Photocatalytic H2 Evolution, CO2 Reduction, and NOx Oxidation by Highly Exfoliated g-C3N4
Abstract
:1. Introduction
2. Results and Discussion
2.1. Physicochemical Properties of Highly Exfoliated g-C3N4
2.1.1. Crystalline Structure
2.1.2. Morphology
2.1.3. Porosity and Specific Surface Area
2.1.4. Chemical Composition
2.1.5. Light Absorbance and Band Gap Characteristics
2.2. Active Species Formation
2.3. Photocatalytic Activity
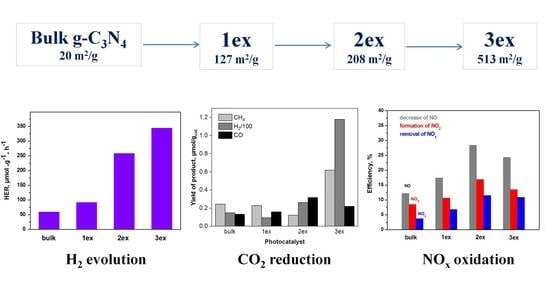
2.3.1. Photocatalytic H2 Production
2.3.2. Photocatalytic Reduction of CO2
2.3.3. Photocatalytic Oxidation of NOx
3. Materials and Methods
3.1. Sample Preparation
3.2. Characterization
3.3. Photocatalytic Activity Evaluation
3.3.1. Photocatalytic H2 Evolution
3.3.2. Photocatalytic CO2 Reduction
3.3.3. Photocatalytic NOx Oxidation
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Li, X.; Xiong, J.; Gao, X.; Huang, J.; Feng, Z.; Chen, Z.; Zhu, Y. Recent advances in 3D g-C3N4 composite photocatalysts for photocatalytic water splitting, degradation of pollutants and CO2 reduction. J. Alloys Comp. 2019, 802, 196–209. [Google Scholar] [CrossRef]
- Christoforidis, K.C.; Fornasiero, P. Photocatalytic Hydrogen production: A rift into the future energy supply. Chem. Cat. Chem. 2017, 9, 1523–1544. [Google Scholar] [CrossRef] [Green Version]
- Fu, J.; Xu, Q.; Low, J.; Jiang, C.; Yu, J. Ultrathin 2D/2D WO3/g-C3N4 step-scheme H2-production photocatalyst. Appl. Catal. B Environ. 2019, 243, 556–565. [Google Scholar] [CrossRef]
- Cao, S.; Yu, J. g-C3N4-Based Photocatalysts for Hydrogen Generation. J. Phys. Chem. Lett. 2014, 5, 2101–2107. [Google Scholar] [CrossRef]
- Cheng, J.; Hu, Z.; Li, Q.; Li, X.; Fang, S.; Wu, X.; Li, M.; Ding, Y.; Liu, B.; Yang, C.; et al. Fabrication of high photoreactive carbon nitride nanosheets by polymerization of amidinourea for hydrogen production. Appl. Catal. B Environ. 2019, 245, 197–206. [Google Scholar] [CrossRef]
- Shehzad, N.; Tahir, M.; Johari, K.; Murugesan, T.; Hussain, M. A critical review on TiO2based photocatalytic CO2reductionsystem: Strategiestoimprove efficiency. J. CO2 Util. 2018, 26, 98–122. [Google Scholar] [CrossRef]
- Abdullah, H.; Khan, M.M.R.; Ong, H.R.; Yaakob, Z. Modified TiO2photocatalyst for CO2 photocatalytic reduction: An overview. J. CO2 Util. 2017, 22, 15–32. [Google Scholar] [CrossRef]
- Lingampalli, S.R.; Ayyub, M.M.; Rao, C.N.R. Recent Progress in the Photocatalytic Reduction of Carbon Dioxide. ACS Omega 2017, 2, 2740–2748. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.; Tan, Q.; Li, Q.; Zhou, J.; Fan, J.; Li, B.; Sun, J.; Lv, K. 2D/2D Ti3C2MXene/g-C3N4nanosheetsheterojunction for high efficient CO2 reduction photocatalyst: Dual effects of urea. Appl. Catal. B Environ. 2020, 268, 118738. [Google Scholar] [CrossRef]
- Cao, S.; Low, J.; Yu, J.; Jaroniec, M. Polymeric Photocatalysts Based on Graphitic Carbon Nitride. Adv. Mater. 2015, 27, 2150–2176. [Google Scholar] [CrossRef]
