structure. Appl. Catal. A Gen. 2013, 458, 28–38. [Google Scholar] [CrossRef]
- Wang, X.C.; Song, Y.J.; Huang, L.; Wang, H.; Huang, C.P.; Li, C.Q. Tin Modified Nb2O5 as an efficient solid acid catalyst for the catalytic conversion of triose sugars to lactic acid. Catal. Sci. Technol. 2019, 9, 1669–1679. [Google Scholar]
- Ammari, F.; Milone, C.; Touroude, R. Selective hydrogenation of crotonaldehyde on Pt/ZnCl2/SiO2 catalysts. J. Catal. 2005, 235, 1–9. [Google Scholar] [CrossRef]
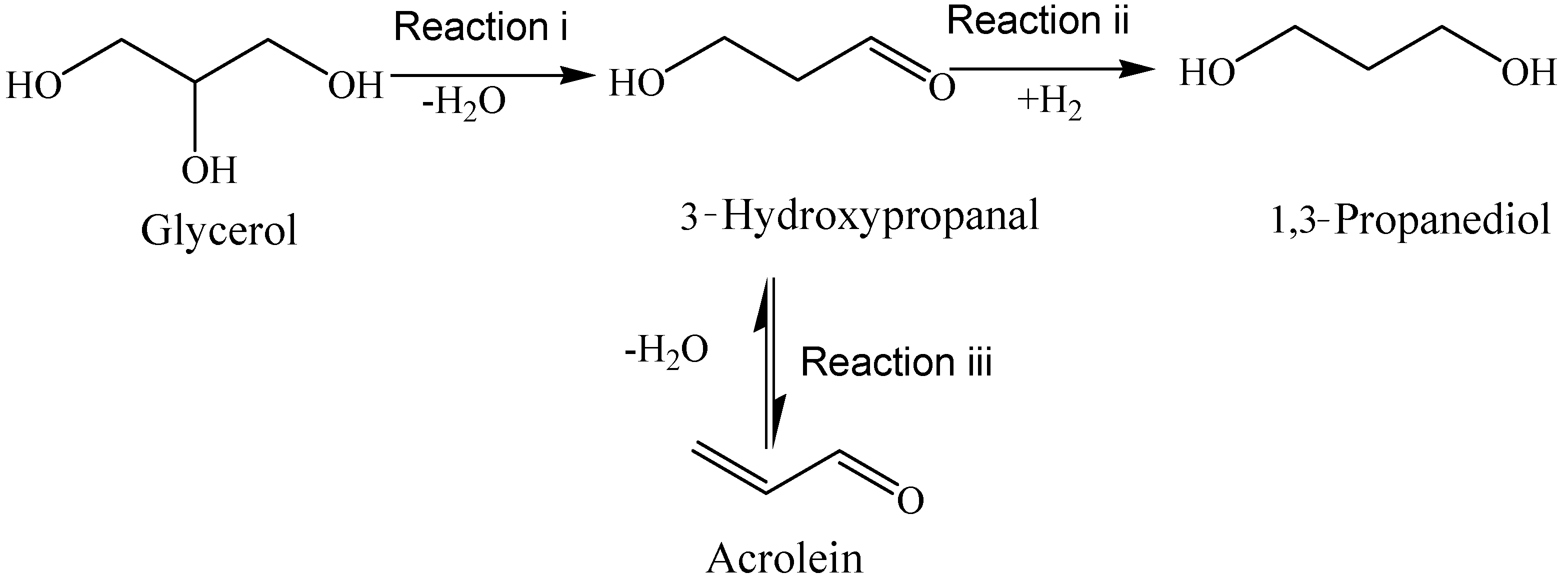

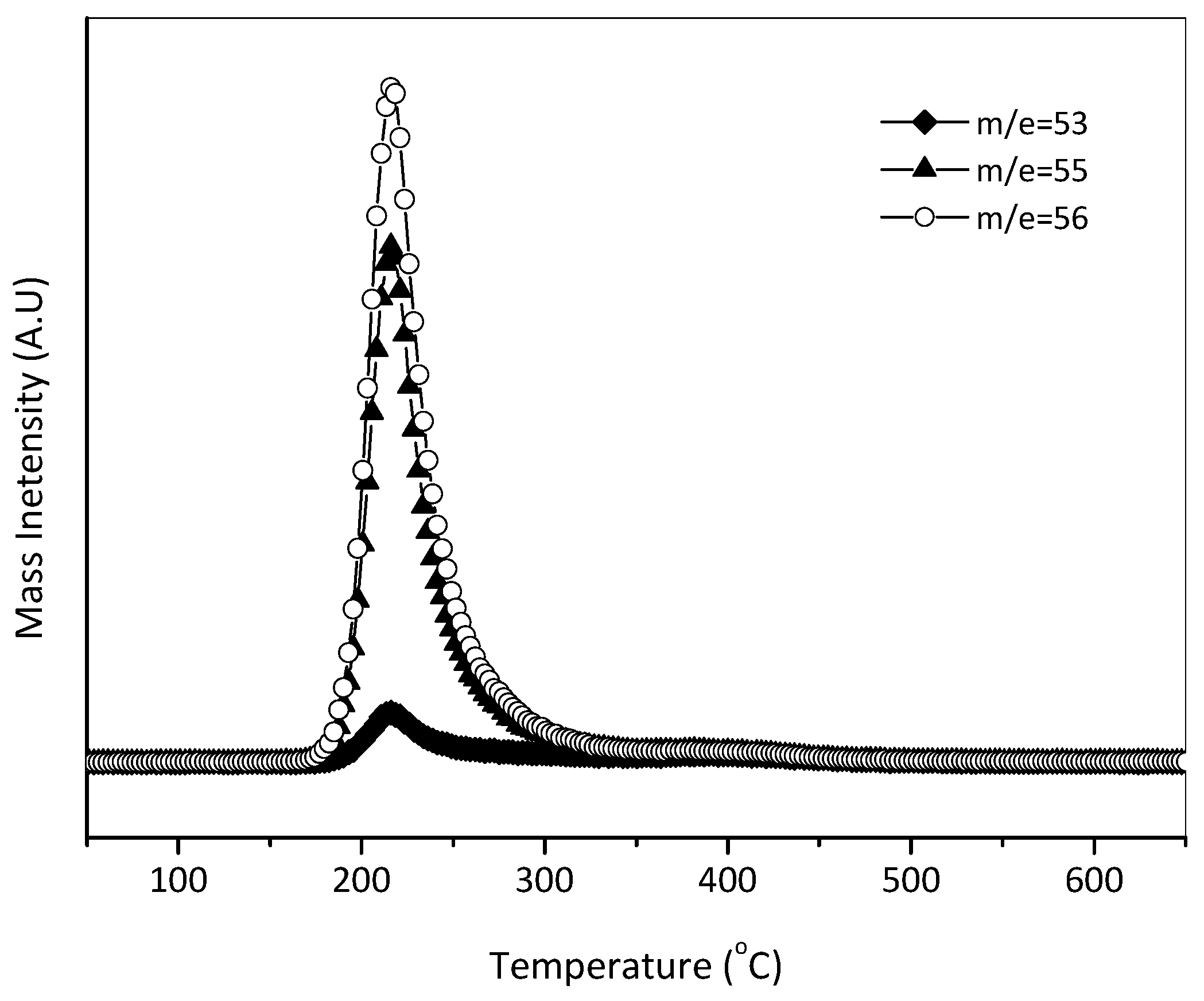
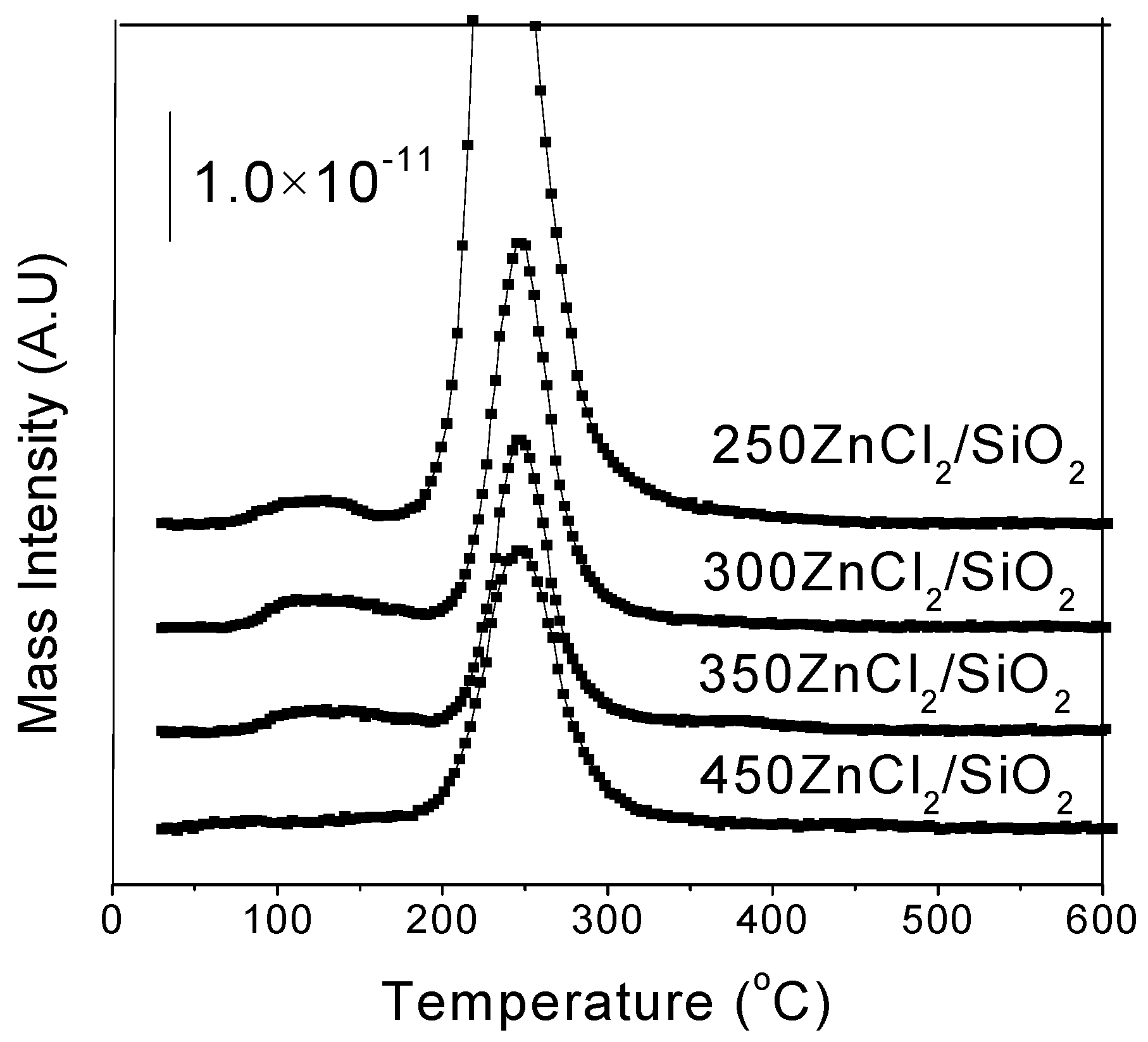
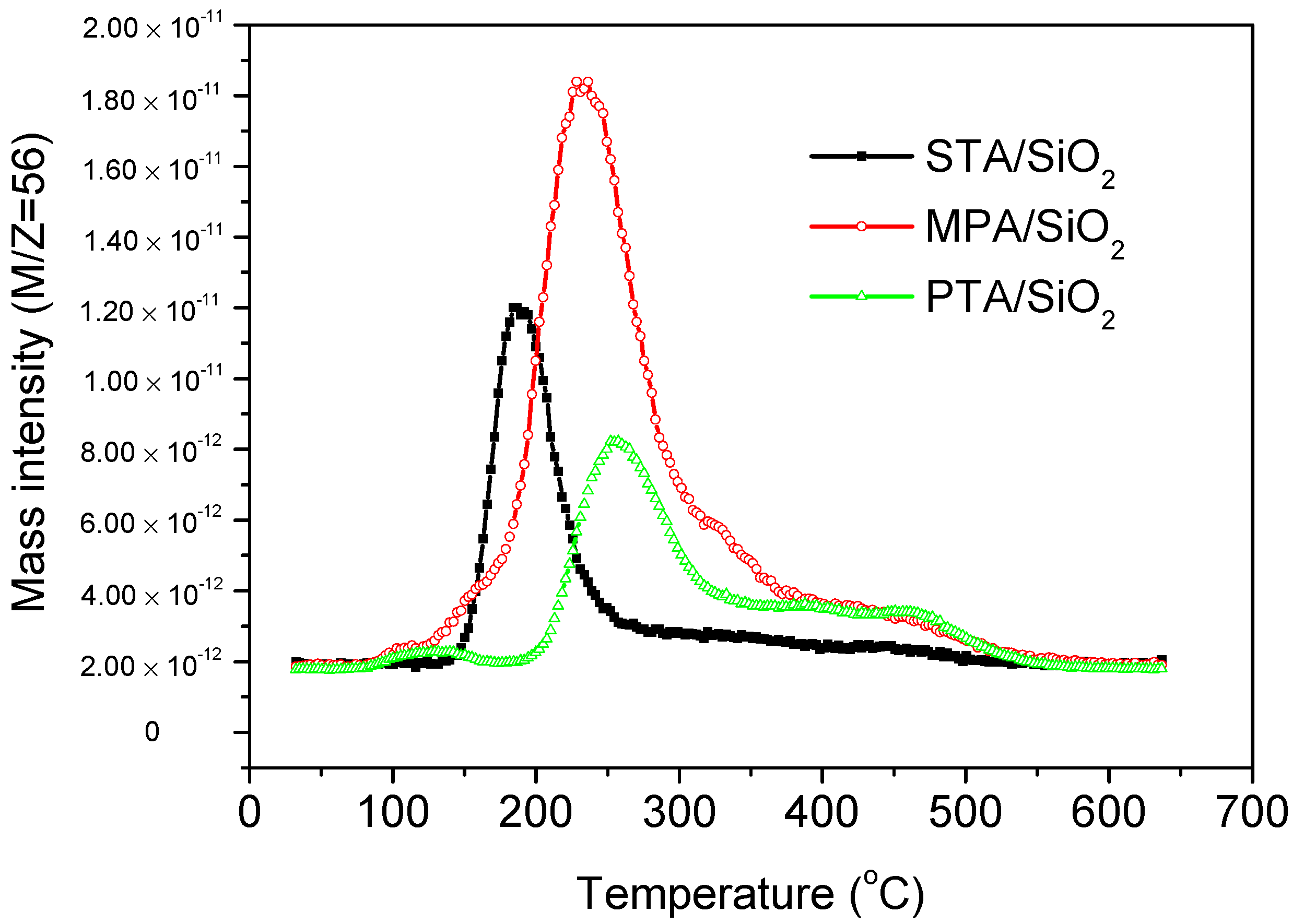
| Substance | Molecular Weight | Main Fragment Ions (Relative Intensity) |