- Prasad, C.; Tang, H.; Bahadur, I. Graphitic carbon nitride based ternary nanocomposites: From synthesis to their applications in photocatalysis: A recent review. J. Mol. Liq. 2019, 281, 634–654. [Google Scholar] [CrossRef]
- Shayegan, Z.; Lee, C.-S.; Haghighat, F. TiO2photocatalyst for removal of volatile organic compounds in gas phase—A review. Chem. Eng. J. 2018, 334, 2408–2439. [Google Scholar] [CrossRef]
- Fu, J.; Yu, J.; Jiang, C.; Cheng, B. g-C3N4-Based Hetero structured Photocatalysts. Adv. Energy Mater. 2017, 1701503, 1–31. [Google Scholar]
- Kumar, S.; Karthikeyan, S.; Lee, A. g-C3N4-Based Nanomaterials for Visible Light-Driven Photocatalysis. Catalysts 2018, 8, 74. [Google Scholar] [CrossRef] [Green Version]
- Ma, J.; Wang, C.; He, H. Enhanced photocatalytic oxidation of NO over g-C3N4-TiO2 under UV and visible light. Appl. Catal. B Environ. 2016, 184, 28–34. [Google Scholar] [CrossRef] [Green Version]
- Papailias, I.; Todorova, N.; Giannakopoulou, T.; Yu, J.; Dimotikali, D.; Trapalis, C. Photocatalytic activity of modified g-C3N4/TiO2 nanocomposites for NOx removal. Catalysis Today 2017, 280, 37–44. [Google Scholar] [CrossRef]
- Wen, J.; Xie, J.; Chen, X.; Li, X. A review on g-C3N4-based photocatalysts. Appl. Surf. Sci. 2017, 391 Pt B, 72–123. [Google Scholar] [CrossRef]
- Ma, Y.; Liu, E.; Hu, X.; Tang, C.; Wan, J.; Li, J.; Fan, J. A simple process to prepare few-layer g-C3N4nanosheets with enhanced photocatalytic activities. Appl. Surf. Sci. 2015, 358, 246–251. [Google Scholar] [CrossRef]
- Papailias, I.; Todorova, N.; Giannakopoulou, T.; Ioannidis, N.; Boukos, N.; Athanasekou, C.P.; Dimotikali, D.; Trapalis, C. Chemical vs thermal exfoliation of g-C3N4 for NOx removal under visible light irradiation. Appl. Catal. B Environ. 2018, 239, 16–26. [Google Scholar] [CrossRef]
- Dong, F.; Sun, Y.J.; Wu, L.W.; Fu, M.; Wu, Z. Facile transformation of low cost thiourea into nitrogen-rich graphitic carbon nitride nanocatalyst with high visible light photocatalytic performance. Catal. Sci. Technol. 2012, 2, 1332–1335. [Google Scholar] [CrossRef]
- Li, Y.; Wang, M.-Q.; Bao, S.-J.; Lu, S.; Xu, M.; Long, D.; Pu, S. Tuning and thermal exfoliation graphene-like carbon nitride nanosheets for superior photocatalytic activity. Ceramics Int. 2016, 42, 18521–18528. [Google Scholar] [CrossRef]
- Cheng, J.; Hu, Z.; Lv, K.; Wu, X.; Li, Q.; Li, Y.; Li, X.; Sun, J. Drastic promoting the visible photoreactivity of layered carbon nitride by polymerization of dicyandiamide at high pressure. Appl. Catal. B Environ. 2018, 232, 330–339. [Google Scholar] [CrossRef]
- Han, D.; Liu, J.; Cai, H.; Zhou, X.; Kong, L.; Wang, J.; Shi, H.; Guo, Q.; Fan, X. High-yield and low-cost method to synthesize large-area porous g-C3N4nanosheets with improved photocatalytic activity for gaseous nitric oxide and 2-propanol photodegradation. Appl. Surf. Sci. 2019, 464, 577–585. [Google Scholar] [CrossRef]
- Papailias, I.; Giannakopoulou, T.; Todorova, N.; Dimotikali, D.; Vaimakis, T.; Trapalis, C. Effect of processing temperature on structure and photocatalytic properties of g-C3N4. Appl. Surf. Sci. 2015, 358, 278–286. [Google Scholar] [CrossRef]
- Zhao, Z.; Sun, Y.; Luo, Q.; Dong, F.; Li, H.; Ho, W.-K. Mass-Controlled Direct Synthesis of Graphene-like Carbon Nitride Nanosheets with Exceptional High Visible Light Activity. Less is Better. Sci. Rep. 2015, 5, 14643. [Google Scholar] [CrossRef]
- Zhu, K.; Lv, Y.; Liu, J.; Wang, W.; Wang, C.; Wang, P.; Meng, A.; Li, Z.; Li, Q. Explosive thermal exfoliation of intercalated graphitic carbon nitride for enhanced photocatalytic degradation properties. Ceram. Int. 2019, 45, 3643–3647. [Google Scholar] [CrossRef]
- Wu, X.; Cheng, J.; Li, X.; Li, Y.; Lv, K. Enhanced visible photocatalytic oxidation of NO by repeated calcination of g-C3N4. Appl. Surf. Sci. 2019, 465, 1037–1046. [Google Scholar] [CrossRef]
- She, X.; Wu, J.; Zhong, J.; Xu, H.; Yang, Y.; Vajtai, R.; Lou, J.; Liu, Y.; Du, D.; Li, H.; et al. Oxygenated monolayer carbon nitride for excellent photocatalytic Hydrogen evolution and external quantum efficiency. Nano Energy 2016, 27, 138–146. [Google Scholar] [CrossRef]
- Niu, P.; Zhang, L.; Liu, G.; Cheng, H.-M. Graphene-Like Carbon Nitride Nanosheets for Improved Photocatalytic Activities. Adv. Funct. Mater. 2012, 22, 4763–4770. [Google Scholar] [CrossRef]
- Zhao, H.; Yu, H.; Quan, X.; Chen, S.; Zhang, Y.; Zhao, H.; Wang, H. Fabrication of atomic single layer graphitic-C3N4 and its high performance of photocatalytic disinfection under visible light irradiation. Appl. Catal. B Environ. 2014, 152–153, 46–50. [Google Scholar] [CrossRef]
- Song, X.; Yang, Q.; Jiang, X.; Yin, M.; Zhou, L. Porous graphitic carbon nitride nanosheets prepared under self-producing atmosphere for highly improved photocatalytic activity. Appl. Catal. B Environ. 2017, 217, 322–330. [Google Scholar] [CrossRef]
- Zhu, B.; Xia, P.; Ho, W.K.; Yu, J.G. Isoelectric point and adsorption activity of porous g-C3N4. Appl. Surf. Sci. 2015, 344, 188–195. [Google Scholar] [CrossRef]
- Cao, Y.; Lia, Q.; Wang, W. Construction of a crossed-layer-structure MoS2/g-C3N4heterojunction with enhanced photocatalytic performance. RSC Adv. 2017, 7, 6131–6139. [Google Scholar] [CrossRef] [Green Version]
- Jürgens, B.; Irran, E.; Senker, J.; Kroll, P.; Müller, H.; Schnick, W. Melem (2,5,8-triamino-tri-s-triazine), an important intermediate during condensation of melamine rings to graphitic carbon nitride: Synthesis, structure determination by X-ray powder diffraction, solid-state NMR, and theoretical studies. J. Am. Chem. Soc. 2003, 125, 10288–10300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas, A.; Fischer, A.; Goettmann, F.; Antonietti, M.; Mueller, J.O.; Schloegl, R.; Carlsson, J.M. Graphitic carbon nitride materials: Variation of structure and morphology and their use as metal-free catalysts. J. Mater. Chem. 2008, 18, 4893–4908. [Google Scholar] [CrossRef] [Green Version]
- Dong, F.; Wu, L.W.; Sun, Y.J.; Fu, M.; Wu, Z.B.; Lee, S.C. Efficient synthesis of polymeric g-C3N4 layered materials as novel efficient visible light driven photocatalysts. J. Mater. Chem. 2011, 21, 15171–15174. [Google Scholar] [CrossRef]