|---|---|---|
| Glycerol | 92 | 61(100), 44(43), 43(78), 31(39), 29(30) |
| Acrolein | 56 | 56(100), 55(72), 53(11), 29(56) |
| 3-Hydroxypropanal | 74 | 45, 44, 30, 29 |
| Acetol | 74 | 43(100), 31(17) |
| Acetaldehyde | 44 | 44(83), 43(47), 29(100) |
| Formaldehyde | 30 | 30(58), 29(100), 28(24) |
| Glycidol | 74 | 44(100), 43(90), 31(59), 29(42), 28(44) |
| Water | 18 | 18(100), 17(21) |
| CO2 | 44 | 44(100) |
| CO | 28 | 28(100) |
| CH4 | 16 | 16(100), 15(89), 14(20) |
| Group | Catalysts | Peak 1 | Peak 2 | Peak 3 | |||
|---|---|---|---|---|---|---|---|
| Range | Maximum | Range | Maximum | Range | Maximum | ||
| Heteropolyacid | PW/SiO2 | 75–162 | 120 | 170–351 | 245 | 322–507 | 376 |
| SiW/SiO2 | 141–261 | 189 | |||||
| PMo/SiO2 | 85–320 | 231 | 224–409 | 312 | |||
| Zeolites/Al2O3 | SAPO-11 | 165 | 220 | ||||
| HZSM-5 | 275 | 400 | |||||
| Al2O3 | 190–320 | 260 | |||||
| SiO2-Al2O3 | 145–260 | 175 | 223–460 | 341 | |||
| HY | 116–237 | 160 | 150–414 | 250 | |||
| Hβ | 180–207 | 195 | 180 | 235 | 180 | 305 | |
| Lewis acid | 250ZnCl2/SiO2 | 72–160 | 115 | 183–315 | 216 | ||
| 300ZnCl2/SiO2 | 73–170 | 119 | 185–310 | 220 | |||
| 350ZnCl2/SiO2 | 175–306 | 220 | |||||
| 450ZnCl2/SiO2 | 183–319 | 220 | |||||
| Super acid | SO42−/TiO2 | 129–265 | 205 | ||||
| SO42−/ZrO2 | 126–233 | 190 | |||||
| Catalyst | Conv. (%) | Yield (%) | Selectivity (%) | |||
|---|---|---|---|---|---|---|
| Acrolein | Acetol | Acetaldehyde | Others | |||