- Yuan, X.; Zhou, C.; Jin, Y.; Jing, Q.; Yang, Y.; Shen, X.; Tang, Q.; Mu, Y.; Du, A.-K. Facile synthesis of 3D porous thermally exfoliated g-C3N4nanosheet with enhanced photocatalytic degradation of organic dye. J. Colloid Interface Sci. 2016, 468, 211–219. [Google Scholar] [CrossRef] [Green Version]
- Nosaka, Y.; Nosaka, A.Y. Identification and roles of the active species generated on various photocatalysts. In Photocatalysis and Water Purification: From Fundamentals to Recent Applications; Pichat, P., Ed.; Wiley-VCH: Weinheim, Germany, 2013; pp. 3–24. [Google Scholar]
- Brezova, V.; Gabcova, S.; Dvoranova, D.; Stasko, A. Reactive oxygen species produced upon photoexcitation of sunscreens containing titanium dioxide (An EPR study). J. Photochem. Photobiol. B Biol. 2005, 79, 121–134. [Google Scholar] [CrossRef]
- Toumazatou, A.; Antoniadou, M.; Sakellis, E.; Tsoutsou, D.; Gardelis, S.; Romanos, G.E.; Ioannidis, N.; Boukos, N.; Dimoulas, A.; Falaras, P.; et al. Boosting visible light harvesting and charge separation in surface modified TiO2 photonic crystal catalysts with CoOx nanoclusters. Mater. Adv. 2020. [Google Scholar] [CrossRef]
- Dvoranová, D.; Mazúr, M.; Papailias, I.; Giannakopoulou, T.; Trapalis, C.; Brezová, V. EPR Investigations of G-C3N4/TiO2Nanocomposites. Catalysts 2018, 8, 47. [Google Scholar] [CrossRef] [Green Version]
- Schneider, J.; Bahnemann, D.W. Undesired Role of Sacrificial Reagents in Photocatalysis. J. Phys. Chem. Lett. 2013, 4, 3479–3483. [Google Scholar] [CrossRef]
- Papailias, I.; Todorova, N.; Giannakopoulou, T.; Ioannidis, N.; Dallas, P.; Dimotikali, D.; Trapalis, C. Novel torus shaped g-C3N4photocatalysts. Appl. Catal. B Environ. 2020, 268, 118733. [Google Scholar] [CrossRef]
- Chang, F.; Zhang, J.; Xie, Y.; Chen, J.; Li, C.; Wang, J.; Luo, J.; Deng, B.; Hu, X. Fabrication, characterization, and photocatalytic performance of exfoliated g-C3N4–TiO2 hybrids. Appl. Surf. Sci. 2014, 311, 574–581. [Google Scholar] [CrossRef]
- Zang, M.; Shi, L.; Liang, L.; Li, D.; Sun, J. Heterostructured g-C3N4/Ag–TiO2 composites with efficient photocatalytic performance under visible-light irradiation. J. RSC Adv. 2015, 5, 56136–56144. [Google Scholar] [CrossRef]
- Koci, K.; Reli, M.; Kozak, O.; Lacny, Z.; Placha, D.; Praus, P.; Obalova, L. Influence of reactor geometry on the yield of CO2 photocatalytic reduction. Catal. Today 2011, 176, 212–214. [Google Scholar] [CrossRef]
- Tasbihi, M.; Koci, K.; Edelmannova, M.; Troppova, I.; Reli, M.; Schomacker, R. Pt/TiO2photocatalysts deposited on commercial support for photocatalytic reduction of CO2. J. Photochem. Photobiol. A Chem. 2018, 366, 72–80. [Google Scholar] [CrossRef]
- Todorova, N.; Giannakopoulou, T.; Karapati, S.; Petridis, D.; Vaimakis, T.; Trapalis, C. Composite TiO2/clays materials for photocatalytic NOx oxidation. Appl. Surf. Sci. 2014, 319, 113–120. [Google Scholar] [CrossRef]
- Ou, M.; Wan, S.; Zhong, Q.; Zhang, S.; Song, Y.; Guo, L.; Cai, W.; Xu, Y. Hierarchical Z-scheme photocatalyst of g-C3N4@Ag/BiVO4 (040) with enhanced visible-light-induced photocatalytic oxidation performance. Appl. Catal. B Environ. 2018, 221, 97–107. [Google Scholar] [CrossRef]
- Dong, F.; Li, Q.; Sun, Y.; Ho, W.K. Noble metal-like behavior of plasmonic Bi particles as a cocatalyst deposited on (BiO)2CO3 microspheres for efficient visible light photocatalysis. ACS Catal. 2014, 4, 4341–4350. [Google Scholar] [CrossRef]
- Giannakopoulou, T.; Papailias, I.; Todorova, N.; Boukos, N.; Liu, Y.; Yu, J.; Trapalis, C. Tailoring the energy band gap and edges’ potentials of g-C3N4/TiO2 composite photocatalysts for NOx removal. Chem. Eng. J. 2017, 310, 571–580. [Google Scholar] [CrossRef]


















| Sample | d (nm) | SSABET (m2/g) | Vp (cm3/g) | Sp (nm) | Pore Size Distribution |
|---|---|---|---|---|---|
| Bulk | 0.323 | 20 | 0.141 | 2 | Narrow |
| 1ex | 0.32 | 127 | 0.821 | 1.8 | Narrow |
| 2ex | 0.319 | 208 | 0.582 | 1.6 | Wide |
| 3ex | 0.318 | 513 | 1.952 | 1.7 | Wide |
| Sample | XPS | Elemental Analysis | |||||
|---|---|---|---|---|---|---|---|
| C (at.%) | N (at.%) | O (at.%) | C (%) | N (%) | H (%) | Atomic Ratio C/N | |
| Bulk | 43.37 | 52.11 | 4.52 | 33.81 | 62.36 | 0.75 | 0.6325 |
| 1ex | 44.14 | 52.23 | 3.62 | 34.2 | 63.25 | 0.77 | 0.6305 |
| 2ex | 45.05 | 51.24 | 3.71 | 34.32 | 63.71 | 0.93 | 0.6286 |
| 3ex | 44.31 | 51.22 | 4.46 | 34.63 | 62.41 | 0.8 | 0.648 |
| Sample | Band Gap Eg (eV) | Conduction Band Potential (V) | Valence Band Potential (V) | |
|---|---|---|---|---|
| vs. Ag/AgCl, pH 5.8 | vs. NHE, pH 0 | vs. NHE, pH 0 | ||
| Bulk | 2.70 | −1.43 | −0.88 | 1.82 |
| 1ex | 2.82 | −1.38 | −0.83 | 1.99 |
| 2ex | 2.92 | −1.35 | −0.80 | 2.12 |
| 3ex | 3.04 | −1.32 | −0.77 | 2.27 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Todorova, N.; Papailias, I.; Giannakopoulou, T.; Ioannidis, N.; Boukos, N.; Dallas, P.; Edelmannová, M.; Reli, M.; Kočí, K.; Trapalis, C. Photocatalytic H2 Evolution, CO2 Reduction, and NOx Oxidation by Highly Exfoliated g-C3N4. Catalysts 2020, 10, 1147. https://doi.org/10.3390/catal10101147
Todorova N, Papailias I, Giannakopoulou T, Ioannidis N, Boukos N, Dallas P, Edelmannová M, Reli M, Kočí K, Trapalis C. Photocatalytic H2 Evolution, CO2 Reduction, and NOx Oxidation by Highly Exfoliated g-C3N4. Catalysts. 2020; 10(10):1147. https://doi.org/10.3390/catal10101147
Chicago/Turabian StyleTodorova, Nadia, Ilias Papailias, Tatiana Giannakopoulou, Nikolaos Ioannidis, Nikos Boukos, Panagiotis Dallas, Miroslava Edelmannová, Martin Reli, Kamila Kočí, and Christos Trapalis. 2020. "Photocatalytic H2 Evolution, CO2 Reduction, and NOx Oxidation by Highly Exfoliated g-C3N4" Catalysts 10, no. 10: 1147. https://doi.org/10.3390/catal10101147
APA StyleTodorova, N., Papailias, I., Giannakopoulou, T., Ioannidis, N., Boukos, N., Dallas, P., Edelmannová, M., Reli, M., Kočí, K., & Trapalis, C. (2020). Photocatalytic H2 Evolution, CO2 Reduction, and NOx Oxidation by Highly Exfoliated g-C3N4. Catalysts, 10(10), 1147. https://doi.org/10.3390/catal10101147