| 30STA/SiO2 | 65.5 | 58.1 | 88.7 | 2.6 | 1.8 | 6.6 |
| 66.3 | 59.6 | 89.9 | 1.3 | 2.2 | 5.8 | |
| - | 44.8 | - | - | - | - | |
| - | 17.4 | - | - | - | - | |
| HY | - | 17.0 | - | - | - | - |
| SO4/TiO2 | 86.9 | 76.7 | 88.2 | 6.2 | 0.5 | 5.1 |
| 77.4 | 64.4 | 83.2 | 6.3 | 0.5 | 10.0 | |
| - | 15.6 | - | - | - | - | |
| SO42−/ZrO2 | - | 5.1 | - | - | - | - |
| 250ZnCl2/SiO2 | 33.1 | 25.1 | 75.8 | |||
| 300ZnCl2/SiO2 | 42.1 | 36.9 | 87.6 | |||
| 350ZnCl2/SiO2 | 54.9 | 48.3 | 88.0 | |||
| 350ZnCl2/SiO2 * | 94.4 | 84.1 | 89.1 | |||
| 450ZnCl2/SiO2 | 40.9 | 35.3 | 86.3 | |||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Zhao, F.; Huang, L. Low Temperature Dehydration of Glycerol to Acrolein in Vapor Phase with Hydrogen as Dilution: From Catalyst Screening via TPSR to Real-Time Reaction in a Fixed-Bed. Catalysts 2020, 10, 43. https://doi.org/10.3390/catal10010043
Wang X, Zhao F, Huang L. Low Temperature Dehydration of Glycerol to Acrolein in Vapor Phase with Hydrogen as Dilution: From Catalyst Screening via TPSR to Real-Time Reaction in a Fixed-Bed. Catalysts. 2020; 10(1):43. https://doi.org/10.3390/catal10010043
Chicago/Turabian StyleWang, Xincheng, Fenghe Zhao, and Long Huang. 2020. "Low Temperature Dehydration of Glycerol to Acrolein in Vapor Phase with Hydrogen as Dilution: From Catalyst Screening via TPSR to Real-Time Reaction in a Fixed-Bed" Catalysts 10, no. 1: 43. https://doi.org/10.3390/catal10010043




